Gamma-Aminobutyrate Transaminase Protects against Lipid Overload-Triggered Cardiac Injury in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Experimental Protocol
2.3. Intraperitoneal Glucose Tolerance Test
2.4. Echocardiography
2.5. Histological Analysis
2.6. Analysis of Differentially Expressed Genes
2.7. Triglyceride in Heart Tissues
2.8. Determination of Oxidative Stress in Heart Tissues
2.9. Isolation and Cultures of Neonatal Cardiomyocytes
2.10. Bovine Serum Albumin (BSA)-Free Fatty Acid (FFA) Conjugation
2.11. Adenoviral Infection of Cardiomyocytes
2.12. Measurement of Mitochondrial Superoxide Generation in Cardiomyocytes
2.13. ABAT’s GABA Catabolic Activity
2.14. Analysis of Apoptosis
2.15. Real-Time RT-PCR
2.16. Western Blotting
2.17. Statistical Analysis
3. Results
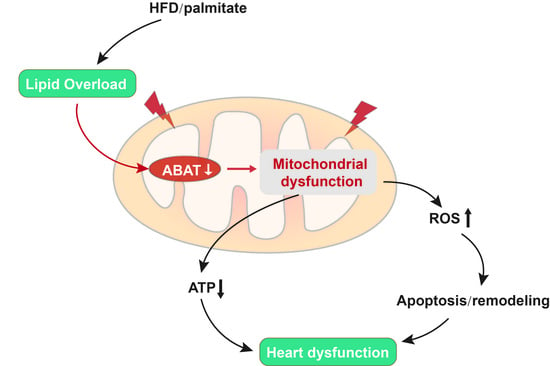
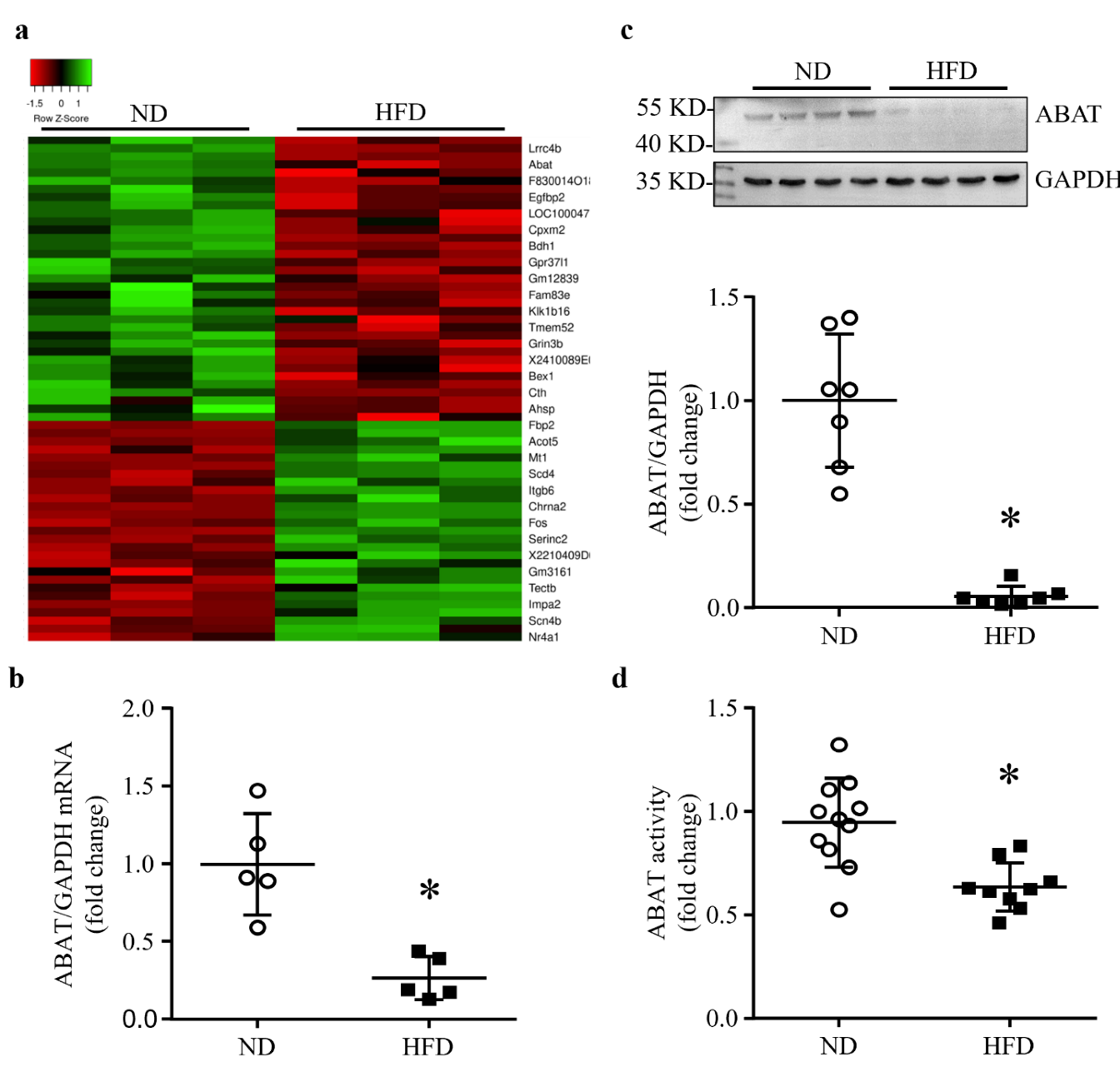
3.1. HFD Feeding Results in a Down-Regulation of ABAT in Mouse Hearts
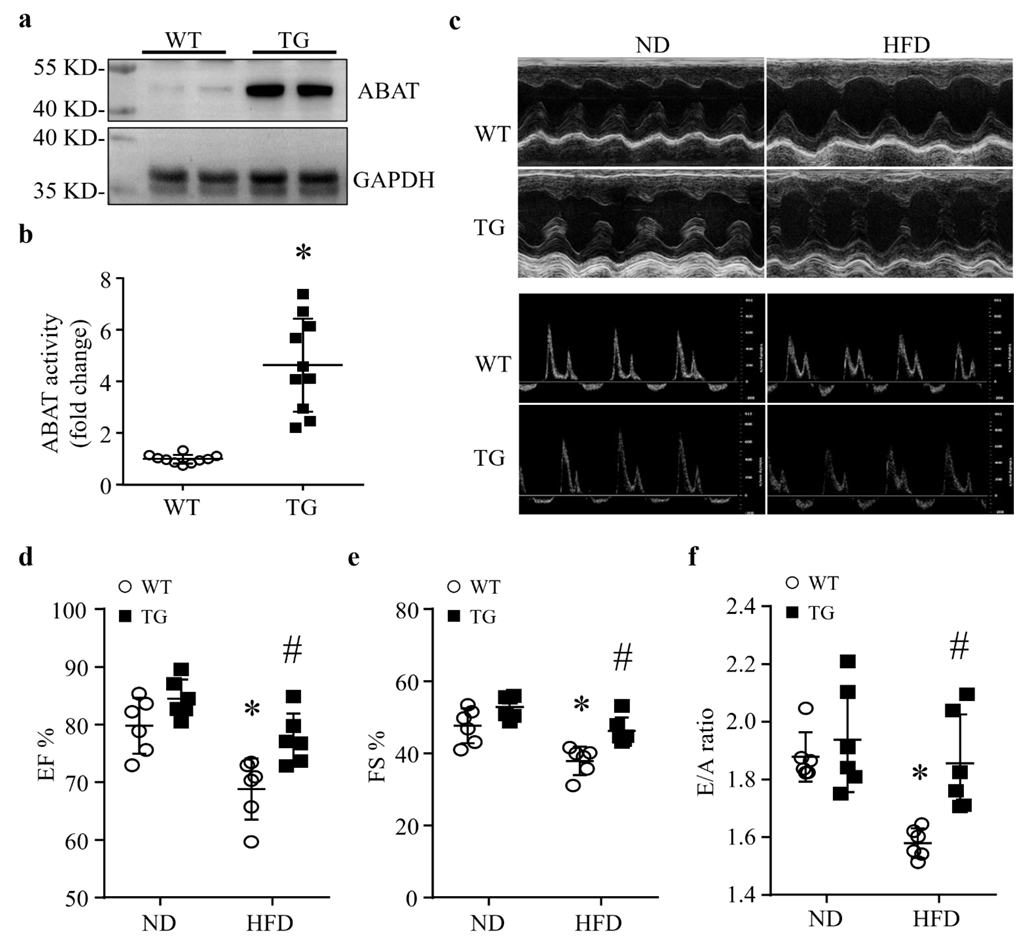
3.2. Cardiomyocyte-Specific Over-Expression of ABAT Reduces Myocardial Dysfunction without Affecting the Systemic Metabolism in Mice Fed a HFD
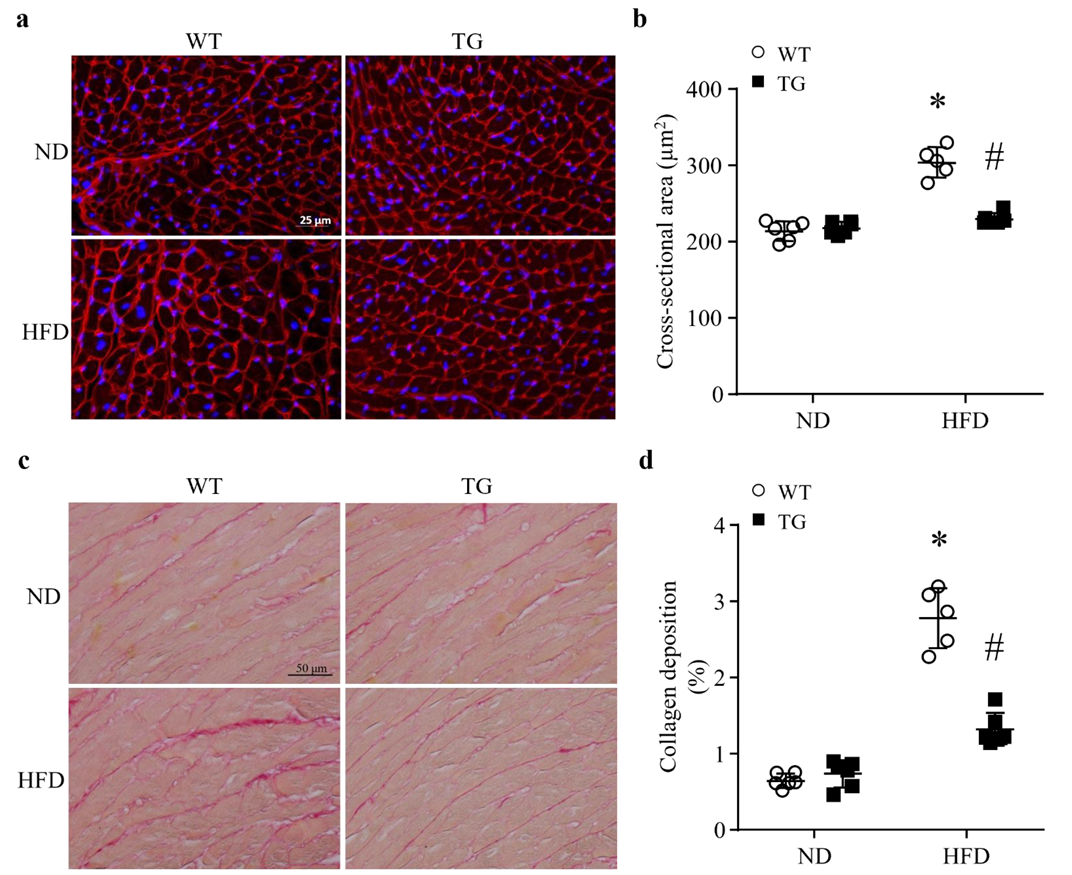
3.3. Lipid Overload-Induced Myocardial Remodeling Is Attenuated by Transgenic ABAT Over-Expression in Mice
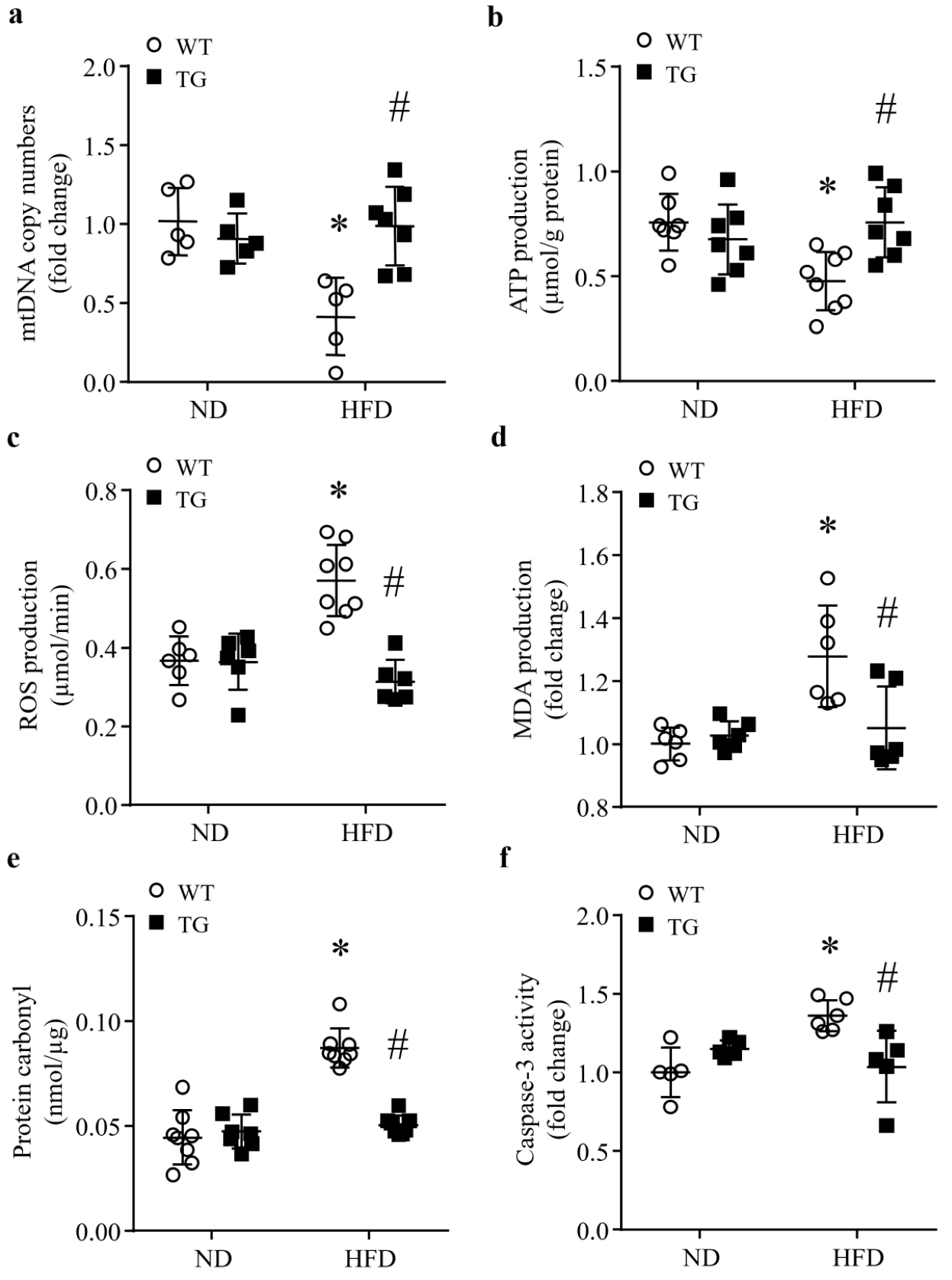
3.4. Transgenic ABAT Over-Expression Improves Mitochondrial Function and Attenuates Oxidative Stress and Apoptosis in HFD-Fed Mouse Hearts
3.5. Over-Expression of ABAT Attenuates Palmitate-Induced Cardiomyocyte Injury In Vitro
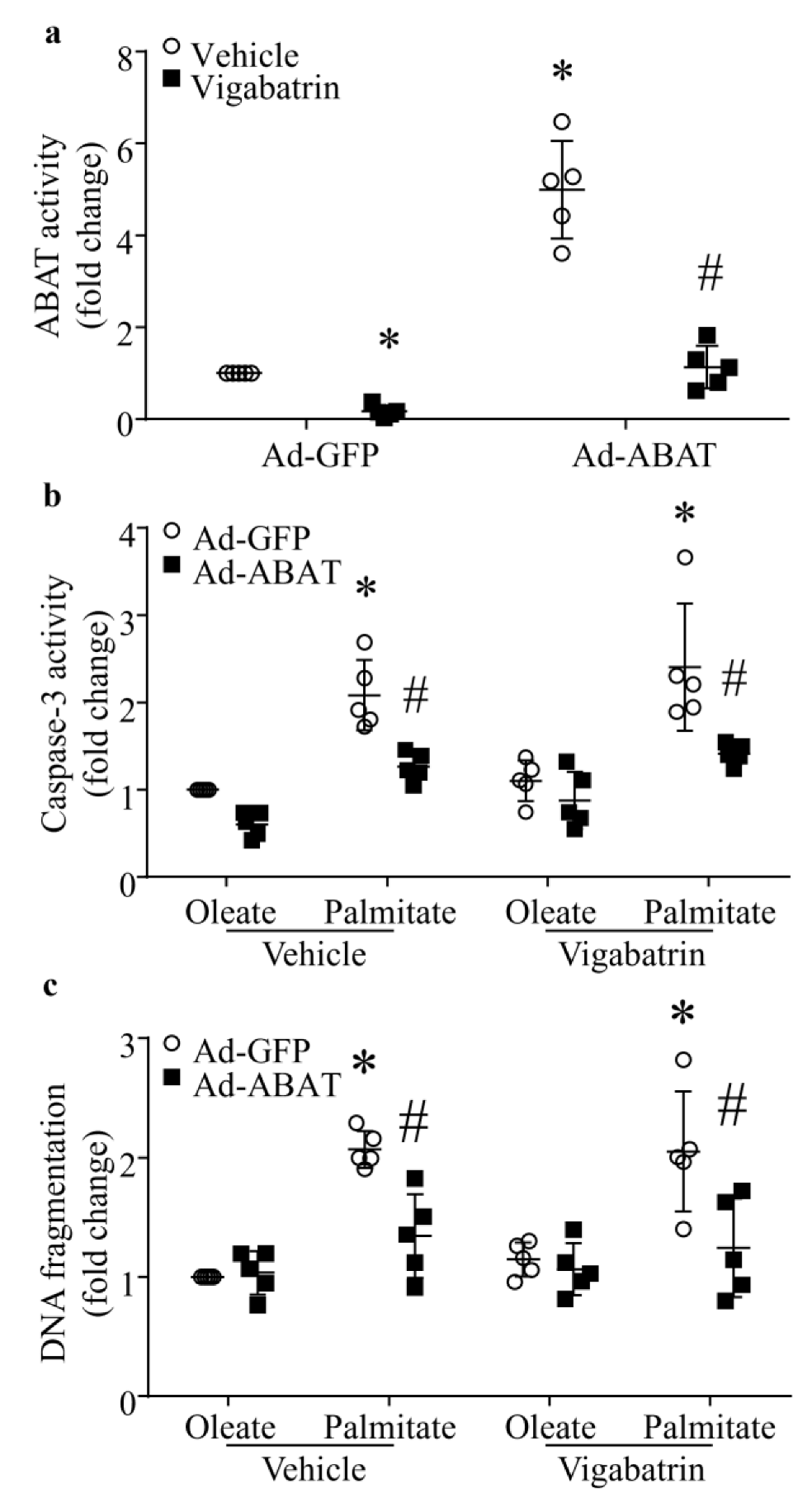
3.6. The Protective Effect of ABAT Over-Expression on Palmitate-Induced Apoptosis Is Independent of Its GABA Catabolic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Lond. Engl. 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Zhang, H.; Cortés, N.G.; Wu, D.; Wang, P.; Zhang, J.; Mattison, J.A.; Smith, E.; Bettcher, L.F.; Wang, M.; et al. Increased Drp1 Acetylation by Lipid Overload Induces Cardiomyocyte Death and Heart Dysfunction. Circ. Res. 2020, 126, 456–470. [Google Scholar] [CrossRef]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar]
- Makrecka-Kuka, M.; Liepinsh, E.; Murray, A.; Lemieux, H.; Dambrova, M.; Tepp, K.; Puurand, M.; Käämbre, T.; Han, W.H.; De Goede, P.; et al. Altered mitochondrial metabolism in the insulin-resistant heart. Acta Physiol. Oxf. Engl. 2019, 228, e13430. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, L.; Ni, R.; Cao, T.; Zheng, D.; Xiong, S.; Greer, P.A.; Fan, G.-C.; Peng, T. Disruption of calpain reduces lipotoxicity-induced cardiac injury by preventing endoplasmic reticulum stress. Biochim. Biophys. Acta 2016, 1862, 2023–2033. [Google Scholar] [CrossRef]
- Chen, X.; Cao, Q.; Liao, R.; Wu, X.; Xun, S.; Huang, J.; Dong, C. Loss of ABAT-mediated GABAergic system promotes basal-like breast cancer progression by activating Ca2+-NFAT1 axis. Theranostics 2019, 9, 34–47. [Google Scholar] [CrossRef]
- Besse, A.; Wu, P.; Bruni, F.; Donti, T.; Graham, B.; Craigen, W.J.; McFarland, R.; Moretti, P.; Lalani, S.; Scott, K.L.; et al. The GABA transaminase, ABAT, is essential for mitochondrial nucleoside metabolism. Cell Metab. 2015, 21, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Ruiz-Velasco, A.; Wang, S.; Khan, S.; Zi, M.; Jungmann, A.; Camacho-Muñoz, M.D.; Guo, J.; Du, G.; Xie, L.; et al. Metabolic stress-induced cardiomyopathy is caused by mitochondrial dysfunction due to attenuated Erk5 signaling. Nat. Commun. 2017, 8, 494. [Google Scholar] [CrossRef]
- Sundquist, K.; Sundquist, J.; Palmer, K.; Memon, A.A. Role of mitochondrial DNA copy number in incident cardiovascular diseases and the association between cardiovascular disease and type 2 diabetes: A follow-up study on middle-aged women. Atherosclerosis 2022, 341, 58–62. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Yuan, K.; Yuan, J. mtDNA in the pathogenesis of cardiovascular diseases. Dis. Markers 2021, 2021, 7157109. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Wang, Y.; Ding, T.; Zhang, X.; Wu, N. Pharmacological postconditioning with sappanone A ameliorates myocardial ischemia reperfusion injury and mitochondrial dysfunction via AMPK-mediated mitochondrial quality control. Toxicol. Appl. Pharmacol. 2021, 427, 115668. [Google Scholar] [CrossRef]
- Sanbe, A.; Gulick, J.; Hanks, M.C.; Liang, Q.; Osinska, H.; Robbins, J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ. Res. 2003, 92, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.; Fan, S.; Zheng, D.; Wang, G.; Yu, Y.; Chen, R.; Song, L.-S.; Fan, G.-C.; Zhang, Z.; Peng, T. Increased calpain-1 in mitochondria induces dilated heart failure in mice: Role of mitochondrial superoxide anion. Basic Res. Cardiol. 2019, 114, 17. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, N.; Wei, M.; Ma, J.; Yu, Y.; Chen, R.; Lacefield, J.C.; Xu, H.; Peng, T. Over-expression of calpastatin aggravates cardiotoxicity induced by doxorubicin. Cardiovasc. Res. 2013, 98, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Takahashi, H.; Fukuda, M.; Hino, H.; Kobayashi, K.; Tanaka, J.; Ishii, E. β-hydroxybutyrate alters GABA-transaminase activity in cultured astrocytes. Brain Res. 2009, 1268, 17–23. [Google Scholar] [CrossRef]
- Teng, X.; Ji, C.; Zhong, H.; Zheng, D.; Ni, R.; Hill, D.J.; Xiong, S.; Fan, G.C.; Greer, P.A.; Shen, Z.; et al. Selective deletion of endothelial cell calpain in mice reduces diabetic cardiomyopathy by improving angiogenesis. Diabetologia 2019, 62, 860–872. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Ma, J.; Yu, Y.; Li, M.; Ni, R.; Wang, G.; Chen, R.; Li, J.; Fan, G.-C.; Lacefield, J.C.; et al. Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia 2015, 58, 1949–1958. [Google Scholar] [CrossRef]
- Liang, L.; Li, H.; Cao, T.; Qu, L.; Zhang, L.; Fan, G.C.; Greer, P.A.; Li, J.; Jones, D.L.; Peng, T. Calpain activation mediates microgravity-induced myocardial abnormalities in mice via p38 and ERK1/2 MAPK pathways. J. Biol. Chem. 2020, 295, 16840–16851. [Google Scholar] [CrossRef]
- Feng, W.; Lei, T.; Wang, Y.; Feng, R.; Yuan, J.; Shen, X.; Wu, Y.; Gao, J.; Ding, W.; Lu, Z. GCN2 deficiency ameliorates cardiac dysfunction in diabetic mice by reducing lipotoxicity and oxidative stress. Free Radic. Biol. Med. 2019, 130, 128–139. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and oleic acid: The Yin and Yang of fatty acids in type 2 diabetes mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Singh, R.; Carson, R.P. Vigabatrin in StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Koenig, M.K.; Hodgeman, R.; Riviello, J.J.; Chung, W.; Bain, J.; Chiriboga, C.A.; Ichikawa, K.; Osaka, H.; Tsuji, M.; Gibson, K.M.; et al. Phenotype of GABA-transaminase deficiency. Neurology 2017, 88, 1919–1924. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Chen, Z.; Zhao, H.; Dong, H.; Zhu, L.; Zhang, Y.; Wang, J.; Zhu, H.; Cui, Q.; Qi, C.; et al. ABAT and ALDH6A1, regulated by transcription factor HNF4A, suppress tumorigenic capability in clear cell renal cell carcinoma. J. Transl. Med. 2020, 18, 101. [Google Scholar] [CrossRef]
- Nesterov, S.V.; Skorobogatova, Y.A.; Panteleeva, A.A.; Pavlik, L.L.; Mikheeva, I.B.; Yaguzhinsky, L.S.; Nartsissov, Y.R. NMDA and GABA receptor presence in rat heart mitochondria. Chem. Biol. Interact. 2018, 291, 40–46. [Google Scholar] [CrossRef]
- Parviz, M.; Vogel, K.; Gibson, K.M.; Pearl, P.L. Disorders of GABA metabolism: SSADH and GABA-transaminase deficiencies. J. Pediatr. Epilepsy 2014, 3, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Bönnighausen, J.; Gebhard, D.; Kröger, C.; Hadeler, B.; Tumforde, T.; Lieberei, R.; Bergemann, J.; Schäfer, W.; Bormann, J. Disruption of the GABA shunt affects mitochondrial respiration and virulence in the cereal pathogen Fusarium graminearum. Mol. Microbiol. 2015, 98, 1115–1132. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Wang, C.; Ying, J.; Xu, T.; McAlinden, A.; O’Keefe, R.J. Inhibition of 4-aminobutyrate aminotransferase protects against injury-induced osteoarthritis in mice. JCI Insight 2019, 4, e128568. [Google Scholar] [CrossRef]
- Besse, A.; Petersen, A.K.; Hunter, J.V.; Appadurai, V.; Lalani, S.R.; Bonnen, P.E. Personalized medicine approach confirms a milder case of ABAT deficiency. Mol. Brain 2016, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Bagul, P.K.; Katare, P.B.; Bugga, P.; Dinda, A.K.; Banerjee, S.K. SIRT-3 Modulation by resveratrol improves mitochondrial oxidative phosphorylation in diabetic heart through deacetylation of TFAM. Cells 2018, 7, 235. [Google Scholar] [CrossRef] [Green Version]
- Yuzefovych, L.V.; Solodushko, V.A.; Wilson, G.L.; Rachek, L.I. Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis, and impaired insulin signaling in rat L6 skeletal muscle cells. Endocrinology 2012, 153, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Paull, T.T. Mitochondria at the crossroads of ATM-mediated stress signaling and regulation of reactive oxygen species. Redox Biol. 2020, 32, 101511. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhong, H.; Cao, T.; Huang, Y.; Ji, X.; Fan, G.-C.; Peng, T. Gamma-Aminobutyrate Transaminase Protects against Lipid Overload-Triggered Cardiac Injury in Mice. Int. J. Mol. Sci. 2022, 23, 2182. https://doi.org/10.3390/ijms23042182
Zhang M, Zhong H, Cao T, Huang Y, Ji X, Fan G-C, Peng T. Gamma-Aminobutyrate Transaminase Protects against Lipid Overload-Triggered Cardiac Injury in Mice. International Journal of Molecular Sciences. 2022; 23(4):2182. https://doi.org/10.3390/ijms23042182
Chicago/Turabian StyleZhang, Mengxiao, Huiting Zhong, Ting Cao, Yifan Huang, Xiaoyun Ji, Guo-Chang Fan, and Tianqing Peng. 2022. "Gamma-Aminobutyrate Transaminase Protects against Lipid Overload-Triggered Cardiac Injury in Mice" International Journal of Molecular Sciences 23, no. 4: 2182. https://doi.org/10.3390/ijms23042182
APA StyleZhang, M., Zhong, H., Cao, T., Huang, Y., Ji, X., Fan, G.-C., & Peng, T. (2022). Gamma-Aminobutyrate Transaminase Protects against Lipid Overload-Triggered Cardiac Injury in Mice. International Journal of Molecular Sciences, 23(4), 2182. https://doi.org/10.3390/ijms23042182