Pro- and Anti-Inflammatory Cytokines in the Context of NK Cell–Trophoblast Interactions
Abstract
:1. Introduction
2. Results
2.1. Influence of Cytokines on the Phenotype of the NK-92 Cell Line in Monoculture and under Co-Cultivation Conditions with the JEG-3 Cell Line
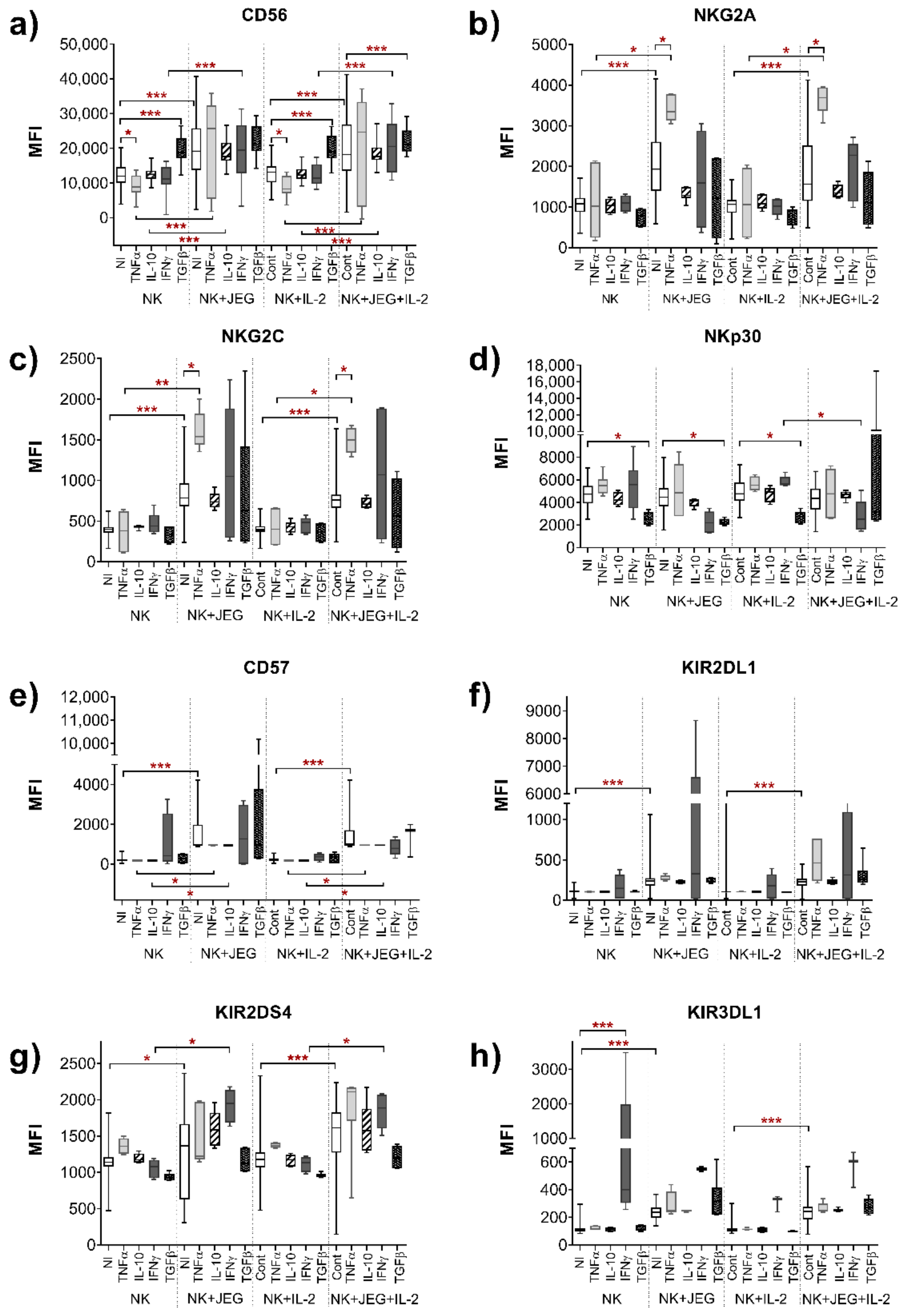
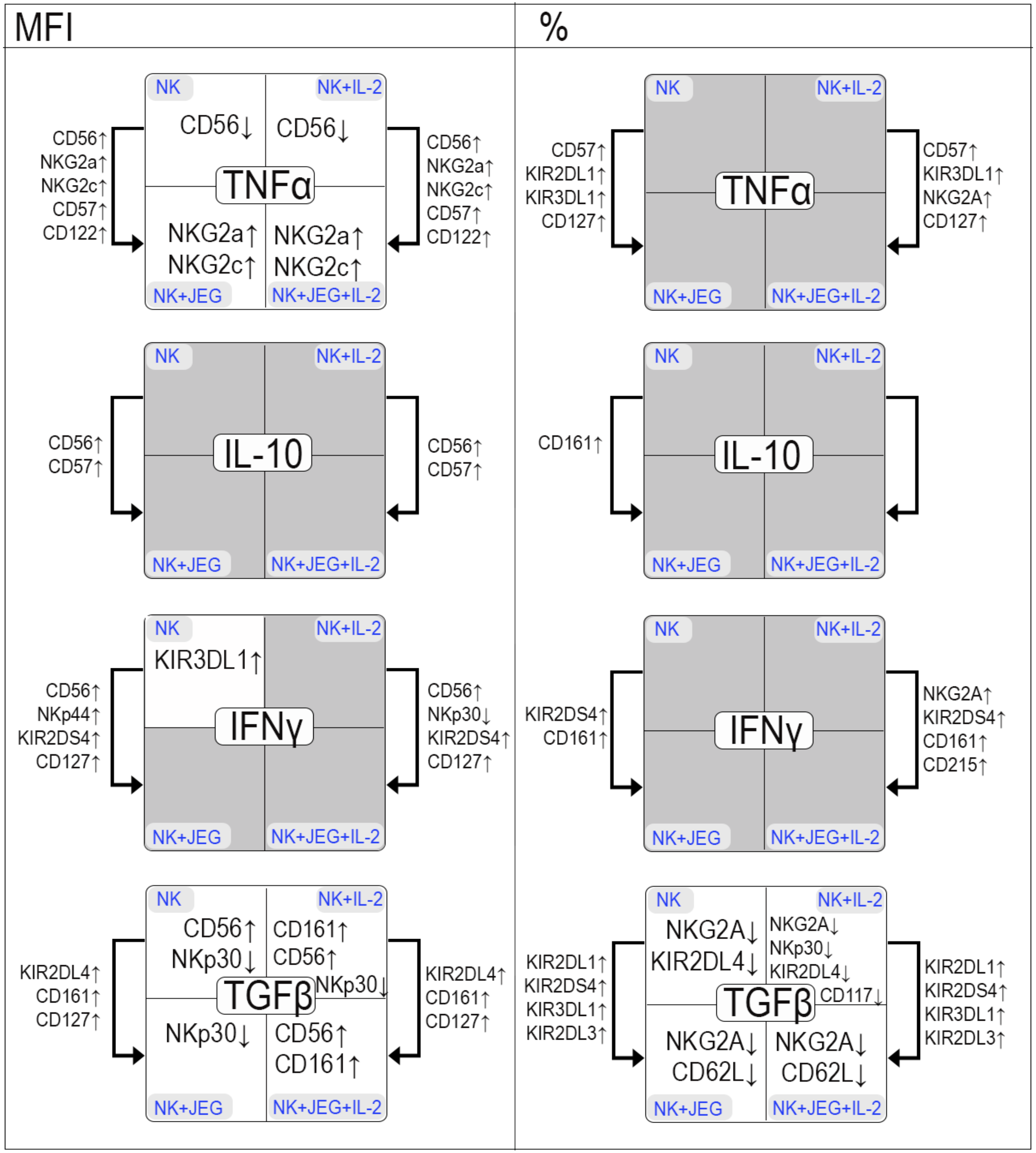
2.1.1. Effect of TNFα on the Phenotype of the NK-92 Cell Line in Monoculture and under Co-Cultivation Conditions with the JEG-3 Cell Line
2.1.2. Effect of IL-10 on the Phenotype of the NK-92 Cell Line in Monoculture and under Co-Cultivation Conditions with the JEG-3 Cell Line
2.1.3. Influence of IFNγ on the Phenotype of the NK-92 Cell Line in Monoculture and under Co-Cultivation Conditions with the JEG-3 Cell Line
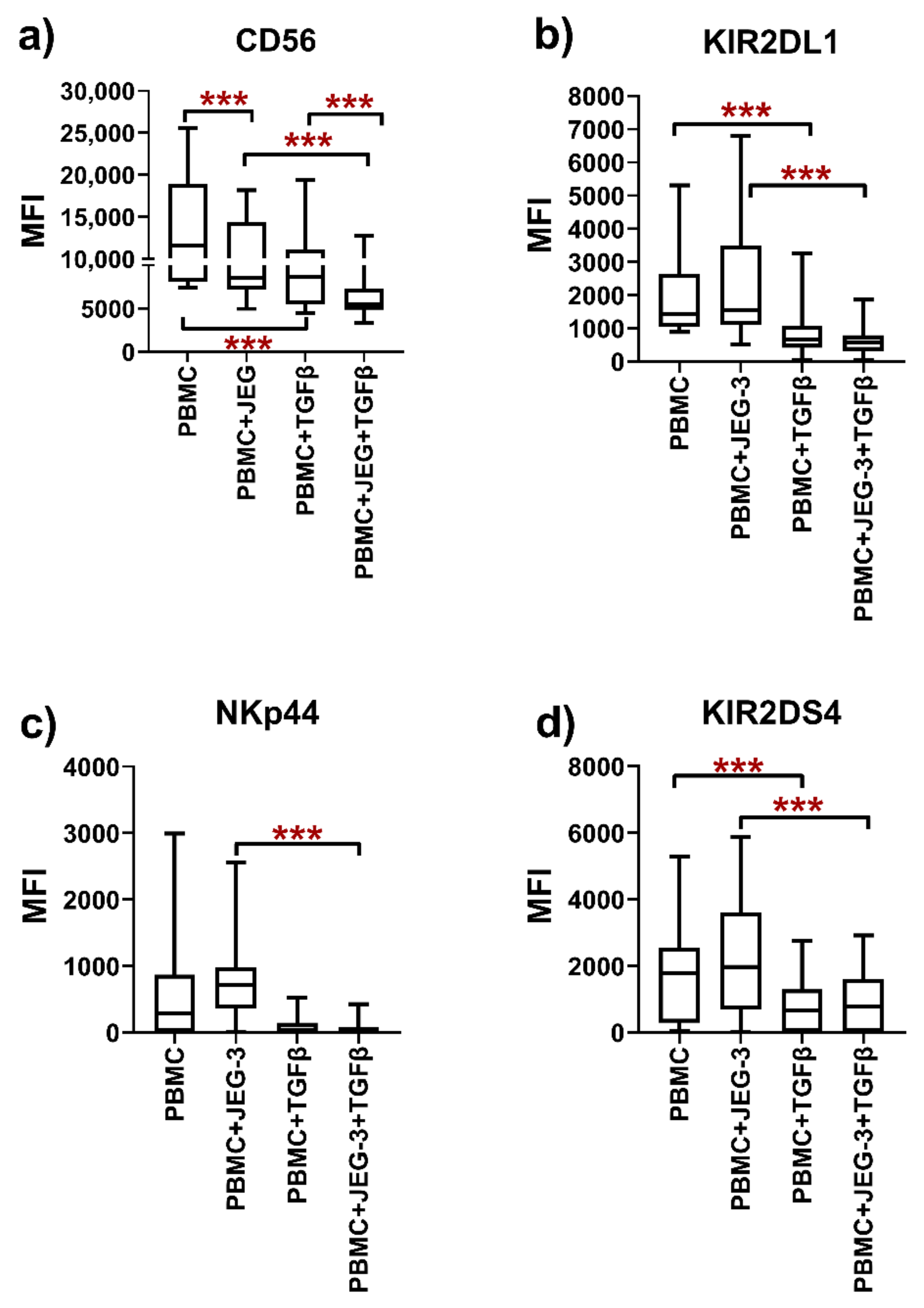
2.1.4. Effect of TGFβ on the Phenotype of the NK-92 Cell Line in Monoculture and under Co-Cultivation Conditions with the JEG-3 Cell Line
2.2. Cytotoxicity of NK-92 Cell Line after Culture with Cytokines
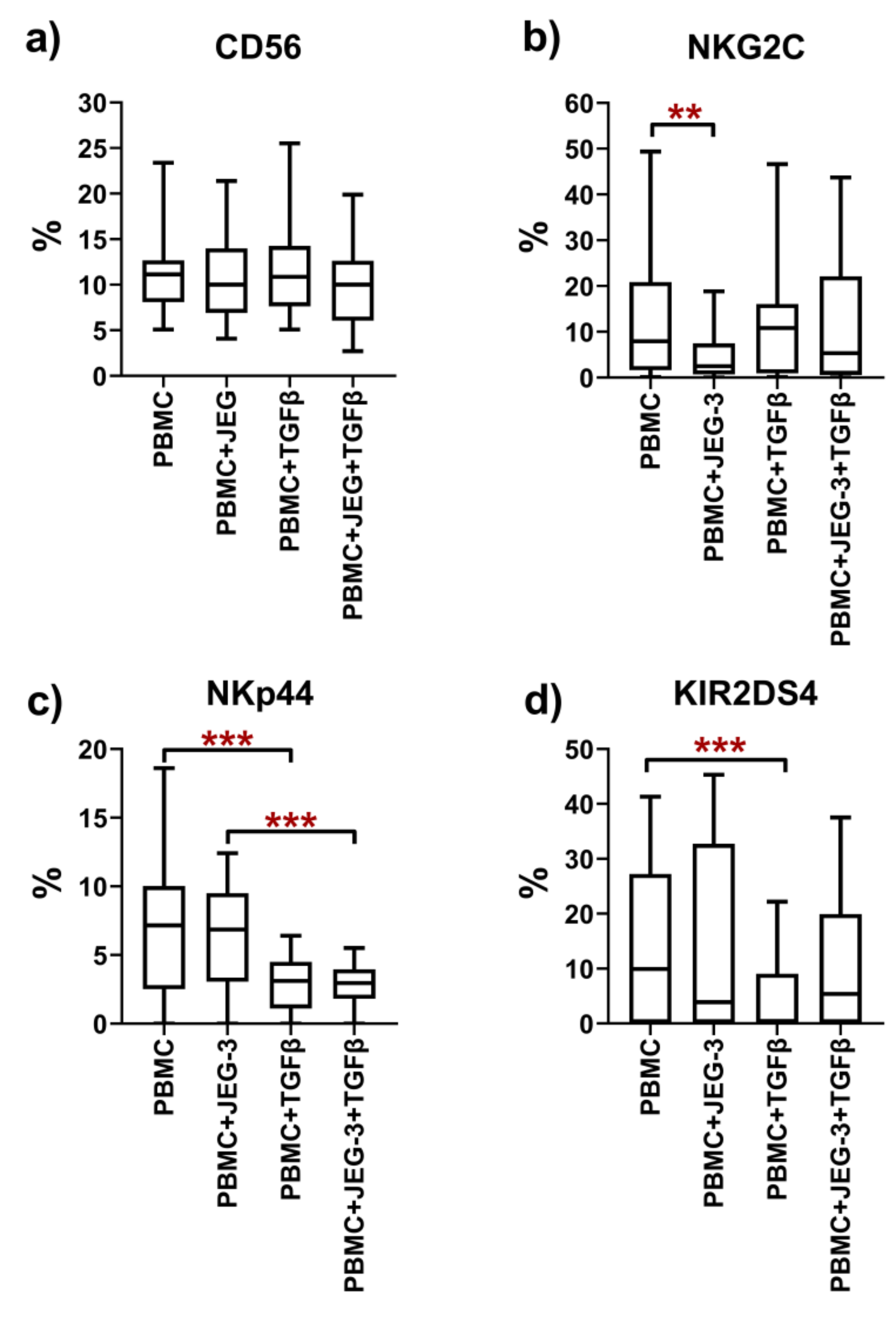
2.3. Phenotype of Peripheral Blood NK Cells in Monoculture and under Co-Cultivation Conditions with the JEG-3 Cell Line
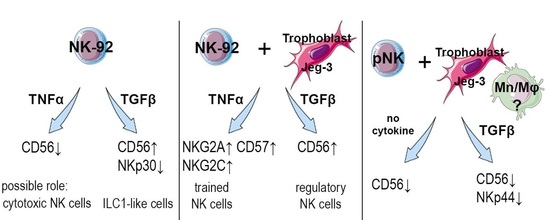
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Peripheral Blood Mononuclear Cells
4.3. Cytokines
4.4. Assessment of the Phenotype of the NK-92 Cell Line after Cultivation with JEG-3 Trophoblast Cells and Cytokines
4.5. Evaluation of the Cytotoxicity of the NK-92 Cell Line after Pre-Cultivation with Cytokines
4.6. Evaluation of the Phenotype of pNK Cells after Cultivation with JEG-3 Trophoblast Cells and Cytokines
4.7. Statistical Data Analysis
4.8. Ethical Approval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Veluchamy, J.P.; Delso-Vallejo, M.; Kok, N.; Bohme, F.; Seggewiss-Bernhardt, R.; van der Vliet, H.J.; de Gruijl, T.D.; Huppert, V.; Spanholtz, J. Standardized and flexible eight colour flow cytometry panels harmonized between different laboratories to study human NK cell phenotype and function. Sci. Rep. 2017, 7, 43873. [Google Scholar] [CrossRef]
- Sojka, D.K.; Plougastel-Douglas, B.; Yang, L.; Pak-Wittel, M.A.; Artyomov, M.N.; Ivanova, Y.; Zhong, C.; Chase, J.M.; Rothman, P.B.; Yu, J.; et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 2014, 3, e01659. [Google Scholar] [CrossRef]
- Sun, H.; Sun, C.; Tian, Z.; Xiao, W. NK cells in immunotolerant organs. Cell. Mol. Immunol. 2013, 10, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Santoni, A.; Carlino, C.; Stabile, H.; Gismondi, A. Mechanisms underlying recruitment and accumulation of decidual NK cells in uterus during pregnancy. Am. J. Reprod. Immunol. 2008, 59, 417–424. [Google Scholar] [CrossRef]
- Kennedy, P.R.; Chazara, O.; Gardner, L.; Ivarsson, M.A.; Farrell, L.E.; Xiong, S.; Hiby, S.E.; Colucci, F.; Sharkey, A.M.; Moffett, A. Activating KIR2DS4 Is Expressed by Uterine NK Cells and Contributes to Successful Pregnancy. J. Immunol. 2016, 197, 4292–4300. [Google Scholar] [CrossRef] [Green Version]
- Male, V.; Sharkey, A.; Masters, L.; Kennedy, P.R.; Farrell, L.E.; Moffett, A. The effect of pregnancy on the uterine NK cell KIR repertoire. Eur. J. Immunol. 2011, 41, 3017–3027. [Google Scholar] [CrossRef] [Green Version]
- Le Gars, M.; Seiler, C.; Kay, A.W.; Bayless, N.L.; Starosvetsky, E.; Moore, L.; Shen-Orr, S.S.; Aziz, N.; Khatri, P.; Dekker, C.L.; et al. Pregnancy-Induced Alterations in NK Cell Phenotype and Function. Front. Immunol. 2019, 10, 2469. [Google Scholar] [CrossRef] [Green Version]
- Otun, H.A.; Lash, G.E.; Innes, B.A.; Bulmer, J.N.; Naruse, K.; Hannon, T.; Searle, R.F.; Robson, S.C. Effect of tumour necrosis factor-alpha in combination with interferon-gamma on first trimester extravillous trophoblast invasion. J. Reprod. Immunol. 2011, 88, 1–11. [Google Scholar] [CrossRef]
- Prossler, J.; Chen, Q.; Chamley, L.; James, J.L. The relationship between TGFbeta, low oxygen and the outgrowth of extravillous trophoblasts from anchoring villi during the first trimester of pregnancy. Cytokine 2014, 68, 9–15. [Google Scholar] [CrossRef]
- Aldo, P.B.; Racicot, K.; Craviero, V.; Guller, S.; Romero, R.; Mor, G. Trophoblast induces monocyte differentiation into CD14+/CD16+ macrophages. Am. J. Reprod. Immunol. 2014, 72, 270–284. [Google Scholar] [CrossRef] [Green Version]
- Co, E.C.; Gormley, M.; Kapidzic, M.; Rosen, D.B.; Scott, M.A.; Stolp, H.A.; McMaster, M.; Lanier, L.L.; Barcena, A.; Fisher, S.J. Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy. Biol. Reprod. 2013, 88, 155. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, M.; Ban, Y.; Gao, W.; Song, B.; Wang, Y.; Zhang, Y.; Shao, Q.; Kong, B.; Qu, X. Tim-3 Is Upregulated in NK Cells during Early Pregnancy and Inhibits NK Cytotoxicity toward Trophoblast in Galectin-9 Dependent Pathway. PLoS ONE 2016, 11, e0147186. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Massague, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Fu, B.; Gao, Y.; Liao, X.; Sun, R.; Tian, Z.; Wei, H. TGF-beta1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012, 8, e1002594. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Cantoni, C.; Della Chiesa, M.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef] [Green Version]
- Mezouar, S.; Mege, J.L. Changing the paradigm of IFN-gamma at the interface between innate and adaptive immunity: Macrophage-derived IFN-gamma. J. Leukoc. Biol. 2020, 108, 419–426. [Google Scholar] [CrossRef]
- Lash, G.E.; Pitman, H.; Morgan, H.L.; Innes, B.A.; Agwu, C.N.; Bulmer, J.N. Decidual macrophages: Key regulators of vascular remodeling in human pregnancy. J. Leukoc. Biol. 2016, 100, 315–325. [Google Scholar] [CrossRef] [Green Version]
- McIntire, R.H.; Ganacias, K.G.; Hunt, J.S. Programming of human monocytes by the uteroplacental environment. Reprod. Sci. 2008, 15, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Driessler, F.; Venstrom, K.; Sabat, R.; Asadullah, K.; Schottelius, A.J. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: A role for p50. Clin. Exp. Immunol. 2004, 135, 64–73. [Google Scholar] [CrossRef]
- Li, Z.Y.; Song, Z.H.; Meng, C.Y.; Yang, D.D.; Yang, Y.; Peng, J.P. IFN-gamma modulates Ly-49 receptors on NK cells in IFN-gamma-induced pregnancy failure. Sci. Rep. 2015, 5, 18159. [Google Scholar] [CrossRef] [Green Version]
- Chiba, H.; Fukui, A.; Fuchinoue, K.; Funamizu, A.; Tanaka, K.; Mizunuma, H. Expression of Natural Cytotoxicity Receptors on and Intracellular Cytokine Production by NK Cells in Women with Gestational Diabetes Mellitus. Am. J. Reprod. Immunol. 2016, 75, 529–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Dong, P.; Jia, N.; Wen, X.; Luo, L.; Wang, S.; Li, J. The expression of intracellular cytokines of decidual natural killer cells in unexplained recurrent pregnancy loss. J. Matern. Fetal Neonatal Med. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dutz, J.P.; MacCalman, C.D.; Yong, P.; Tan, R.; von Dadelszen, P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: A role for IFN-gamma. J. Immunol. 2006, 177, 8522–8530. [Google Scholar] [CrossRef] [Green Version]
- Valencia-Ortega, J.; Zarate, A.; Saucedo, R.; Hernandez-Valencia, M.; Cruz, J.G.; Puello, E. Placental Proinflammatory State and Maternal Endothelial Dysfunction in Preeclampsia. Gynecol. Obstet. Investig. 2018, 84, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, W.; Liu, C. Increased expression of IFN-gamma in preeclampsia impairs human trophoblast invasion via a SOCS1/JAK/STAT1 feedback loop. Exp. Ther. Med. 2021, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, V.; Khokhlova, E.; Grebenkina, P.; Salloum, Z.; Nikolaenkov, I.; Markova, K.; Davidova, A.; Selkov, S.; Sokolov, D. NK-92 cells change their phenotype and function when cocultured with IL-15, IL-18 and trophoblast cells. Immunobiology 2021, 226, 152125. [Google Scholar] [CrossRef]
- Zelova, H.; Hosek, J. TNF-alpha signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Favrot, M.; Combaret, V.; Blay, J.Y.; Capdeville, R.; Zhou, D.C.; Clapisson, G.; Chouaib, S.; Franks, C.R.; Philip, T. TNF alpha enhancement of NK and LAK cell functions induced by high-dose IL-2 in human peripheral blood mononuclear cells from patients pretreated with alpha IFN + IL-2. Eur. Cytokine Netw. 1990, 1, 221–227. [Google Scholar]
- Mason, A.T.; McVicar, D.W.; Smith, C.A.; Young, H.A.; Ware, C.F.; Ortaldo, J.R. Regulation of NK cells through the 80-kDa TNFR (CD120b). J. Leukoc. Biol. 1995, 58, 249–255. [Google Scholar] [CrossRef]
- Kobyzeva, P.A.; Streltsova, M.A.; Erokhina, S.A.; Kanevskiy, L.M.; Telford, W.G.; Sapozhnikov, A.M.; Kovalenko, E.I. CD56(dim) CD57(−) NKG2C(+) NK cells retaining proliferative potential are possible precursors of CD57(+) NKG2C(+) memory-like NK cells. J. Leukoc. Biol. 2020, 108, 1379–1395. [Google Scholar] [CrossRef] [PubMed]
- Gamliel, M.; Goldman-Wohl, D.; Isaacson, B.; Gur, C.; Stein, N.; Yamin, R.; Berger, M.; Grunewald, M.; Keshet, E.; Rais, Y.; et al. Trained Memory of Human Uterine NK Cells Enhances Their Function in Subsequent Pregnancies. Immunity 2018, 48, 951–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feyaerts, D.; Kuret, T.; van Cranenbroek, B.; van der Zeeuw-Hingrez, S.; van der Heijden, O.W.H.; van der Meer, A.; Joosten, I.; van der Molen, R.G. Endometrial natural killer (NK) cells reveal a tissue-specific receptor repertoire. Hum. Reprod. 2018, 33, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Mourik, M.S.; Macklon, N.S.; Heijnen, C.J. Embryonic implantation: Cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J. Leukoc. Biol. 2009, 85, 4–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Xu, C.; Wu, X.; Zhao, X.; Fan, J.; Meng, S. The potential markers of NK-92 associated to cytotoxicity against K562 cells. Biologicals 2020, 68, 46–53. [Google Scholar] [CrossRef]
- Bazhenov, D.; Mikhailova, V.; Nikolaenkov, I.; Markova, K.; Salloum, Z.; Kogan, I.; Gzgzyan, A.; Selkov, S.; Sokolov, D. The uteroplacental contact zone cytokine influence on NK cell cytotoxicity to trophoblasts. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2020, 36, S1–S6. [Google Scholar] [CrossRef]
- Persson, G.; Bork, J.B.S.; Isgaard, C.; Larsen, T.G.; Bordoy, A.M.; Bengtsson, M.S.; Hviid, T.V.F. Cytokine stimulation of the choriocarcinoma cell line JEG-3 leads to alterations in the HLA-G expression profile. Cell. Immunol. 2020, 352, 104110. [Google Scholar] [CrossRef]
- Li, T.; Cui, L.; Xu, X.; Zhang, H.; Jiang, Y.; Ren, L.; Yang, C.; Liu, X.; Hu, X. The Role of Tim-3 on dNK Cells Dysfunction During Abnormal Pregnancy With Toxoplasma gondii Infection. Front. Cell Infect Microbiol. 2021, 11, 587150. [Google Scholar] [CrossRef]
- Blois, S.M.; Freitag, N.; Tirado-Gonzalez, I.; Cheng, S.B.; Heimesaat, M.M.; Bereswill, S.; Rose, M.; Conrad, M.L.; Barrientos, G.; Sharma, S. NK cell-derived IL-10 is critical for DC-NK cell dialogue at the maternal-fetal interface. Sci. Rep. 2017, 7, 2189. [Google Scholar] [CrossRef] [Green Version]
- Schulz, U.; Kreutz, M.; Multhoff, G.; Stoelcker, B.; Kohler, M.; Andreesen, R.; Holler, E. Interleukin-10 promotes NK cell killing of autologous macrophages by stimulating expression of NKG2D ligands. Scand. J. Immunol. 2010, 72, 319–331. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, D.; Huo, J.; Biswas, S.K.; Huang, Y.; Yang, Y.; Xu, S.; Lam, K.P. IL-10 Enhances Human Natural Killer Cell Effector Functions via Metabolic Reprogramming Regulated by mTORC1 Signaling. Front. Immunol. 2021, 12, 619195. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, W.; Han, P.; Yang, B.; Feng, X.; Zhou, P.; Zhu, X.; Zhou, B.; Chen, W.; Qian, J.; et al. MST4 Regulates Epithelial-Mesenchymal Transition of Choriocarcinoma by Mediating TGF-beta1 Expression. Onco Targets Ther. 2020, 13, 11935–11946. [Google Scholar] [CrossRef]
- Mikhailova, V.A.; Kudryavtsev, I.V.; Serebryakova, M.K.; Milyutina, Y.P.; Demidova, E.S.; Panina, A.N.; Bazhenov, D.O.; Belikova, M.E.; Selkov, S.A.; Sokolov, D.I. Trophoblast cell influence on peripheral blood natural killer cell proliferation and phenotype in non-pregnant women and women in early pregnancy. Immunobiology 2020, 225, 151910. [Google Scholar] [CrossRef] [PubMed]
- Allan, D.S.; Rybalov, B.; Awong, G.; Zuniga-Pflucker, J.C.; Kopcow, H.D.; Carlyle, J.R.; Strominger, J.L. TGF-beta affects development and differentiation of human natural killer cell subsets. Eur. J. Immunol. 2010, 40, 2289–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawke, L.G.; Mitchell, B.Z.; Ormiston, M.L. TGF-beta and IL-15 Synergize through MAPK Pathways to Drive the Conversion of Human NK Cells to an Innate Lymphoid Cell 1-like Phenotype. J. Immunol. 2020, 204, 3171–3181. [Google Scholar] [CrossRef]
- Huang, Z.; Li, S.; Fan, W.; Ma, Q. Transforming growth factor beta1 promotes invasion of human JEG-3 trophoblast cells via TGF-beta/Smad3 signaling pathway. Oncotarget 2017, 8, 33560–33570. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, B.; Kunihs, V.; Haider, S.; Pollheimer, J.; Knofler, M. 3-Dimensional JEG-3 choriocarcinoma cell organoids as a model for trophoblast expansion and differentiation. Placenta 2021, 104, 243–246. [Google Scholar] [CrossRef]
- Yi, Y.; Cheng, J.C.; Klausen, C.; Leung, P.C.K. TGF-beta1 inhibits human trophoblast cell invasion by upregulating cyclooxygenase-2. Placenta 2018, 68, 44–51. [Google Scholar] [CrossRef]
- Ramhorst, R.E.; Giribaldi, L.; Fraccaroli, L.; Toscano, M.A.; Stupirski, J.C.; Romero, M.D.; Durand, E.S.; Rubinstein, N.; Blaschitz, A.; Sedlmayr, P.; et al. Galectin-1 confers immune privilege to human trophoblast: Implications in recurrent fetal loss. Glycobiology 2012, 22, 1374–1386. [Google Scholar] [CrossRef] [Green Version]
- Cortez, V.S.; Cervantes-Barragan, L.; Robinette, M.L.; Bando, J.K.; Wang, Y.; Geiger, T.L.; Gilfillan, S.; Fuchs, A.; Vivier, E.; Sun, J.C.; et al. Transforming Growth Factor-beta Signaling Guides the Differentiation of Innate Lymphoid Cells in Salivary Glands. Immunity 2016, 44, 1127–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlie, D.; Doughty-Shenton, D.; Soong, D.Y.; Nixon, C.; Carragher, N.O.; Carlin, L.M.; Kitamura, T. Metastasis-associated macrophages constrain antitumor capability of natural killer cells in the metastatic site at least partially by membrane bound transforming growth factor beta. J. Immunother. Cancer 2021, 9, e001740. [Google Scholar] [CrossRef] [PubMed]
- Nunez, S.Y.; Ziblat, A.; Secchiari, F.; Torres, N.I.; Sierra, J.M.; Raffo Iraolagoitia, X.L.; Araya, R.E.; Domaica, C.I.; Fuertes, M.B.; Zwirner, N.W. Human M2 Macrophages Limit NK Cell Effector Functions through Secretion of TGF-beta and Engagement of CD85j. J. Immunol. 2018, 200, 1008–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awoyemi, T.; Motta-Mejia, C.; Zhang, W.; Kouser, L.; White, K.; Kandzija, N.; Alhamlan, F.S.; Cribbs, A.P.; Tannetta, D.; Mazey, E.; et al. Syncytiotrophoblast Extracellular Vesicles From Late-Onset Preeclampsia Placentae Suppress Pro-Inflammatory Immune Response in THP-1 Macrophages. Front. Immunol. 2021, 12, 676056. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, V.A.; Bazhenov, D.O.; Viazmina, L.P.; Agnaeva, A.O.; Bespalova, O.N.; Selkov, S.A.; Sokolov, D.I. Cytotoxic Activity of Peripheral Blood NK Cells towards Trophoblast Cells during Pregnancy. Bull. Exp. Biol. Med. 2019, 166, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Yu, H.; Wu, D.; Peng, C. Transforming growth factor-beta1 inhibits steroidogenesis in human trophoblast cells. Mol. Hum. Reprod. 2002, 8, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Cabrera, M.I.; Navarro-Hernandez, R.E.; Santerre, A.; Ortiz-Lazareno, P.C.; Pereira-Suarez, A.L.; Estrada-Chavez, C. Effect of pregnancy hormone mixtures on cytokine production and surface marker expression in naive and LPS-activated THP-1 differentiated monocytes/macrophages. Innate Immun. 2020, 26, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, D.I.; Mikhailova, V.A.; Agnayeva, A.O.; Bazhenov, D.I.; Khokhlova, E.V.; Bespalova, O.N.; Gzgzyan, A.M.; Selkov, S.A. NK and trophoblast cells interaction: Cytotoxic activity on recurrent pregnancy loss. Gynecol. Endocrinol. 2019, 35, 5–10. [Google Scholar] [CrossRef]
- Bazhenov, D.O.; Khokhlova, E.V.; Viazmina, L.P.; Furaeva, K.N.; Mikhailova, V.A.; Kostin, N.A.; Selkov, S.A.; Sokolov, D.I. Characteristics of Natural Killer Cell Interaction with Trophoblast Cells during Pregnancy. Curr. Mol. Med. 2020, 20, 202–219. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, V.; Grebenkina, P.; Khokhlova, E.; Davydova, A.; Salloum, Z.; Tyshchuk, E.; Zagainova, V.; Markova, K.; Kogan, I.; Selkov, S.; et al. Pro- and Anti-Inflammatory Cytokines in the Context of NK Cell–Trophoblast Interactions. Int. J. Mol. Sci. 2022, 23, 2387. https://doi.org/10.3390/ijms23042387
Mikhailova V, Grebenkina P, Khokhlova E, Davydova A, Salloum Z, Tyshchuk E, Zagainova V, Markova K, Kogan I, Selkov S, et al. Pro- and Anti-Inflammatory Cytokines in the Context of NK Cell–Trophoblast Interactions. International Journal of Molecular Sciences. 2022; 23(4):2387. https://doi.org/10.3390/ijms23042387
Chicago/Turabian StyleMikhailova, Valentina, Polina Grebenkina, Evgeniia Khokhlova, Alina Davydova, Zeina Salloum, Elizaveta Tyshchuk, Valeria Zagainova, Kseniia Markova, Igor Kogan, Sergey Selkov, and et al. 2022. "Pro- and Anti-Inflammatory Cytokines in the Context of NK Cell–Trophoblast Interactions" International Journal of Molecular Sciences 23, no. 4: 2387. https://doi.org/10.3390/ijms23042387
APA StyleMikhailova, V., Grebenkina, P., Khokhlova, E., Davydova, A., Salloum, Z., Tyshchuk, E., Zagainova, V., Markova, K., Kogan, I., Selkov, S., & Sokolov, D. (2022). Pro- and Anti-Inflammatory Cytokines in the Context of NK Cell–Trophoblast Interactions. International Journal of Molecular Sciences, 23(4), 2387. https://doi.org/10.3390/ijms23042387