Development of Bactericidal Peptides against Multidrug-Resistant Acinetobacter baumannii with Enhanced Stability and Low Toxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Peptide Design
2.2. Antimicrobial Activity of the Peptides in Normal Media, Serum and Lung Surfactant
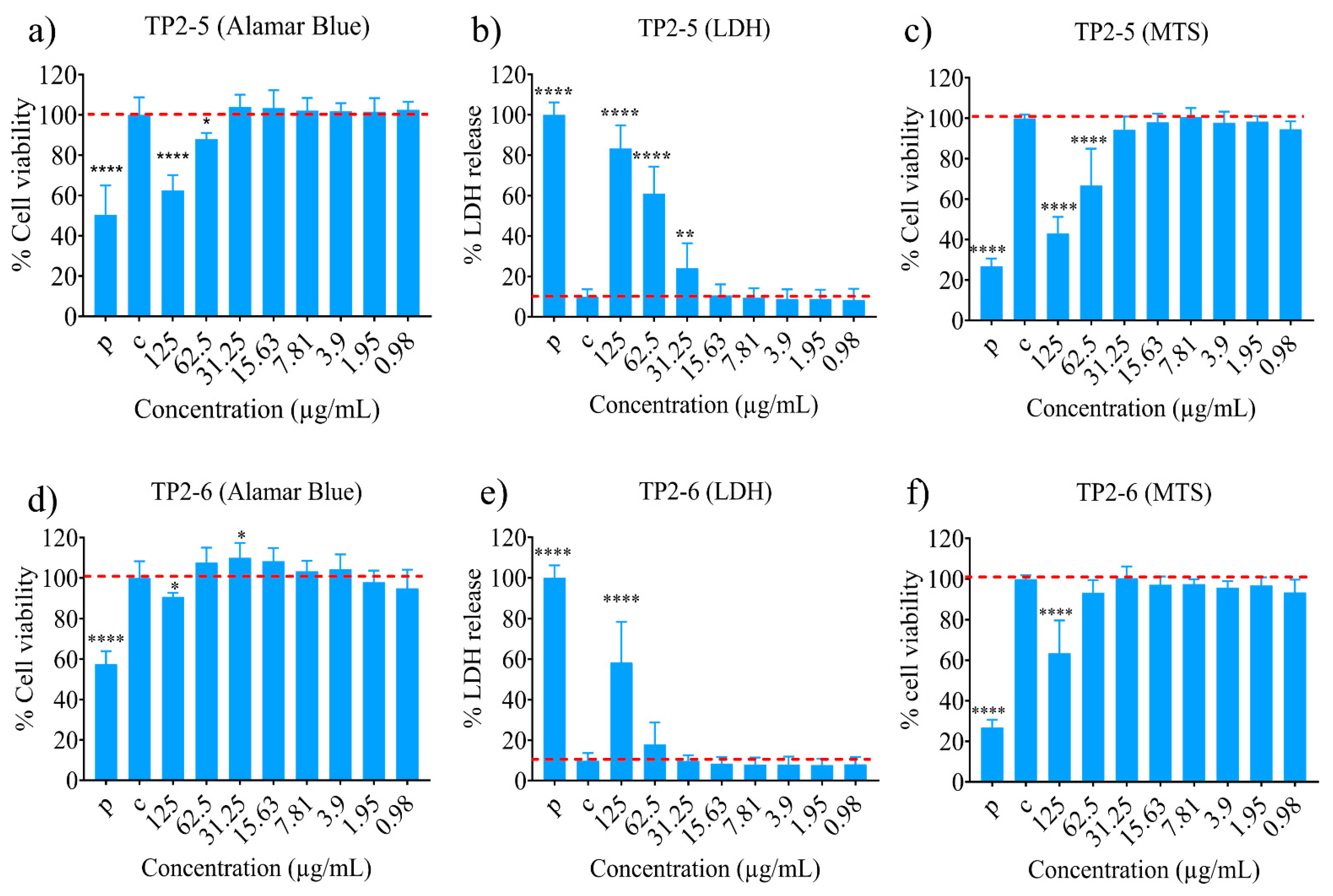
2.3. Cytotoxicity Potential of the Peptides
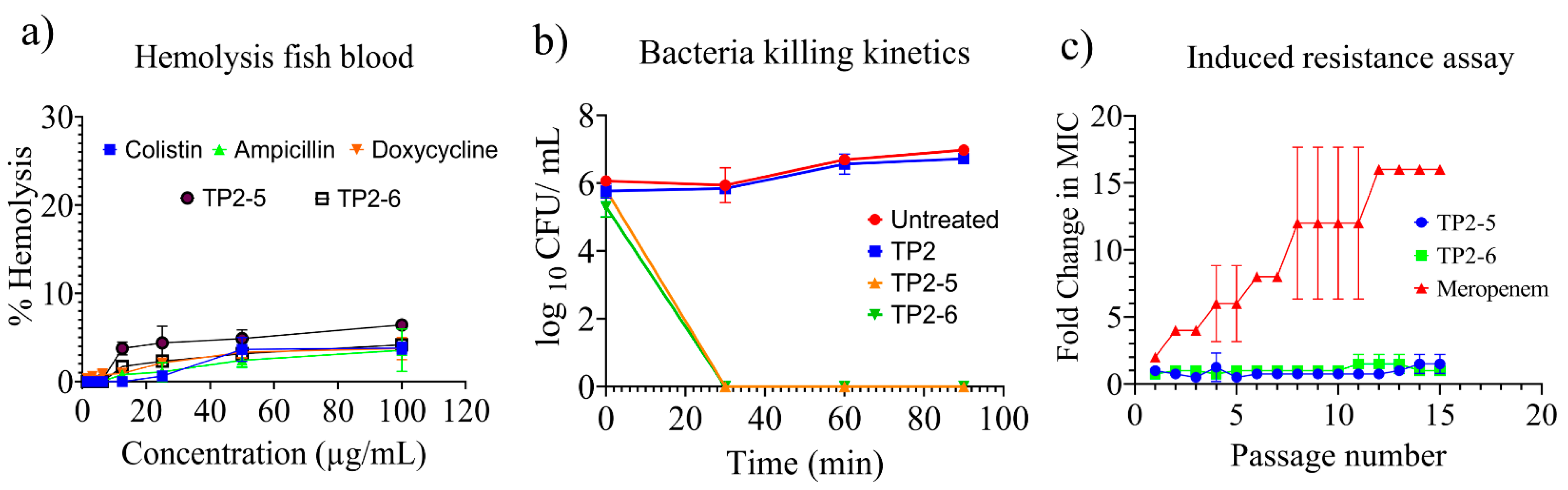
2.4. Haemolysis, Bacterial Killing Kinetics and Induced Resistance Assay
2.5. Antimicrobial Activity of the Peptides in the Presence of Variable Temperature and Physiological Ions
2.6. FIC Index of the Peptides and Antibiotics
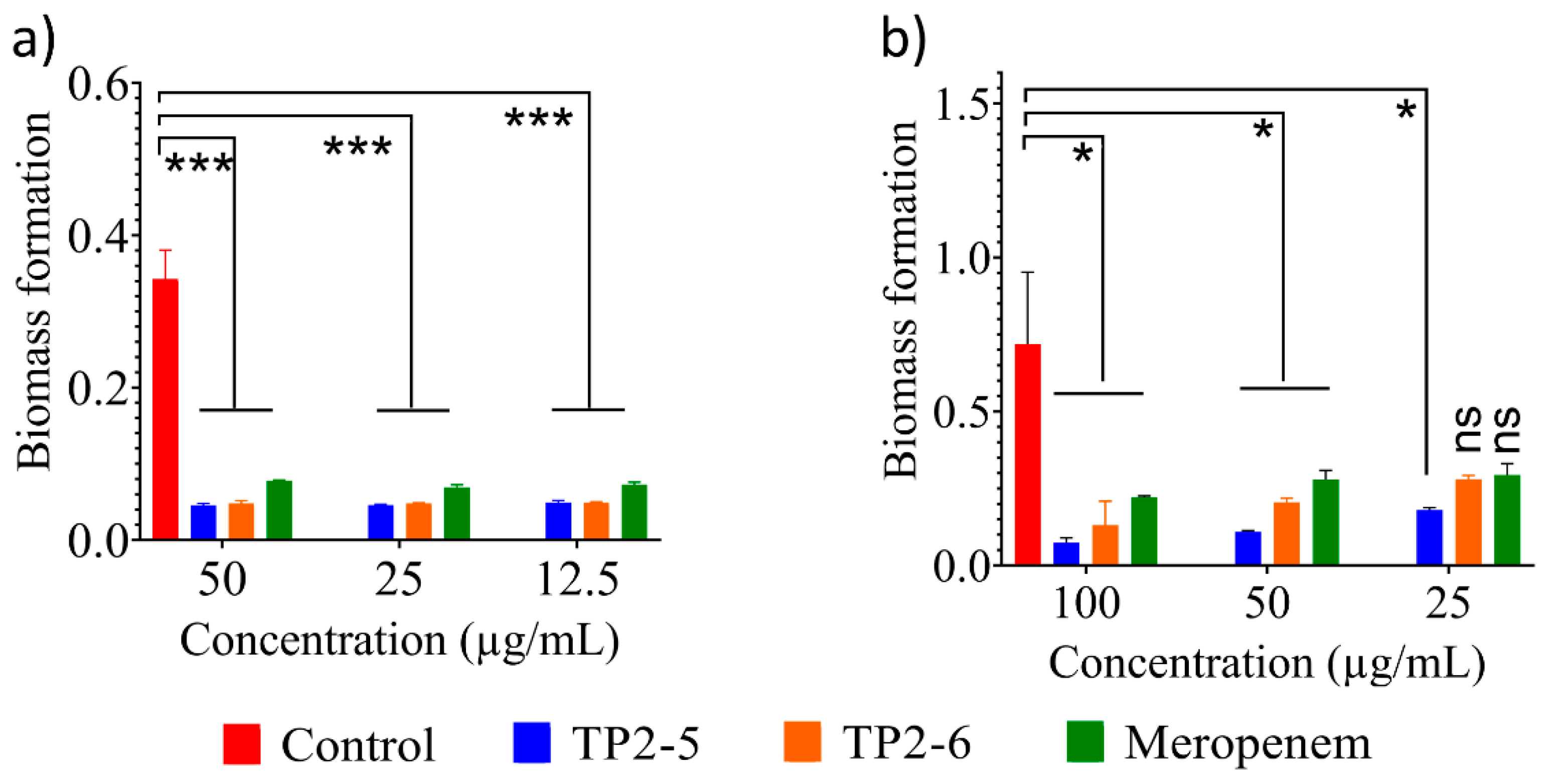
2.7. Antibiofilm Activity of the Peptides
2.8. Membranolytic Activity of the Peptides
3. Materials and Methods
3.1. Peptide Synthesis and Characterization
3.2. Antimicrobial Assay and Bacterial Killing Kinetics
3.3. Fish Blood Haemolysis
3.4. Cytotoxicity Assay
3.5. Antibiofilm Assay
3.6. Peptide Sensitivity to Temperature
3.7. Induced Resistance Assay
3.8. Fractional Inhibitory Concentration (FIC) Index Assay
3.9. Membrane Lysis Assay
3.10. Outer Membrane Permeabilization Assay
3.11. Membrane Depolarization Assay
3.12. Field Emission Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, M.S.; Paterson, D.L. Antibiotics in the clinical pipeline in October 2019. J. Antibiot. 2020, 73, 329–364. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Feldhaus, I.; Özçelik, E.; Hashiguchi, T.C.O.; Cecchini, M. Financial strategies targeting healthcare providers to promote the prudent use of antibiotics: A systematic review of the evidence. Int. J. Antimicrob. Agents 2021, 58, 106446. [Google Scholar] [CrossRef]
- Tan, R.; Huang, Z.; Guo, H.; Weng, Y.; Chow, A. Antibiotic expectations of emergency department patients with upper respiratory tract infections: Clinical and antibiotic use behavioral determinants. Int. J. Antimicrob. Agents 2021, 106511. [Google Scholar] [CrossRef]
- Venkatesan, P. WHO 2020 report on the antibacterial production and development pipeline. Lancet Microbe 2021, 2, e239. [Google Scholar] [CrossRef]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef]
- Elliott, A.G.; Huang, J.X.; Neve, S.; Zuegg, J.; Edwards, I.A.; Cain, A.K.; Boinett, C.J.; Barquist, L.; Lundberg, C.V.; Steen, J.; et al. An amphipathic peptide with antibiotic activity against multidrug-resistant Gram-negative bacteria. Nat. Commun. 2020, 11, 3184. [Google Scholar] [CrossRef]
- Hazam, P.K.; Cheng, C.C.; Lin, W.C.; Hsieh, C.Y.; Chen, J.Y. Strategic modification of low-active natural antimicrobial peptides confers ability to neutralize pathogens in vitro and in vivo. 2022; Unpublished. [Google Scholar]
- Hazam, P.K.; Chen, J.Y. Therapeutic utility of the antimicrobial peptide Tilapia Piscidin 4 (TP4). Aquac. Rep. 2020, 17, 100409. [Google Scholar] [CrossRef]
- Peng, K.C.; Lee, S.H.; Hour, A.L.; Pan, C.-Y.; Lee, L.H.; Chen, J.Y. Five different piscidins from Nile Tilapia, Oreochromis niloticus: Analysis of their expressions and biological functions. PLoS ONE 2012, 7, e50263. [Google Scholar] [CrossRef]
- de la Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E.W. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.W.; Su, B.C.; Chen, J.Y. Tilapia piscidin 4 (TP4) reprograms M1 macrophages to m2 phenotypes in cell models of Gardnerella vaginalis-induced vaginosis. Front. Immunol. 2021, 12, 773013. [Google Scholar] [CrossRef]
- Petersen, P.J.; Jones, C.H.; Bradford, P.A. In vitro antibacterial activities of tigecycline and comparative agents by time-kill kinetic studies in fresh Mueller-Hinton broth. Diagn. Microbiol. Infect. Dis. 2007, 59, 347–349. [Google Scholar] [CrossRef]
- Hazam, P.K.; Singh, A.; Chaudhary, N.; Ramakrishnan, V. Bactericidal potency and extended serum life of stereo-chemically engineered peptides against Mycobacterium. Int. J. Pept. Res. Ther. 2019, 25, 465–472. [Google Scholar] [CrossRef]
- Arechabala, B.; Coiffard, C.; Rivalland, P.; Coiffard, L.J.; de Roeck-Holtzhauer, Y. Comparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH release. J. Appl. Toxicol. 1999, 19, 163–165. [Google Scholar] [CrossRef]
- Nkenfou, C.N.; Mawabo, I.K.; Notedji, A.; Nkenfou, J.; Fokou, P.V.T.; Jouda, J.B.; Kuiate, J.R. In vitro antimycobacterial activity of six Cameroonian medicinal plants using microplate alamarBlue assay. Int. J. Mycobact. 2015, 4, 306–311. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, C.J.; Holt, S.J.; Downes, S.; Marshall, N.J. Microculture tetrazolium assays: A comparison between two new tetrazolium salts, XTT and MTS. J. Immunol. Methods. 1995, 179, 95–103. [Google Scholar] [CrossRef]
- Au-O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar]
- Gopal, R.; Kim, Y.G.; Lee, J.H.; Lee, S.K.; Chae, J.D.; Son, B.K.; Seo, C.H.; Park, Y. Synergistic effects and antibiofilm properties of chimeric peptides against multidrug-resistant Acinetobacter baumannii strains. Antimicrob. Agents Chemother. 2014, 58, 1622–1629. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.Y.; Tsai, T.Y.; Su, B.C.; Hui, C.F.; Chen, J.Y. Study of the antimicrobial activity of tilapia piscidin 3 (TP3) and TP4 and their effects on immune functions in hybrid tilapia (Oreochromis spp.). PLoS ONE 2017, 12, e0169678. [Google Scholar] [CrossRef] [Green Version]
- Ebbensgaard, A.; Mordhorst, H.; Overgaard, M.T.; Nielsen, C.G.; Aarestrup, F.M.; Hansen, E.B. Comparative evaluation of the antimicrobial activity of different antimicrobial peptides against a range of pathogenic bacteria. PLoS ONE 2015, 10, e0144611. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhu, C.; Ren, B.; Yin, X.; Shim, S.H.; Gao, Y.; Zhu, J.; Zhao, P.; Liu, C.; Yu, R.; et al. Two optimized antimicrobial peptides with therapeutic potential for clinical antibiotic-resistant Staphylococcus aureus. Eur. J. Med. Chem. 2019, 183, 111686. [Google Scholar] [CrossRef]
- Li, Q.; Cebrián, R.; Montalbán-López, M.; Ren, H.; Wu, W.; Kuipers, O.P. Outer-membrane-acting peptides and lipid II-targeting antibiotics cooperatively kill Gram-negative pathogens. Commun. Biol. 2021, 4, 31. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Kim, M.K.; Mereuta, L.; Seo, C.H.; Luchian, T.; Park, Y. Mechanism of action of antimicrobial peptide P5 truncations against Pseudomonas aeruginosa and Staphylococcus aureus. AMB Express. 2019, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.D.T.; Voskian, S.; Brown, P.; Liu, A.; Lu, T.K.; Hatton, T.A.; de la Fuente-Nunez, C. Coatable and Resistance-Proof Ionic Liquid for Pathogen Eradication. ACS Nano 2021, 15, 966–978. [Google Scholar] [CrossRef]
- Clementi, E.A.; Marks, L.R.; Roche-Håkansson, H.; Håkansson, A.P. Monitoring changes in membrane polarity, membrane integrity, and intracellular ion concentrations in Streptococcus pneumoniae using fluorescent dyes. J. Vis. Exp. 2014, 84, e51008. [Google Scholar] [CrossRef] [Green Version]

| Peptides/Drugs | TP2-5 (µg/mL) | TP2-6 (µg/mL) | LL-37 (µg/mL) | Meropenem (µg/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial Strain | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Wild strain | A. baumannii 10591 a | 3.125 | 3.125 | 3.125 | 3.125 | >100 | >100 | 1.56 | 1.56 |
| A. baumannii 10591 b | 3.125 | 3.125 | 3.125 | 3.125 | >100 | >100 | 1.56 | 1.56 | |
| MDR strain | 14B0091 | 3.125 | 3.125 | 3.125–6.25 | 3.125–6.25 | 12.5–25 | 12.5–25 | >100 | >100 |
| 2088 | 3.125 | 3.125 | 3.125 | 3.125 | 25 | 25 | >100 | >100 | |
| 921 | 3.125 | 3.125 | 6.25 | 6.25 | 12.5 | 12.5 | 100 | >100 | |
| 1019 | 3.125 | 3.125 | 6.25–12.5 | 6.25–12.5 | 12.5 | 12.5 | 25 | 25 | |
| 1033 | 3.125 | 3.125 | 6.25 | 6.25 | 25 | 25 | 50 | 100 | |
| 1607 | 1.56 | 1.56 | 6.25 | 6..25 | 12.5 | 12.5 | 50 | 50 | |
| 1702 | 3.125 | 3.125 | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 | |
| 2962 | 3.125–6.25 | 6.25 | 3.125–6.25 | 6.25 | 25 | 25 | 50 | 50 | |
| 2982 | 3.125 | 3.125–6.25 | 3.125 | 3.125 | 25 | 25–50 | >100 | >100 | |
| 2997 | 1.56–3.125 | 1.56–3.125 | 6.25 | 6.25 | 12.5 | 12.5 | 50–100 | 50–100 | |
| 2998 | 1.56–3.125 | 1.56–3.125 | 3.125–6.25 | 3.125–6.25 | 25 | 50 | >100 | >100 | |
| 3618 | 3.125 | 3.125 | 6.25 | 6.25 | 12.5 | 12.5 | 50–100 | 50–100 | |
| 14B0091 c | 12.5 | 12.5 | 50 | 50 | >100 | >100 | >100 | >100 | |
| A. baumannii | TP2-5 (µg/mL) | TP2-6 (µg/mL) | Meropenem (µg/mL) | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| 40 °C | 6.25 | 6.25 | 12.5 | 12.5 | 1.56 | 1.56 |
| 60 °C | 6.25 | 6.25 | 12.5 | 12.5 | 1.56 | 1.56 |
| 80 °C | 6.25 | 6.25 | 12.5 | 12.5 | 3.125 | 3.125 |
| 100 °C | 6.25 | 6.25 | 12.5 | 12.5 | 50 | 50 |
| A. baumannii | TP2-5 (µg/mL) | TP2-6 (µg/mL) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| CaCl2 | 12.5 | 12.5 | 50 | 50 |
| MgCl2 | 3.125 | 3.125 | 12.5 | 12.5 |
| NH4Cl | 1.56 | 1.56 | 3.125 | 3.125 |
| KCl | 3.125 | 3.125 | 3.125 | 3.125 |
| NaCl | 6.25 | 6.25 | 12.5 | 12.5 |
| Glucose | 1.56 | 6.25 | 6.25 | 6.25 |
| Bacteria | MIC Alone (µg/mL) | MIC in Combination (µg/mL) | FICI | Interaction | ||
|---|---|---|---|---|---|---|
| TP2-5 | Meropenem | TP2-5 | Meropenem | |||
| 14B001 | 3.125 | 400 | 1.56 | 200 | 1 | Additive |
| 2088 | 3.125 | 200 | 1.56 | 100 | 1 | Additive |
| 921 | 3.125 | 100 | 0.0976 | 100 | 0.53 | Additive |
| 1019 | 3.125 | 25 | 0.78 | 12.5 | 0.75 | Additive |
| 1033 | 3.125 | 50 | 0.0976 | 50 | 1.03 | Indifferent |
| 1607 | 1.56 | 25 | 0.0488 | 25 | 0.53 | Additive |
| 1702 | 3.125 | 12.5 | 0.0976 | 12.5 | 0.53 | Additive |
| 2962 | 6.25 | 50 | (0.19 | 25 | 0.53 | Additive |
| 2982 | 3.125 | 100 | 1.56 | 100 | 1.5 | Indifferent |
| 2997 | 3.125 | 100 | 1.56 | 50 | 1 | Additive |
| 2998 | 3.125 | 200 | 0.096 | 200 | 1.03125 | Indifferent |
| 3618 | 3.125 | 100 | 0.096 | 50 | 0.28125 | Synergistic |
| Bacteria | MIC Alone (µg/mL) | MIC in Combination (µg/mL) | FICI | Interaction | ||
|---|---|---|---|---|---|---|
| TP2-6 | Meropenem | TP2-6 | Meropenem | |||
| 14B001 | 6.25 | 400 | 3.125 | 200 | 1 | Additive |
| 2088 | 3.125 | 200 | 1.56 | 100 | 1 | Additive |
| 921 | 6.25 | 100 | 0.39 | 50 | 0.5625 | Additive |
| 1019 | 12.5 | 25 | 3.125 | 12.5 | 0.375 | Synergistic |
| 1033 | 6.25 | 50 | 3.125 | 25 | 1 | Additive |
| 1607 | 6.25 | 25 | 1.56 | 25 | 0.75 | Additive |
| 1702 | 6.25 | 12.5 | 3.125 | 1.56 | 0.625 | Additive |
| 2962 | 6.25 | 50 | 0.78 | 25 | 0.625 | Additive |
| 2982 | 3.125 | 100 | 0.097 | 100 | 1.03125 | Indifferent |
| 2997 | 6.25 | 100 | 1.56/3.125 | 50/25 | 0.75 | Additive |
| 2998 | 6.25 | 200 | 3.125 | 100 | 1 | Additive |
| 3618 | 6.25 | 100 | 3.125 | 25 | 0.625 | Additive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazam, P.K.; Cheng, C.-C.; Hsieh, C.-Y.; Lin, W.-C.; Hsu, P.-H.; Chen, T.-L.; Lee, Y.-T.; Chen, J.-Y. Development of Bactericidal Peptides against Multidrug-Resistant Acinetobacter baumannii with Enhanced Stability and Low Toxicity. Int. J. Mol. Sci. 2022, 23, 2191. https://doi.org/10.3390/ijms23042191
Hazam PK, Cheng C-C, Hsieh C-Y, Lin W-C, Hsu P-H, Chen T-L, Lee Y-T, Chen J-Y. Development of Bactericidal Peptides against Multidrug-Resistant Acinetobacter baumannii with Enhanced Stability and Low Toxicity. International Journal of Molecular Sciences. 2022; 23(4):2191. https://doi.org/10.3390/ijms23042191
Chicago/Turabian StyleHazam, Prakash Kishore, Chin-Cheng Cheng, Chu-Yi Hsieh, Wen-Chun Lin, Po-Hsien Hsu, Te-Li Chen, Yi-Tzu Lee, and Jyh-Yih Chen. 2022. "Development of Bactericidal Peptides against Multidrug-Resistant Acinetobacter baumannii with Enhanced Stability and Low Toxicity" International Journal of Molecular Sciences 23, no. 4: 2191. https://doi.org/10.3390/ijms23042191






