Smart Lipid–Polysaccharide Nanoparticles for Targeted Delivery of Doxorubicin to Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
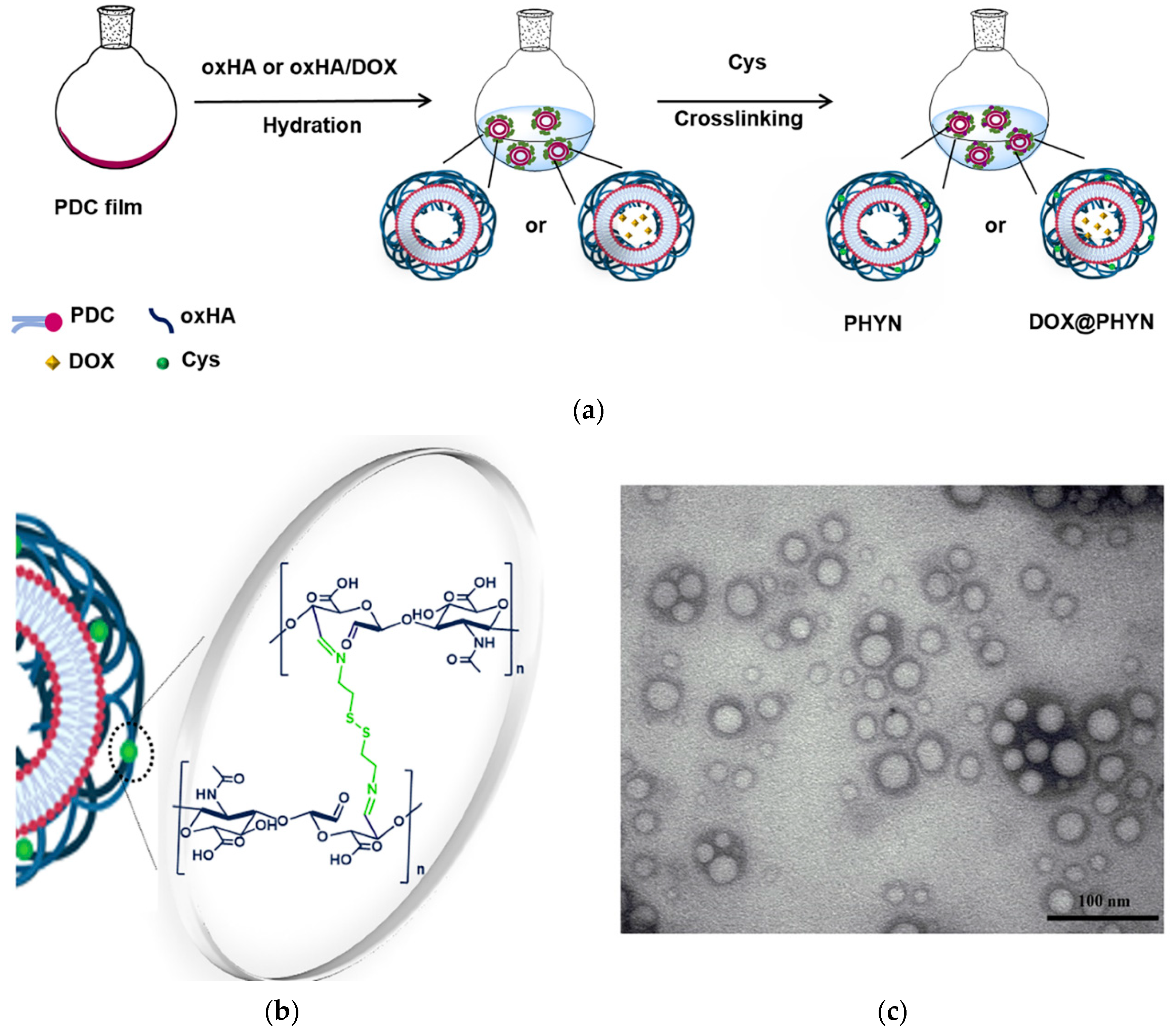
2.1. Synthesis and Characterization of Nanoparticles
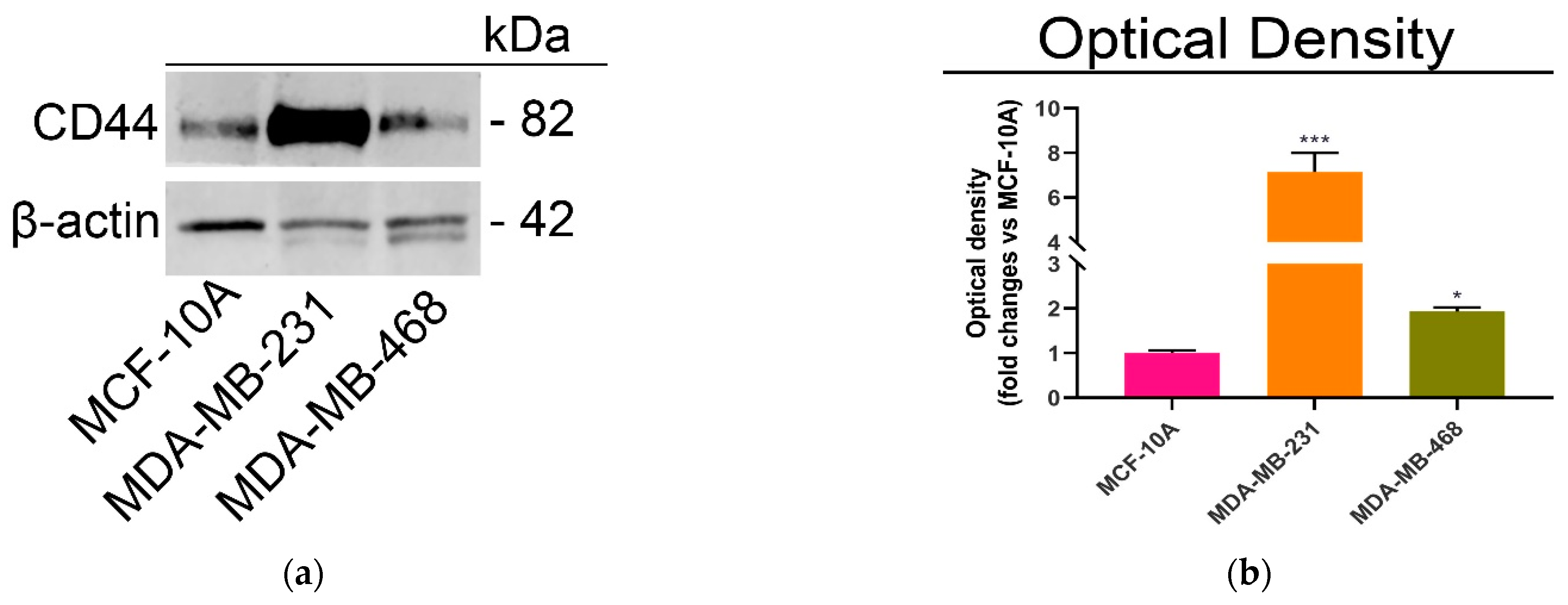
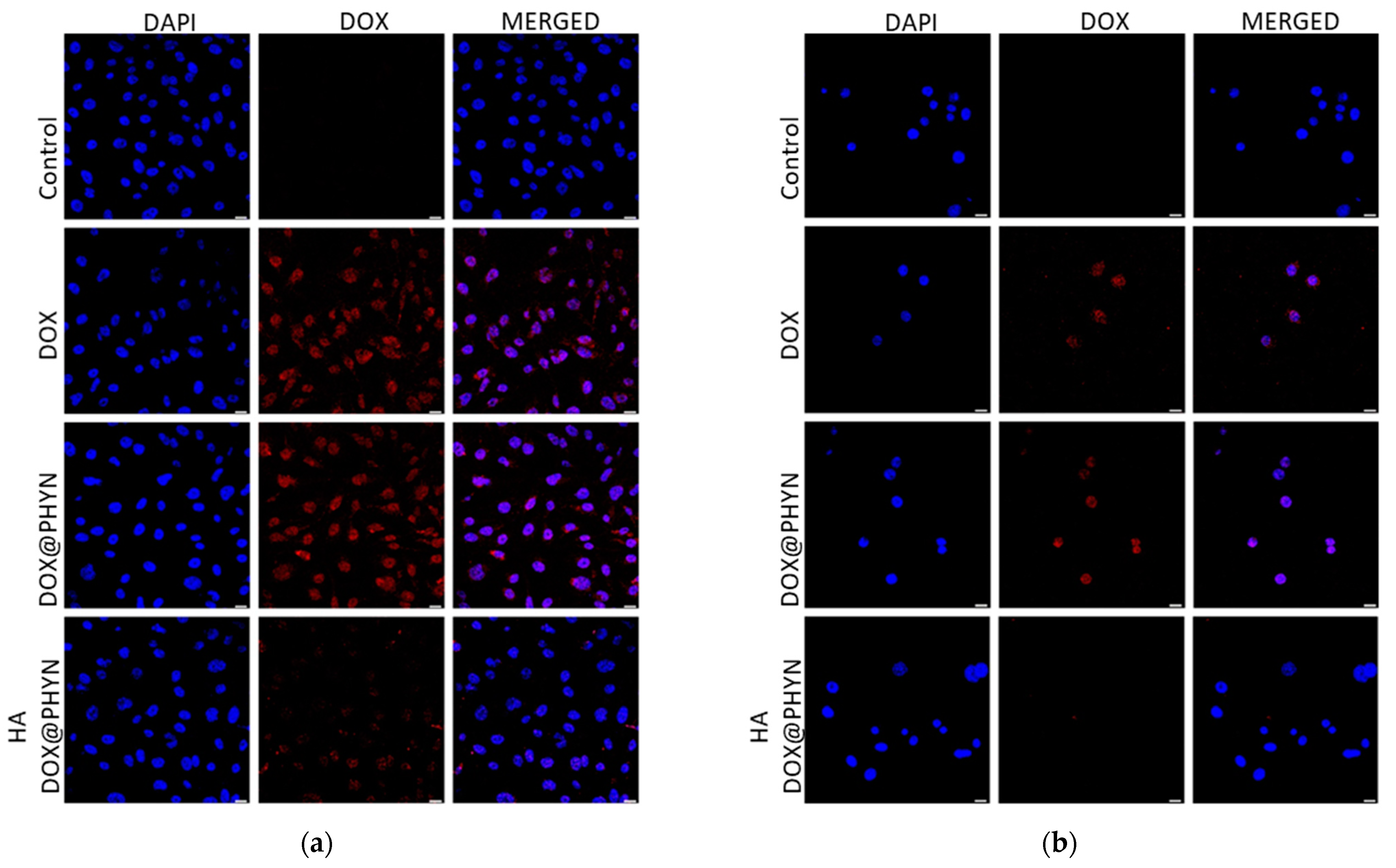
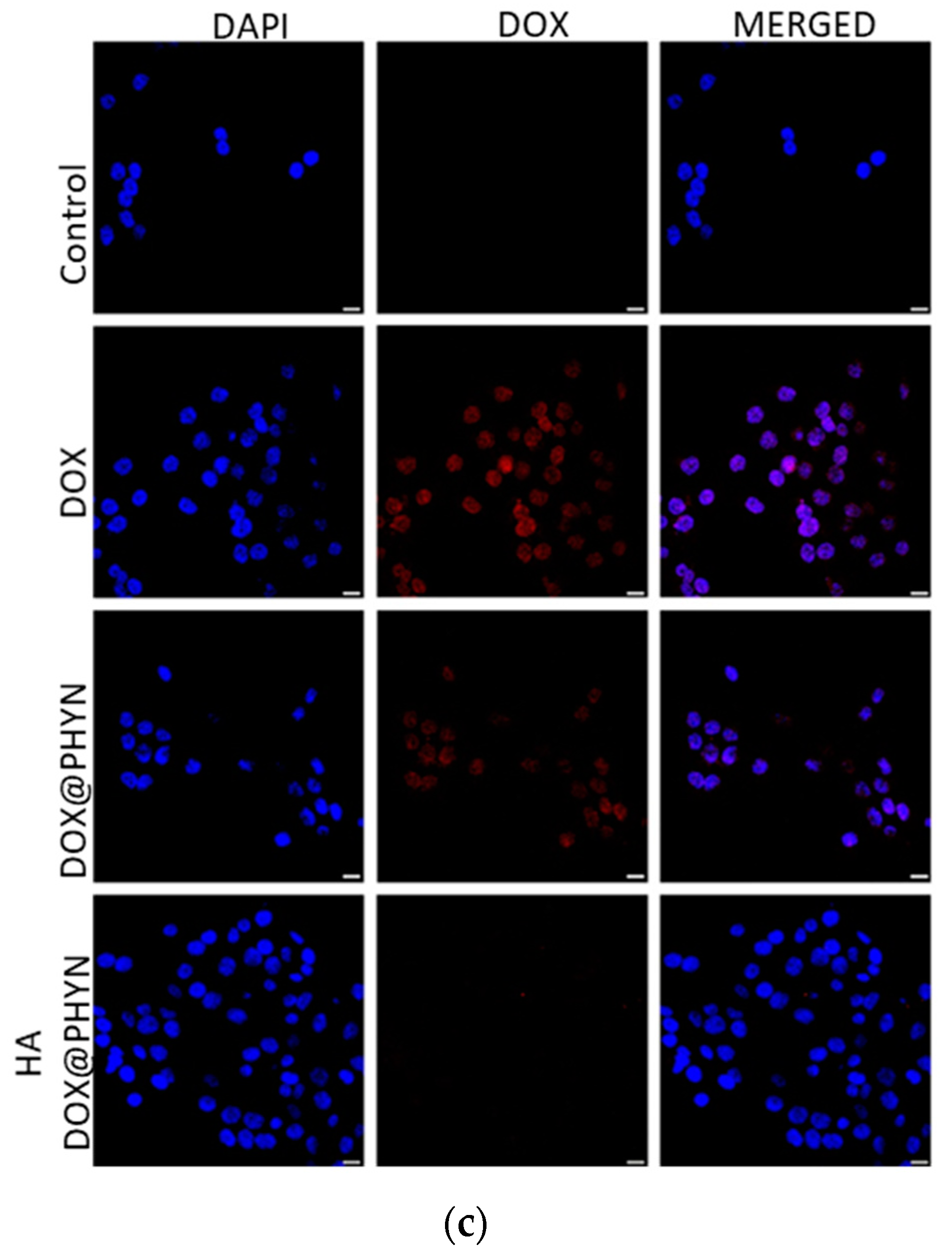
2.2. CD44-Mediated Cellular Uptake of Nanoparticles
2.3. DOX Release Experiments
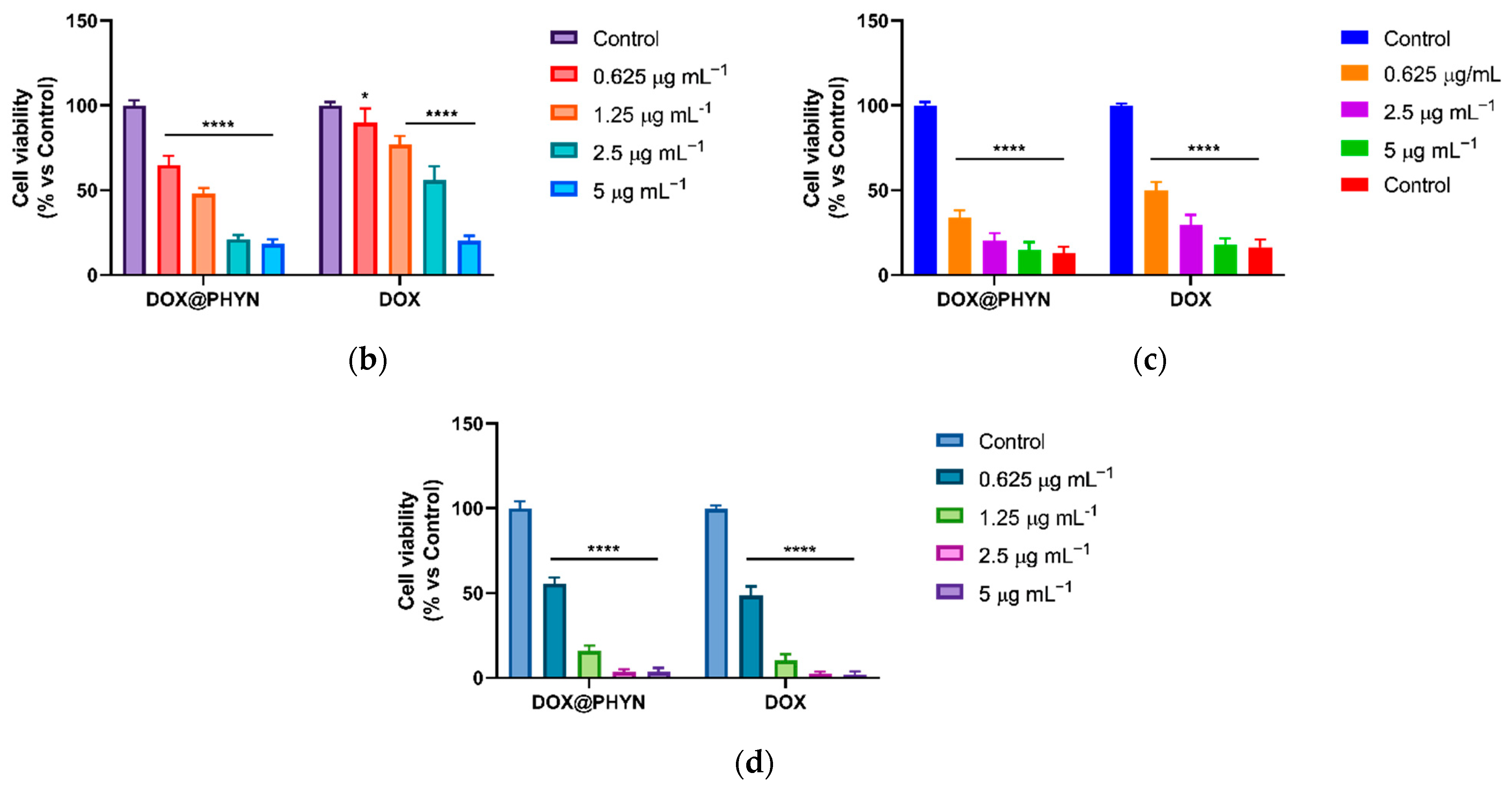
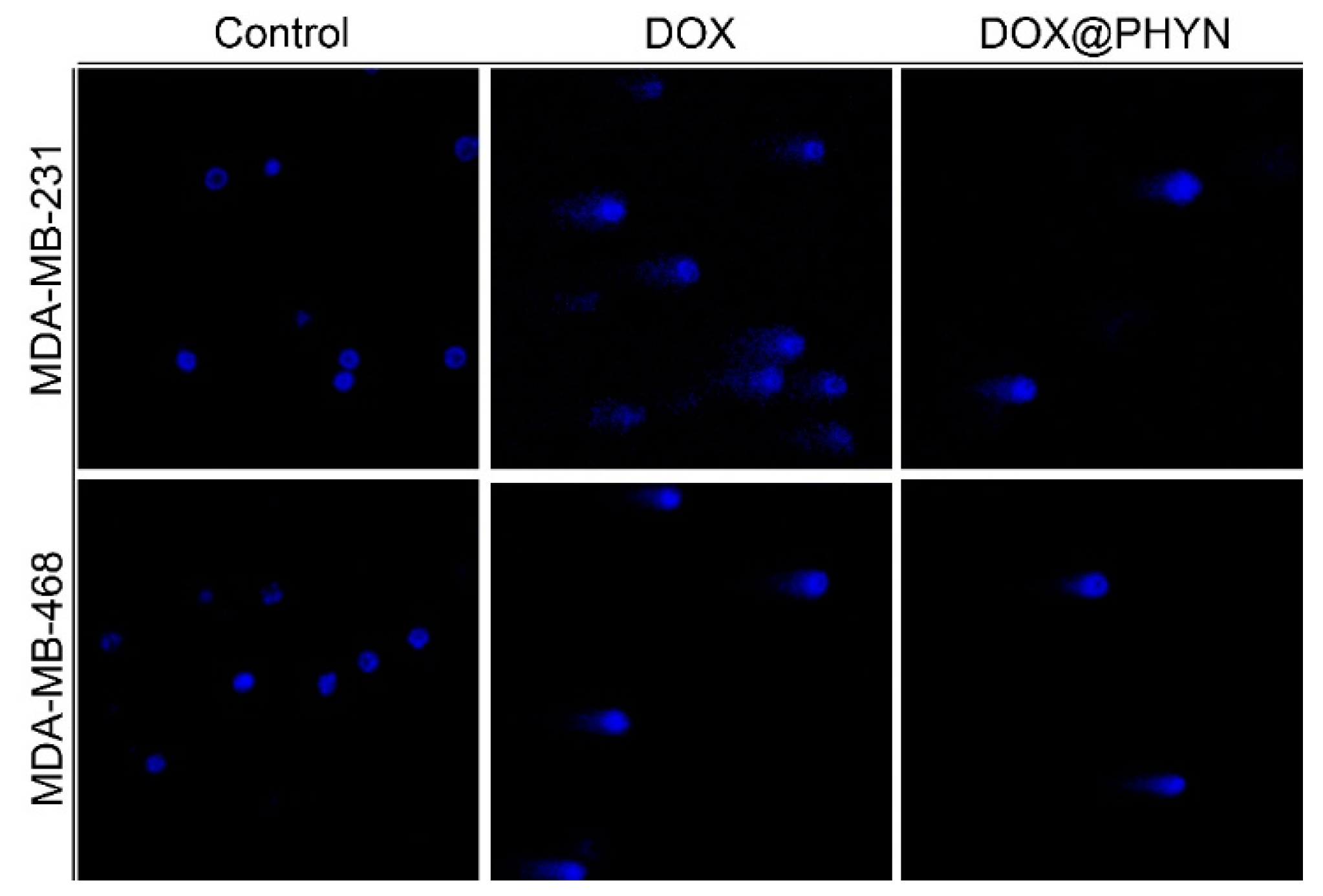
2.4. Cell Viability Experiments and Apoptotic Assay
3. Materials and Methods
3.1. Synthesis of Oxidized Hyaluronic Acid
3.2. Preparation of Unloaded and Loaded Nanoparticles (PHYN and DOX@PHYN)
3.3. Cell Cultures
3.4. Immunoblotting Analysis
3.5. Confocal Microscopy Analysis
3.6. Release Experiments
3.7. Cell Viability and Growth Curves
3.8. Comet Assay
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dang, V.T.; Dao, T.V.; Tran, T.V.; Tran, H.T. Abstract 1618: Breast Cancer Screening in Low and Lower–Middle Income Country: A Program in Vietnam. Cancer Res. 2019, 79, 1618. [Google Scholar] [CrossRef]
- Tomlinson, D.; Martin, H.; Smith, L. Multidrug-resistant breast cancer: Current perspectives. Breast Cancer Targets Ther. 2014, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigro, A.; Frattaruolo, L.; Fava, M.; De Napoli, I.; Greco, M.; Comandè, A.; De Santo, M.; Pellegrino, M.; Ricci, E.; Giordano, F.; et al. Bortezomib-Loaded Mesoporous Silica Nanoparticles Selectively Alter Metabolism and Induce Death in Multiple Myeloma Cells. Cancers 2020, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Clemons, T.D.; Singh, R.; Sorolla, A.; Chaudhari, N.; Hubbard, A.; Iyer, K.S. Distinction Between Active and Passive Targeting of Nanoparticles Dictate Their Overall Therapeutic Efficacy. Langmuir 2018, 34, 15343–15349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Pearce, A.K.; O’Reilly, R.K. Insights into Active Targeting of Nanoparticles in Drug Delivery: Advances in Clinical Studies and Design Considerations for Cancer Nanomedicine. Bioconjug. Chem. 2019, 30, 2300–2311. [Google Scholar] [CrossRef]
- Kampen, K.R. Membrane Proteins: The Key Players of a Cancer Cell. J. Membr. Biol. 2011, 242, 69–74. [Google Scholar] [CrossRef]
- Tang, Q.; Yu, B.; Gao, L.; Cong, H.; Song, N.; Lu, C. Stimuli Responsive Nanoparticles for Controlled Anti-cancer Drug Release. Curr. Med. Chem. 2018, 25, 1837–1866. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. Semin. Orig. Investig. 2008, 26, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymer 2021, 13, 1027. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [Green Version]
- Hayward, S.L.; Wilson, C.L.; Kidambi, S. Hyaluronic acid-conjugated liposome nanoparticles for targeted delivery to CD44 overexpressing glioblastoma cells. Oncotarget 2016, 7, 34158–34171. [Google Scholar] [CrossRef]
- Bartheldyová, E.; Effenberg, R.; Mašek, J.; Procházka, L.; Knötigová, P.T.; Kulich, P.; Hubatka, F.; Velínská, K.; Zelníčková, J.; Zouharová, D.; et al. Hyaluronic Acid Surface Modified Liposomes Prepared via Orthogonal Aminoxy Coupling: Synthesis of Nontoxic Aminoxylipids Based on Symmetrically α-Branched Fatty Acids, Preparation of Liposomes by Microfluidic Mixing, and Targeting to Cancer Cells Expressing CD44. Bioconjug. Chem. 2018, 29, 2343–2356. [Google Scholar] [CrossRef]
- Kari, O.K.; Tavakoli, S.; Parkkila, P.; Baan, S.; Savolainen, R.; Ruoslahti, T.; Johansson, N.G.; Ndika, J.; Alenius, H.; Viitala, T.; et al. Light-Activated Liposomes Coated with Hyaluronic Acid as a Potential Drug Delivery System. Pharmaceutics 2020, 12, 763. [Google Scholar] [CrossRef]
- Ravar, F.; Saadat, E.; Gholami, M.; Dehghankelishadi, P.; Mahdavi, M.; Azami, S.; Dorkoosh, F.A. Hyaluronic acid-coated liposomes for targeted delivery of paclitaxel, in-vitro characterization and in-vivo evaluation. J. Control. Release 2016, 229, 10–22. [Google Scholar] [CrossRef]
- Chi, Y.; Yin, X.; Sun, K.; Feng, S.; Liu, J.; Chen, D.; Guo, C.; Wu, Z. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J. Control. Release 2017, 261, 113–125. [Google Scholar] [CrossRef]
- Rao, N.; Rho, J.G.; Um, W.; Ek, P.K.; Nguyen, V.Q.; Oh, B.H.; Kim, W.; Park, J.H. Hyaluronic Acid Nanoparticles as Nanomedicine for Treatment of Inflammatory Diseases. Pharmaceutics 2020, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Diaz-Gomez, L.; Cirillo, G.; Nicoletta, F.P.; Leggio, A.; Iemma, F. Dual-Targeted Hyaluronic Acid/Albumin Micelle-Like Nanoparticles for the Vectorization of Doxorubicin. Pharmaceutics 2021, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, J.; Zhu, W.; Sun, C.; Di, D.; Zhang, Y.; Wang, P.; Wang, Z.; Wang, S. Dual-stimuli responsive hyaluronic acid-conjugated mesoporous silica for targeted delivery to CD44-overexpressing cancer cells. Acta Biomater. 2015, 23, 147–156. [Google Scholar] [CrossRef]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic Acid–Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Adv. Mater. 2019, 31, e1803549. [Google Scholar] [CrossRef]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer Res. Ther. 2014, 10, 853–858. [Google Scholar] [CrossRef]
- Persano, F.; Gigli, G.; Leporatti, S. Lipid-polymer hybrid nanoparticles in cancer therapy: Current overview and future directions. Nano Express 2021, 2, 012006. [Google Scholar] [CrossRef]
- Ma, J.; Deng, H.; Zhao, F.; Deng, L.; Wang, W.; Dong, A.; Zhang, J. Liposomes-Camouflaged Redox-Responsive Nanogels to Resolve the Dilemma between Extracellular Stability and Intracellular Drug Release. Macromol. Biosci. 2018, 18, e1800049. [Google Scholar] [CrossRef]
- Pandit, A.H.; Mazumdar, N.; Ahmad, S. Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2019, 137, 853–869. [Google Scholar] [CrossRef]
- Dahlmann, J.; Krause, A.; Möller, L.; Kensah, G.; Möwes, M.; Diekmann, A.; Martin, U.; Kirschning, A.; Gruh, I.; Dräger, G. Fully defined in situ cross-linkable alginate and hyaluronic acid hydrogels for myocardial tissue engineering. Biomaterials 2013, 34, 940–951. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Q.; Wang, J.; Guan, S.; Cai, D.; Liu, J. Inhibition of growth and metastasis of breast cancer by targeted delivery of 17-hydroxy-jolkinolide B via hyaluronic acid-coated liposomes. Carbohydr. Polym. 2021, 257, 117572. [Google Scholar] [CrossRef]
- Hanieh, P.; Forte, J.; Di Meo, C.; Ammendolia, M.; Del Favero, E.; Cantù, L.; Rinaldi, F.; Marianecci, C.; Carafa, M. Hyaluronic Acid Derivative Effect on Niosomal Coating and Interaction with Cellular Mimetic Membranes. Molecules 2021, 26, 3434. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, E.; Dorati, R.; Conti, B.; Modena, T.; Cova, E.; Meloni, F.; Genta, I. Hyaluronic Acid-Decorated Chitosan Nanoparticles for CD44-Targeted Delivery of Everolimus. Int. J. Mol. Sci. 2018, 19, 2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosco, D.; Tsapis, N.; Nascimento, T.L.; Fresta, M.; Chapron, D.; Taverna, M.; Arpicco, S.; Fattal, E. Polysaccharide-coated liposomes by post-insertion of a hyaluronan-lipid conjugate. Colloids Surf. B Biointerfaces 2017, 158, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewurum, A.; Alur, A.A.; Glenn, M.; Schnepf, A.; Borchman, D. Hyaluronic Acid-Lipid Binding. BMC Chem. 2021, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Blanco-Fernandez, B.; Diaz-Gomez, L.; Concheiro, A.; Alvarez-Lorenzo, C. Hydrophobically Modified Keratin Vesicles for GSH-Responsive Intracellular Drug Release. Bioconjug. Chem. 2015, 26, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, Y.; Wang, L.; Liang, Z.; Li, D.; Xu, X.; Chen, Y.; Yang, X.; Zhang, H.; Niu, H. Self-Crosslinkable Chitosan-Hyaluronic Acid Dialdehyde Nanoparticles for Cd44-Targeted Sirna Delivery to Treat Bladder Cancer. Bioact. Mater. 2021, 6, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef]
- Kanamala, M.; Wilson, W.R.; Yang, M.M.; Palmer, B.D.; Wu, Z.M. Mechanisms and Biomaterials in Ph-Responsive Tumour Targeted Drug Delivery: A Review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Xu, H.; Paxton, J.W.; Wu, Z. Enhanced pH-Responsiveness, Cellular Trafficking, Cytotoxicity and Long-circulation of PEGylated Liposomes with Post-insertion Technique Using Gemcitabine as a Model Drug. Pharm. Res. 2015, 32, 2428–2438. [Google Scholar] [CrossRef]
- Yan, Y.; Ding, H. Ph-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials 2020, 10, 1613. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Curcio, M.; Cirillo, G.; Paoli, A.; Naimo, G.D.; Mauro, L.; Amantea, D.; Leggio, A.; Nicoletta, F.P.; Lemma, F. Self-Assembling Dextran Prodrug for Redox-and Ph-Responsive Co-Delivery of Therapeutics in Cancer Cells. Colloids Surf. B-Biointerfaces 2020, 185, 110537. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Diaz-Gomez, L.; Cirillo, G.; Concheiro, A.; Iemma, F.; Alvarez-Lorenzo, C. pH/redox dual-sensitive dextran nanogels for enhanced intracellular drug delivery. Eur. J. Pharm. Biopharm. 2017, 117, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.V.; Guilherme, M.R.; Rubira, A.; Muniz, E. Mathematical model for the prediction of the overall profile of in vitro solute release from polymer networks. J. Colloid Interface Sci. 2007, 310, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Lee, K.-M.; Kim, S.-H.; Kwon, Y.-J.; Chun, Y.-J.; Choi, H.-K. Comparative metabolic and lipidomic profiling of human breast cancer cells with different metastatic potentials. Oncotarget 2016, 7, 67111–67128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.-H.; Tseng, Y.-M.; Wu, S.-H.; Tsai, S.-M.; Tsai, L.-Y. Whey Protein Concentrate Renders Mda-Mb-231 Cells Sensitive to Rapamycin by Altering Cellular Redox State and Activating Gsk3beta/Mtor Signaling. Sci. Rep. 2017, 7, 15976. [Google Scholar] [CrossRef]
- Green, P.S.; Leeuwenburgh, C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2002, 1588, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.; Dusinska, M.; Franklin, M.; Somorovska, M.; Petrovska, H.; Duthie, S.; Fillion, L.; Panayiotidis, M.; Raslova, K.; Vaughan, N. Comet Assay in Human Biomonitoring Studies: Reliability, Validation, and Applications. Environ. Mol. Mutagenesis 1997, 30, 139–146. [Google Scholar] [CrossRef]
- AshokKumar, T.; Prabhu, D.; Geetha, R.; Govindaraju, K.; Manikandan, R.; Arulvasu, C.; Singaravelu, G. Apoptosis in liver cancer (HepG2) cells induced by functionalized gold nanoparticles. Colloids Surf. B Biointerfaces 2014, 123, 549–556. [Google Scholar] [CrossRef]
- Joo, H.; Park, J.; Sutthiwanjampa, C.; Kim, H.; Bae, T.; Kim, W.; Choi, J.; Kim, M.; Kang, S.; Park, H. Surface Coating with Hyaluronic Acid-Gelatin-Crosslinked Hydrogel on Gelatin-Conjugated Poly(dimethylsiloxane) for Implantable Medical Device-Induced Fibrosis. Pharmaceutics 2021, 13, 269. [Google Scholar] [CrossRef]
- Dasari, B.C.; Cashman, S.M.; Kumar-Singh, R. Reducible Peg-Pod/DNA Nanoparticles for Gene Transfer in Vitro and in Vivo: Application in a Mousemodel of Age-Relatedmacular Degeneration. Mol. Ther. Nucleic Acids 2017, 8, 77–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavano, L.; Muzzalupo, R.; Trombino, S.; Cassano, R.; Pingitore, A.; Picci, N. Effect of formulations variables on the in vitro percutaneous permeation of Sodium Diclofenac from new vesicular systems obtained from Pluronic triblock copolymers. Colloids Surf. B Biointerfaces 2010, 79, 227–234. [Google Scholar] [CrossRef]
- Provencher, S.W. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput. Phys. Commun. 1982, 27, 213–227. [Google Scholar] [CrossRef]
- Armentano, B.; Curcio, R.; Brindisi, M.; Mancuso, R.; Rago, V.; Ziccarelli, I.; Frattaruolo, L.; Fiorillo, M.; Dolce, V.; Gabriele, B.; et al. 5-(Carbamoylmethylene)-oxazolidin-2-ones as a Promising Class of Heterocycles Inducing Apoptosis Triggered by Increased ROS Levels and Mitochondrial Dysfunction in Breast and Cervical Cancer. Biomed. 2020, 8, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacopetta, D.; Lappano, R.; Cappello, A.R.; Madeo, M.; De Francesco, E.M.; Santoro, A.; Curcio, R.; Capobianco, L.; Pezzi, V.; Maggiolini, M.; et al. SLC37A1 Gene expression is up-regulated by epidermal growth factor in breast cancer cells. Breast Cancer Res. Treat. 2009, 122, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Brindisi, M.C.; Bouzidi, L.; Frattaruolo, M.R.; Loizzo, R.; Tundis, A.; Dugay, B.; Deguin, A.R.; Cappello, A.R.; Cappello, M.S. Chemical Profile, Antioxidant, Anti-Inflammatory, and Anti-Cancer Effects of Italian Salvia Rosmarinus Spenn. Methanol Leaves Extracts. Antioxidants 2020, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, P.; Yang, X.; Li, C.; Guo, Y.; Sun, K. Self-assembly of a disulfide-containing core/shell nanocomplex with intracellular environment-sensitive facilitated endo-lysosomal escape for enhanced antitumor efficacy. J. Mater. Sci. 2020, 56, 4380–4395. [Google Scholar] [CrossRef]
- Park, H.-K.; Lee, S.J.; Oh, J.-S.; Lee, S.-G.; Jeong, Y.-I.; Lee, H.C. Smart Nanoparticles Based on Hyaluronic Acid for Redox-Responsive and CD44 Receptor-Mediated Targeting of Tumor. Nanoscale Res. Lett. 2015, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.; Cappello, M.; Dugay, A.; Deguin, B.; Lauria, G.; Cappello, A.; Tundis, R. New Insights into the Antioxidant and Anti-Inflammatory Effects of Italian Salvia officinalis Leaf and Flower Extracts in Lipopolysaccharide and Tumor-Mediated Inflammation Models. Antioxidants 2021, 10, 311. [Google Scholar] [CrossRef]
- Brindisi, M.; Frattaruolo, L.; Mancuso, R.; Palumbo Piccionello, A.; Ziccarelli, I.; Catto, M.; Nicolotti, O.; Altomare, C.D.; Gabriele, B.; Cappello, A.R. Anticancer Potential of Novel Alpha, Beta-Unsaturated Gamma-Lactam Derivatives Targeting the Pi3k/Akt Signaling Pathway. Biochem. Pharm. 2021, 190, 114659. [Google Scholar] [CrossRef]
- Olive, P.L.; Banáth, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Speit, G.; Hartmann, A. The Comet Assay. In DNA Repair Protocols: Mammalian Systems; Henderson, D.S., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 275–286. [Google Scholar]

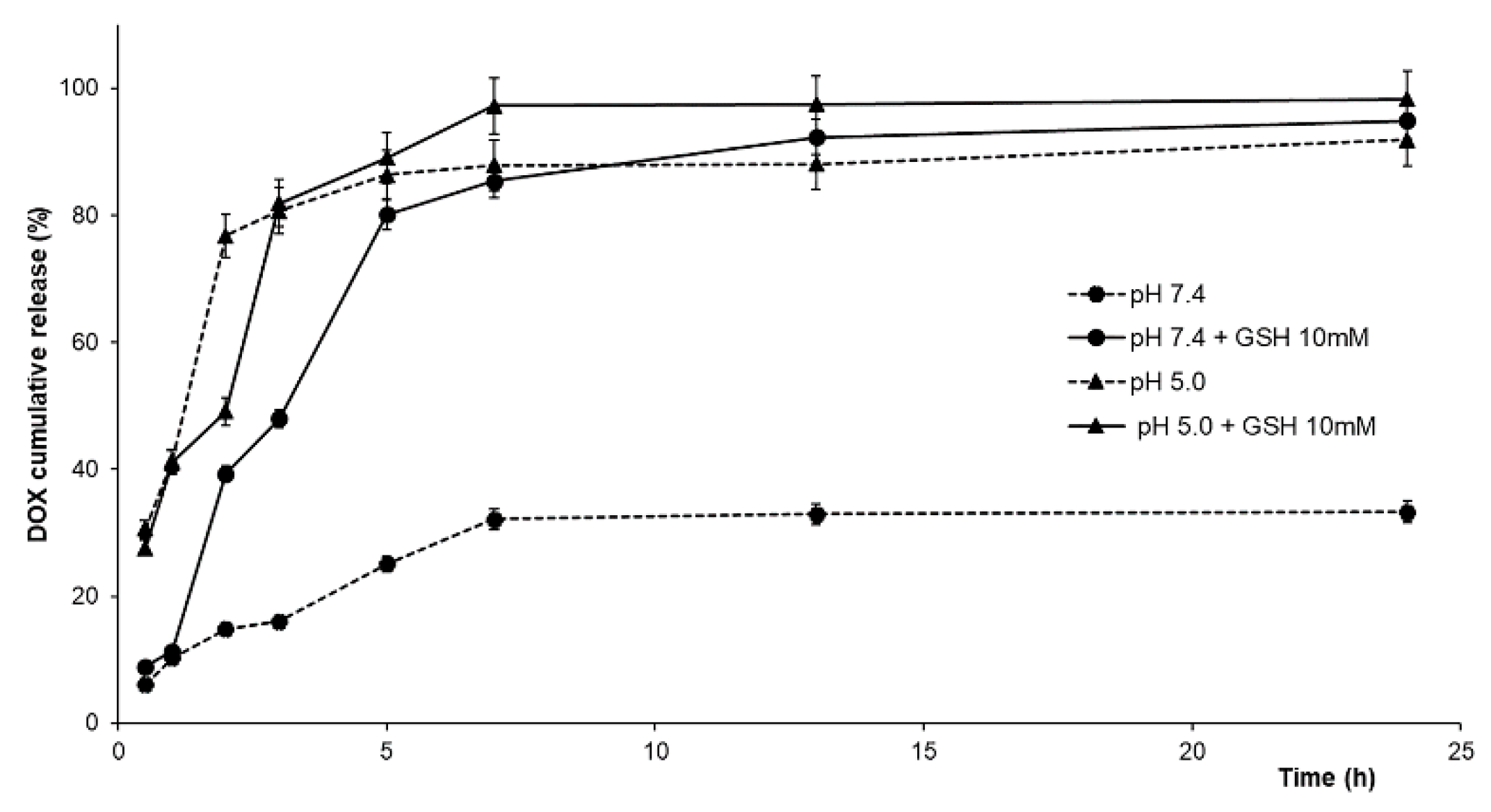


| Mathematical Model | Parameter | DOX | |||
|---|---|---|---|---|---|
| pH 7.4 | pH 5.0 | ||||
| (GSH) 0 mM | (GSH) 10 mM | (GSH) 0 mM | (GSH) 10 mM | ||
| Equation (2) | R2 | 0.97518 | 0.98428 | 0.98652 | 0.98273 |
| Fmax | 0.33989 | 0.96906 | 0.93295 | 0.96913 | |
| α | 0.51 | 31.32 | 13.91 | 31.39 | |
| kR (10−2) | 0.472 | 0.408 | 1.174 | 0.996 | |
| Equation (3) | R2 | 0.96441 | 0.38416 | 0.96811 | 0.95466 |
| Cell Line | IC50 (μg mL−1) | |
|---|---|---|
| DOX | DOX@PHYN | |
| MCF-10A | 0.3377 0.2404 to 0.4529 (a) | 0.4310 (b) 0.3160 to 0.5688 (a) |
| MDA-MB-231 | 2.808 2.214 to 3.584 (a) | 1.022 (c) 0.9022 to 1.156 (a) |
| MDA-MB-468 | 0.5947 0.5158 to 0.6820 (a) | 0.3508 (c) 0.2972 to 0.4093 (a) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curcio, M.; Brindisi, M.; Cirillo, G.; Frattaruolo, L.; Leggio, A.; Rago, V.; Nicoletta, F.P.; Cappello, A.R.; Iemma, F. Smart Lipid–Polysaccharide Nanoparticles for Targeted Delivery of Doxorubicin to Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 2386. https://doi.org/10.3390/ijms23042386
Curcio M, Brindisi M, Cirillo G, Frattaruolo L, Leggio A, Rago V, Nicoletta FP, Cappello AR, Iemma F. Smart Lipid–Polysaccharide Nanoparticles for Targeted Delivery of Doxorubicin to Breast Cancer Cells. International Journal of Molecular Sciences. 2022; 23(4):2386. https://doi.org/10.3390/ijms23042386
Chicago/Turabian StyleCurcio, Manuela, Matteo Brindisi, Giuseppe Cirillo, Luca Frattaruolo, Antonella Leggio, Vittoria Rago, Fiore Pasquale Nicoletta, Anna Rita Cappello, and Francesca Iemma. 2022. "Smart Lipid–Polysaccharide Nanoparticles for Targeted Delivery of Doxorubicin to Breast Cancer Cells" International Journal of Molecular Sciences 23, no. 4: 2386. https://doi.org/10.3390/ijms23042386
APA StyleCurcio, M., Brindisi, M., Cirillo, G., Frattaruolo, L., Leggio, A., Rago, V., Nicoletta, F. P., Cappello, A. R., & Iemma, F. (2022). Smart Lipid–Polysaccharide Nanoparticles for Targeted Delivery of Doxorubicin to Breast Cancer Cells. International Journal of Molecular Sciences, 23(4), 2386. https://doi.org/10.3390/ijms23042386










