Divergent Regulation of Decidual Oxidative-Stress Response by NRF2 and KEAP1 in Preeclampsia with and without Fetal Growth Restriction
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Non-Enzymatic Antioxidant Capacity and Oxidative-Stress Levels in the Decidua
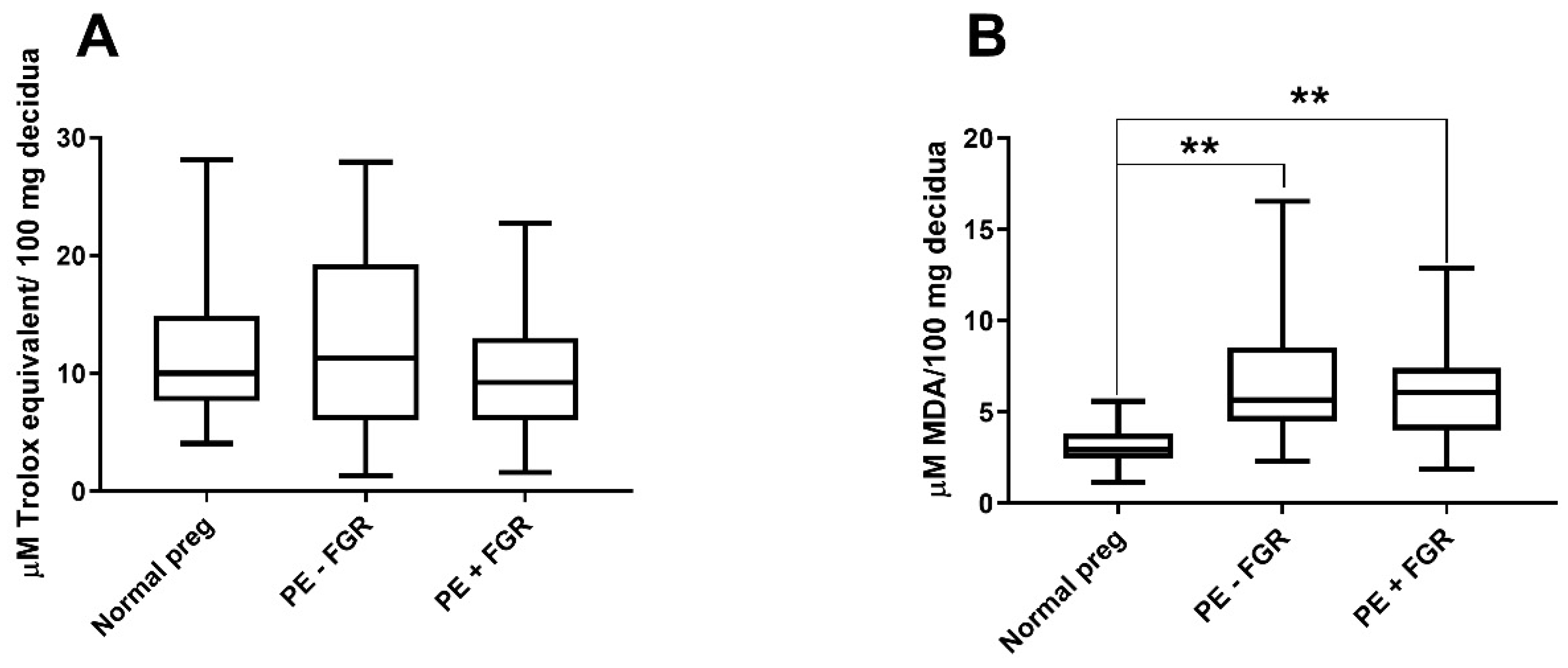
2.2.1. Non-Enzymatic Antioxidant Capacity in the Decidua
2.2.2. Decidual Oxidative-Stress Levels
2.3. NRF2-Regulated Transcriptional Activation
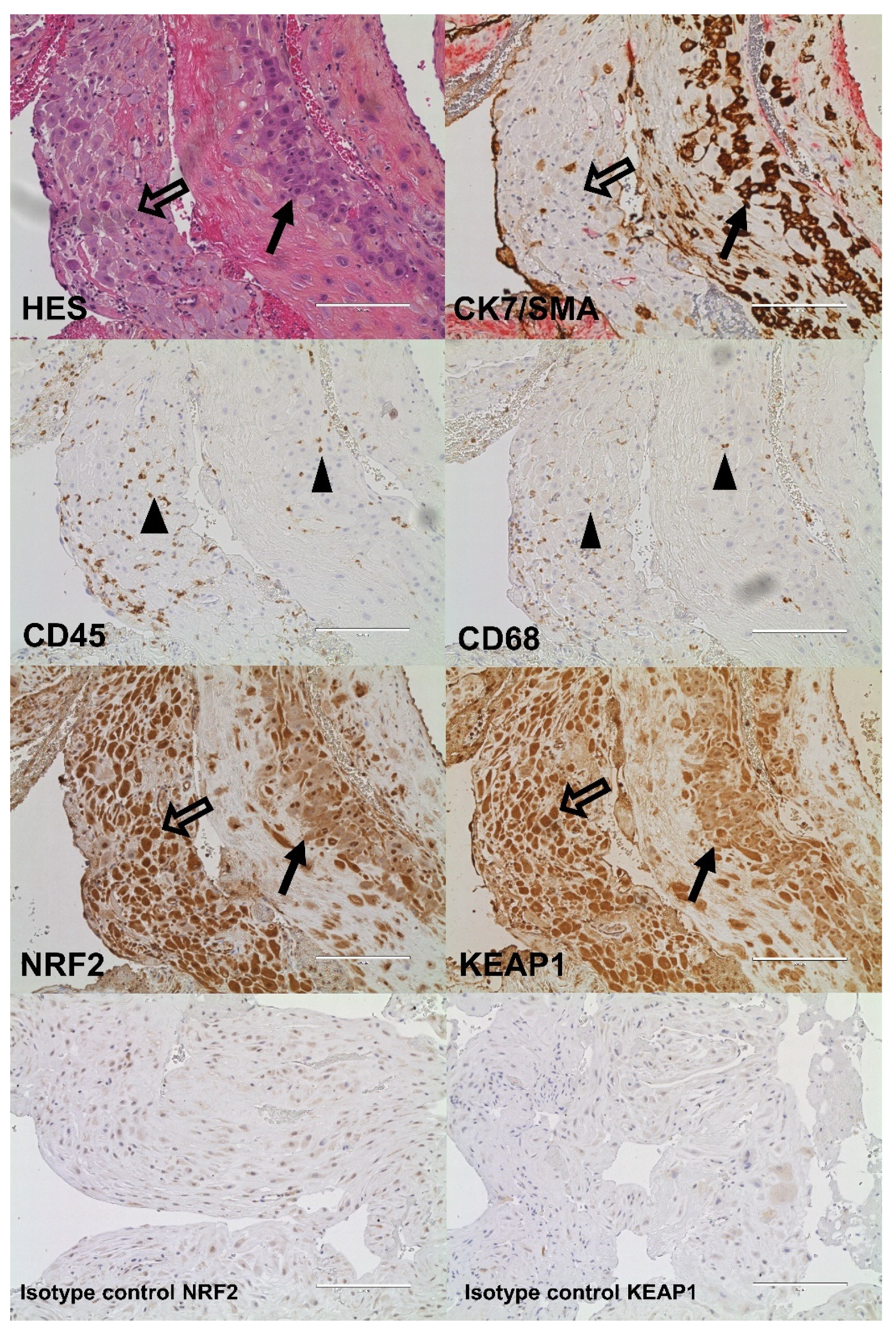
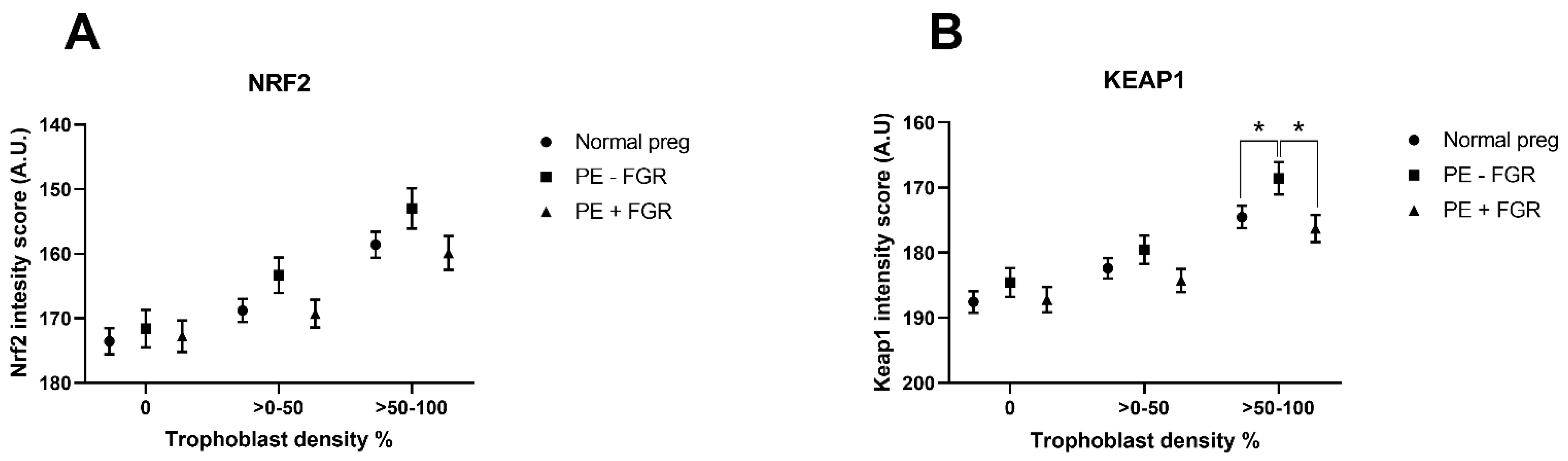
2.4. Cellular Quantitative Decidual NRF2 and KEAP1 Expression
3. Discussion
4. Materials and Methods
4.1. Study Participants and Decidual Biopsies
4.2. Non-Enzymatic Antioxidant-Capacity Assay
4.3. Measuring Oxidative-Stress Levels by a Malondialdehyde (MDA) Assay
4.4. NRF2-Regulated Transcriptional Activation
4.5. Immunohistochemistry
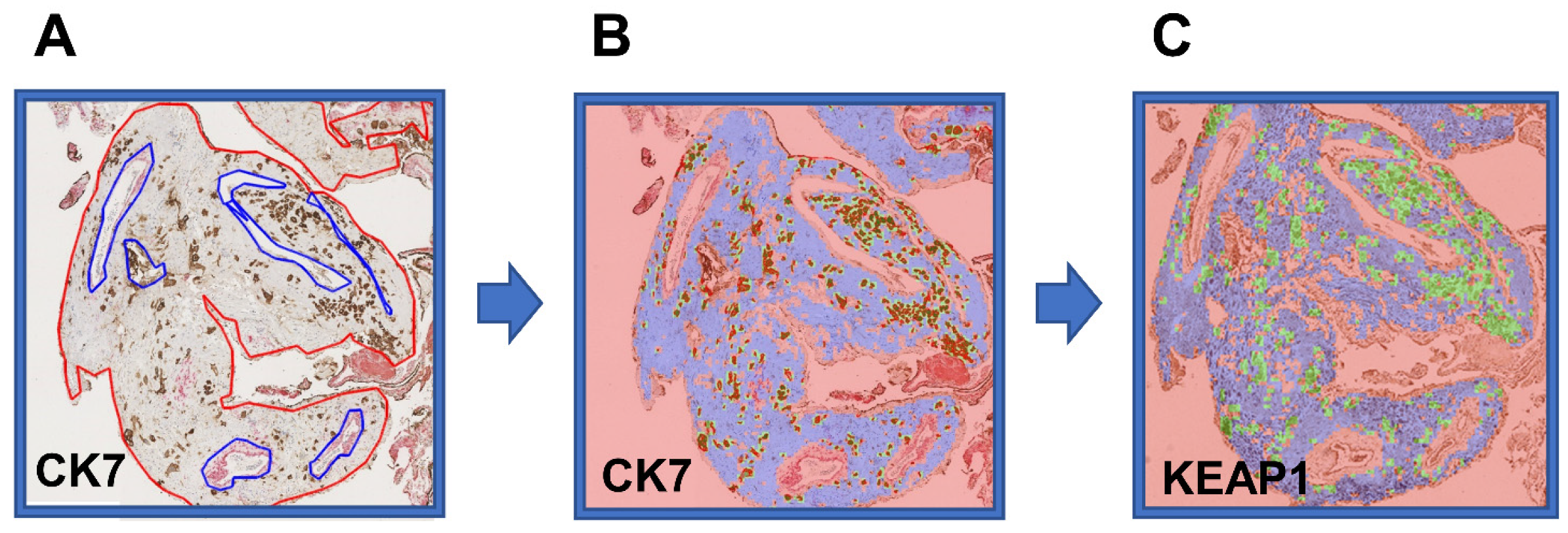
4.6. Automated Quantification of Protein Expression
4.7. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 3-ethylbenzothiazoline-6-sulphonic acid |
| ARE | Antioxidant response element |
| CK7 | Cytokeratin 7 |
| ER | Endoplasmic reticulum |
| FGR | Fetal growth restriction |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| MDA | Malondialdehyde |
| HO-1 | Heme oxygenase 1 |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| ROS | Reactive oxygen species |
References
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obs. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef] [Green Version]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [Green Version]
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Nonvillous Parts and Trophoblast Invasion. In Pathology of the Human Placenta; Springer: Berlin/Heidelberg, Germany, 2012; pp. 157–240. [Google Scholar]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pr. Res. Clin. Obs. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Dayalan Naidu, S.; Kostov, R.V.; Dinkova-Kostova, A.T. Transcription factors Hsf1 and Nrf2 engage in crosstalk for cytoprotection. Trends Pharm. Sci. 2015, 36, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochem. Pharm. 2013, 85, 705–717. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharm. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Lv, Y.F.; Zhao, J.L.; You, Q.D.; Jiang, Z.Y. Regulation of Nrf2 by phosphorylation: Consequences for biological function and therapeutic implications. Free. Radic. Biol. Med. 2021, 168, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wu, T.; Zhao, F.; Lau, A.; Birch, C.M.; Zhang, D.D. KPNA6 (Importin {alpha}7)-mediated nuclear import of Keap1 represses the Nrf2-dependent antioxidant response. Mol. Cell Biol. 2011, 31, 1800–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; Leon, R.; Lopez, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharm. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Zhang, S.; Chan, J.Y.; Zhang, D.D. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol. Cell Biol. 2007, 27, 6334–6349. [Google Scholar] [CrossRef] [Green Version]
- Szczesny-Malysiak, E.; Stojak, M.; Campagna, R.; Grosicki, M.; Jamrozik, M.; Kaczara, P.; Chlopicki, S. Bardoxolone Methyl Displays Detrimental Effects on Endothelial Bioenergetics, Suppresses Endothelial ET-1 Release, and Increases Endothelial Permeability in Human Microvascular Endothelium. Oxid. Med. Cell Longev. 2020, 2020, 4678252. [Google Scholar] [CrossRef]
- Lee, S.; Hu, L. Nrf2 activation through the inhibition of Keap1-Nrf2 protein-protein interaction. Med. Chem. Res. 2020, 29, 846–867. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obs. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staff, A.C.; Ranheim, T.; Khoury, J.; Henriksen, T. Increased contents of phospholipids, cholesterol, and lipid peroxides in decidua basalis in women with preeclampsia. Am. J. Obs. Gynecol. 1999, 180, 587–592. [Google Scholar] [CrossRef]
- Zusterzeel, P.L.; Rutten, H.; Roelofs, H.M.; Peters, W.H.; Steegers, E.A. Protein carbonyls in decidua and placenta of pre-eclamptic women as markers for oxidative stress. Placenta 2001, 22, 213–219. [Google Scholar] [CrossRef]
- Staff, A.C.; Halvorsen, B.; Ranheim, T.; Henriksen, T. Elevated level of free 8-iso-prostaglandin F2alpha in the decidua basalis of women with preeclampsia. Am. J. Obs. Gynecol. 1999, 181, 1211–1215. [Google Scholar] [CrossRef]
- Wruck, C.J.; Huppertz, B.; Bose, P.; Brandenburg, L.O.; Pufe, T.; Kadyrov, M. Role of a fetal defence mechanism against oxidative stress in the aetiology of preeclampsia. Histopathology 2009, 55, 102–106. [Google Scholar] [CrossRef]
- Feng, H.; Wang, L.; Zhang, G.; Zhang, Z.; Guo, W. Oxidative stress activated by Keap-1/Nrf2 signaling pathway in pathogenesis of preeclampsia. Int. J. Clin. Exp. Pathol. 2020, 13, 382–392. [Google Scholar]
- Chigusa, Y.; Tatsumi, K.; Kondoh, E.; Fujita, K.; Nishimura, F.; Mogami, H.; Konishi, I. Decreased lectin-like oxidized LDL receptor 1 (LOX-1) and low Nrf2 activation in placenta are involved in preeclampsia. J. Clin. Endocrinol. Metab. 2012, 97, E1862–E1870. [Google Scholar] [CrossRef] [Green Version]
- Kweider, N.; Huppertz, B.; Wruck, C.J.; Beckmann, R.; Rath, W.; Pufe, T.; Kadyrov, M. A role for Nrf2 in redox signalling of the invasive extravillous trophoblast in severe early onset IUGR associated with preeclampsia. PLoS ONE 2012, 7, e47055. [Google Scholar] [CrossRef] [PubMed]
- Acar, N.; Soylu, H.; Edizer, I.; Ozbey, O.; Er, H.; Akkoyunlu, G.; Gemici, B.; Ustunel, I. Expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and peroxiredoxin 6 (Prdx6) proteins in healthy and pathologic placentas of human and rat. Acta Histochem. 2014, 116, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Loset, M.; Mundal, S.B.; Johnson, M.P.; Fenstad, M.H.; Freed, K.A.; Lian, I.A.; Eide, I.P.; Bjorge, L.; Blangero, J.; Moses, E.K.; et al. A transcriptional profile of the decidua in preeclampsia. Am. J. Obs. Gynecol. 2011, 204, 84.e1–84.e27. [Google Scholar] [CrossRef] [Green Version]
- Staff, A.; Henriksen, T.; Langsætre, E.; Magnussen, E.; Michelsen, T. Hypertensive svangerskapskomplikasjoner og eklampsi. In Den Norske Legeforening; Legeforeningen: Oslo, Norway, 2014. [Google Scholar]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Thompson, J.M.; Irgens, L.M.; Skjaerven, R.; Rasmussen, S. Placenta weight percentile curves for singleton deliveries. BJOG 2007, 114, 715–720. [Google Scholar] [CrossRef]
- Eide, I.P.; Isaksen, C.V.; Salvesen, K.A.; Langaas, M.; Schonberg, S.A.; Austgulen, R. Decidual expression and maternal serum levels of heme oxygenase 1 are increased in pre-eclampsia. Acta Obs. Gynecol. Scand. 2008, 87, 272–279. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Velichkova, M.; Hasson, T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol. Cell Biol. 2005, 25, 4501–4513. [Google Scholar] [CrossRef] [Green Version]
- van Uitert, M.; Moerland, P.D.; Enquobahrie, D.A.; Laivuori, H.; van der Post, J.A.; Ris-Stalpers, C.; Afink, G.B. Meta-Analysis of Placental Transcriptome Data Identifies a Novel Molecular Pathway Related to Preeclampsia. PLoS ONE 2015, 10, e0132468. [Google Scholar]
- Madazli, R.; Benian, A.; Aydin, S.; Uzun, H.; Tolun, N. The plasma and placental levels of malondialdehyde, glutathione and superoxide dismutase in pre-eclampsia. J. Obs. Gynaecol. 2002, 22, 477–480. [Google Scholar] [CrossRef]
- Mutlu-Turkoglu, U.; Ademoglu, E.; Ibrahimoglu, L.; Aykac-Toker, G.; Uysal, M. Imbalance between lipid peroxidation and antioxidant status in preeclampsia. Gynecol. Obs. Inves. 1998, 46, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Walsh, S.W. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J. Soc. Gynecol. Investig. 1996, 3, 179–184. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in pre-eclampsia. Placenta 2001, 22, 206–212. [Google Scholar] [CrossRef]
- Tong, J.; Niu, Y.; Chen, Z.J.; Zhang, C. Comparison of the transcriptional profile in the decidua of early-onset and late-onset pre-eclampsia. J. Obs. Gynaecol. Res. 2020, 46, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Q.; Peng, Q.; Xie, Y.; Wang, W.; Pei, C.; Zhao, Y.; Liu, R.; Huang, L.; Li, T.; et al. Single-cell RNA sequencing reveals heterogeneity and differential expression of decidual tissues during the peripartum period. Cell Prolif. 2021, 54, e12967. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Zhao, W.; Lv, H.; Li, W.P.; Chen, Z.J.; Zhang, C. Transcriptomic Profiling in Human Decidua of Severe Preeclampsia Detected by RNA Sequencing. J. Cell Biochem. 2018, 119, 607–615. [Google Scholar] [CrossRef]
- Lian, I.A.; Loset, M.; Mundal, S.B.; Fenstad, M.H.; Johnson, M.P.; Eide, I.P.; Bjorge, L.; Freed, K.A.; Moses, E.K.; Austgulen, R. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta 2011, 32, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Yung, H.W.; Murray, A.J. Mitochondrial—Endoplasmic reticulum interactions in the trophoblast: Stress and senescence. Placenta 2017, 52, 146–155. [Google Scholar]
- Nezu, M.; Souma, T.; Yu, L.; Sekine, H.; Takahashi, N.; Wei, A.Z.; Ito, S.; Fukamizu, A.; Zsengeller, Z.K.; Nakamura, T.; et al. Nrf2 inactivation enhances placental angiogenesis in a preeclampsia mouse model and improves maternal and fetal outcomes. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H.; Xue, J.; Chen, P.; Zhou, Q.; Zhang, C. Nanoparticle-Mediated Simultaneous Downregulation of Placental Nrf2 and sFlt1 Improves Maternal and Fetal Outcomes in a Preeclampsia Mouse Model. ACS Biomater. Sci. Eng. 2020, 6, 5866–5873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, B.; Meng, F.; Li, H. Effects of Nrf-2 expression in trophoblast cells and vascular endothelial cells in preeclampsia. Am. J. Transl. Res. 2021, 13, 1006–1021. [Google Scholar]
- Yarosz, E.L.; Chang, C.H. The Role of Reactive Oxygen Species in Regulating T Cell-mediated Immunity and Disease. Immune Netw. 2018, 18, e14. [Google Scholar] [CrossRef]
- Qin, Q.; Qu, C.; Niu, T.; Zang, H.; Qi, L.; Lyu, L.; Wang, X.; Nagarkatti, M.; Nagarkatti, P.; Janicki, J.S.; et al. Nrf2-Mediated Cardiac Maladaptive Remodeling and Dysfunction in a Setting of Autophagy Insufficiency. Hypertension 2016, 67, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Ghosh, A.; Lo, C.S.; Chenier, I.; Scholey, J.W.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S.D. Nrf2 Deficiency Upregulates Intrarenal Angiotensin-Converting Enzyme-2 and Angiotensin 1-7 Receptor Expression and Attenuates Hypertension and Nephropathy in Diabetic Mice. Endocrinology 2018, 159, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.; Shi, Y.; Otoukesh, A.; Ghosh, A.; Lo, C.S.; Chenier, I.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S. Catalase overexpression prevents nuclear factor erythroid 2-related factor 2 stimulation of renal angiotensinogen gene expression, hypertension, and kidney injury in diabetic mice. Diabetes 2014, 63, 3483–3496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaan, J.J.; Brown, M.A. Renin-angiotensin system in pre-eclampsia: Everything old is new again. Obs. Med. 2012, 5, 147–153. [Google Scholar] [CrossRef]
- Anton, L.; Merrill, D.C.; Neves, L.A.; Diz, D.I.; Corthorn, J.; Valdes, G.; Stovall, K.; Gallagher, P.E.; Moorefield, C.; Gruver, C.; et al. The uterine placental bed Renin-Angiotensin system in normal and preeclamptic pregnancy. Endocrinology 2009, 150, 4316–4325. [Google Scholar] [CrossRef] [PubMed]
- Anton, L.; Merrill, D.C.; Neves, L.A.A.; Stovall, K.; Gallagher, P.E.; Diz, D.I.; Moorefield, C.; Gruver, C.; Ferrario, C.M.; Brosnihan, K.B. Activation of local chorionic villi angiotensin II levels but not angiotensin (1-7) in preeclampsia. Hypertension 2008, 51, 1066–1072. [Google Scholar] [CrossRef] [Green Version]
- Mistry, H.D.; Kurlak, L.O.; Broughton Pipkin, F. The placental renin-angiotensin system and oxidative stress in pre-eclampsia. Placenta 2013, 34, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.L.; Rasmussen, S.; Wilsgaard, T.; Sollien, R.; Kiserud, T. Longitudinal reference ranges for estimated fetal weight. Acta Obs. Gynecol. Scand. 2006, 85, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Harsem, N.K.; Staff, A.C.; He, L.; Roald, B. The decidual suction method: A new way of collecting decidual tissue for functional and morphological studies. Acta Obs. Gynecol. Scand. 2004, 83, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Joraholmen, M.W.; Skalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Songstad, N.T.; Kaspersen, K.H.; Hafstad, A.D.; Basnet, P.; Ytrehus, K.; Acharya, G. Effects of High Intensity Interval Training on Pregnant Rats, and the Placenta, Heart and Liver of Their Fetuses. PLoS ONE 2015, 10, e0143095. [Google Scholar] [CrossRef] [Green Version]
- Almasy, L.; Blangero, J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 1998, 62, 1198–1211. [Google Scholar] [CrossRef] [Green Version]
- Brazma, A.; Hingamp, P.; Quackenbush, J.; Sherlock, G.; Spellman, P.; Stoeckert, C.; Aach, J.; Ansorge, W.; Ball, C.A.; Causton, H.C.; et al. Minimum information about a microarray experiment (MIAME)—Toward standards for microarray data. Nat. Genet. 2001, 29, 365–371. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009, 25, 1463–1465. [Google Scholar] [CrossRef]
- Gierman, L.M.; Silva, G.B.; Pervaiz, Z.; Rakner, J.J.; Mundal, S.B.; Thaning, A.J.; Nervik, I.; Elschot, M.; Mathew, S.; Thomsen, L.C.V.; et al. TLR3 expression by maternal and fetal cells at the maternal-fetal interface in normal and preeclamptic pregnancies. J. Leukoc. Biol. 2020, 109, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.B.; Gierman, L.M.; Rakner, J.J.; Stødle, G.S.; Mundal, S.B.; Thaning, A.J.; Sporsheim, B.; Elschot, M.; Collett, K.; Bjørge, L.; et al. Cholesterol Crystals and NLRP3 Mediated Inflammation in the Uterine Wall Decidua in Normal and Preeclamptic Pregnancies. Front. Immunol. 2020, 11, 564712. [Google Scholar] [CrossRef] [PubMed]
- Rakner, J.J.; Silva, G.B.; Mundal, S.B.; Thaning, A.J.; Elschot, M.; Ostrop, J.; Thomsen, L.C.V.; Bjørge, L.; Gierman, L.M.; Iversen, A.C. Decidual and placental NOD1 is associated with inflammation in normal and preeclamptic pregnancies. Placenta 2021, 105, 23–31. [Google Scholar] [CrossRef] [PubMed]

| Normal Pregnancies (n = 70) | PE without FGR (n = 28) | PE with FGR (n = 47) | |
|---|---|---|---|
| Baseline characteristics | |||
| Maternal age, years | 31.8 (±5.1) | 30.5 (±4.9) | 30.9 (±5.5) |
| BMI, kg/m2 * | 25.2 (±4.2) | 26.5 (±6.6) | 27.2 (±5.5) † |
| Primipara, n (%) | 12 (17) | 17 (61) ‡ | 26 (55) ‡ |
| Characteristics at delivery | |||
| Systolic BP, mmHg § | 121.1 (±12.8) | 165.1 (±21.2) ‡ | 161.6 (±19.8) ‡ |
| Diastolic BP, mmHg § | 73.4 (±8.6) | 102.0 (±12.4) ‡ | 99.6 (±8.9) ‡ |
| Gestational age, weeks | 38.5 (±0.9) | 33.1 (±3.9) ‡ | 30.1 (±3) ‡# |
| Severe PE ||, n (%) | n.a. | 23 (82) | 34 (72) |
| Early onset PE (<34 weeks), n (%) | n.a. | 15 (54) | 40 (85) † |
| Placental weight, g | 620 (193) | 450 (238) ‡ | 275 (140) ‡# |
| Fetal birth weight, g | 3621 (±474) | 2208 (±955) ‡ | 1182 (±456) ‡ |
| Placental weight ratio ** | 1.04 (0.35) | 0.90 (0.35) ‡ | 0.60 (0.27) ‡# |
| Comparison between Diagnostic Groups | Gene-Set Description | No. of Transcr. | ES | NES | p-Value | |
|---|---|---|---|---|---|---|
| PE − FGR | Normal preg | Antioxidant proteins | 18 | −0.47 | −1.39 | 0.08 |
| PE − FGR | Normal preg | Phase I and II metabolizing enzymes | 48 | −0.25 | −0.85 | 0.70 |
| PE − FGR | Normal preg | Chaperone and stress response proteins | 43 | −0.23 | −0.82 | 0.76 |
| PE − FGR | Normal preg | Phase III detoxifying proteins | 4 | 0.31 | 0.59 | 0.90 |
| PE − FGR | Normal preg | Ubiquitination/proteasomal degradation | 5 | 0.37 | 0.74 | 0.77 |
| PE − FGR | Normal preg | All five gene sets | 118 | −0.24 | −1.01 | 0.44 |
| PE + FGR | Normal preg | Antioxidant proteins | 18 | 0.46 | 1.38 | 0.10 |
| PE + FGR | Normal preg | Phase I and II metabolizing enzymes | 48 | −0.33 | −1.23 | 0.24 |
| PE + FGR | Normal preg | Chaperone and stress response proteins | 43 | 0.45 | 1.73 | <0.001 |
| PE + FGR | Normal preg | Phase III detoxifying proteins | 4 | 0.55 | 1.07 | 0.45 |
| PE + FGR | Normal preg | Ubiquitination/proteasomal degradation | 5 | 0.71 | 1.43 | 0.07 |
| PE + FGR | Normal preg | All five gene sets | 118 | 0.32 | 1.32 | 0.08 |
| PE − FGR | PE + FGR | Antioxidant proteins | 18 | −0.60 | −1.66 | 0.02 |
| PE − FGR | PE + FGR | Phase I and II metabolizing enzymes | 48 | 0.31 | 0.99 | 0.55 |
| PE − FGR | PE + FGR | Chaperone and stress response proteins | 43 | −0.44 | −1.59 | <0.05 |
| PE − FGR | PE + FGR | Phase III detoxifying proteins | 4 | −0.60 | −1.06 | 0.45 |
| PE − FGR | PE + FGR | Ubiquitination/proteasomal degradation | 5 | −0.71 | −1.43 | 0.07 |
| PE − FGR | PE + FGR | All five gene sets | 118 | −0.36 | −1.41 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundal, S.B.; Rakner, J.J.; Silva, G.B.; Gierman, L.M.; Austdal, M.; Basnet, P.; Elschot, M.; Bakke, S.S.; Ostrop, J.; Thomsen, L.C.V.; et al. Divergent Regulation of Decidual Oxidative-Stress Response by NRF2 and KEAP1 in Preeclampsia with and without Fetal Growth Restriction. Int. J. Mol. Sci. 2022, 23, 1966. https://doi.org/10.3390/ijms23041966
Mundal SB, Rakner JJ, Silva GB, Gierman LM, Austdal M, Basnet P, Elschot M, Bakke SS, Ostrop J, Thomsen LCV, et al. Divergent Regulation of Decidual Oxidative-Stress Response by NRF2 and KEAP1 in Preeclampsia with and without Fetal Growth Restriction. International Journal of Molecular Sciences. 2022; 23(4):1966. https://doi.org/10.3390/ijms23041966
Chicago/Turabian StyleMundal, Siv Boon, Johanne Johnsen Rakner, Gabriela Brettas Silva, Lobke Marijn Gierman, Marie Austdal, Purusotam Basnet, Mattijs Elschot, Siril Skaret Bakke, Jenny Ostrop, Liv Cecilie Vestrheim Thomsen, and et al. 2022. "Divergent Regulation of Decidual Oxidative-Stress Response by NRF2 and KEAP1 in Preeclampsia with and without Fetal Growth Restriction" International Journal of Molecular Sciences 23, no. 4: 1966. https://doi.org/10.3390/ijms23041966
APA StyleMundal, S. B., Rakner, J. J., Silva, G. B., Gierman, L. M., Austdal, M., Basnet, P., Elschot, M., Bakke, S. S., Ostrop, J., Thomsen, L. C. V., Moses, E. K., Acharya, G., Bjørge, L., & Iversen, A.-C. (2022). Divergent Regulation of Decidual Oxidative-Stress Response by NRF2 and KEAP1 in Preeclampsia with and without Fetal Growth Restriction. International Journal of Molecular Sciences, 23(4), 1966. https://doi.org/10.3390/ijms23041966








