Effect of Multiple Sclerosis Cerebrospinal Fluid and Oligodendroglia Cell Line Environment on Human Wharton’s Jelly Mesenchymal Stem Cells Secretome
Abstract
:1. Introduction
2. Results
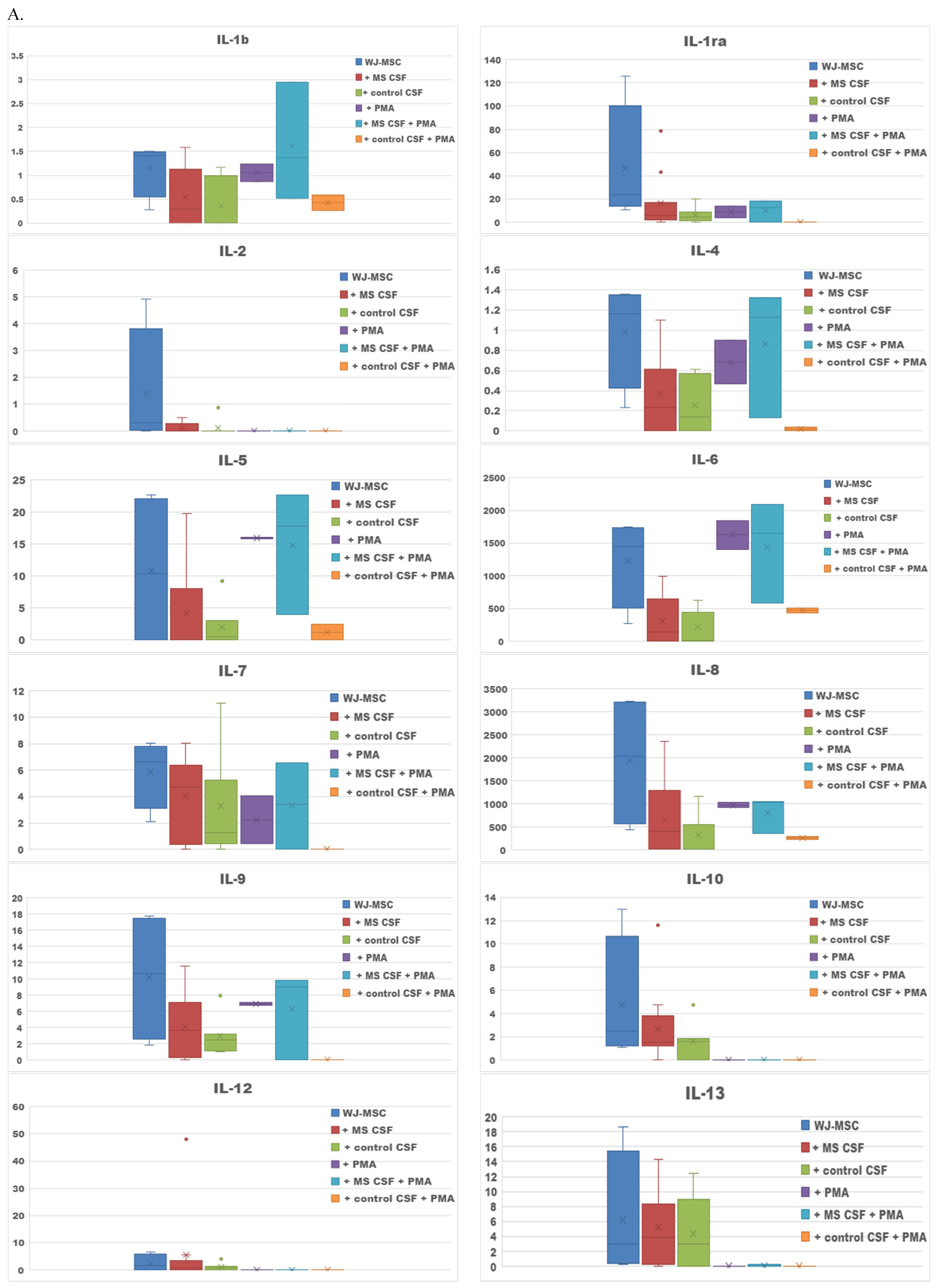
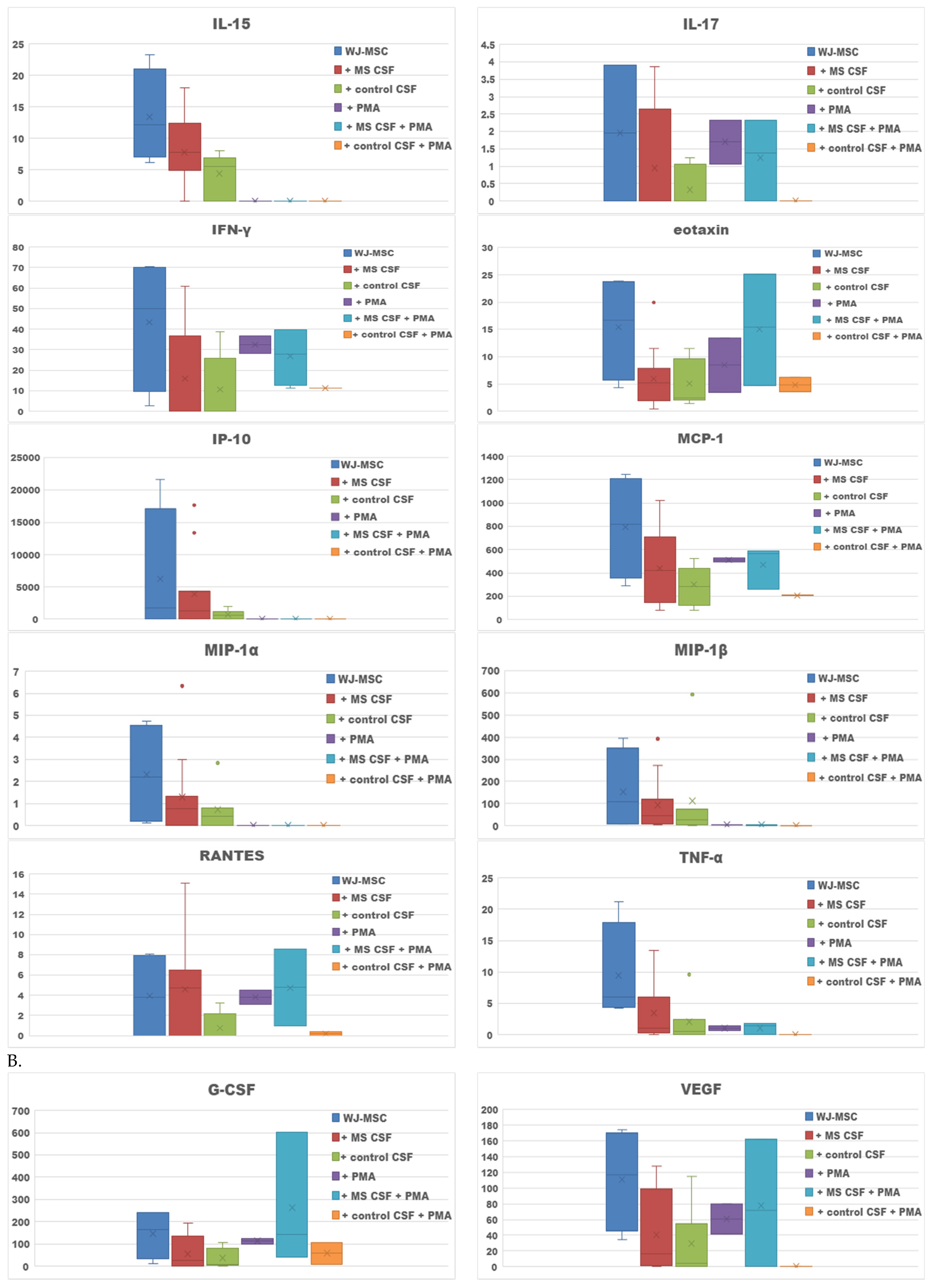
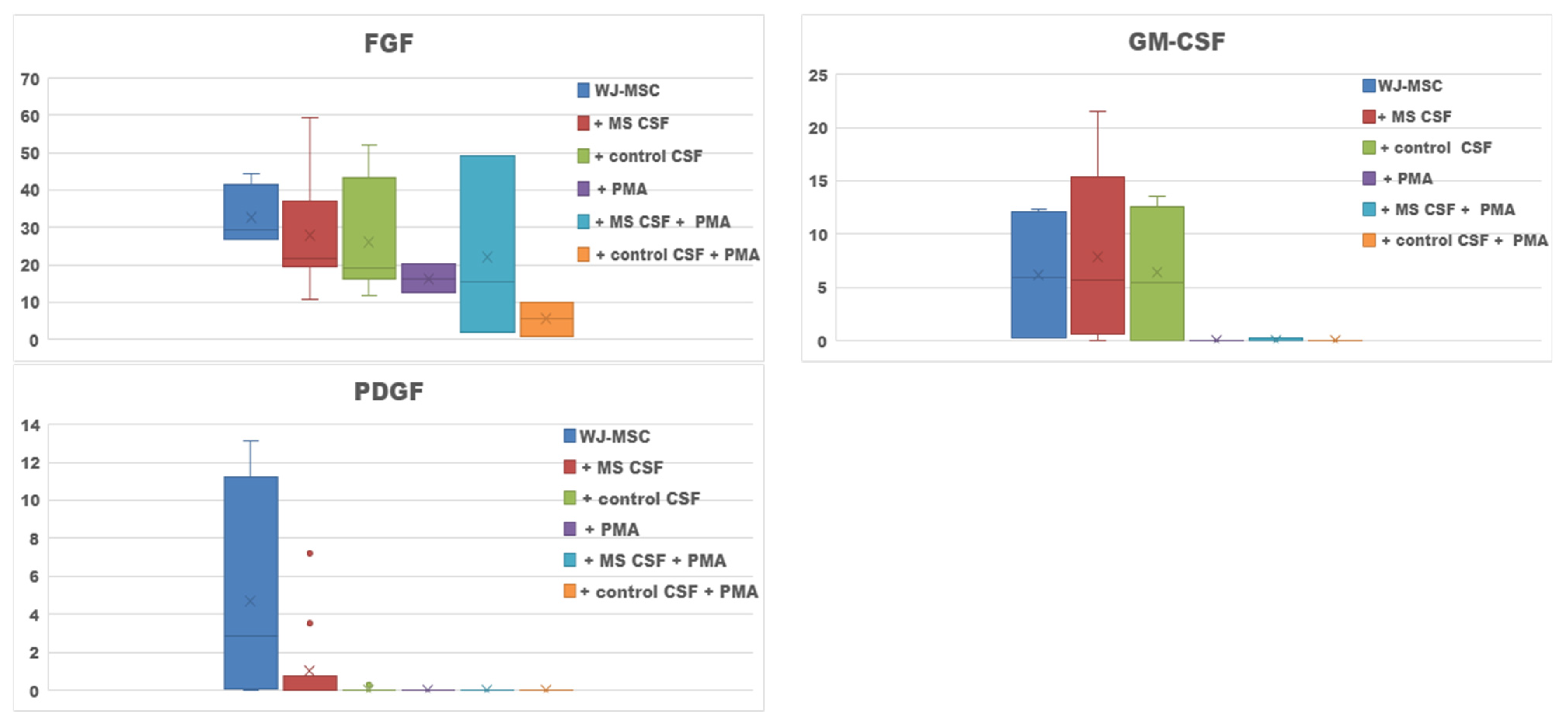
2.1. Secretome of WJ-MSCs Conditioned with MS and Control CSF
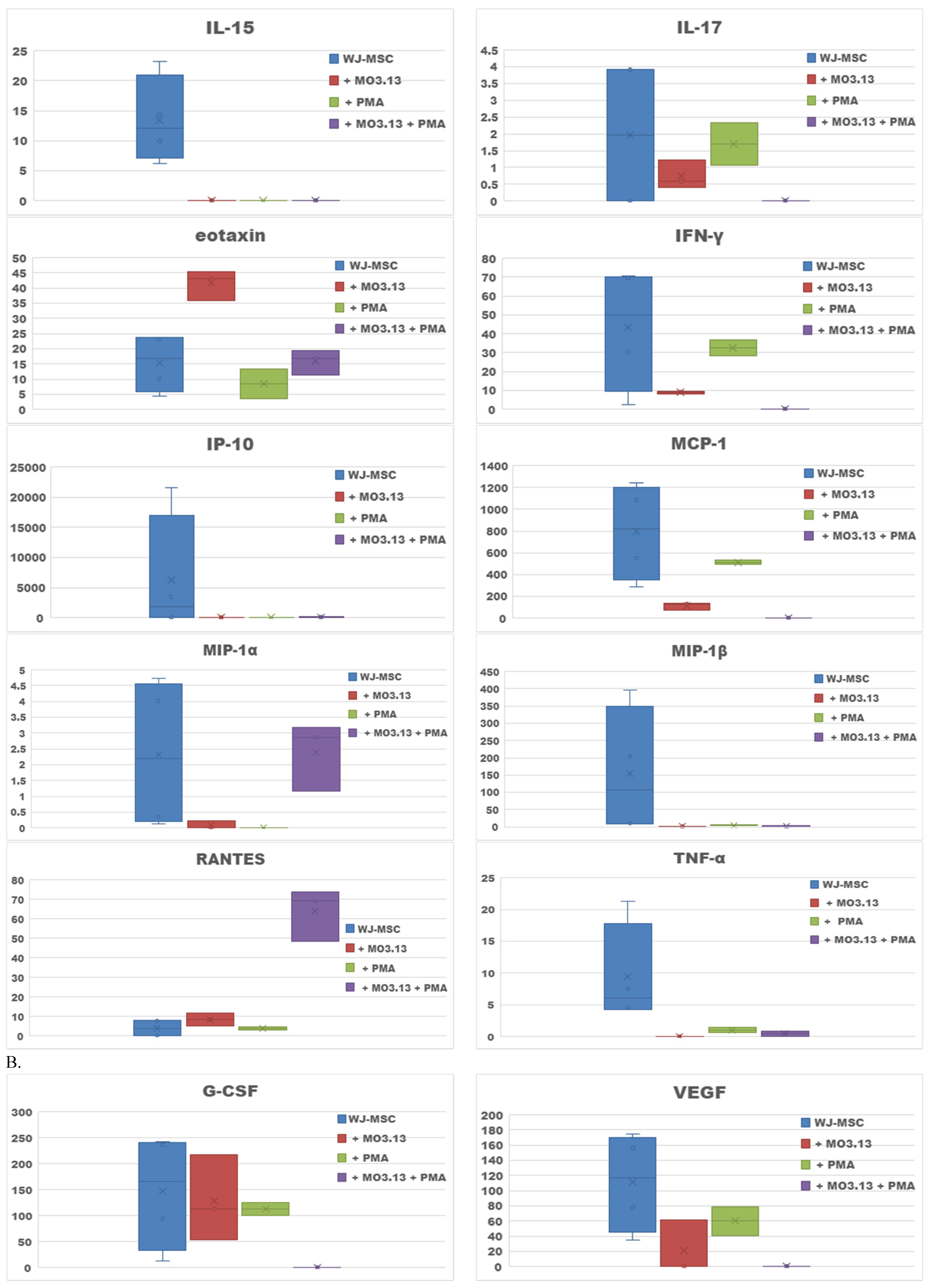
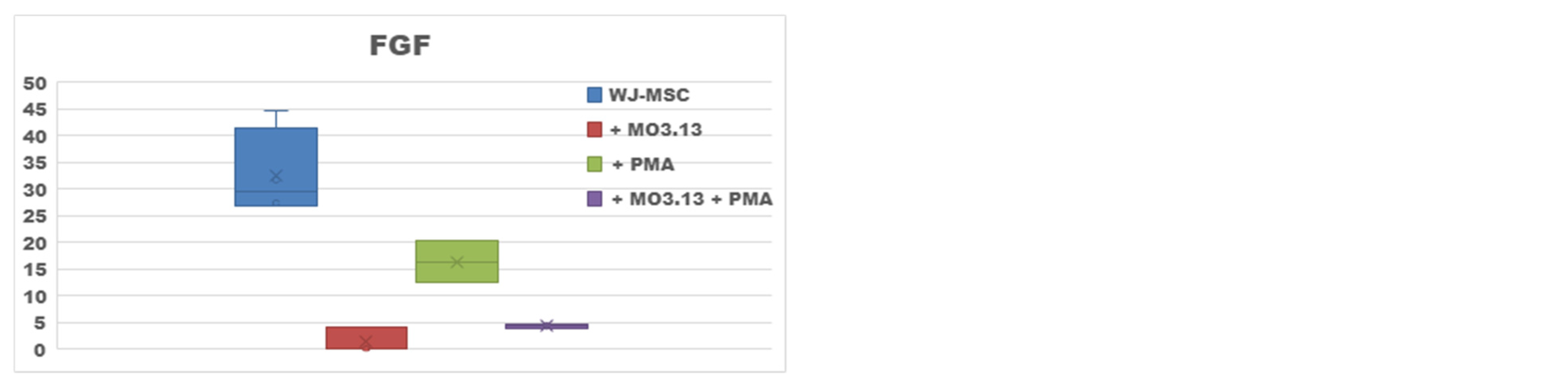
2.2. Effect of Oligodendrocytes Precursor Cell Line Co-Culture on WJ-MSC Secretome
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. WJ-MSCs Culture Conditioning with MS and Healthy Control Cerebrospinal Fluid
4.3. Human Cytokine Multiple Profiling Assay in WJ-MSCs Cultures
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frischer, J.M.; Bramow, S.; Dal-Bianco, A.; Lucchinetti, C.F.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Lassmann, H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009, 132 Pt 5, 1175–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassmann, H. Cortical lesions in multiple sclerosis: Inflammation versus neurodegeneration. Brain 2012, 135 Pt 10, 2904–2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassmann, H. Stem cell and progenitor cell transplantation in multiple sclerosis: The discrepancy between neurobiological attraction and clinical feasibility. J. Neurol. Sci. 2005, 233, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Chen, J.; Cui, Y.; Lu, M.; Elias, S.B.; Mitchell, J.B.; Hammill, L.; Vanguri, P.; Chopp, M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp. Neurol. 2005, 19, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Laterza, C.; Merlini, A.; De Feo, D.; Ruffini, F.; Menon, R.; Onorati, M.; Fredrickx, E.; Muzio, L.; Lombardo, A.; Comi, G.; et al. iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nat. Commun. 2013, 4, 2597. [Google Scholar] [CrossRef] [Green Version]
- Matysiak, M.; Stasiołek, M.; Orłowski, W.; Jurewicz, A.; Janczar, S.; Raine, C.S.; Selmaj, K. Stem cells ameliorate EAE via an indoleamine 2,3-dioxygenase (IDO) mechanism. J. Neuroimmunol. 2008, 193, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Buller, B.A.; Zhang, Z.G.; Zhang, Y.; Lu, M.; Rosene, D.L.; Medalla, M.; Moore, T.L.; Chopp, M. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp. Neurol. 2021, 347, 113895. [Google Scholar] [CrossRef]
- Blakemore, W.F.; Franklin, R.J. Transplantation options for therapeu-tic central nervous system remyelination. Cell Transpl. 2000, 9, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Chari, D.M.; Blakemore, W.F. New insights into remyelination faillure inmultiple sclerosis: Implications for glial cell transplantation. Mult. Scler. J. 2002, 8, 271–277. [Google Scholar] [CrossRef]
- Pluchino, S.; Quattrini, A.; Brambilla, E.; Gritti, A.; Salani, G.; Dina, G.; Galli, R.; Del Carro, U.; Amadio, S.; Bergami, A.; et al. Injection of adult neurospheres induces recovery in a chronic model ofmultiple sclerosis. Nature 2003, 422, 688–694. [Google Scholar] [CrossRef]
- Harris, V.K.; Stark, J.; Vyshkina, T.; Blackshear, L.; Joo, G.; Stefanova, V.; Sara, G.; Sadiq, S.A. Phase I Trial of Intrathecal Mesenchymal Stem Cell-derived Neural Progenitors in Progressive Multiple Sclerosis. EBioMedicine 2018, 29, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karussis, D.; Karageorgiou, C.; Vaknin-Dembinsky, A.; Gowda-Kurkalli, B.; Gomori, J.M.; Kassis, I.; Bulte, J.W.; Petrou, P.; Ben-Hur, T.; Abramsky, O.; et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010, 67, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Petrou, P.; Kassis, I.; Levin, N.; Paul, F.; Backner, Y.; Benoliel, T.; Oertel, F.C.; Scheel, M.; Hallimi, M.; Yaghmour, N.; et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 2020, 143, 3574–3588. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Immunoregulatory function of mesenchymal stem cells. Eur. J. Immunol. 2006, 36, 2566–2573. [Google Scholar] [CrossRef] [PubMed]
- Buddensiek, J.; Dressel, A.; Kowalski, M.; Storch, A.; Sabolek, M. Adult cerebrospinal fluid inhibits neurogenesis but facilitates gliogenesis from fetal rat neural stem cells. J. Neurosci. Res. 2009, 87, 3054–3066. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Cymring, B.; Lu, A.; Rosenthal, H.; Sadiq, S.A. Cerebrospinal fluid derived from progressive multiple sclerosis patients promotes neuronal and oligodendroglial differentiation of human neural precursor cells in vitro. Neuroscience 2013, 250, 614–621. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, D.J.; Geng, T.; Chen, L.; Huang, H.; Yin, H.L.; Zhang, Y.Z.; Lou, J.Y.; Cao, B.; Wang, Y.L. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transpl. 2014, 23 (Suppl. S1), S113–S122. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Wang, H.S.; Hung, S.C.; Peng, S.T.; Huang, C.C.; Wei, H.M.; Guo, Y.J.; Fu, Y.S.; Lai, M.C.; Chen, C.C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.A.; Fraga, J.S.; Grãos, M.; Neves, N.M.; Reis, R.L.; Gimble, J.M.; Sousa, N.; Salgado, A.J. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res. Ther. 2012, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, T.C.; Okamura, L.H.; Baptistella, J.C.; Gameiro, R.; Ferreira, H.L.; Marinho, M.; Flores, E.F. Isolation, characterization and immunomodulatory-associated gene transcription of Wharton’s jelly-derived multipotent mesenchymal stromal cells at different trimesters of cow pregnancy. Cell Tissue Res. 2017, 367, 243–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lublin, F.D.; Bowen, J.D.; Huddlestone, J.; Kremenchutzky, M.; Carpenter, A.; Corboy, J.R.; Freedman, M.S.; Krupp, L.; Paulo, C.; Hariri, R.J.; et al. Human placenta-derived cells (PDA-001) for the treatment of adults with multiple sclerosis: A randomized, placebo-controlled, multiple-dose study. Mult. Scler. Relat. Disord. 2014, 3, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.J.; Zhang, J.F.; Sun, B.; Peng, H.S.; Kong, Q.F.; Bai, S.S.; Liu, Y.M.; Wang, G.Y.; Wang, J.H.; Li, H.L. Reciprocal effect of mesenchymal stem cell on experimental autoimmune encephalomyelitis is mediated by transforming growth factor-beta and interleukin-6. Clin. Exp. Immunol. 2009, 158, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Donders, R.; Vanheusden, M.; Bogie, J.F.; Ravanidis, S.; Thewissen, K.; Stinissen, P.; Gyselaers, W.; Hendriks, J.J.; Hellings, N. Human Wharton’s Jelly-Derived Stem Cells Display Immunomodulatory Properties and Transiently Improve Rat Experimental Autoimmune Encephalomyelitis. Cell Transpl. 2015, 24, 2077–2098. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, F.G.; Carvalho, M.M.; Neves-Carvalho, A.; Panchalingam, K.M.; Behie, L.A.; Pinto, L.; Sousa, N.; Salgado, A.J. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev. Rep. 2015, 11, 288–297. [Google Scholar] [CrossRef]
- Kiiski, H.; Aänismaa, R.; Tenhunen, J.; Hagman, S.; Ylä-Outinen, L.; Aho, A.; Yli-Hankala, A.; Bendel, S.; Skottman, H.; Narkilahti, S. Healthy human CSF promotes glial differentiation of hESC-derived neural cells while retaining spontaneous activity in existing neuronal networks. Biol. Open. 2013, 2, 605–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, C.; Yin, P.; Ren, N.; Wang, Z.; Wang, J.; Zhang, C.; Ge, W.; Geng, D.; Wang, X. Cerebrospinal fluid-stem cell interactions may pave the path for cell-based therapy in neurological diseases. Stem Cell Res. Ther. 2018, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Zeng, Y.M.; Wan, M.R.; Lu, X.F. Induction of human bone marrow mesenchymal stem cells differentiation into neural-like cells using cerebrospinal fluid. Cell Biochem. Biophys. 2011, 59, 179–184. [Google Scholar] [CrossRef]
- de Sonnaville, S.F.; van Strien, M.E.; Middeldorp, J.; Sluijs, J.A.; van den Berge, S.A.; Moeton, M.; Donega, V.; van Berkel, A.; Deering, T.; De Filippis, L.; et al. The adult human subventricular zone: Partial ependymal coverage and proliferative capacity of cerebrospinal fluid. Brain Commun. 2020, 2, fcaa150. [Google Scholar] [CrossRef]
- Hagman, S.; Mäkinen, A.; Ylä-Outinen, L.; Huhtala, H.; Elovaara, I.; Narkilahti, S. Effects of inflammatory cytokines IFN-γ, TNF-α and IL-6 on the viability and functionality of human pluripotent stem cell-derived neural cells. J. Neuroimmunol. 2019, 331, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Ben-Hur, T.; Einstein, O.; Mizrachi-Kol, R.; Ben-Menachem, O.; Reinhartz, E.; Karussis, D.; Abramsky, O. Transplanted multipotential neural precursor cells migrate into the inflamed white matter in response to experimental autoimmune encephalomyelitis. Glia 2003, 41, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.S.; Hu, S.; Ni, H.T.; Rowen, T.N.; Lokensgard, J.R.; Peterson, P.K. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J. Leukoc. Biol. 2005, 78, 1233–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, J.; Honsek, S.D.; Illes, S.; Wellen, J.M.; Hartung, H.P.; Rose, C.R.; Dihné, M. A new role for interferon gamma in neural stem/precursor cell dysregulation. Mol. Neurodegener. 2011, 6, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Huang, Y.; Zhao, L.; Li, Y.; Sun, L.; Zhou, Y.; Qian, G.; Zheng, J.C. IL-1β and TNF-α induce neurotoxicity through glutamate production: A potential role for neuronal glutaminase. J. Neurochem. 2013, 125, 897–908. [Google Scholar] [CrossRef]
- Janssens, K.; Slaets, H.; Hellings, N. Immunomodulatory properties of the IL-6 cytokine family in multiple sclerosis. Ann. N. Y. Acad. Sci. 2015, 1351, 52–60. [Google Scholar] [CrossRef]
- Petković, F.; Campbell, I.L.; Gonzalez, B.; Castellano, B. Astrocyte-targeted production of interleukin-6 reduces astroglial and microglial activation in the cuprizone demyelination model: Implications for myelin clearance and oligodendrocyte maturation. Glia 2016, 64, 2104–2119. [Google Scholar] [CrossRef]
- Sørensen, T.L.; Tani, M.; Jensen, J.; Pierce, V.; Lucchinetti, C.; Folcik, V.A.; Qin, S.; Rottman, J.; Sellebjerg, F.; Strieter, R.M.; et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Investig. 1999, 103, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Bartosik-Psujek, H.; Stelmasiak, Z. The levels of chemokines CXCL8, CCL2 and CCL5 in multiple sclerosis patients are linked to the activity of the disease. Eur. J. Neurol. 2005, 12, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Donninelli, G.; Studer, V.; Brambilla, L.; Zecca, C.; Peluso, D.; Laroni, A.; Michelis, D.; Mantegazza, R.; Confalonieri, P.; Volpe, E. Immune Soluble Factors in the Cerebrospinal Fluid of Progressive Multiple Sclerosis Patients Segregate Into Two Groups. Front. Immunol. 2021, 12, 633167. [Google Scholar] [CrossRef]
- de Haas, A.H.; van Weering, H.R.; de Jong, E.K.; Boddeke, H.W.; Biber, K.P. Neuronal chemokines: Versatile messengers in central nervous system cell interaction. Mol. Neurobiol. 2007, 36, 137–151. [Google Scholar] [CrossRef] [Green Version]
- Pugazhenthi, S.; Zhang, Y.; Bouchard, R.; Mahaffey, G. Induction of an inflammatory loop by interleukin-1β and tumor necrosis factor-α involves NF-kB and STAT-1 in differentiated human neuroprogenitor cells. PLoS ONE 2013, 8, e69585. [Google Scholar] [CrossRef] [Green Version]
- Widera, D.; Holtkamp, W.; Entschladen, F.; Niggemann, B.; Zänker, K.; Kaltschmidt, B.; Kaltschmidt, C. MCP-1 induces migration of adult neural stem cells. Eur. J. Cell Biol. 2004, 83, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; Fife, B.T.; Begolka, W.S.; Miller, S.D.; Karpus, W.J. Central nervous system chemokine expression during Theiler’s virus-induced demyelinating disease. J. Neurovirol. 1999, 5, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Izikson, L.; Klein, R.S.; Charo, I.F.; Weiner, H.L.; Luster, A.D. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 2000, 192, 1075–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, T.L.; Ransohoff, R.M.; Strieter, R.M.; Sellebjerg, F. Chemokine CCL2 and chemokine receptor CCR2 in early active multiple sclerosis. Eur. J. Neurol. 2004, 11, 445–449. [Google Scholar] [CrossRef]
- Mahad, D.J.; Ransohoff, R.M. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2003; Volume 15, pp. 23–32. [Google Scholar] [CrossRef]
- Sørensen, T.L.; Sellebjerg, F.; Jensen, C.V.; Strieter, R.M.; Ransohoff, R.M. Chemokines CXCL10 and CCL2: Differential involvement in intrathecal inflammation in multiple sclerosis. Eur. J. Neurol. 2001, 8, 665–672. [Google Scholar] [CrossRef]
- Bartosik-Psujek, H.; Stelmasiak, Z. Steroid therapy altered serum levels of CCL2 and CCL5 chemokines in multiple sclerosis patients during relapse. Eur. Neurol. 2004, 52, 237–241. [Google Scholar] [CrossRef]
- Harris, V.K.; Stark, J.W.; Yang, S.; Zanker, S.; Tuddenham, J.; Sadiq, S.A. Mesenchymal stem cell-derived neural progenitors in progressive MS: Two-year follow-up of a phase I study. Neurol. Neuroimmunol. Neuroinflamm. 2020, 8, e928. [Google Scholar] [CrossRef]
- Ge, W.; Ren, C.; Duan, X.; Geng, D.; Zhang, C.; Liu, X.; Chen, H.; Wan, M.; Geng, R. Differentiation of mesenchymal stem cells into neural stem cells using cerebrospinal fluid. Cell Biochem. Biophys. 2015, 71, 449–455. [Google Scholar] [CrossRef]
- Sabolek, M.; Herborg, A.; Schwarz, J.; Storch, A. Dexamethasone blocks astroglial differentiation from neural precursor cells. Neuroreport 2006, 17, 1719–1723. [Google Scholar] [CrossRef]
- Bai, H.; Suzuki, Y.; Noda, T.; Wu, S.; Kataoka, K.; Kitada, M.; Ohta, M.; Chou, H.; Ide, C. Dissemination and proliferation of neural stem cells on the spinal cord by injection into the fourth ventricle of the rat: A method for cell transplantation. J. Neurosci. Methods 2003, 124, 181–187. [Google Scholar] [CrossRef]
- Rodgers, J.M.; Miller, S.D. Cytokine control of inflammation and repair in the pathology of multiple sclerosis. Yale J. Biol. Med. 2012, 85, 447–468. [Google Scholar]
- Joerger-Messerli, M.S.; Thomi, G.; Haesler, V.; Keller, I.; Renz, P.; Surbek, D.; Schoeberlein, A. Human Wharton’s Jelly Mesenchymal Stromal Cell-Derived Small Extracellular Visicles Derive Oligodendroglial Maturation by Restraining MAPK/ERK and Notch Signaling Pathways. Front. Cell Dev. Biol. 2021, 9, 622539. [Google Scholar] [CrossRef] [PubMed]
- Agrelo, I.; Schira-Heinen, J.; Beyer, F.; Groh, J.; Butermann, C.; Estrada, V.; Poschmann, G.; Bribian, A.; Jadasz, J.; Lopez-Mascaraque, L.; et al. Secretome Analysis of Mesenchymal Stem Cell Factors Fostering Oligodendroglial Differentiation of Neural Stem Cells In Vivo. Int. J. Mol. Sci. 2020, 21, 4350. [Google Scholar] [CrossRef] [PubMed]
- Valitsky, M.; Benhamron, S.; Nitzan, K.; Karussis, D.; Ella, E.; Abramsky, O.; Kassism, I.; Rosenmann, H. Cerebrospinal Fluid (CSF) Exchange with Artificial CSF Enriched with Mesenchymal Stem Cell Secretions Ameliorates Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2019, 20, 1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Culture Type | Increased Secretion |
|---|---|
| WJ-MSCs + MS CSF v. WJ-MSCs | Il-12, RANTES, GM-CSF |
| WJ-MSCs + control CSF v. WJ-MSCs | - |
| WJ-MSCs + PMA v. WJ-MSCs | Il-5, Il-6 |
| WJ-MSCs + PMA + MS CSF v. WJ-MSCs + MS CSF | IL-1b, IL-1ra, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-17, IFN-γ, eotaxin, MCP-1, RANTES; G-CSF, VEGF |
| WJ-MSCs + PMA + control CSF v. WJ-MSCs + control CSF | IL-1b, IL-6, IL-8, IFN-γ, eotaxin and RANTES; G-CSF |
| WJ-MSCs + MO3.13 v. WJ-MSCs | Eotaxin, RANTES |
| WJ-MSCs + PMA + MO3.13 v. WJ-MSCs + MO3.13 | MIP-1α, RANTES |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salwierak-Głośna, K.; Piątek, P.; Domowicz, M.; Świderek-Matysiak, M. Effect of Multiple Sclerosis Cerebrospinal Fluid and Oligodendroglia Cell Line Environment on Human Wharton’s Jelly Mesenchymal Stem Cells Secretome. Int. J. Mol. Sci. 2022, 23, 2177. https://doi.org/10.3390/ijms23042177
Salwierak-Głośna K, Piątek P, Domowicz M, Świderek-Matysiak M. Effect of Multiple Sclerosis Cerebrospinal Fluid and Oligodendroglia Cell Line Environment on Human Wharton’s Jelly Mesenchymal Stem Cells Secretome. International Journal of Molecular Sciences. 2022; 23(4):2177. https://doi.org/10.3390/ijms23042177
Chicago/Turabian StyleSalwierak-Głośna, Karolina, Paweł Piątek, Małgorzata Domowicz, and Mariola Świderek-Matysiak. 2022. "Effect of Multiple Sclerosis Cerebrospinal Fluid and Oligodendroglia Cell Line Environment on Human Wharton’s Jelly Mesenchymal Stem Cells Secretome" International Journal of Molecular Sciences 23, no. 4: 2177. https://doi.org/10.3390/ijms23042177






