Salicylic Acid in Root Growth and Development
Abstract
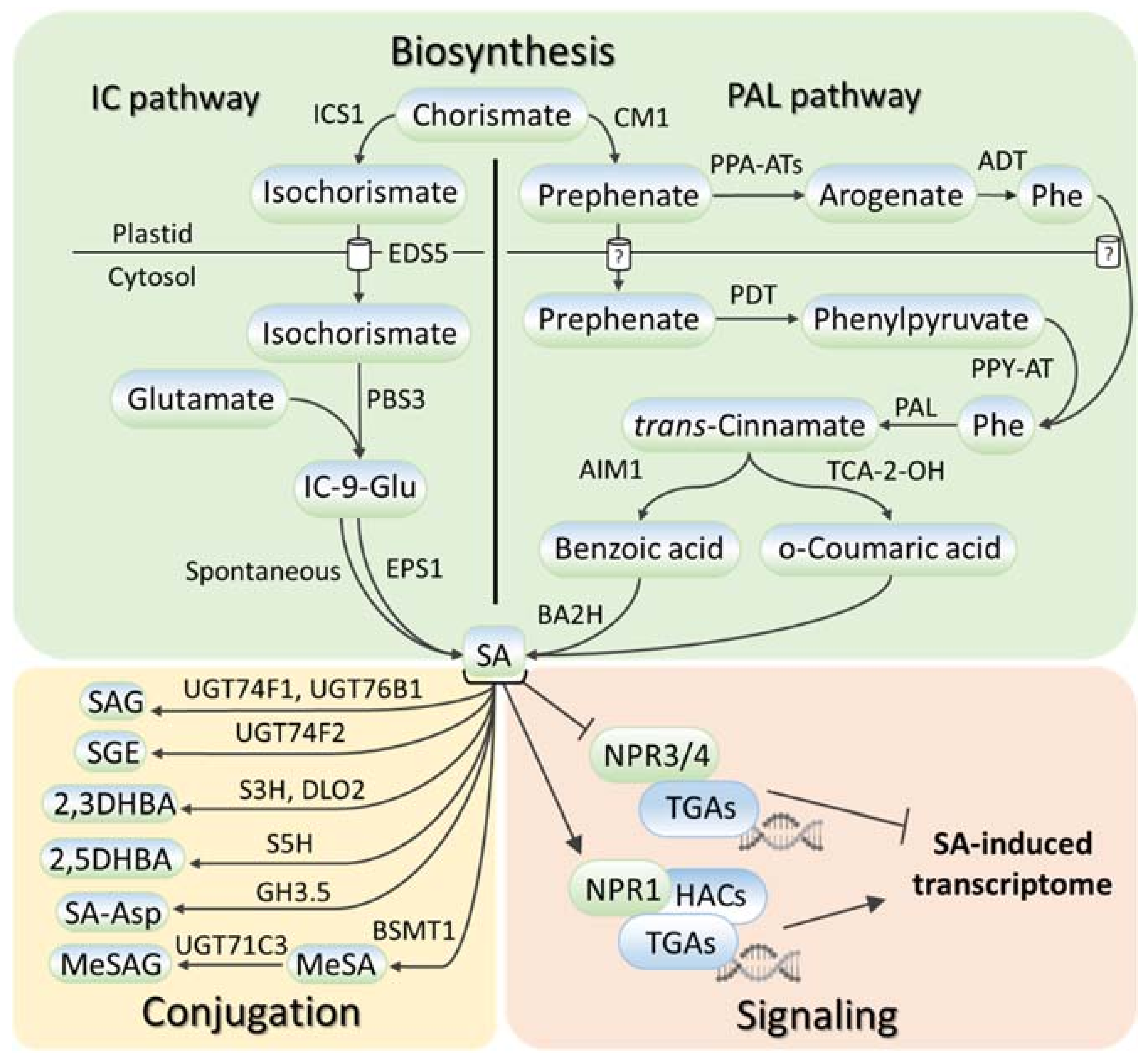
:1. Introduction
2. SA Metabolism and Signaling in Plants
3. Modulation of Endogenous SA Levels in Roots
4. SA Regulates Root Morphology in a Concentration-Dependent Manner
4.1. Regulation of Radicle Emergence
4.2. SA Impact on Root Length
4.3. SA Regulates the Development of Lateral Roots
4.4. SA Regulates the Development of Adventitious Roots
5. SA Acts Mainly via the Regulation of Auxin Distribution in the Root
6. SA Regulates Columella Development
7. SA Controls Radial Root Patterning
8. SA Alleviates Changes in Root System Morphology Induced by Abiotic Stresses
9. SA Couples Root Morphology and Plant–Soil Biota Interactions
10. Conclusions: SA Links Stress Response and Development
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Raskin, I. Salicylate, A New Plant Hormone. Plant Physiol. 1992, 99, 799–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Kong, M.; Freeman, A.; Chen, H.; Liu, F. More stories to tell: NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1, a salicylic acid receptor. Plant Cell Environ. 2021, 44, 1716–1727. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Iyer, S.; Caplan, A.; Klessig, D.F.; Fan, B. Differential accumulation of salicylic acid and salicylic acid-sensitive catalase in different rice tissues. Plant Physiol. 1997, 114, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakhmankulova, Z.F.; Fedyaev, V.V.; Rakhmatulina, S.R.; Ivanov, C.P.; Gilvanova, I.R.; Usmanov, I.Y. The effect of wheat seed presowing treatment with salicylic acid on its endogenous content, activities of respiratory pathways, and plant antioxidant status. Russ. J. Plant Physiol. 2010, 57, 778–783. [Google Scholar] [CrossRef]
- Sašek, V.; Janda, M.; Delage, E.; Puyaubert, J.; Guivarc’h, A.; López Maseda, E.; Dobrev, P.I.; Caius, J.; Bóka, K.; Valentová, O.; et al. Constitutive salicylic acid accumulation in pi4kIIIβ1β2 Arabidopsis plants stunts rosette but not root growth. New Phytol. 2014, 203, 805–816. [Google Scholar] [CrossRef]
- Janda, M.; Šašek, V.; Ruelland, E. The Arabidopsis pi4kIIIβ1β2 double mutant is salicylic acid-overaccumulating: A new example of salicylic acid influence on plant stature. Plant Signal Behav. 2014, 9, e977210. [Google Scholar] [CrossRef] [Green Version]
- Armengot, L.; Marquès-Bueno, M.M.; Soria-Garcia, A.; Müller, M.; Munné-Bosch, S.; Martínez, M.C. Functional interplay between protein kinase CK 2 and salicylic acid sustains PIN transcriptional expression and root development. Plant J. 2014, 78, 411–423. [Google Scholar] [CrossRef] [Green Version]
- König, S.; Feussner, K.; Schwarz, M.; Kaever, A.; Iven, T.; Landesfeind, M.; Ternes, P.; Karlovsky, P.; Lipka, V.; Feussner, I. Arabidopsis mutants of sphingolipid fatty acid α-hydroxylases accumulate ceramides and salicylates. New Phytol. 2012, 196, 1086–1097. [Google Scholar] [CrossRef]
- Meng, Z.; Ruberti, C.; Gong, Z.; Brandizzi, F. CPR5 modulates salicylic acid and the unfolded protein response to manage tradeoffs between plant growth and stress responses. Plant J. 2017, 89, 486–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Zhao, H.; Ruan, W.; Deng, M.; Wang, F.; Peng, J.; Luo, J.; Chen, Z.; Yi, K. ABNORMAL INFLORESCENCE MERISTEM1 Functions in Salicylic Acid Biosynthesis to Maintain Proper Reactive Oxygen Species Levels for Root Meristem Activity in Rice. Plant Cell 2017, 29, 560–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusumi, K.; Yaeno, T.; Kojo, K.; Hirayama, M.; Hirokawa, D.; Yara, A.; Iba, K. The role of salicylic acid in the glutathione-mediated protection against photooxidative stress in rice. Physiol. Plant. 2006, 128, 651–661. [Google Scholar] [CrossRef]
- Stacey, G.; McAlvin, C.B.; Kim, S.Y.; Olivares, J.; Soto, M.J. Effects of endogenous salicylic acid on nodulation in the model legumes Lotus japonicus and Medicago truncatula. Plant Physiol. 2006, 141, 1473–1481. [Google Scholar] [CrossRef] [Green Version]
- Raskin, I.; Skubatz, H.; Tang, W.; Meeuse, B.J. Salicylic acid levels in thermogenic and non-thermogenic plants. Ann. Bot. 1990, 66, 369–373. [Google Scholar] [CrossRef]
- Mishra, A.K.; Baek, K.H. Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria. Biomolecules 2021, 11, 705. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic Acid: Biosynthesis and Signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Hartmann, M.; Zeier, J. N-hydroxypipecolic acid and salicylic acid: A metabolic duo for systemic acquired resistance. Curr. Opin. Plant Biol. 2019, 50, 44–57. [Google Scholar] [CrossRef]
- Li, P.; Cai, Q.; Wang, H.; Li, S.; Cheng, J.; Li, H.; Yu, Q.; Wu, S. Hydrogen peroxide homeostasis provides beneficial micro-environment for SHR-mediated periclinal division in Arabidopsis root. New Phytol. 2020, 228, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Takács, Z.; Poór, P.; Borbély, P.; Czékus, Z.; Szalai, G.; Tari, I. H2O2 homeostasis in wild-type and ethylene-insensitive Never ripe tomato in response to salicylic acid treatment in normal photoperiod and in prolonged darkness. Plant Physiol. Biochem. 2018, 126, 74–85. [Google Scholar] [CrossRef]
- Xia, X.J.; Zhou, Y.H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and Extracellular Journey of the Phytohormone Salicylic Acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic Acid Affects Root Meristem Patterning via Auxin Distribution in a Concentration-Dependent Manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef]
- Tan, S.; Abas, M.; Verstraeten, I.; Glanc, M.; Molnár, G.; Hajný, J.; Lasák, P.; Petřík, I.; Russinova, E.; Petrášek, J.; et al. Salicylic Acid Targets Protein Phosphatase 2A to Attenuate Growth in Plants. Curr. Biol. 2020, 30, 381–395. [Google Scholar] [CrossRef] [Green Version]
- Klessig, D.F.; Tian, M.; Choi, H.W. Multiple Targets of Salicylic Acid and Its Derivatives in Plants and Animals. Front. Immunol. 2016, 7, 206. [Google Scholar] [CrossRef] [Green Version]
- Slaymaker, D.H.; Navarre, D.A.; Clark, D.; Del Pozo, O.; Martin, G.B.; Klessig, D.F. The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 11640–11645. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.W.; Manohar, M.; Manosalva, P.; Tian, M.; Moreau, M.; Klessig, D.F. Activation of Plant Innate Immunity by Extracellular High Mobility Group Box 3 and Its Inhibition by Salicylic Acid. PLoS Pathog. 2016, 12, e1005518. [Google Scholar] [CrossRef] [Green Version]
- Manohar, M.; Wang, D.; Manosalva, P.M.; Choi, H.W.; Kombrink, E.; Klessig, D.F. Members of the abscisic acid co-receptor PP2C protein family mediate salicylic acid-abscisic acid crosstalk. Plant Direct. 2017, 1, e00020. [Google Scholar] [CrossRef] [Green Version]
- Innes, R. The Positives and Negatives of NPR: A Unifying Model for Salicylic Acid Signaling in Plants. Cell 2018, 173, 1314–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokotylo, I.; Kravets, V.; Ruelland, E. Salicylic Acid Binding Proteins (SABPs): The Hidden Forefront of Salicylic Acid Signalling. Int. J. Mol. Sci. 2019, 20, 4377. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xu, S.; Ding, P.; Wang, D.; Cheng, Y.T.; He, J.; Gao, M.; Xu, F.; Li, Y.; Zhu, Z.; et al. Control of salicylic acid synthesis and systemic acquired resistance by two members of a plant-specific family of transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18220–18225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef] [PubMed]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Del Rio, T.G.; Jones, C.D.; Tringe, S.G.; et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Coninck, B.; Timmermans, P.; Vos, C.; Cammue, B.P.; Kazan, K. What lies beneath: Belowground defense strategies in plants. Trends Plant Sci. 2015, 20, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Segarra, G.; Jáuregui, O.; Casanova, E.; Trillas, I. Simultaneous quantitative LC-ESI-MS/MS analyses of salicylic acid and jasmonic acid in crude extracts of Cucumis sativus under biotic stress. Phytochemistry 2006, 67, 395–401. [Google Scholar] [CrossRef]
- Branch, C.; Hwang, C.F.; Navarre, D.A.; Williamson, V.M. Salicylic acid is part of the Mi-1-mediated defense response to root-knot nematode in tomato. Mol. Plant Microbe Interact. 2004, 17, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Wubben, M.J.E.; Jin, J.; Baum, T.J. Cyst nematode parasitism of Arabidopsis thaliana is inhibited by salicylic acid (SA) and elicits uncoupled SA-independent pathogenesis-related gene expression in roots. Mol. Plant Microbe Interact. 2008, 21, 424–432. [Google Scholar] [CrossRef] [Green Version]
- De Vleesschauwer, D.; Van Buyten, E.; Satoh, K.; Balidion, J.; Mauleon, R.; Choi, I.R.; Vera-Cruz, C.; Kikuchi, S.; Höfte, M. Brassinosteroids antagonize gibberellin- and salicylate-mediated root immunity in rice. Plant Physiol. 2012, 158, 1833–1846. [Google Scholar] [CrossRef] [Green Version]
- Denancé, N.; Ranocha, P.; Oria, N.; Barlet, X.; Rivière, M.P.; Yadeta, K.A.; Hoffmann, L.; Perreau, F.; Clément, G.; Maia-Grondard, A.; et al. Arabidopsis wat1 (walls are thin1)-mediated resistance to the bacterial vascular pathogen, Ralstonia solanacearum, is accompanied by cross-regulation of salicylic acid and tryptophan metabolism. Plant J. 2013, 73, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Agtuca, B.; Rieger, E.; Hilger, K.; Song, L.; Robert, C.A.; Erb, M.; Karve, A.; Ferrieri, R.A. Carbon-11 reveals opposing roles of auxin and salicylic acid in regulating leaf physiology, leaf metabolism, and resource allocation patterns that impact root growth in Zea mays. J. Plant Growth Regul. 2014, 33, 328–339. [Google Scholar] [CrossRef]
- Lemarié, S.; Robert-Seilaniantz, A.; Lariagon, C.; Lemoine, J.; Marnet, N.; Jubault, M.; Manzanares-Dauleux, M.J.; Gravot, A. Both the Jasmonic Acid and the Salicylic Acid Pathways Contribute to Resistance to the Biotrophic Clubroot Agent Plasmodiophora brassicae in Arabidopsis. Plant Cell Physiol. 2015, 56, 2158–2168. [Google Scholar]
- Yang, Z.M.; Wang, J.; Wang, S.H.; Xu, L.L. Salicylic acid-induced aluminum tolerance by modulation of citrate efflux from roots of Cassia tora L. Planta 2003, 217, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Song, F.; Zhu, X.; You, J.; Yang, Z.; Li, X. Salicylic acid alleviates aluminum toxicity in soybean roots through modulation of reactive oxygen species metabolism. Front. Chem. 2017, 5, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajti, J.; Németh, E.; Glatz, G.; Janda, T.; Pál, M. Pattern of changes in salicylic acid-induced protein kinase (SIPK) gene expression and salicylic acid accumulation in wheat under cadmium exposure. Plant Biol. 2019, 21, 1176–1180. [Google Scholar] [CrossRef]
- Zhao, Q.; Gu, C.; Sun, Y.; Li, G.; Li, L.L.; Hao, L. Root defense in salicylic acid-altering Arabidopsis plants in responses to cadmium stress. J. Plant Growth Regul. 2021, 40, 1764–1776. [Google Scholar] [CrossRef]
- Wang, D.H.; Li, X.X.; Su, Z.K.; Ren, H.X. The role of salicylic acid in response of two rice cultivars to chilling stress. Biol. Plant. 2009, 53, 545. [Google Scholar] [CrossRef]
- Dong, C.J.; Li, L.; Shang, Q.M.; Liu, X.Y.; Zhang, Z.G. Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 2014, 240, 687–700. [Google Scholar] [CrossRef]
- Bandurska, H.; Stroiński, A. The effect of salicylic acid on barley response to water deficit. Acta Physiol. Plant. 2005, 27, 379–386. [Google Scholar] [CrossRef]
- Bandurska, H.; Cieślak, M. The interactive effect of water deficit and UV-B radiation on salicylic acid accumulation in barley roots and leaves. Environ. Exp. Bot. 2013, 94, 9–18. [Google Scholar] [CrossRef]
- Su, H.; Song, S.; Yan, X.; Fang, L.; Zeng, B.; Zhu, Y. Endogenous salicylic acid shows different correlation with baicalin and baicalein in the medicinal plant Scutellaria baicalensis Georgi subjected to stress and exogenous salicylic acid. PLoS ONE 2018, 13, e0192114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Yang, Y.; Liu, K.; Zhang, L.; Guo, H.; Sun, T.; Wang, H. Involvement of endogenous salicylic acid in iron-deficiency responses in Arabidopsis. J. Exp. Bot. 2016, 67, 4179–4193. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, S.; Zhang, S.; Ge, C.; Shen, Q.; Ma, H.; Zhang, X.; Dong, H.; Zhao, X.; Pang, C. Nitrogen modulates cotton root morphology by affecting abscisic acid (ABA) and salicylic acid (SA) content. Arch. Agron. Soil Sci. 2020, 67, 1722–1738. [Google Scholar] [CrossRef]
- Khan, A.; Kamran, M.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Al-Amri, I.; Lee, I.J.; Khan, A.L. Silicon and salicylic acid confer high-pH stress tolerance in tomato seedlings. Sci. Rep. 2019, 9, 1–16. [Google Scholar]
- Guo, B.; Liu, C.; Liang, Y.; Li, N.; Fu, Q. Salicylic Acid Signals Plant Defence against Cadmium Toxicity. Int, J. Mol. Sci. 2019, 20, 2960. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.T.; Liu, Y.P.; Huang, W.D. Root-fed salicylic acid in grape involves the response caused by aboveground high temperature. J. Integr. Plant Biol. 2008, 50, 761–767. [Google Scholar] [CrossRef]
- Dong, C.J.; Liu, X.Y.; Xie, L.L.; Wang, L.L.; Shang, Q.M. Salicylic acid regulates adventitious root formation via competitive inhibition of the auxin conjugation enzyme CsGH3. 5 in cucumber hypocotyls. Planta 2020, 252, 1–15. [Google Scholar] [CrossRef]
- Szalai, G.; Horgosi, S.; Soós, V.; Majláth, I.; Balázs, E.; Janda, T. Salicylic acid treatment of pea seeds induces its de novo synthesis. J. Plant Physiol. 2011, 168, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Cai, S.Y.; Ruan, X.L.; Chen, S.Y.; Mei, G.F.; Ruan, G.H.; Cao, D.D. Salicylic acid enhances sunflower seed germination under Zn2+ stress via involvement in Zn2+ metabolic balance and phytohormone interactions. Sci. Hortic. 2021, 275, 109702. [Google Scholar] [CrossRef]
- Moravcová, Š.; Tůma, J.; Dučaiová, Z.K.; Waligórski, P.; Kula, M.; Saja, D.; Słomka, A.; Bąba, W.; Libik-Konieczny, M. Influence of salicylic acid pretreatment on seeds germination and some defence mechanisms of Zea mays plants under copper stress. Plant Physiol. Biochem. 2018, 122, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Bowling, S.A.; Clarke, J.D.; Liu, Y.; Klessig, D.F.; Dong, X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 1997, 9, 1573–1584. [Google Scholar]
- Clough, S.J.; Fengler, K.A.; Yu, I.C.; Lippok, B.; Smith, R.K., Jr.; Bent, A.F. The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA 2000, 97, 9323–9328. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, Y.; Xu, T.; Srivastava, A.K.; Wang, D.; Zeng, L.; Yang, L.; He, L.; Zhang, H.; Zheng, Z.; et al. The chromatin remodeler DDM1 promotes hybrid vigor by regulating salicylic acid metabolism. Cell Discov. 2016, 2, 6027. [Google Scholar] [CrossRef]
- Obroucheva, N.V.; Lityagina, S.V.; Sinkevich, I.A. Activation and activity of plasma membrane H+-ATPase: Key events in germinating Vicia faba seeds. Seed Sci. Res. 2021, 31, 76–82. [Google Scholar] [CrossRef]
- Sliwinska, E.; Bassel, G.W.; Bewley, J.D. Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocotyl. J. Exp. Bot. 2009, 60, 3587–3594. [Google Scholar] [CrossRef] [Green Version]
- Rajasekaran, L.R.; Claude, A.S.; Caldwell, D. Stand establishment in processing carrots—Effects of various temperature regimes on germination and the role of salicylates in promoting germination at low temperatures. Can. J. Plant Sci. 2002, 82, 443–450. [Google Scholar] [CrossRef]
- Singh, P.K.; Chaturvedi, V.K.; Bose, B. Effects of salicylic acid on seedling growth and nitrogen metabolism in cucumber (Cucumis sativus L.). J. Stress Physiol. Biochem. 2010, 6, 102–113. [Google Scholar]
- Bahrani, A.; Pourreza, J. Gibberellic acid and salicylic acid effects on seed germination and seedlings growth of wheat (Triticum aestivum L.) under salt stress condition. World Appl. Sci. J. 2012, 18, 633–641. [Google Scholar]
- Dolatabadian, A.; Modarres Sanavy, S.A.M.; Sharifi, M. Effect of salicylic acid and salt on wheat seed germination. Acta Agric. Scand. Sect. B Soil Plant Sci. 2009, 59, 456–464. [Google Scholar] [CrossRef]
- Pasternak, T.; Rudas, V.; Potters, G.; Jansen, M.A. Morphogenic effects of abiotic stress: Reorientation of growth in Arabidopsis thaliana seedlings. Environ. Exp. Bot. 2005, 53, 299–314. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.G.; Park, C.M. Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytologist. 2010, 188, 626–637. [Google Scholar] [CrossRef]
- Rajjou, L.; Belghazi, M.; Huguet, R.; Robin, C.; Moreau, A.; Job, C.; Job, D. Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol. 2006, 141, 910–923. [Google Scholar] [CrossRef] [Green Version]
- Guan, L.; Scandalios, J.G. Developmentally related responses of maize catalase genes to salicylic acid. Proc. Natl. Acad. Sci. USA 1995, 92, 5930–5934. [Google Scholar] [CrossRef] [Green Version]
- Pour, A.P.; Farahbakhsh, H.; Saffari, M.; Keramat, B. Effects of seed priming on germination and seedling growth under salinity stress in fenugreek. Int. J. Agric. Crop Sci. 2012, 4, 779–786. [Google Scholar]
- Liu, J.; Li, L.; Yuan, F.; Chen, M. Exogenous salicylic acid improves the germination of Limonium bicolor seeds under salt stress. Plant Signal. Behav. 2019, 14, e1644595. [Google Scholar] [CrossRef]
- Balouchi, H.; Dehkordi, S.A.; Dehnavi, M.M.; Benzadi, B. Effect of priming types on germination. South-West. J. Hortic. Biol. Environ. 2015, 6, 1–20. [Google Scholar]
- Kalaivani, K.; Kalaiselvi, M.M.; Senthil-Nathan, S. Effect of methyl salicylate (MeSA), an elicitor on growth, physiology and pathology of resistant and susceptible rice varieties. Sci. Rep. 2016, 6, 34498. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chen, Q.; Hussain, S.; Mei, J.; Dong, H.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Pre-sowing seed treatments in direct-seeded early rice: Consequences for emergence, seedling growth and associated metabolic events under chilling stress. Sci. Rep. 2016, 6, 19637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saberi, M.; Shahriari, A.; Da Silva, J.A.T.; Tarnian, F.; Tavili, A. Influence of salicylic acid on Bromus tomentellus germination and initial growth properties under cadmium stress. Plant Stress 2012, 6, 44–48. [Google Scholar]
- Kuiters, A.T. Effects of phenolic acids on germination and early growth of herbaceous woodland plants. J. Chem. Ecol. 1989, 15, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Dutt, M.; Kester, S.; Geneve, R. Elevated levels of ethylene during germination reduces the time to emergence in impatiens. In Proceedings of the XXVI International Horticultural Congress: Issues and Advances in Transplant Production and Stand Establishment Research, Toronto, ON, Canada, 11 August 2002; Volume 631, pp. 43–47. [Google Scholar]
- Hermann, K.; Meinhard, J.; Dobrev, P.; Linkies, A.; Pesek, B.; Heß, B.; Macháčková, I.; Fischer, U.; Leubner-Metzger, G. 1-Aminocyclopropane-1-carboxylic acid and abscisic acid during the germination of sugar beet (Beta vulgaris L.): A comparative study of fruits and seeds. J. Exp. Bot. 2007, 58, 3047–3060. [Google Scholar] [CrossRef] [PubMed]
- Siriwitayawan, G.; Dutt, M.; Kester, S.; Downie, B.; Geneve, R.; Nicolás, G.; Pritchard, H.W. Ageing in tomato reduces the capacity of seeds to produce ethylene, whyle priming increases ethylene evolution during germination. Biol. Seeds Recent Res. Adv. 2003, 441–446. [Google Scholar]
- Nojavan-Asghari, M.; Ishizawa, K. Inhibitory effects of methyl jasmonate on the germination and ethylene production in cocklebur seeds. J. Plant Growth Regul. 1998, 17, 13–18. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Zalewski, K.; Głowacka, K.; Nitkiewicz, B.; Amarowicz, R. Effect of Methyl Jasmonate on Carbohydrate Composition, α-Amylase Activity and Growth of Triticale (Triticosecale Witmmack) Seedlings. J. Agr. Sci. Techol. 2017, 19, 1127–1137. [Google Scholar]
- Norastehnia, A.; Sajedi, R.H.; Nojavan-Asghari, M. Inhibitory effects of methyl jasmonate on seed germination in maize (Zea mays): Effect on α-amylase activity and ethylene production. Gen. Appl. Plant Physiol. 2007, 33, 13–23. [Google Scholar]
- Huang, Y.; Sun, M.M.; Ye, Q.; Wu, X.Q.; Wu, W.H.; Chen, Y.F. Abscisic acid modulates seed germination via ABA INSENSITIVE5-mediated PHOSPHATE1. Plant Physiol. 2017, 175, 1661–1668. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, Y.S.; Kim, S.G.; Jung, J.H.; Woo, J.C.; Park, C.M. Integration of auxin and salt signals by the NAC transcription factor NTM2 during seed germination in Arabidopsis. Plant Physiol. 2011, 156, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Ghassemian, M.; Nambara, E.; Cutler, S.; Kawaide, H.; Kamiya, Y.; McCourt, P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant. Cell 2000, 12, 1117–11126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaudoin, N.; Serizet, C.; Gosti, F.; Giraudat, J. Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 2000, 12, 1103–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, W.; Ye, T.; Yao, X.; Liu, X.; Ma, S.; Chen, X.; Chen, M.L.; Wu, Y. The dioxygenase GIM2 functions in seed germination by altering gibberellin production in Arabidopsis. J. Integr. Plant Biol. 2018, 60, 276–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkelstein, R.R.; Lynch, T.J. Abscisic acid inhibition of radicle emergence but not seedling growth is suppressed by sugars. Plant Physiol. 2000, 122, 1179–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abley, K.; Formosa-Jordan, P.; Tavares, H.; Chan, E.Y.; Afsharinafar, M.; Leyser, O.; Locke, J.C. An ABA-GA bistable switch can account for natural variation in the variability of Arabidopsis seed germination time. eLife 2021, 10, e59485. [Google Scholar] [CrossRef] [PubMed]
- Safari, H.; Hosseini, S.M.; Azari, A.; Rafsanjani, M.H. Effects of seed priming with ABA and SA on seed germination and seedling growth of sesame (Sesamum indicum L.) under saline condition. Aust. J. Crop Sci. 2018, 12, 1385–1392. [Google Scholar] [CrossRef]
- Song, D.; Zhou, J.; Lai, L.; Alarcon, I.; Tar’an, B.; Abrams, S. Development of ABA antagonists to overcome ABA-and low temperature-induced inhibition of seed germination in canola, lentil, and soybean. J. Plant Growth Regul. 2020, 39, 1403–1413. [Google Scholar] [CrossRef]
- Graeber, K.; Linkies, A.; Müller, K.; Wunchova, A.; Rott, A.; Leubner-Metzger, G. Cross-species approaches to seed dormancy and germination: Conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes. Plant Mol. Biol. 2010, 73, 67–87. [Google Scholar] [CrossRef]
- Sarath, G.; Hou, G.; Baird, L.M.; Mitchell, R.B. ABA, ROS and NO are key players during switchgrass seed germination. Plant Signal. Behav. 2007, 2, 492–493. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Zhang, Z.L.; Hanzlik, S.; Cook, E.; Shen, Q.J. Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Mol. Biol. 2007, 64, 293–303. [Google Scholar] [CrossRef]
- Chakma, S.P.; Chileshe, S.M.; Thomas, R.; Krishna, P. Cotton Seed Priming with Brassinosteroid Promotes Germination and Seedling Growth. Agronomy 2021, 11, 566. [Google Scholar] [CrossRef]
- Wang, H.; Van Staden, J. Seedling establishment characteristics of Paeonia ostii var. lishizhenii. S. Afr. J. Bot. 2002, 68, 382–385. [Google Scholar] [CrossRef] [Green Version]
- Shuai, H.; Meng, Y.; Luo, X.; Chen, F.; Zhou, W.; Dai, Y.; Qi, Y.; Du, J.; Yang, F.; Liu, J.; et al. Exogenous auxin represses soybean seed germination through decreasing the gibberellin/abscisic acid (GA/ABA) ratio. Sci. Rep. 2017, 7, 12620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.A.; Tolbert, N.E. Inhibition of lettuce seed germination and root elongation by derivatives of auxin and reversal by derivatives of cycocel. Physiol. Plant. 1966, 19, 81–86. [Google Scholar] [CrossRef]
- Saeedipour, S. Effects of Seed Priming Phytohormone on Germination and Seedling Growth of Maize with Different Duration of Treatment. Sci. J. Agron. Plant Breed. 2013, 1, 37–43. [Google Scholar]
- Li, Z.; Zhang, J.; Liu, Y.; Zhao, J.; Fu, J.; Ren, X.; Wang, G.; Wang, J. Exogenous auxin regulates multi-metabolic network and embryo development, controlling seed secondary dormancy and germination in Nicotiana tabacum L. BMC Plant Biol. 2016, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Gimeno-Gilles, C.; Lelièvre, E.; Viau, L.; Malik-Ghulam, M.; Ricoult, C.; Niebel, A.; Leduc, N.; Limami, A.M. ABA-mediated inhibition of germination is related to the inhibition of genes encoding cell-wall biosynthetic and architecture: Modifying enzymes and structural proteins in Medicago truncatula embryo axis. Mol. Plant 2009, 2, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Pawela, A.; Banasiak, J.; Biała, W.; Martinoia, E.; Jasiński, M. Mt ABCG 20 is an ABA exporter influencing root morphology and seed germination of Medicago truncatula. Plant J. 2019, 98, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Hu, Y.; Wang, H.; Guo, Q.; Chen, Y.; Howe, G.A.; Yu, D. Molecular mechanism underlying the synergetic effect of jasmonate on abscisic acid signaling during seed germination in Arabidopsis. Plant Cell 2020, 32, 3846–3865. [Google Scholar] [CrossRef]
- Sawan, Z.M.; Mohamed, A.A.; Sakr, R.A.; Tarrad, A.M. Effect of kinetin concentration and methods of application on seed germination, yield components, yield and fiber properties of the Egyptian cotton (Gossypium barbadense). Environ. Exp. Bot. 2000, 44, 59–68. [Google Scholar] [CrossRef]
- Mensah, S.I.; Ekeke, C.; Ibeagi, N.K. Effect of Gibberellic Acid (GA3) and Kinetin on Seed Germination of Sesbania sesban L. and Sesbania rostrata L. (Fabaceae). As. J. Agric. Hortic. Res. 2020, 5, 32–41. [Google Scholar] [CrossRef]
- Araújo, S.; Pagano, A.; Dondi, D.; Lazzaroni, S.; Pinela, E.; Macovei, A.; Balestrazzi, A. Metabolic signatures of germination triggered by kinetin in Medicago truncatula. Sci. Rep. 2019, 9, 10466. [Google Scholar]
- Alonso-Ramírez, A.; Rodríguez, D.; Reyes, D.; Jiménez, J.A.; Nicolás, G.; López-Climent, M.; Gomez-Cadenas, A.; Nicolás, C. Evidence for a role of gibberellins in salicylic acid-modulated early plant responses to abiotic stress in Arabidopsis seeds. Plant Physiol. 2009, 150, 1335–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, L.; Ye, T.; Zhao, S.; Liu, Z.; Feng, Y.Q.; Wu, Y. Cytokinin antagonizes ABA suppression to seed germination of Arabidopsis by downregulating ABI5 expression. Plant J. 2011, 68, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Tahaei, A.; Soleymani, A.; Shams, M. Seed germination of medicinal plant, fennel (Foeniculum vulgare Mill), as affected by different priming techniques. Appl. Biochem. Biotechnol. 2016, 180, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Xu, H.; Ye, S.; Wang, S.; Li, L.; Zhang, S.; Wang, X. Gibberellic acid-stimulated Arabidopsis 6 serves as an integrator of gibberellin, abscisic acid, and glucose signaling during seed germination in Arabidopsis. Plant Physiol. 2015, 169, 2288–2303. [Google Scholar]
- Zhong, C.; Patra, B.; Tang, Y.; Li, X.; Yuan, L.; Wang, X. A transcriptional hub integrating gibberellin–brassinosteroid signals to promote seed germination in Arabidopsis. J. Exp. Bot. 2021, 72, 4708–4720. [Google Scholar] [CrossRef]
- Bouallègue, A.; Souissi, F.; Nouairi, I.; Souibgui, M.; Abbes, Z.; Mhadhbi, H. Salicylic acid and hydrogen peroxide pretreatments alleviate salt stress in faba bean (Vicia faba) seeds during germination. Seed Sci. Technol. 2017, 45, 675–690. [Google Scholar] [CrossRef]
- Bouallègue, A.; Souissi, F.; Nouairi, I.; Souibgui, M.; Abbes, Z.; Mhadhbi, H. Physiological and biochemicals changes modulated by seeds’ priming of lentil (Lens culinaris L.) under salt stress at germination stage. Acta Sci. Pol. Hortorum Cultus 2019, 18, 27–38. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.M.A.; Ahmad, N.; Farooq, M. Optimization of hormonal priming techniques for alleviation of salinity stress in wheat (Triticum aestivum L.). Cad. Pesqui. 2005, 17, 95–109. [Google Scholar]
- Sultana, R.; Adnan, M.Y.; Ali, H. Salicylic acid seed priming modulates some biochemical parametrs to improve germination and seedling growth of salt stressed wheat (Triticum aestivum L.). Pak. J. Bot. 2019, 51, 385–391. [Google Scholar]
- Ngom, B.; Sarr, I.; Kimatu, J.; Mamati, E.; Kane, N.A. Genome-wide analysis of cytosine DNA methylation revealed salicylic acid promotes defense pathways over seedling development in pearl millet. Plant Signal. Behav. 2017, 12, e1356967. [Google Scholar] [CrossRef] [PubMed]
- Horváth, E.; Csiszár, J.; Gallé, Á.; Poór, P.; Szepesi, Á.; Tari, I. Hardening with salicylic acid induces concentration-dependent changes in abscisic acid biosynthesis of tomato under salt stress. J. Plant Physiol. 2015, 183, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Larqué-Saavedra, A.; Martín-Mex, R.; Nexticapan-Garcéz, Á.; Vergara-Yoisura, S.; Gutiérrez-Rendón, M. Efecto del ácido salicílico en el crecimiento de plántulas de tomate (Lycopersicon esculentum Mill.). Rev. Chapingo. Ser. Hortic. 2010, 16, 183–187. [Google Scholar] [CrossRef]
- Jini, D.; Joseph, B. Salicylic acid mediated salt tolerance at different growth stages of Oryza sativa L. and its effect on salicylic acid biosynthetic pathway genes. Biotechnol. Indian J. 2017, 13, 1–18. [Google Scholar]
- Bauer, S.; Mekonnen, D.W.; Geist, B.; Lange, B.; Ghirardo, A.; Zhang, W.; Schäffner, A.R. The isoleucic acid triad: Distinct impacts on plant defense, root growth, and formation of reactive oxygen species. J. Exp. Bot. 2020, 71, 4258–4270. [Google Scholar] [CrossRef]
- Conesa, C.M.; Saez, A.; Navarro-Neila, S.; De Lorenzo, L.; Hunt, A.G.; Sepúlveda, E.B.; Baigorri, R.; Garcia-Mina, J.N.; Zamarreño, A.M.; Sacristán, S.; et al. Alternative polyadenylation and salicylic acid modulate root responses to low nitrogen availability. Plants 2020, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Tejos, R.; Beck, M.; Himschoot, E.; Li, H.; Robatzek, S.; Vanneste, S.; Friml, J. Salicylic acid interferes with clathrin-mediated endocytic protein trafficking. Proc. Natl. Acad. Sci. USA 2013, 110, 7946–7951. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Roy, S.; Singh, S.; Das, S.S.; Gautam, V.; Yadav, S.; Kumar, A.; Singh, A.; Sarkar, A.K.; Sarkar, A.K. Phytohormonal crosstalk modulates the expression of miR166/165s, target Class III HD-ZIPs, and KANADI genes during root growth in Arabidopsis thaliana. Sci. Rep. 2017, 7, 3408. [Google Scholar] [CrossRef] [Green Version]
- San-Miguel, R.; Gutiérrez, M.; Larqué-Saavedra, A. Salicylic acid increases the biomass accumulation of Pinus patula. South. J. Appl. For. 2003, 27, 52–54. [Google Scholar] [CrossRef] [Green Version]
- Tucuch-Haas, C.J.; Pérez-Balam, J.V.; Díaz-Magaña, K.B.; Castillo-Chuc, J.M.; Dzib-Ek, M.G.; Alcántar-González, G.; Vergara-Yoisura, S.; Larqué-Saavedra, A. Role of Salicylic Acid in the Control of General Plant Growth, Development, and Productivity. In Salicylic Acid: A Multifaceted Hormone; Springer: Singapore, 2017; pp. 1–15. [Google Scholar]
- Demirci, T.; Ascı, Ö.A.; Baydar, N.G. Influence of salicylic acid and L-phenylalanine on the accumulation of anthraquinone and phenolic compounds in adventitious root cultures of madder (Rubia tinctorum L.). Plant Cell Tissue Organ Cult. 2021, 144, 313–324. [Google Scholar] [CrossRef]
- Gutierrez-Coronado, M.A.; Trejo-López, C.; Larqué-Saavedra, A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 1998, 36, 563–565. [Google Scholar] [CrossRef]
- Al-Badri, M.K.K.; Rasheed, A.A.H.; Ahmed, S.A.H. Effect of the seed priming and duration on the seed germination and seedling vigor of sunflower crop. Plant Arch. 2021, 21, 1122–1127. [Google Scholar] [CrossRef]
- Nasiri, Y.; Feyzi, P.; Javanmard, A. Effects of hydro and hormonal seed priming on seed germination of Milk thistle under saline stress condition. Not. Sci. Biol. 2014, 6, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Husen, A.; Iqbal, M.; Sohrab, S.S.; Ansari, M.K.A. Salicylic acid alleviates salinity-caused damage to foliar functions, plant growth and antioxidant system in Ethiopian mustard (Brassica carinata A. Br.). Agric. Food Secur. 2018, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Moori, S.; Ahmadi-Lahijani, M.J. Hormopriming instigates defense mechanisms in Thyme (Thymus vulgaris L.) seeds under cadmium stress. J. Appl. Res. Med. Aromat. Plants 2020, 19, 100268. [Google Scholar] [CrossRef]
- Jorjandi, M.; Sharifi Sirchi, G.R. The effect of priming on germination and seedling growth of alfalfa (Medicago sativa L.) under salinity stress. J. Stress Physiol. Biochem. 2012, 8, 234–239. [Google Scholar]
- Sardoei, A.S.; Shahdadneghad, M. Effect of Salicylic Acid Synergists on Rooting Softwood Cuttings of Poinsettia (Euphorbia pulcherrima). J. Plant Sci. 2015, 10, 206–209. [Google Scholar] [CrossRef] [Green Version]
- Pino, M.R.; Muñiz, S.; Val, J.; Navarro, E. Phytotoxicity of 15 common pharmaceuticals on the germination of Lactuca sativa and photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Pollut. Res. 2016, 23, 22530–22541. [Google Scholar] [CrossRef]
- Hou, P.C.; Lin, K.H.; Huang, Y.J.; Wu, C.W.; Chang, Y.S. Evaluation of vegetation indices and plant growth regulator use on the rooting of azalea cuttings. Hortic. Bras. 2020, 38, 153–159. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Evans, M.L.; Ishikawa, H.; Estelle, M.A. Responses of Arabidopsis roots to auxin studied with high temporal resolution: Comparison of wild type and auxin-response mutants. Planta 1994, 194, 215–222. [Google Scholar] [CrossRef]
- Clouse, S.D.; Langford, M.; McMorris, T.C. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996, 111, 671–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Song, H.; Li, B.; Kronzucker, H.J.; Shi, W. Auxin resistant1 and PIN-FORMED2 protect lateral root formation in Arabidopsis under iron stress. Plant Physiol. 2015, 169, 2608–2623. [Google Scholar]
- Pilet, P.E.; Saugy, M. Effect on root growth of endogenous and applied IAA and ABA: A critical reexamination. Plant Physiol. 1987, 83, 33–38. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The biphasic root growth response to abscisic acid in Arabidopsis involves interaction with ethylene and auxin signalling pathways. Front. Plant Sci. 2017, 8, 1493. [Google Scholar] [CrossRef] [Green Version]
- González-García, M.P.; Vilarrasa-Blasi, J.; Zhiponova, M.; Divol, F.; Mora-García, S.; Russinova, E.; Caño-Delgado, A.I. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 2011, 138, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Fendrych, M.; Akhmanova, M.; Merrin, J.; Glanc, M.; Hagihara, S.; Takahashi, K.; Uchida, N.; Torii, K.U.; Friml, J. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nat. Plants 2018, 4, 453–459. [Google Scholar] [CrossRef]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 2007, 17, 1784–1790. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, M.J.; Terrile, M.C.; Casalongué, C.A. Auxin and salicylic acid signalings counteract the regulation of adaptive responses to stress. Plant Signal. Behav. 2011, 6, 452–454. [Google Scholar] [CrossRef]
- Barbez, E.; Dünser, K.; Gaidora, A.; Lendl, T.; Busch, W. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, E4884–E4893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, L.; Dietrich, D.; Ng, C.H.; Chan, P.M.Y.; Bhalerao, R.; Bennett, M.J.; Dinneny, J.R. Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 2013, 25, 324–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thole, J.M.; Beisner, E.R.; Liu, J.; Venkova, S.V.; Strader, L.C. Abscisic acid regulates root elongation through the activities of auxin and ethylene in Arabidopsis thaliana. G3 Genes Genomes Genet. 2014, 4, 1259–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussig, C.; Shin, G.H.; Altmann, T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 2003, 133, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- Le, J.; Vandenbussche, F.; Van Der Straeten, D.; Verbelen, J.P. In the early response of Arabidopsis roots to ethylene, cell elongation is up-and down-regulated and uncoupled from differentiation. Plant Physiol. 2001, 125, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Ruzicka, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef] [Green Version]
- Ivanchenko, M.G.; Muday, G.K.; Dubrovsky, J.G. Ethylene–auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J. 2008, 55, 335–347. [Google Scholar] [CrossRef]
- Ma, B.; He, S.J.; Duan, K.X.; Yin, C.C.; Chen, H.; Yang, C.; Xiong, Q.; Song, Q.X.; Lu, X.; Chen, H.W.; et al. Identification of rice ethylene-response mutants and characterization of MHZ7/OsEIN2 in distinct ethylene response and yield trait regulation. Mol. Plant 2013, 6, 1830–1848. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Zhang, Z.; Wang, J.; Chen, X.; Wei, P.; Huang, R. The activation of OsEIL1 on YUC8 transcription and auxin biosynthesis is required for ethylene-inhibited root elongation in rice early seedling development. PLoS Genet. 2017, 13, e1006955. [Google Scholar] [CrossRef]
- Chen, H.; Ma, B.; Zhou, Y.; He, S.J.; Tang, S.Y.; Lu, X.; Xie, Q.; Chen, S.Y.; Zhang, J.S. E3 ubiquitin ligase SOR1 regulates ethylene response in rice root by modulating stability of Aux/IAA protein. Proc. Natl. Acad. Sci. USA 2018, 115, 4513–4518. [Google Scholar] [CrossRef] [Green Version]
- Kudo, T.; Makita, N.; Kojima, M.; Tokunaga, H.; Sakakibara, H. Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-zeatin-O-glucosyltransferase in rice. Plant Physiol. 2012, 160, 319–331. [Google Scholar] [PubMed] [Green Version]
- Li, X.; Mo, X.; Shou, H.; Wu, P. Cytokinin-mediated cell cycling arrest of pericycle founder cells in lateral root initiation of Arabidopsis. Plant Cell Physiol. 2006, 47, 1112–1123. [Google Scholar]
- Márquez, G.; Alarcón, M.V.; Salguero, J. Cytokinin inhibits lateral root development at the earliest stages of lateral root primordium initiation in maize primary root. J. Plant Growth Regul. 2019, 38, 83–92. [Google Scholar]
- Argueso, C.T.; Ferreira, F.J.; Epple, P.; To, J.P.; Hutchison, C.E.; Schaller, G.E.; Dangl, J.L.; Kieber, J.J. Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genet. 2012, 8, e1002448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laplaze, L.; Benkova, E.; Casimiro, I.; Maes, L.; Vanneste, S.; Swarup, R.; Weijers, D.; Calvo, V.; Parizot, B.; Herrera-Rodriguez, M.B.; et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 2007, 19, 3889–3900. [Google Scholar]
- Blackman, S.A.; Brown, K.L.; Manalo, J.R.; Roos, E.E. Embryo culture as a means to rescue deteriorated maize seeds. Crop Sci. 1996, 36, 1693–1698. [Google Scholar]
- Raya-González, J.; Pelagio-Flores, R.; López-Bucio, J. The jasmonate receptor COI1 plays a role in jasmonate-induced lateral root formation and lateral root positioning in Arabidopsis thaliana. J. Plant Physiol. 2012, 169, 1348–1358. [Google Scholar]
- Chen, Q.; Sun, J.; Zhai, Q.; Zhou, W.; Qi, L.; Xu, L.; Wang, B.; Chen, R.; Jiang, H.; Qi, J.; et al. The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 2011, 23, 3335–3352. [Google Scholar] [CrossRef] [Green Version]
- Staswick, P.E.; Su, W.; Howell, S.H. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc. Natl. Acad. Sci. USA 1992, 89, 6837–6840. [Google Scholar]
- Tung, P.; Hooker, T.S.; Tampe, P.A.; Reid, D.M.; Thorpe, T.A. Jasmonic acid: Effects on growth and development of isolated tomato roots cultured in vitro. Int. J. Plant Sci. 1996, 157, 713–721. [Google Scholar] [CrossRef]
- Gasperini, D.; Chételat, A.; Acosta, I.F.; Goossens, J.; Pauwels, L.; Goossens, A.; Dreos, R.; Alfonso, E.; Farmer, E.E. Multilayered organization of jasmonate signalling in the regulation of root growth. PLoS Genet. 2015, 11, e1005300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thatcher, L.F.; Cevik, V.; Grant, M.; Zhai, B.; Jones, J.D.; Manners, J.M.; Kazan, K. Characterization of a JAZ7 activation-tagged Arabidopsis mutant with increased susceptibility to the fungal pathogen Fusarium oxysporum. J. Exp. Bot. 2016, 67, 2367–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosblech, A.; Thurow, C.; Gatz, C.; Feussner, I.; Heilmann, I. Jasmonic acid perception by COI1 involves inositol polyphosphates in Arabidopsis thaliana. Plant J. 2011, 65, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Acosta, I.F.; Gasperini, D.; Chételat, A.; Stolz, S.; Santuari, L.; Farmer, E.E. Role of NINJA in root jasmonate signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 15473–15478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyu, C.; Figueroa, P.; DePew, C.L.; Cooke, T.F.; Sheard, L.B.; Moreno, J.E.; Katsir, L.; Zheng, N.; Browse, J.; Howe, G.A. JAZ8 lacks a canonical degron and has an EAR motif that mediates transcriptional repression of jasmonate responses in Arabidopsis. Plant Cell 2012, 24, 536–550. [Google Scholar] [CrossRef] [Green Version]
- Remakanthan, A.; Menon, T.G.; Soniya, E.V. Somatic embryogenesis in banana (Musa acuminata AAA cv. Grand Naine): Effect of explant and culture conditions. In Vitro Cell. Dev. Biol. Plant 2014, 50, 127–136. [Google Scholar] [CrossRef]
- Paleg, L.; Aspinall, D.; Coombe, B.; Nicholls, P. Physiological effects of gibberellic acid. VI. Other gibberellins in three test systems. Plant Physiol. 1964, 39, 286. [Google Scholar]
- Rhie, Y.H.; Lee, S.Y.; Walck, J.L.; Hidayati, S.N. Seed dormancy and germination of Asarum sieboldii, a disjunct relict species in East Asia. Plant Biol. 2021, 23, 300–306. [Google Scholar]
- Li, Q.; Li, C.; Yu, X.; Shi, Q. Gibberellin A3 pretreatment increased antioxidative capacity of cucumber radicles and hypocotyls under suboptimal temperature. Afr. J. Agric. Res. 2011, 6, 4091–4098. [Google Scholar]
- Arefi, I.H.; Nejad, S.K.; Kafi, M. Roles of duration and concentration of priming agents on dormancy breaking and germination of caper (Capparis spinosa L.) for the protection of arid degraded areas. Pak. J. Bot. 2012, 44, 225–230. [Google Scholar]
- Fateh, E.; Malekpourlashkariani, N.; Gerami, F. Effects of various treatments on Echinacea purpurea L. seed dormancy breaking and germination. Int. J. Plant Anim. Environ. Sci. 2012, 2, 188–194. [Google Scholar]
- Motte, H.; Vanneste, S.; Beeckman, T. Molecular and environmental regulation of root development. Annu. Rev. Plant Biol. 2019, 70, 465–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Risueno, M.A.; Van Norman, J.M.; Moreno, A.; Zhang, J.; Ahnert, S.E.; Benfey, P.N. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 2010, 329, 1306–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, L.; Roberts, I.; De Rycke, R.; Beeckman, T. Phloem-associated auxin response maxima determine radial positioning of lateral roots in maize. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1525–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Tian, H.; Yue, K.; Liu, J.; Zhang, B.; Li, X.; Ding, Z. A P-loop NTPase regulates quiescent center cell division and distal stem cell identity through the regulation of ROS homeostasis in Arabidopsis root. PLoS Genet. 2016, 12, e1006175. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, C.; Zheng, H.; Sun, M.; Zhang, F.; Zhang, M.; Cui, F.; Lv, D.; Liu, L.; Guo, S.; et al. Antagonistic Interaction between Auxin and SA Signaling Pathways Regulates Bacterial Infection through Lateral Root in Arabidopsis. Cell Rep. 2020, 32, 108060. [Google Scholar] [CrossRef]
- Echevarría-Machado, I.; Escobedo-GM, R.M.; Larqué-Saavedra, A. Responses of transformed Catharanthus roseus roots to femtomolar concentrations of salicylic acid. Plant Physiol. Biochem. 2007, 45, 501–507. [Google Scholar] [CrossRef]
- Ivanchenko, M.G.; Napsucialy-Mendivil, S.; Dubrovsky, J.G. Auxin-induced inhibition of lateral root initiation contributes to root system shaping in Arabidopsis thaliana. Plant J. 2010, 64, 740–752. [Google Scholar] [CrossRef]
- Blakely, L.M.; Blakely, R.M.; Colowit, P.M.; Elliott, D.S. Experimental studies on lateral root formation in radish seedling roots: II. Analysis of the dose-response to exogenous auxin. Plant Physiol. 1988, 87, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Negi, S.; Ivanchenko, M.G.; Muday, G.K. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008, 55, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Harris, J.M. Response of root branching to abscisic acid is correlated with nodule formation both in legumes and nonlegumes. Am. J. Bot. 2005, 92, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.; Strauss, S.H.; Tsai, C.J.; Fang, K.; Chen, Y.; Jiang, X.; Busov, V.B. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant. Cell 2010, 22, 623–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Zhu, C.; Ma, X.; Li, G.; Gan, L.; Ng, D.; Xia, K. Hydrogen peroxide is a second messenger in the salicylic acid-triggered adventitious rooting process in mung bean seedlings. PLoS ONE 2013, 8, e84580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agulló-Antón, M.Á.; Ferrández-Ayela, A.; Fernández-García, N.; Nicolás, C.; Albacete, A.; Pérez-Alfocea, F.; Sanchez-Bravo, J.; Perez-Perez, J.M.; Acosta, M. Early steps of adventitious rooting: Morphology, hormonal profiling and carbohydrate turnover in carnation stem cuttings. Physiol. Plant. 2014, 150, 446–462. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, G.J.; Keppel, M.; Brugge, J.T.; Meekes, H. Timing of the phases in adventitous root formation in apple microcuttings. J. Exp. Bot. 1995, 46, 965–972. [Google Scholar] [CrossRef]
- De Klerk, G.J.; Guan, H.; Huisman, P.; Marinova, S. Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork 9’. Plant Growth Regul. 2011, 63, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Park, J.Y.; Kim, Y.S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.Y.; Kim, J.; Lee, Y.H.; Park, C.M. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, L.; Mongelard, G.; Floková, K.; Pacurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Pacurar, M.; Demailly, H.; Geiss, G.; et al. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef] [Green Version]
- Lischweski, S.; Muchow, A.; Guthörl, D.; Hause, B. Jasmonates act positively in adventitious root formation in petunia cuttings. BMC Plant Biol. 2015, 15, 229. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Zhang, D.; Meng, Y.; Li, K.; Wang, H.; Han, M. Inhibition of adventitious root development in apple rootstocks by cytokinin is based on its suppression of adventitious root primordia formation. Physiol. Plant. 2019, 166, 663–676. [Google Scholar] [CrossRef]
- Michniewicz, M.; Zago, M.K.; Abas, L.; Weijers, D.; Schweighofer, A.; Meskiene, I.; Heisler, M.G.; Ohno, C.; Zhang, J.; Huang, F.; et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 2007, 130, 1044–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Hu, T.; Yan, X.; Meng, T.; Wang, Y.; Wang, Q.; Zhang, X.; Gu, Y.; Sánchez-Rodríguez, C.; Gadeyne, A.; et al. Differential Regulation of Clathrin and Its Adaptor Proteins during Membrane Recruitment for Endocytosis. Plant Physiol. 2016, 171, 215–229. [Google Scholar] [CrossRef]
- Ke, M.; Ma, Z.; Wang, D.; Sun, Y.; Wen, C.; Huang, D.; Chen, Z.; Yang, L.; Tan, S.; Li, R.; et al. Salicylic acid regulates PIN2 auxin transporter hyperclustering and root gravitropic growth via Remorin-dependent lipid nanodomain organisation in Arabidopsis thaliana. New Phytol. 2021, 229, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, J.; Yuan, J.; Wang, X.L.; Zhao, Q.P.; Kong, P.T.; Zhang, X. NITRIC OXIDE-ASSOCIATED PROTEIN1 (AtNOA1) is essential for salicylic acid-induced root waving in Arabidopsis thaliana. New Phytol. 2015, 207, 211–224. [Google Scholar] [CrossRef]
- Guo, F.Q.; Okamoto, M.; Crawford, N.M. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 2003, 302, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Lee, G.I.; Wang, Y.; Crane, B.R.; Klessig, D.F. AtNOS/AtNOA1 is a functional Arabidopsis thaliana cGTPase and not a nitric-oxide synthase. J. Biol. Chem. 2008, 283, 32957–32967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zottini, M.; Costa, A.; De Michele, R.; Ruzzene, M.; Carimi, F.L.; Schiavo, F. Salicylic acid activates nitric oxide synthesis in Arabidopsis. J. Exp. Bot. 2007, 58, 1397–1405. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Rong, H.; Pilbeam, D. Signalling mechanisms underlying the morphological responses of the root system to nitrogen in Arabidopsis thaliana. J. Exp. Bot. 2007, 58, 2329–2338. [Google Scholar] [CrossRef] [Green Version]
- Van Den Berg, C.; Willemsen, V.; Hage, W.; Weisbeek, P.; Scheres, B. Cell fate in the Arabidopsis root meristem determined by directional signalling. Nature 1995, 378, 62–65. [Google Scholar] [CrossRef] [Green Version]
- Van Den Berg, C.; Willemsen, V.; Hendriks, G.; Weisbeek, P.; Scheres, B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 1997, 390, 287–289. [Google Scholar] [CrossRef]
- Strotmann, V.I.; Stahl, Y. At the root of quiescence: Function and regulation of the quiescent center. J. Exp. Bot. 2021, 72, 6716–6726. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.; Scheres, B. Root development-two meristems for the price of one? Curr. Top Dev. Biol. 2010, 91, 67–102. [Google Scholar] [PubMed]
- Wang, Z.; Rong, D.; Chen, D.; Xiao, Y.; Liu, R.; Wu, S.; Yamamuro, C. Salicylic acid promotes quiescent center cell division through ROS accumulation and down-regulation of PLT1, PLT2, and WOX5. J. Integr. Plant Biol. 2021, 63, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Morita-Yamamuro, C.; Tsutsui, T.; Sato, M.; Yoshioka, H.; Tamaoki, M.; Ogawa, D.; Matsuura, H.; Yoshihara, T.; Ikeda, A.; Uyeda, I.; et al. The Arabidopsis gene CAD1 controls programmed cell death in the plant immune system and encodes a protein containing a MACPF domain. Plant Cell Physiol. 2005, 46, 902–912. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Tian, H.; Yu, Q.; Zhang, F.; Wang, R.; Gao, S.; Xu, W.; Liu, J.; Shani, E.; Fu, C.; et al. PHB3 Maintains Root Stem Cell Niche Identity through ROS-Responsive AP2/ERF Transcription Factors in Arabidopsis. Cell Rep. 2018, 22, 1350–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Friml, J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2010, 107, 12046–12051. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Martínez, O.; Pernas, M.; Carol, R.J.; Dolan, L. Ethylene modulates stem cell division in the Arabidopsis thaliana root. Science 2007, 317, 507–510. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y.; Pham, G.; Kim, J.W.; Song, J.H.; Lee, Y.; Roux, S.J.; Hwang, Y.S.; Kim, S.H. Brassinazole resistant 1 (BZR1)-dependent brassinosteroid signalling pathway leads to ectopic activation of quiescent cell division and suppresses columella stem cell differentiation. J. Exp. Bot. 2015, 66, 4835–4849. [Google Scholar] [CrossRef] [Green Version]
- Baum, S.F.; Dubrovsky, J.G.; Rost, T.L. Apical organization and maturation of the cortex and vascular cylinder in Arabidopsis thaliana (Brassicaceae) roots. Am. J. Bot. 2002, 89, 908–920. [Google Scholar] [CrossRef]
- Paquette, A.J.; Benfey, P.N. Maturation of the ground tissue of the root is regulated by gibberellin and SCARECROW and requires SHORT-ROOT. Plant Physiol. 2005, 138, 636–640. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Kong, D.; Wei, P.; Hao, Y.; Torii, K.U.; Lee, J.S.; Li, J. SPINDLY, ERECTA, and its ligand STOMAGEN have a role in redox-mediated cortex proliferation in the Arabidopsis root. Mol. Plant 2014, 7, 1727–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Levesque, M.P.; Vernoux, T.; Jung, J.W.; Paquette, A.J.; Gallagher, K.L.; Wang, J.Y.; Blilou, I.; Scheres, B.; Benfey, P.N. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 2007, 316, 421–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Benfey, P.N. Interplay between SCARECROW, GA and LIKE HETEROCHROMATIN PROTEIN 1 in ground tissue patterning in the Arabidopsis root. Plant J. 2009, 58, 1016–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Benfey, P.N. Cortex proliferation: Simple phenotype, complex regulatory mechanisms. Plant Signal. Behav. 2009, 4, 551–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.A.; Jang, S.; Yoon, E.K.; Heo, J.O.; Chang, K.S.; Choi, J.W.; Dhar, S.; Kim, G.; Choe, J.E.; Heo, J.B.; et al. Interplay between ABA and GA modulates the timing of asymmetric cell divisions in the Arabidopsis root ground tissue. Mol. Plant 2016, 9, 870–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.O.; Chang, K.S.; Kim, I.A.; Lee, M.H.; Lee, S.A.; Song, S.K.; Lee, M.M.; Lim, J. Funneling of gibberellin signaling by the GRAS transcription regulator scarecrow-like 3 in the Arabidopsis root. Proc. Natl. Acad. Sci. USA 2011, 108, 2166–2171. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Flores-Vergara, M.A.; Hong, J.H.; Chu, H.; Lim, J.; Franks, R.G.; Liu, Z.; Xu, J. SEUSS integrates gibberellin signaling with transcriptional inputs from the SHR-SCR-SCL3 module to regulate middle cortex formation in the Arabidopsis root. Plant Physiol. 2016, 170, 1675–1683. [Google Scholar] [CrossRef] [Green Version]
- Cormack, R.G.H. A comparative study of developing epidermal cells in white mustard and tomato roots. Am. J. Bot. 1947, 34, 310–314. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Hayat, Q.; Wijaya, L.; Hayat, S. Effect of salicylic acid on the growth, photosynthetic efficiency and enzyme activities of leguminous plant under cadmium stress. Not. Bot. Horti Agrobot. Cluj Napoca 2014, 42, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Dixit, G.; Mishra, S.; Dwivedi, S.; Tiwari, M.; Mallick, S.; Pandey, V.; Triverdi, P.K.; Chakrabarty, D.; Tripathi, R.D. Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 340. [Google Scholar] [CrossRef] [Green Version]
- Shakirova, F.M.; Bezrukova, M.V.; Allagulova, C.R.; Maslennikova, D.R.; Lubyanova, A.R. Wheat germ agglutinin and dehydrins as ABA-regulated components of SA-induced cadmium resistance in wheat plants. In Salicylic Acid: A Multifaceted Hormone; Springer: Singapore, 2017; pp. 77–96. [Google Scholar]
- El-Ghamery, A.; Mousa, M. Salicylic acid triggers adaptation cadmium cytogenetic toxicity in roots of Nigella sativa L. Egypt. J. Bot. 2018, 58, 297–310. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestik, M.; Skalicky, M.; Landi, M. The role of salicylic acid in plants exposed to heavy metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadehbagheri, M. Salicylic acid priming in corn (Zea mays L. var. Sc. 704) Reinforces NaCl tolerance at germination and the seedling growth stage. Int. J. Biosci. 2014, 4, 187–197. [Google Scholar]
- Pirasteh Anosheh, H.; Maghsoudi, K. Induced salinity tolerance and altered ion storage factor in Hordeum vulgare plants upon salicylic-acid priming. Iran Agric. Res. 2017, 36, 41–48. [Google Scholar]
- Movaghatian, A.; Khorsandi, F. Germination of Carum copticum under salinity stress as affected by salicylic acid application. Ann. Biol. Res. 2014, 5, 105–110. [Google Scholar]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 2013, 64, 2255–2268. [Google Scholar] [CrossRef] [Green Version]
- Espanany, A.; Fallah, S. Seed germination of dill (Anethum graveolens L.) in response to salicylic acid and halopriming under cadmium stress. Iran. J. Plant Physiol. 2016, 6, 1701–1713. [Google Scholar]
- Gémes, K.; Poór, P.; Horváth, E.; Kolbert, Z.; Szopkó, D.; Szepesi, Á.; Tari, I. Cross-talk between salicylic acid and NaCl-generated reactive oxygen species and nitric oxide in tomato during acclimation to high salinity. Physiol. Plant. 2011, 142, 179–1192. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, X.; Dong, Y.; Xu, L.; Zhang, X.; Kong, J.; Liu, S. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J. Plant Growth Regul. 2013, 32, 721–731. [Google Scholar] [CrossRef]
- Tamás, L.; Mistrík, I.; Alemayehu, A.; Zelinová, V.; Bočová, B.; Huttová, J. Salicylic acid alleviates cadmium-induced stress responses through the inhibition of Cd-induced auxin-mediated reactive oxygen species production in barley root tips. J. Plant Physiol. 2015, 173, 1–8. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Y.C.; Zhu, Y.G.; Zhao, F.J. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ. Pollut. 2007, 147, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, Q.; Zhao, S.; Huang, L.; Hao, L. Arabidopsis ein2-1 and npr1-1 response to Al stress. Bull. Environ. Contam. Toxicol. 2014, 93, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Bezrukova, M.V.; Maslennikova, D.R. Endogenous ABA as a hormonal intermediate in the salicylic acid induced protection of wheat plants against toxic ions. In Salicylic Acid; Hayat, S., Ahmad, A., Alyemeni, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 119–140. [Google Scholar]
- Czymmek, K.J.; Fogg, M.; Powell, D.H.; Sweigard, J.; Park, S.Y.; Kang, S. In vivo time-lapse documentation using confocal and multi-photon microscopy reveals the mechanisms of invasion into the Arabidopsis root vascular system by Fusarium oxysporum. Fungal Genet. Biol. 2007, 44, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Lagopodi, A.L.; Ram, A.F.; Lamers, G.E.; Punt, P.J.; Van den Hondel, C.A.; Lugtenberg, B.J.; Bloemberg, G.V. Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp. radicis-lycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Mol. Plant Microbe Interact 2002, 15, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Van Buyten, E.; Höfte, M. Pythium species from rice roots differ in virulence, host colonization and nutritional profile. BMC Plant Biol. 2013, 13, 203. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Sun, Y.; Ma, Z.; Ke, M.; Cui, Y.; Chen, Z.; Chen, C.J.C.; Tran, T.M.; Yang, L.; Lam, S.M.; et al. Salicylic acid-mediated plasmodesmal closure via Remorin-dependent lipid organization. Proc. Natl. Acad. Sci. USA 2019, 116, 21274–21284. [Google Scholar] [CrossRef] [Green Version]
- He, C.Y.; Wolyn, D.J. Potential role for salicylic acid in induced resistance of asparagus roots to Fusarium oxysporum f. sp. asparagi. Plant Pathol. 2005, 54, 227–232. [Google Scholar] [CrossRef]
- McClerklin, S.A.; Lee, S.G.; Harper, C.P.; Nwumeh, R.; Jez, J.M.; Kunkel, B.N. Indole-3-acetaldehyde dehydrogenase-dependent auxin synthesis contributes to virulence of Pseudomonas syringae strain DC3000. PLoS Pathog. 2018, 14, e1006811. [Google Scholar] [CrossRef] [Green Version]
- Armengot, L.; Caldarella, E.; Marquès-Bueno, M.M.; Martínez, M.C. The protein kinase CK2 mediates cross-talk between auxin-and salicylic acid-signaling pathways in the regulation of PINOID transcription. PLoS ONE 2016, 11, e0157168. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshoff, P.M. Legume nodulation: The host controls the party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Concha, C.; Doerner, P. The impact of the rhizobia-legume symbiosis on host root system architecture. J. Exp. Bot. 2020, 71, 3902–3921. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, C.; Yang, J.; Yu, N.; Wang, E. Hormone modulation of legume-rhizobial symbiosis. J. Integr Plant Biol. 2018, 60, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, R.; Richards, L.; Schäfer, P. Hormones as go-betweens in plant microbiome assembly. Plant J. 2021, 105, 518–541. [Google Scholar] [CrossRef]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303. [Google Scholar] [CrossRef]
- Yu, K.; Pieterse, C.M.J.; Bakker, P.A.H.M.; Berendsen, R.L. Beneficial microbes going underground of root immunity. Plant Cell Environ. 2019, 42, 2860–2870. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Abarca, F.; Herrera-Cervera, J.A.; Bueno, P.; Sanjuan, J.; Bisseling, T.; Olivares, J. Involvement of salicylic acid in the establishment of the Rhizobium meliloti-alfalfa symbiosis. Mol. Plant Microbe Interact. 1998, 11, 153–155. [Google Scholar] [CrossRef] [Green Version]
- Van Spronsen, P.C.; Tak, T.; Rood, A.M.; Van Brussel, A.A.; Kijne, J.W.; Boot, K.J. Salicylic acid inhibits indeterminate-type nodulation but not determinate-type nodulation. Mol. Plant Microbe Interact. 2003, 16, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Blilou, I.; Ocampo, J.A.; García-Garrido, J.M. Resistance of pea roots to endomycorrhizal fungus or Rhizobium correlates with enhanced levels of endogenous salicylic acid. J. Exp. Bot. 1999, 50, 1663–1668. [Google Scholar] [CrossRef]
- Ramanujam, M.P.; Jaleel, V.A.; Kumaravelu, G. Effect of salicylic acid on nodulation, nitrogenous compounds and related enzymes of Vigna mungo. Biol. Plant. 1998, 41, 307–311. [Google Scholar] [CrossRef]
- Qiao, Z.; Zogli, P.; Libault, M. Plant Hormones Differentially Control the Sub-Cellular Localization of Plasma Membrane Microdomains during the Early Stage of Soybean Nodulation. Genes 2019, 10, 1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.L.; Park, H.B.; Lee, J.S.; Ryu, S.; Ryu, C.M. Inhibition of primary roots and stimulation of lateral root development in Arabidopsis thaliana by the rhizobacterium Serratia marcescens 90–166 is through both auxin-dependent and-independent signaling pathways. Mol. Cells 2010, 29, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Llorca, M.; Muñoz, P.; Müller, M.; Munné-Bosch, S. Biosynthesis, Metabolism and Function of Auxin, Salicylic Acid and Melatonin in Climacteric and Non-climacteric Fruits. Front. Plant Sci. 2019, 10, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Plant Species | Stress Factor Type | Stress Factor 1 | SA Level | Reference |
|---|---|---|---|---|
| Biotic stress | ||||
| Cucumus sativus L. | Necrotrophic fungus | Rhizoctonia solani | ↑ | [37] |
| Zea mays L. | Root herbivore | Diabrotica virgifera larvae | ↑ | [42] |
| Arabidopsis thaliana L. (Bur-0) | Biotrophic protist | Plasmodiophora brassicae | ↑ | [43] |
| Arabidopsis thaliana L. (Col-0) | Biotrophic protist | Plasmodiophora brassicae | - | [43] |
| Abiotic stress | ||||
| Cassia tora L. | Aluminium | Al (10–50 µM) | ↑ (RT) | [44] |
| Glycine max L. | Aluminium | AlCl3 (30 μM) | ↑ (RT) | [45] |
| Hordeum vulgare L. | Heavy metal | CdCl2 (25 µM) | ↑ (F) | [46] |
| Triticum aestivum L. | Heavy metal | Cd(NO3)2 (250 µM) | ↑ (F) | [47] |
| Arabidopsis thaliana L. (Col) | Heavy metal | CdCl2 (50 μM) | ↑ | [48] |
| Oryza sativa L. | Chilling | 5 °C | ↑ (F + C) | [49] |
| Cucumis sativus L. | Chilling | 8 °C | ↑ (F + C) | [50] |
| Hordeum spontaneum L. | Drought | PEG 6000 (−0.75 to −1.5 MPa) | ↑ | [51] |
| Hordeum vulgare L. | Drought | PEG 6000 (−0.5 MPa) | ↑ | [52] |
| Scutellaria baicalensis Georgi | Drought | PEG 6000 (15%) | ↓ (F + T) | [53] |
| Scutellaria baicalensis Georgi | Salt | NaCl (150 mM) | ↑ (F + T) | [53] |
| Hordeum vulgare L. | UV-B radiation | UV-B (0.84 W m−2) | ↑ | [52] |
| Arabidopsis thaliana L. (Col-0) | Iron deficiency | –Fe (0 µM) | ↑ (F) | [54] |
| Gossypium hirsutum L. | Nitrogen deficiency | –N (0 µM) | ↑ | [55] |
| Solanum lycopersicum L. | Alkalinity | pH 9.0 buffer | ↑ | [56] |
| Plant Species | TP 1 | SA Concent-Ration | TD 2 | Ref 3 | Plant Species | TP 1 | SA Concent-Ration | TD 2 | Ref 3 |
|---|---|---|---|---|---|---|---|---|---|
| SA Increased Germination | SA Decreased Germination | ||||||||
| Daucus carota H. | 1 | 7 μM | 24 h | [69] | Daucus carota H. | 1 | 7 mM | 24 h | [69] |
| Cucumis sativus L. | 2 | 10–50 µM | 2–14 d | [70] | Cucumis sativus L. | 2 | 100 µM–0.5 mM | 2–14 d | [70] |
| Arabidopsis thaliana L. | 2 | 100 µM | 2 d | [73] | Arabidopsis thaliana L. | 1 | 250 μM–1 mM | 24 h | [74] |
| Arabidopsis thaliana L. | 2 | 2.5–5 mM | 70 h | [75] | |||||
| Triticum aestivum L. | 1 | 10–20 μM | 6 h | [71] | Triticum aestivum L. | 1 | 30 μM | 6 h | [71] |
| Triticum aestivum L. | 1 | 0.5 mM | 24 h | [72] | Triticum aestivum L. | 1 | 1 mM | 24 h | [72] |
| Zea mays L. | 3 | 0.5–1.5 mM | 24 h | [76] | Zea mays L. | 3 | 3–5 mM | 24 h | [76] |
| Plant Species | TP 1 | SA Concent-Ration | TD 2 | Plant Species | TP 1 | SA Concent-Ration | TD 2 | Ref 3 |
|---|---|---|---|---|---|---|---|---|
| SA Increased Root Growth | SA Decreased Root Growth | |||||||
| Trigonellafoenum-graceum L. | 2 | 5–10 μM | 8 d | Trigonellafoenum-graceum L. | 2 | 15 μM | 24 h | [77] |
| Cucumis sativus L. | 2 | 10–50 µM | 2–14 d | Cucumis sativus L. | 2 | 0.1–0.5 mM | 2–14 d | [70] |
| Lens culinaris L. | 1 | 0.1–0.5 mM | Lens culinaris L. | 1 | 1 mM | [3] | ||
| Vicia faba L. | 1 | 0.5 mM | Vicia faba L. | 1 | 1 mM | [4] | ||
| Pennisetum glaucum L. | 1 | 0.5 mM | 2 d | Pennisetum glaucum L. | 1 | 0.5–3 mM | 2 d | [5] |
| Pennisetum glaucum L. | 1 | 2–3 mM | 2 d | [5] | ||||
| Triticum aestivum L. | 1 | 10 μM | 6 h | Triticum aestivum L. | 1 | 30 µM | 6 h | [71] |
| Plant Species | TP 1 | SA Concent-Ration | TD 2 | Plant Species | TP 1 | SA Concent-Ration | TD 2 | Ref 3 |
|---|---|---|---|---|---|---|---|---|
| SA Increased Adventitious Rooting | SA Decreased Adventitious Rooting | |||||||
| Arabidopsis thaliana L. | 1 | 3–50 µM | 5 d | Arabidopsis thaliana L. | 1 | 0.1–0.2 mM | 5 d | [25] |
| Rhododendron pulchrum Sw. | 2 | 100 µM | 62 d | Rhododendron pulchrum Sw. | 2 | 10 mM | 62 d | [142] |
| Vigna radiate L. | 3 | 0.2–0.6 mM | 24 h | Vigna radiate L. | 3 | 0.8 mM | 24 h | [195] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic Acid in Root Growth and Development. Int. J. Mol. Sci. 2022, 23, 2228. https://doi.org/10.3390/ijms23042228
Bagautdinova ZZ, Omelyanchuk N, Tyapkin AV, Kovrizhnykh VV, Lavrekha VV, Zemlyanskaya EV. Salicylic Acid in Root Growth and Development. International Journal of Molecular Sciences. 2022; 23(4):2228. https://doi.org/10.3390/ijms23042228
Chicago/Turabian StyleBagautdinova, Zulfira Z., Nadya Omelyanchuk, Aleksandr V. Tyapkin, Vasilina V. Kovrizhnykh, Viktoriya V. Lavrekha, and Elena V. Zemlyanskaya. 2022. "Salicylic Acid in Root Growth and Development" International Journal of Molecular Sciences 23, no. 4: 2228. https://doi.org/10.3390/ijms23042228
APA StyleBagautdinova, Z. Z., Omelyanchuk, N., Tyapkin, A. V., Kovrizhnykh, V. V., Lavrekha, V. V., & Zemlyanskaya, E. V. (2022). Salicylic Acid in Root Growth and Development. International Journal of Molecular Sciences, 23(4), 2228. https://doi.org/10.3390/ijms23042228






