Oxidative Stress Enhances the TGF-β2-RhoA-MRTF-A/B Axis in Cells Entering Endothelial-Mesenchymal Transition
Abstract
:1. Introduction
2. Results
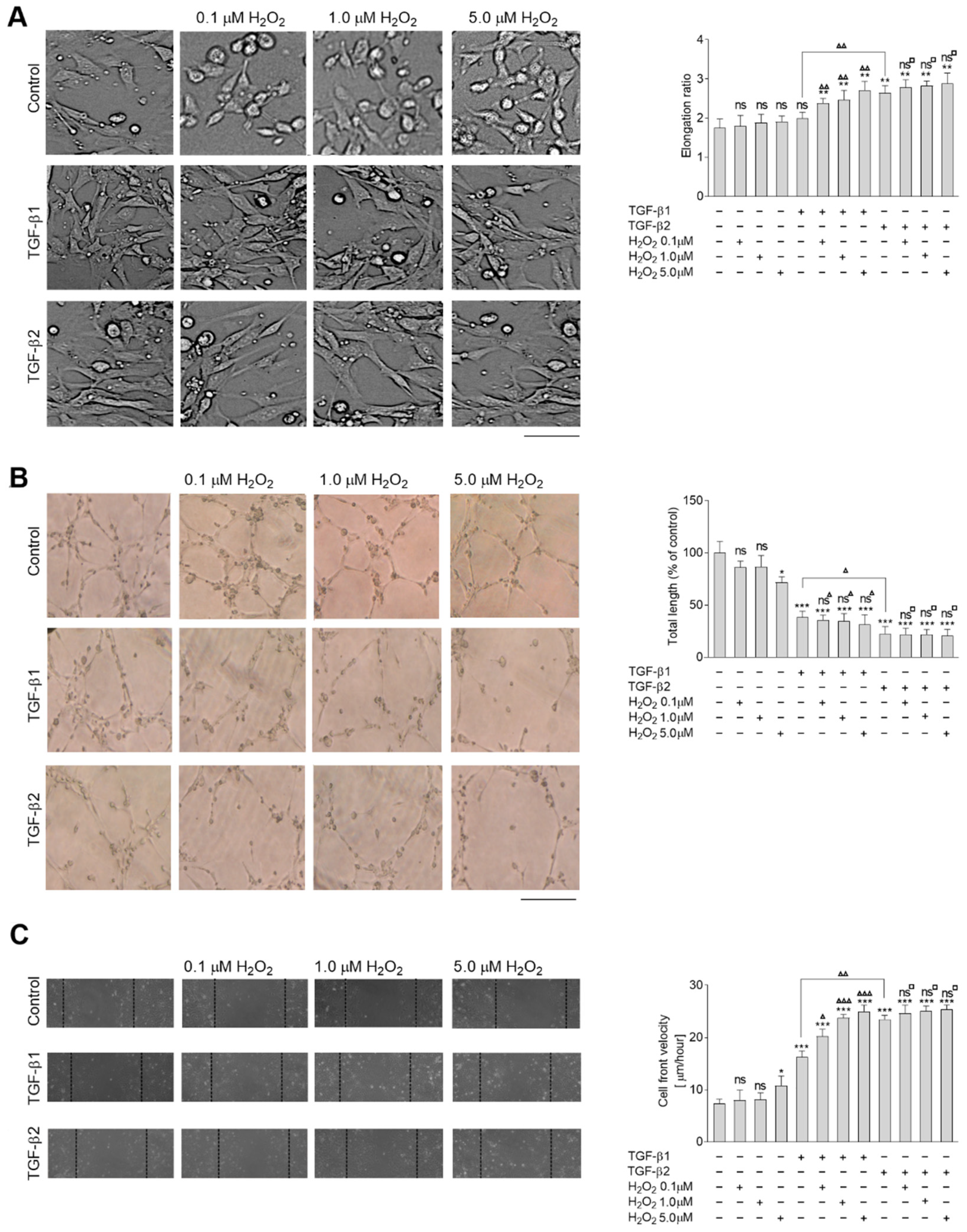
2.1. Hydrogen Peroxide Enhances the TGF-β Effect of EndMT Stimulation HMEC-1 Cells
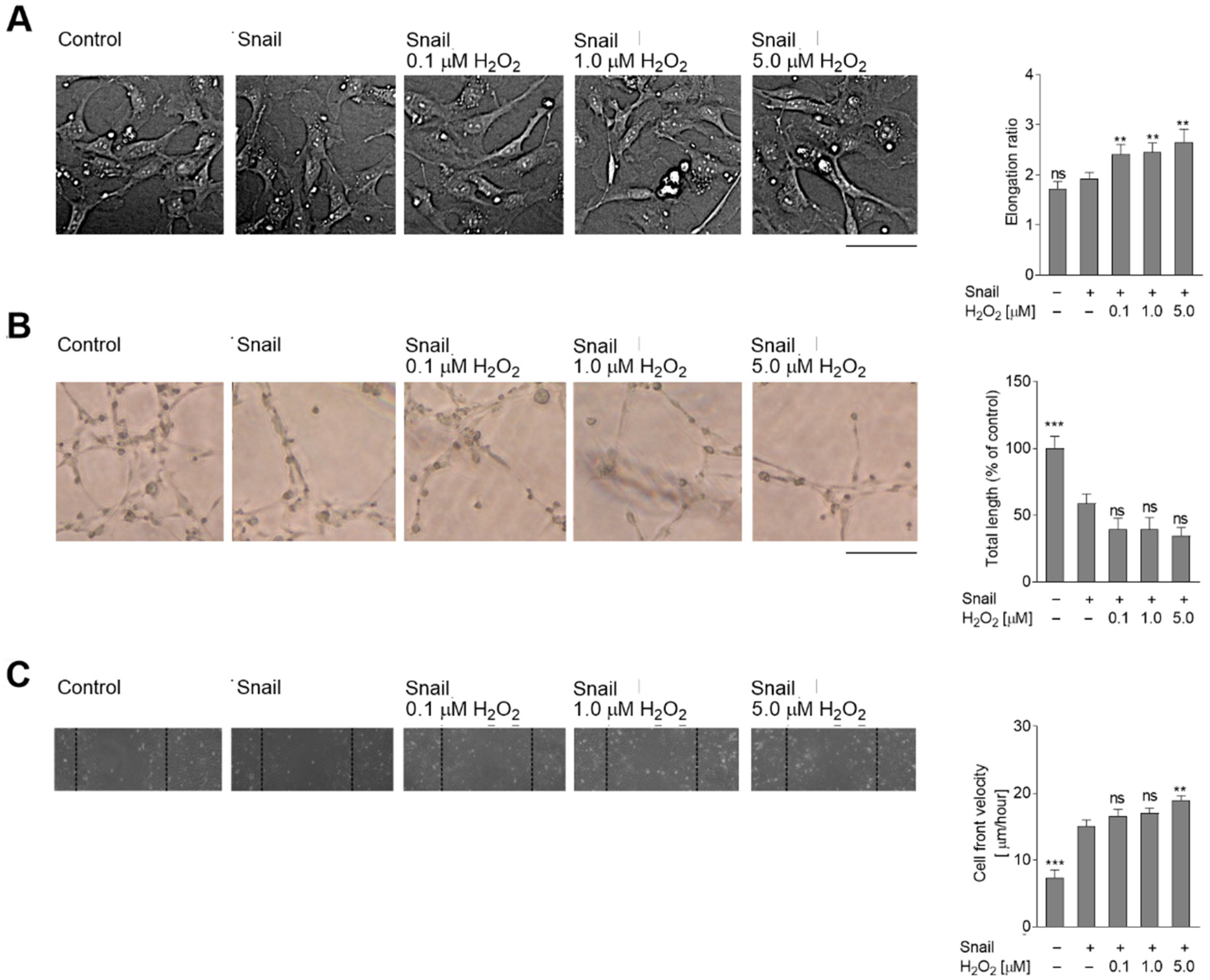
2.2. Oxidative Stress Induces Later Stages of EndMT in Snail-Transfected HMEC-1 Cells
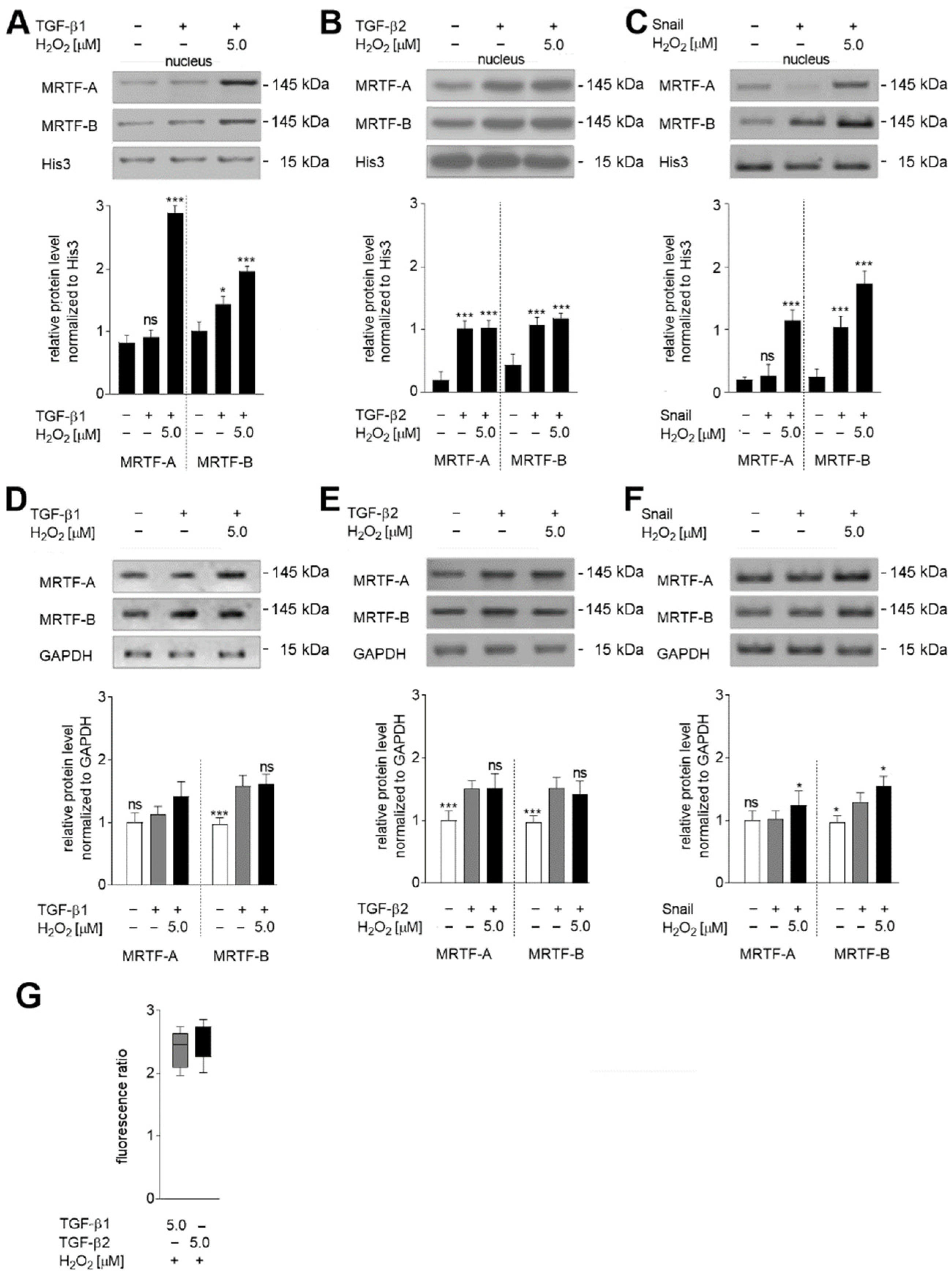
2.3. Hydrogen Peroxide Regulates the Activation of MRTF-A in HMEC-1 Cells
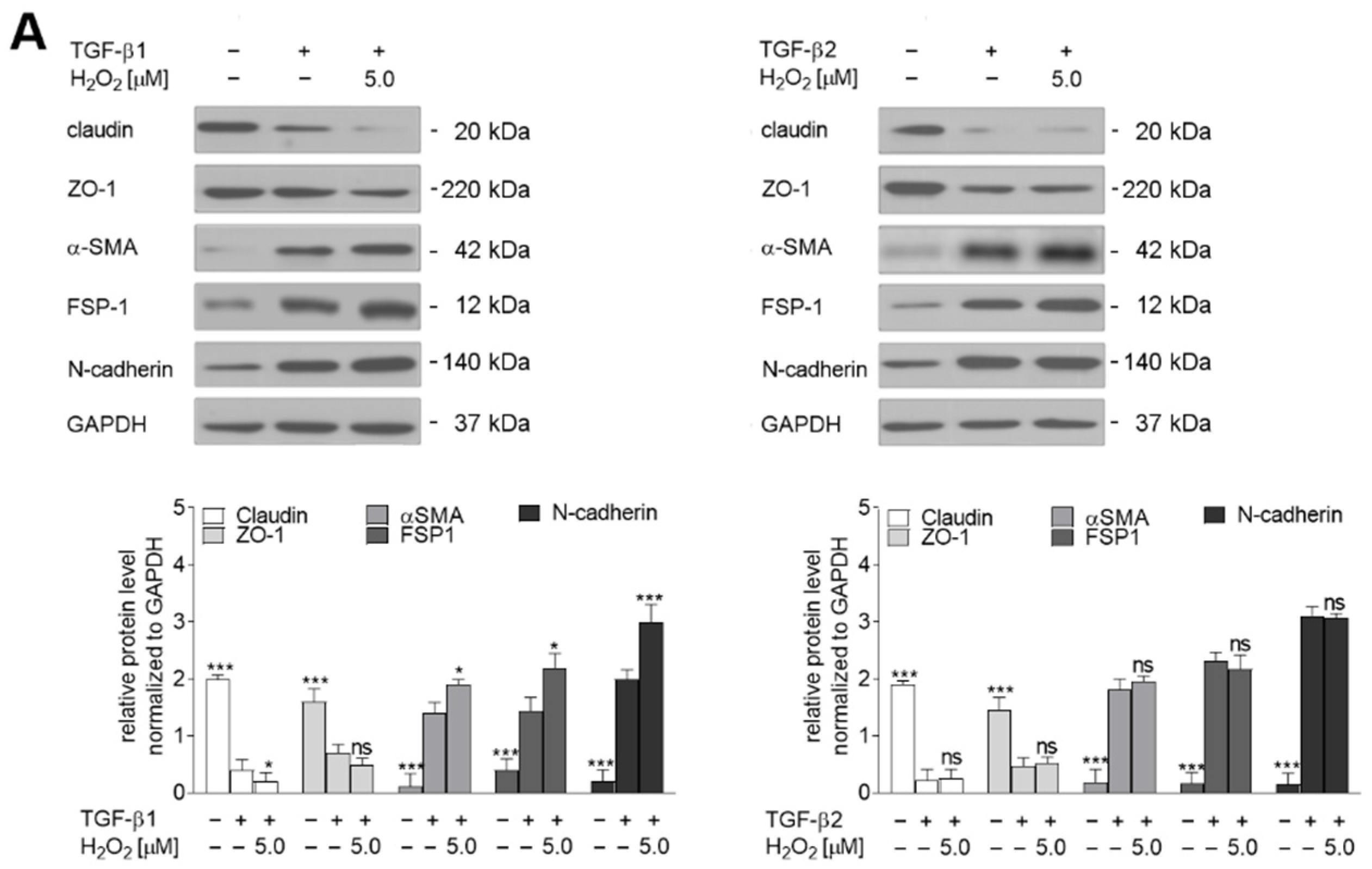
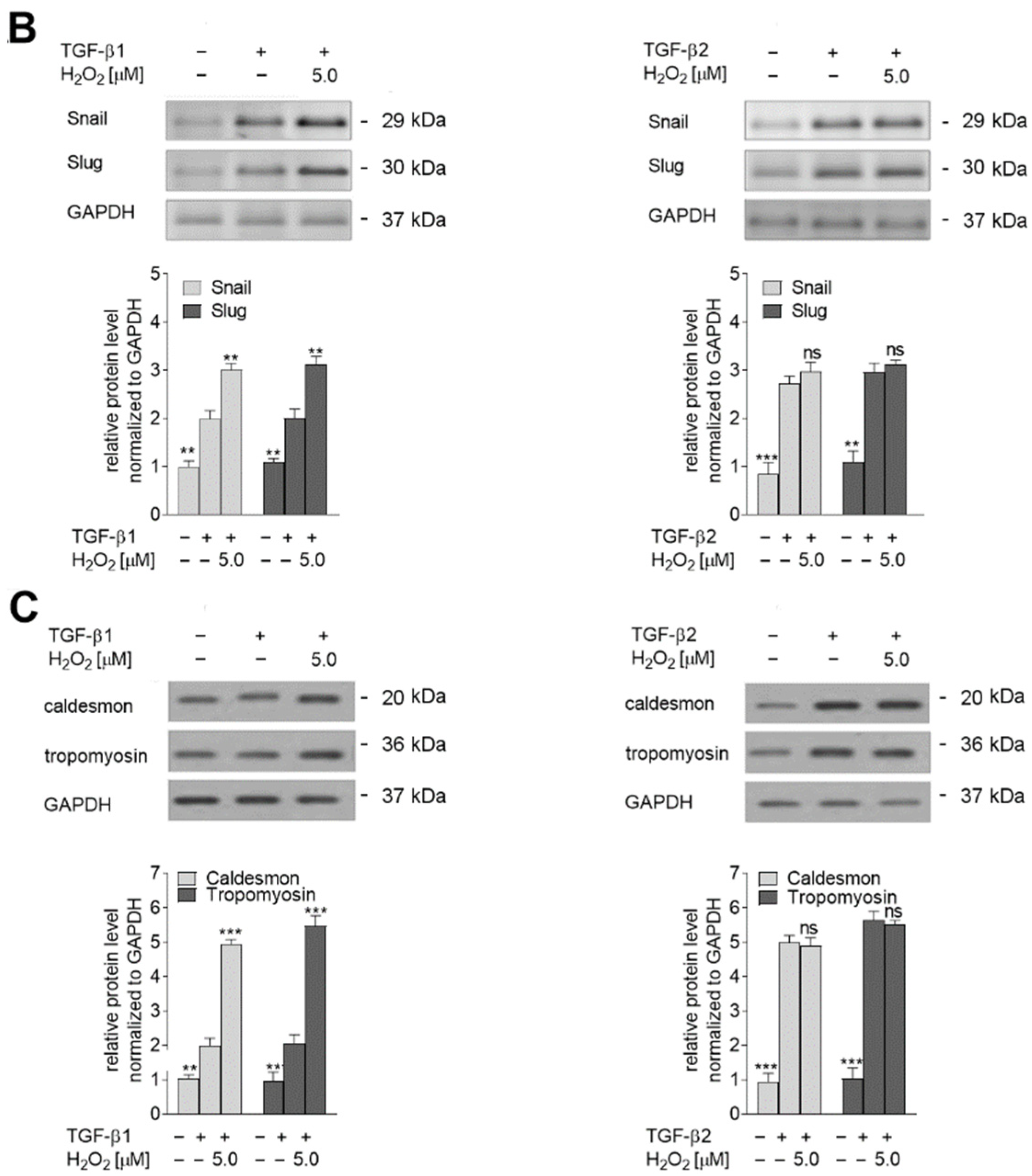
2.4. Oxidative Stress Induces Late Stages of EndMT by Induction of TGF-β2 Upregulation
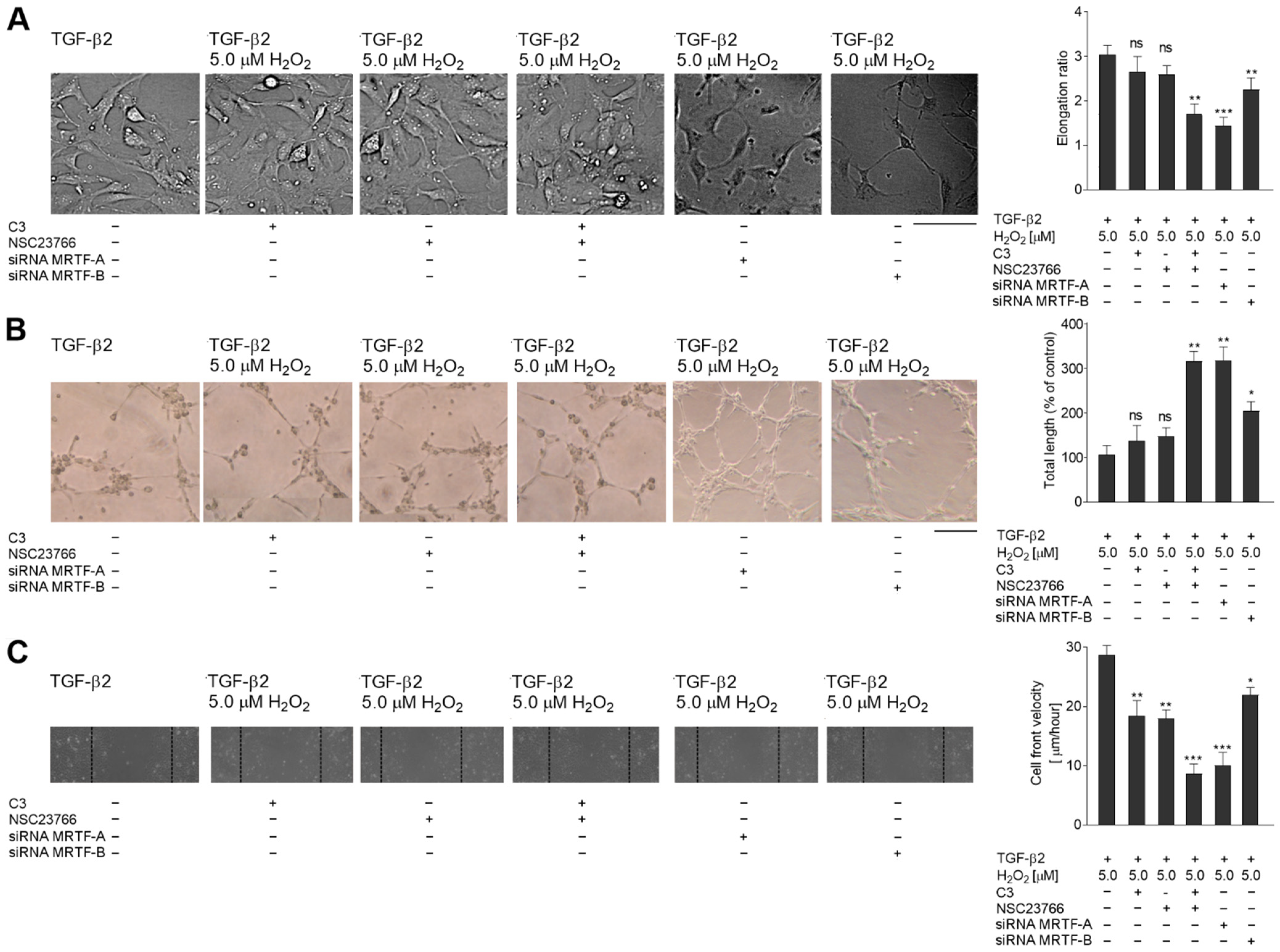
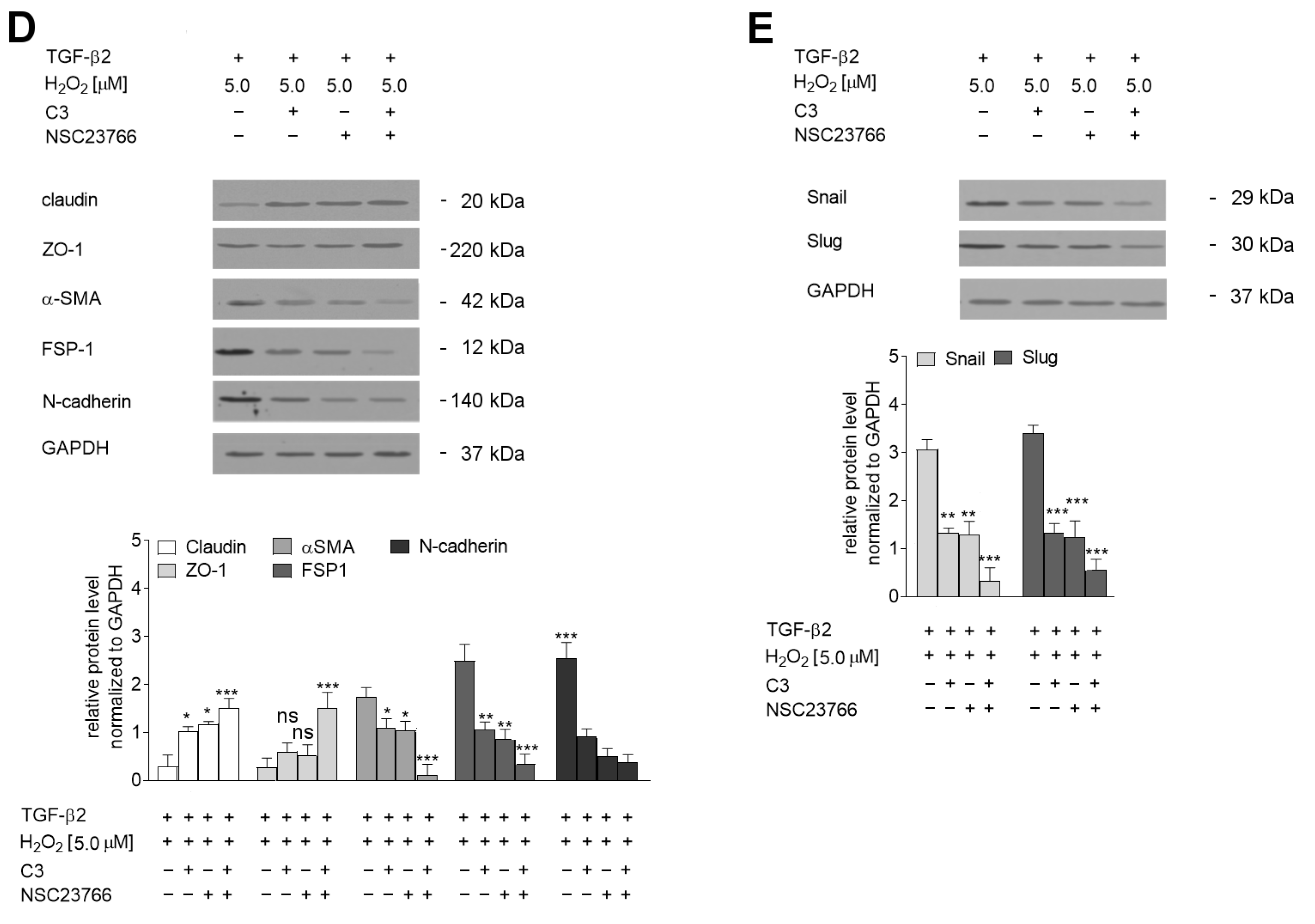
2.5. The TGF-β2-RhoA-MRTF-A/B Axis Is Critical for Later Stages of EndMT
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines
4.3. siRNA Silencing
4.4. Cell Morphology Analysis
4.5. Enzyme-Linked Immunosorbent Assay
4.6. Western Blotting
4.7. Tube Formation Assay
4.8. Wound Healing
4.9. Intracellular ROS Determination
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhurst, R.J. TGF beta signaling in health and disease. Nat. Genet. 2004, 36, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Bierie, B.; Moses, H.L. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006, 17, 2940. [Google Scholar] [CrossRef] [PubMed]
- Ciszewski, W.M.; Sobierajska, K.; Wawro, M.E.; Klopocka, W.; Chefczynska, N.; Muzyczuk, A.; Siekacz, K.; Wujkowska, A.; Niewiarowska, J. The ILK-MMP9-MRTF axis is crucial for EndMT differentiation of endothelial cells in a tumor microenvironment. Biochim. Biophys. Acta 2017, 1864, 2283–2296. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-M.; Desai, L.P. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Krstić, J.; Trivanović, D.; Mojsilović, S.; Santibanez, J.F. Transforming Growth Factor-Beta and Oxidative Stress Interplay: Implications in Tumorigenesis and Cancer Progression. Oxid. Med. Cell. Longev. 2015, 2015, 654594. [Google Scholar] [CrossRef]
- Thuan, D.T.B.; Zayed, H.; Eid, A.H.; Abou-Saleh, H.; Nasrallah, G.K.; Mangoni, A.A.; Pintus, G. A Potential Link Between Oxidative Stress and Endothelial-to-Mesenchymal Transition in Systemic Sclerosis. Front. Immunol. 2018, 9, 1985. [Google Scholar] [CrossRef] [Green Version]
- Richter, K.; Kietzmann, T. Reactive oxygen species and fibrosis: Further evidence of a significant liaison. Cell Tissue Res. 2016, 365, 591–605. [Google Scholar] [CrossRef] [Green Version]
- Birukov, K.G. Cyclic Stretch, Reactive Oxygen Species, and Vascular Remodeling. Antioxid. Redox Signal. 2019, 11, 1651–1667. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.-M.; Pravia, K.A.G. Oxidative stress and glutathione in TGF-β-mediated fibrogenesis. Free Radic. Biol. Med. 2010, 48, 1. [Google Scholar] [CrossRef] [Green Version]
- Maleki, S.; Kjellqvist, S.; Paloschi, V.; Magné, J.; Branca, R.M.; Du, L.; Hultenby, K.; Petrini, J.; Fuxe, J.; Lehtiö, J.; et al. Mesenchymal state of intimal cells may explain higher propensity to ascending aortic aneurysm in bicuspid aortic valves. Sci. Rep. 2016, 6, 35712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustelo, X.R.; Sauzeau, V.; Berenjeno, I.M. GTP-binding proteins of the Rho/Rac family: Regulation, effectors and functions in vivo. Bioessays 2007, 29, 356–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffaghello, L.; Vacca, A.; Pistoia, V.; Ribatti, D. Cancer associated fibroblasts in hematological malignancies. Oncotarget 2015, 6, 2589–2603. [Google Scholar] [CrossRef] [Green Version]
- Wawro, M.E.; Sobierajska, K.; Ciszewski, W.M.; Wagner, W.; Frontczak, M.; Wieczorek, K.; Niewiarowska, J. Tubulin beta 3 and 4 are involved in the generation of early fibrotic stages. Cell Signal. 2017, 38, 26–38. [Google Scholar] [CrossRef]
- Wawro, M.E.; Sobierajska, K.; Ciszewski, W.M.; Niewiarowska, J. Nonsteroidal Anti-Inflammatory Drugs Prevent Vincristine-Dependent Cancer-Associated Fibroblasts Formation. Int. J. Mol. Sci. 2019, 20, 1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobierajska, K.; Ciszewski, W.M.; Macierzynska-Piotrowska, E.; Klopocka, W.; Przygodzka, P.; Karakula, M.; Pestka, K.; Wawro, M.E.; Niewiarowska, J. The New Model of Snail Expression Regulation: The Role of MRTFs in Fast and Slow Endothelial-Mesenchymal Transition. Int. J. Mol. Sci. 2020, 21, 5875. [Google Scholar] [CrossRef]
- Kryczka, J.; Przygodzka, P.; Bogusz, H.; Boncela, J. HMEC-1 adopt the mixed amoeboidmesenchymal migration type during EndM. Eur. J. Cell. Biol. 2017, 96, 289–300. [Google Scholar] [CrossRef]
- Medici, D.; Potenta, S.; Kalluri, R. Transforming growth factor-β2 promotes Snail-mediated endothelial—mesenchymal transition through convergence of Smad-dependent and Smad-independent signaling. Biochem. J. 2011, 437, 515–520. [Google Scholar] [CrossRef]
- Morita, T.; Mayanagi, T.; Sobue, K. Dual roles of myocardin-related transcription factors in epithelial mesenchymal transition via slug induction and actin remodeling. J. Cell Biol. 2007, 179, 1027–1042. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.N.; Nordheim, A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol. 2010, 11, 353–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, Y.C.; Wei, W.C.; Wang, Y.K.; Lin, S.C.; Sung, J.M.; Tang, M.J. Transforming growth factor-β1 induces Smad3-dependent β1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am. J. Pathol. 2010, 177, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Shirotani-Ikejima, H.; Tadokoro, S.; Maeda, Y.; Kinoshita, T.; Tomiyama, Y.; Miyata, T. Integrin-linked kinase associated with integrin activation. Blood 2009, 113, 5304–5313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vi, L.; de Lasa, C.; DiGuglielmo, G.M.; Dagnino, L. Integrin-linked kinase is required for TGF-β1 induction of dermal myofibroblast differentiation. J. Investig. Dermatol. 2011, 131, 586–593. [Google Scholar] [CrossRef] [Green Version]
- Karagiannis, G.S.; Poutahidis, T.; Erdman, S.E.; Kirsch, R.; Riddell, R.H.; Diamandis, E.P. Cancer-Associated Fibroblasts Drive the Progression of Metastasis through both Paracrine and Mechanical Pressure on Cancer Tissue. Mol. Cancer Res. 2012, 10, 1403–1418. [Google Scholar] [CrossRef] [Green Version]
- Korczyński, J.; Sobierajska, K.; Krzemiński, P.; Wasik, A.; Wypych, D.; Pomorski, P.; Kłopocka, W. Is MLC phosphorylation essential for the recovery from ROCK inhibition in glioma C6 cells? Acta Biochim. Pol. 2011, 58, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Sobierajska, K.; Wieczorek, K.; Ciszewski, W.M.; Sacewicz-Hofman, I.; Wawro, M.E.; Wiktorska, M.; Boncela, J.; Papiewska-Pajak, I.; Kwasniak, P.; Wyroba, E.; et al. β-III tubulin modulates the behavior of Snail overexpressed during the epithelial-to-mesenchymal transition in colon cancer cells. Biochim. Biophys. Acta 2016, 1863, 2221–2233. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska, K.; Ciszewski, W.M.; Wawro, M.E.; Wieczorek-Szukała, K.; Boncela, J.; Papiewska-Pajak, I.; Niewiarowska, J.; Kowalska, M.A. TUBB4B Downregulation Is Critical for Increasing Migration of Metastatic Colon Cancer Cells. Cells 2019, 8, 810. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobierajska, K.; Wawro, M.E.; Niewiarowska, J. Oxidative Stress Enhances the TGF-β2-RhoA-MRTF-A/B Axis in Cells Entering Endothelial-Mesenchymal Transition. Int. J. Mol. Sci. 2022, 23, 2062. https://doi.org/10.3390/ijms23042062
Sobierajska K, Wawro ME, Niewiarowska J. Oxidative Stress Enhances the TGF-β2-RhoA-MRTF-A/B Axis in Cells Entering Endothelial-Mesenchymal Transition. International Journal of Molecular Sciences. 2022; 23(4):2062. https://doi.org/10.3390/ijms23042062
Chicago/Turabian StyleSobierajska, Katarzyna, Marta E. Wawro, and Jolanta Niewiarowska. 2022. "Oxidative Stress Enhances the TGF-β2-RhoA-MRTF-A/B Axis in Cells Entering Endothelial-Mesenchymal Transition" International Journal of Molecular Sciences 23, no. 4: 2062. https://doi.org/10.3390/ijms23042062








