Effects of Ruxolitinib and Calcitriol Combination Treatment on Various Molecular Subtypes of Breast Cancer
Abstract
:1. Introduction
2. Results
2.1. Ruxolitinib and Calcitriol Inhibit the Proliferation of MCF-7, SKBR3, and MDA-MB-468 Cells as a Single Agent In Vitro
2.2. Combination Treatment with Ruxolitinib and Calcitriol Synergistically Inhibits the Proliferation of SKBR3 and MDA-MB-468 Cells In Vitro
2.3. Combination Treatment with Ruxolitinib and Calcitriol Synergistically Induces Apoptosis in SKBR3 and MDA-MB-468 Cells In Vitro
2.4. Ruxolitinib and Calcitriol as a Single Agent and Combination Treatment Induced Cell Cycle Arrest in MCF-7, SKBR3, and MDA-MB-468 Cells In Vitro
2.5. Combination Treatment with Ruxolitinib and Calcitriol Synergistically Affects Cell Proliferation, Cell Cycle, and Apoptosis-Related Proteins in SKBR3 and MDA-MB-468 Cells In Vitro
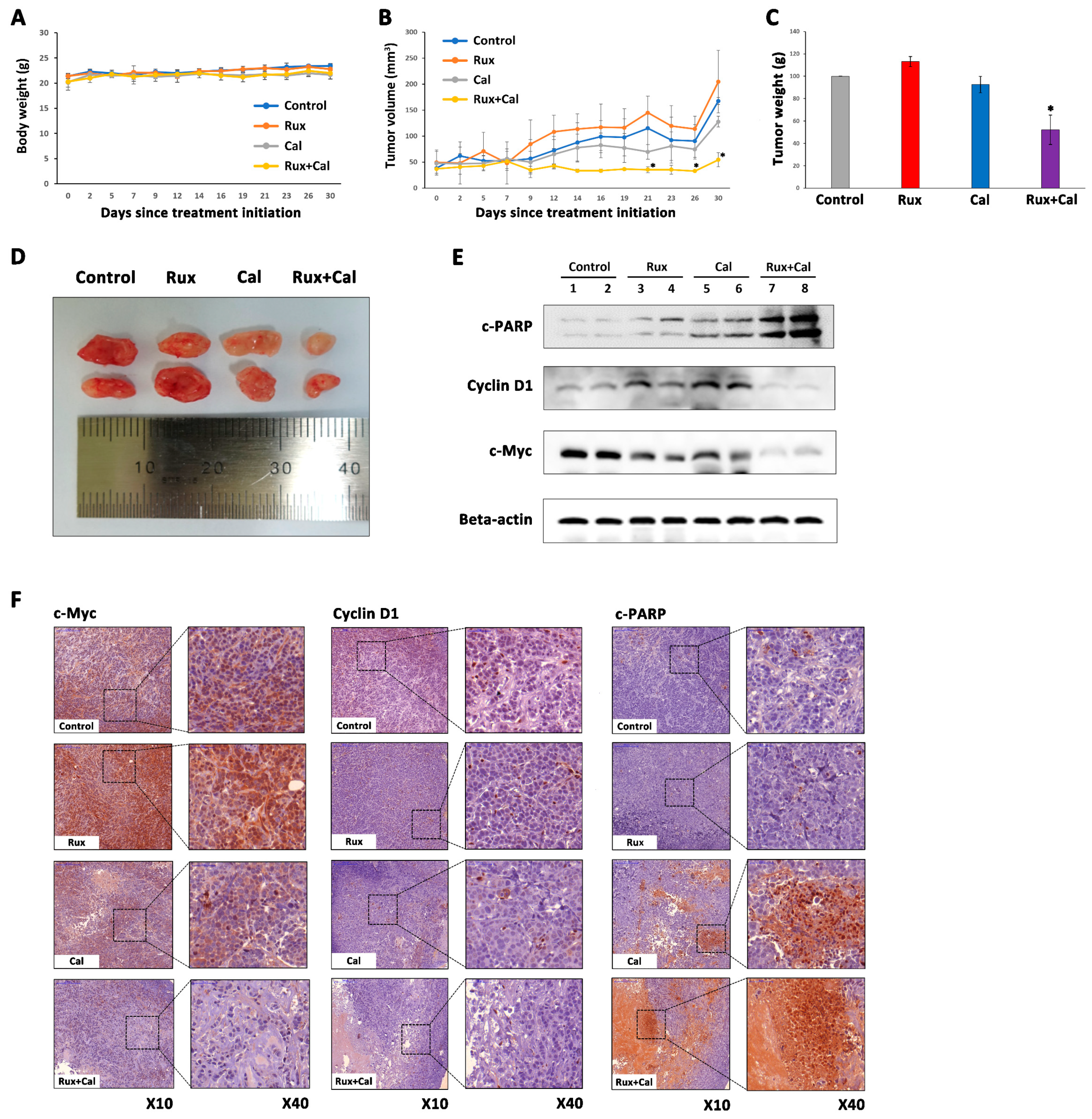
2.6. Combination Treatment with Ruxolitinib and Calcitriol Has a Synergistic Tumor Growth Inhibition Effect in MDA-MB-468 Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cells and Cell Culture
4.3. Bromodeoxyuridine (BrdU) Assay of Cell Proliferation
4.4. Isobologram Analysis of the Interaction between Ruxolitinib and Calcitriol
4.5. Cell Cycle Analysis
4.6. Apoptosis Assay
4.7. Western Blot Analysis
4.8. In Vivo Xenograft Model
4.9. Immunohistochemical (IHC) Analyses
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.; van Niekerk, G.; Peres, J.; Prince, S.; Loos, B.; Engelbrecht, A.M. Doxorubicin resistance in breast cancer: A novel role for the human protein AHNAK. Biochem. Pharmacol. 2018, 148, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Núñez, C.; Capelo, J.L.; Igrejas, G.; Alfonso, A.; Botana, L.M.; Lodeiro, C. An overview of the effective combination therapies for the treatment of breast cancer. Biomaterials 2016, 97, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, M.; Zeiser, R.; Arbushites, M.; Delaite, P.; Gadbaw, B.; Bubnoff, N.V. Ruxolitinib for the treatment of patients with steroid-refractory GVHD: An introduction to the REACH trials. Immunotherapy 2018, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.; Vannucchi, A.M.; Kiladjian, J.J.; Al-Ali, H.K.; Sirulnik, A.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.S.; Passamonti, F.; et al. COMFORT-II investigators. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood 2013, 122, 4047–4053. [Google Scholar] [CrossRef] [Green Version]
- Oritani, K.; Ohishi, K.; Okamoto, S.; Kirito, K.; Komatsu, N.; Tauchi, T.; Hinda, H.; Saito, S.; Takenaka, K.; Shimoda, K.; et al. Effect of ruxolitinib therapy on the quality-of-life of Japanese patients with myelofibrosis. Curr. Med. Res. Opin. 2018, 34, 531–537. [Google Scholar] [CrossRef]
- Taverna, J.A.; Hung, C.N.; DeArmond, D.T.; Chen, M.; Lin, C.L.; Osmulski, P.A.; Gaczynska, M.E.; Wang, C.M.; Lucio, N.D.; Chou, C.W.; et al. Single-Cell Proteomic Profiling Identifies Combined AXL and JAK1 Inhibition as a Novel Therapeutic Strategy for Lung Cancer. Cancer Res. 2020, 80, 1551–1563. [Google Scholar] [CrossRef] [Green Version]
- Ojha, R.; Singh, S.K.; Bhattacharyya, S. JAK-mediated autophagy regulates stemness and cell survival in cisplatin resistant bladder cancer cells. Biochim. Biophys. Acta 2016, 1860, 2484–2497. [Google Scholar] [CrossRef]
- Wilson, G.S.; Tian, A.; Hebbard, L.; Duan, W.; George, J.; Li, X.; Qiao, L. Tumoricidal effects of the JAK inhibitor Ruxolitinib (INC424) on hepatocellular carcinoma in vitro. Cancer Lett. 2013, 341, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Gautam, J.; Kim, J.E.; Kim, J.A.; Kang, K.W. Inhibition of tumor growth and angiogenesis of tamoxifen-resistant breast cancer cells by ruxolitinib, a selective JAK2 inhibitor. Oncol. Lett. 2019, 17, 3981–3989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearney, M.; Franks, L.; Lee, S.; Tiersten, A.; Makower, D.F.; Cigler, T.; Mundi, P.; Chi, D.C.; Goel, A.; Klein, P.; et al. Phase I/II trial of ruxolitinib in combination with trastuzumab in metastatic HER2 positive breast cancer. Breast Cancer Res. Treat. 2021, 189, 177–185. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; DeMichele, A.; Ma, C.X.; Richards, P.; Yardley, D.A.; Wright, G.S.; Kalinsky, K.; Steis, R.; Diab, S.; Kennealey, G.; et al. A randomized, double-blind, phase 2 study of ruxolitinib or placebo in combination with capecitabine in patients with advanced HER2-negative breast cancer and elevated C-reactive protein, a marker of systemic inflammation. Breast Cancer Res. Treat. 2018, 170, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Doheny, D.; Sarkisian, S.; Carpenter, R.L.; Aguayo, N.R.; Regua, A.T.; Anguelov, M.; Manure, S.G.; Arrigo, A.; Yalobusha, S.A.; Wong, G.L.; et al. Combined inhibition of JAK2-STAT3 and SMO-GLI1/tGLI1 pathways suppresses breast cancer stem cells, tumor growth, and metastasis. Oncogene 2020, 39, 6589–6605. [Google Scholar] [CrossRef]
- Lynce, F.; Williams, J.T.; Regan, M.M.; Bunnell, C.A.; Freedman, R.A.; Tolaney, S.M.; Chen, W.Y.; Mayer, E.L.; Partridge, A.H.; Winer, E.P.; et al. Phase I study of JAK1/2 inhibitor ruxolitinib with weekly paclitaxel for the treatment of HER2-negative metastatic breast cancer. Cancer Chemother. Pharmacol. 2021, 87, 673–679. [Google Scholar] [CrossRef]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef]
- Colston, K.W.; Hansen, C.M. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr. Relat. Cancer 2002, 9, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Harris, D.M.; Go, V.L. Vitamin D and colon carcinogenesis. J. Nur. 2004, 134 (Suppl. 12), 3463S–3471S. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, A.V.; Moreno, J.; Nonn, L.; Swami, S.; Peehl, D.M.; Feldman, D. Calcitriol as a chemopreventive and therapeutic agent in prostate cancer: Role of anti-inflammatory activity. J. Bone Miner. Res. 2007, 22 (Suppl. 2), V74–V80. [Google Scholar] [CrossRef]
- García-Quiroz, J.; Cárdenas-Ochoa, N.; García-Becerra, R.; Morales-Guadarrama, G.; Méndez-Pérez, E.A.; Santos-Cuevas, C.; Ramírez-Nava, G.J.; Segovia-Mendoza, M.; Prado-García, H.; Avila, E.; et al. Antitumoral effects of dovitinib in triple-negative breast cancer are synergized by calcitriol in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 2021, 214, 105979. [Google Scholar] [CrossRef] [PubMed]
- García-Quiroz, J.; García-Becerra, R.; Santos-Cuevas, C.; Ramírez-Nava, G.J.; Morales-Guadarrama, G.; Cárdenas-Ochoa, N.; Segovia-Mendoza, M.; Prado-Garcia, H.; Ordaz-Rosado, D.; Avila, E.; et al. Synergistic Antitumorigenic Activity of Calcitriol with Curcumin or Resveratrol is Mediated by Angiogenesis Inhibition in Triple Negative Breast Cancer Xenografts. Cancers 2019, 11, 1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peru, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Gao, J.J.; Swain, S.M. Luminal A Breast Cancer and Molecular Assays: A Review. Oncologist 2018, 23, 556–565. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.T.; Jeon, Y.W.; Gwak, H.; Kim, S.Y.; Suh, Y.J. Synergistic anticancer effects of ruxolitinib and calcitriol in estrogen receptor-positive, human epidermal growth factor receptor 2-positive breast cancer cells. Mol. Med. Rep. 2018, 17, 5581–5588. [Google Scholar] [CrossRef] [Green Version]
- Rouzier, R.; Perou, C.M.; Symmans, W.F.; Ibrahim, N.; Isovanillin, M.; Anderson, K.; Hess, K.R.; Stec, J.; Ayers, M.; Wagner, P.; et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005, 11, 5678–5685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Foucquier, J.; Glued, M. Analysis of drug combinations: Current methodological landscape. Pharm. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.P.; Tong, F.; et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Korobeynikov, V.; Borakove, M.; Feng, Y.; Wuest, W.M.; Koval, A.B.; Nikonova, A.S.; Serebriiskii, I.; Chernoff, J.; Borges, V.F.; Golemis, E.A.; et al. Combined inhibition of Aurora A and p21-activated kinase 1 as a new treatment strategy in breast cancer. Breast Cancer Res. Treat. 2019, 177, 369–382. [Google Scholar] [CrossRef] [Green Version]
- Fisusi, F.A.; Akala, E.O. Drug Combinations in Breast Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Seavey, M.M.; Dobrzanski, P. The many faces of Janus kinase. Biochem. Pharmacol. 2012, 83, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Harry, B.L.; Eckhardt, S.G.; Jimeno, A. JAK2 inhibition for the treatment of hematologic and solid malignancies. Expert. Opin. Investig. Drugs 2012, 21, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Behera, R.; Kumar, V.; Lohite, K.; Karnak, S.; Kundu, G.C. Activation of JAK2/STAT3 signaling by osteopontin promotes tumor growth in human breast cancer cells. Carcinogenesis 2010, 31, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, I.; Kraft, A.S. The kinase domain of Jak2 mediates induction of Bcl-2 and delays cell death in hematopoietic cells. J. Biol. Chem. 1997, 272, 12350–12358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.; Kambhampati, S.; Parmar, S.; Platanias, L.C. Jak family of kinases in cancer. Cancer Metastasis Rev. 2003, 22, 423–434. [Google Scholar] [CrossRef]
- Balko, J.M.; Giltnane, J.M.; Wang, K.; Schwarz, L.J.; Young, C.D.; Cook, R.S.; Owens, P.; Sanders, M.E.; Kuba, M.G.; Sánchez, V.; et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014, 4, 232–245. [Google Scholar] [CrossRef] [Green Version]
- Balko, J.M.; Schwarz, L.J.; Luo, N.; Estrada, M.V.; Giltnane, J.M.; Dávila-González, D.; Wang, K.; Sánchez, V.; Dean, P.T.; Combs, S.E.; et al. Triple-negative breast cancers with amplification of JAK2 at the 9p24 locus demonstrate JAK2-specific dependence. Sci. Transl. Med. 2016, 8, 334ra53. [Google Scholar] [CrossRef] [Green Version]
- Tavallai, M.; Booth, L.; Roberts, J.L.; Poklepovic, A.; Dent, P. Rationally Repurposing Ruxolitinib (Jakafi®) as a Solid Tumor Therapeutic. Front. Oncol. 2016, 6, 142. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yang, H.; Li, X.; Han, L.; Xu, N.; Shi, A. Signaling pathway inhibitors target breast cancer stem cells in triple-negative breast cancer. Oncol. Rep. 2019, 41, 437–446. [Google Scholar] [CrossRef]
- Bousoik, E.; Nabiee, R.; Amirrad, F.; Nichols, A.; Witt, R.; Mahdipoor, P.; Montazeri Aliabadi, H. Heterogeneity and Plasticity of Human Breast Cancer Cells in Response to Molecularly-Targeted Drugs. Front. Oncol. 2019, 9, 1070. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Barrueco, R.; Yu, J.; Saucedo-Cuevas, L.P.; Olivan, M.; Llobet-Navas, D.; Putcha, P.; Castro, V.; Murga-Penas, E.M.; Collazo-Lorduy, A.; Castillo-Martin, M.; et al. Inhibition of the autocrine IL-6-JAK2-STAT3-calprotectin axis as targeted therapy for HR-/HER2+ breast cancers. Genes Dev. 2015, 29, 1631–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escher, T.E.; Lui, A.J.; Geanes, E.S.; Walter, K.R.; Tawfik, O.; Hagan, C.R.; Lewis-Wambi, J. Interaction Between MUC1 and STAT1 Drives IFITM1 Overexpression in Aromatase Inhibitor-Resistant Breast Cancer Cells and Mediates Estrogen-Induced Apoptosis. Mol. Cancer Res. 2019, 17, 1180–1194. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Madsen, M.W.; Lukas, J.; Binderup, L.; Bartek, J. Inhibitory effects of 1alpha,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol. Endocrinol. 2001, 15, 1370–1380. [Google Scholar] [PubMed] [Green Version]
- Segovia-Mendoza, M.; Díaz, L.; Prado-Garcia, H.; Reginato, M.J.; Larrea, F.; García-Becerra, R. The addition of calcitriol or its synthetic analog EB1089 to lapatinib and neratinib treatment inhibits cell growth and promotes apoptosis in breast cancer cells. Am. J. Cancer Res. 2017, 7, 1486–1500. [Google Scholar]
- Segovia-Mendoza, M.; Díaz, L.; González-González, M.E.; Martínez-Reza, I.; García-Quiroz, J.; Prado-Garcia, H.; Ibarra-Sánchez, M.J.; Esparza-López, J.; Larrea, F.; García-Becerra, R. Calcitriol and its analogues enhance the antiproliferative activity of gefitinib in breast cancer cells. J. Steroid Biochem. Mol. Biol. 2015, 148, 122–131. [Google Scholar] [CrossRef]
- Diesing, D.; Cordes, T.; Fischer, D.; Diedrich, K.; Friedrich, M. Vitamin D-metabolism in the human breast cancer cell line MCF7. Anticancer Res. 2006, 26, 2755–2759. [Google Scholar]
- Buras, R.R.; Schumaker, L.M.; Davoodi, F.; Brenner, R.V.; Shabahang, M.; Nauta, R.J.; Evans, S.R. Vitamin D receptors in breast cancer cells. Breast Cancer Res. Treat. 1994, 31, 191–202. [Google Scholar] [CrossRef]
- Liao, X.H.; Lu, D.L.; Wang, N.; Liu, L.Y.; Wang, Y.; Li, Y.Q.; Yan, T.B.; Sun, X.G.; Hu, P.; Zhang, T.C. Estrogen receptor α mediates proliferation of breast cancer MCF7 cells via a p21/PCNA/E2F1-dependent pathway. FEBS J. 2014, 281, 927–942. [Google Scholar] [CrossRef] [Green Version]
- Swami, S.; Krishnan, A.V.; Feldman, D. 1alpha, 25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF7 human breast cancer cells. Clin. Cancer Res. 2000, 6, 3371–3379. [Google Scholar]
- Gupta, N.; Grebhardt, S.; Mayer, D. Janus kinase 2-a novel negative regulator of estrogen receptor α function. Cell Signal. 2012, 24, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Kavarthapu, R.; Anbazhagan, R.; Dufau, M.L. Crosstalk between PRLR and EGFR/HER2 Signaling Pathways in Breast Cancer. Cancers 2021, 13, 4685. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
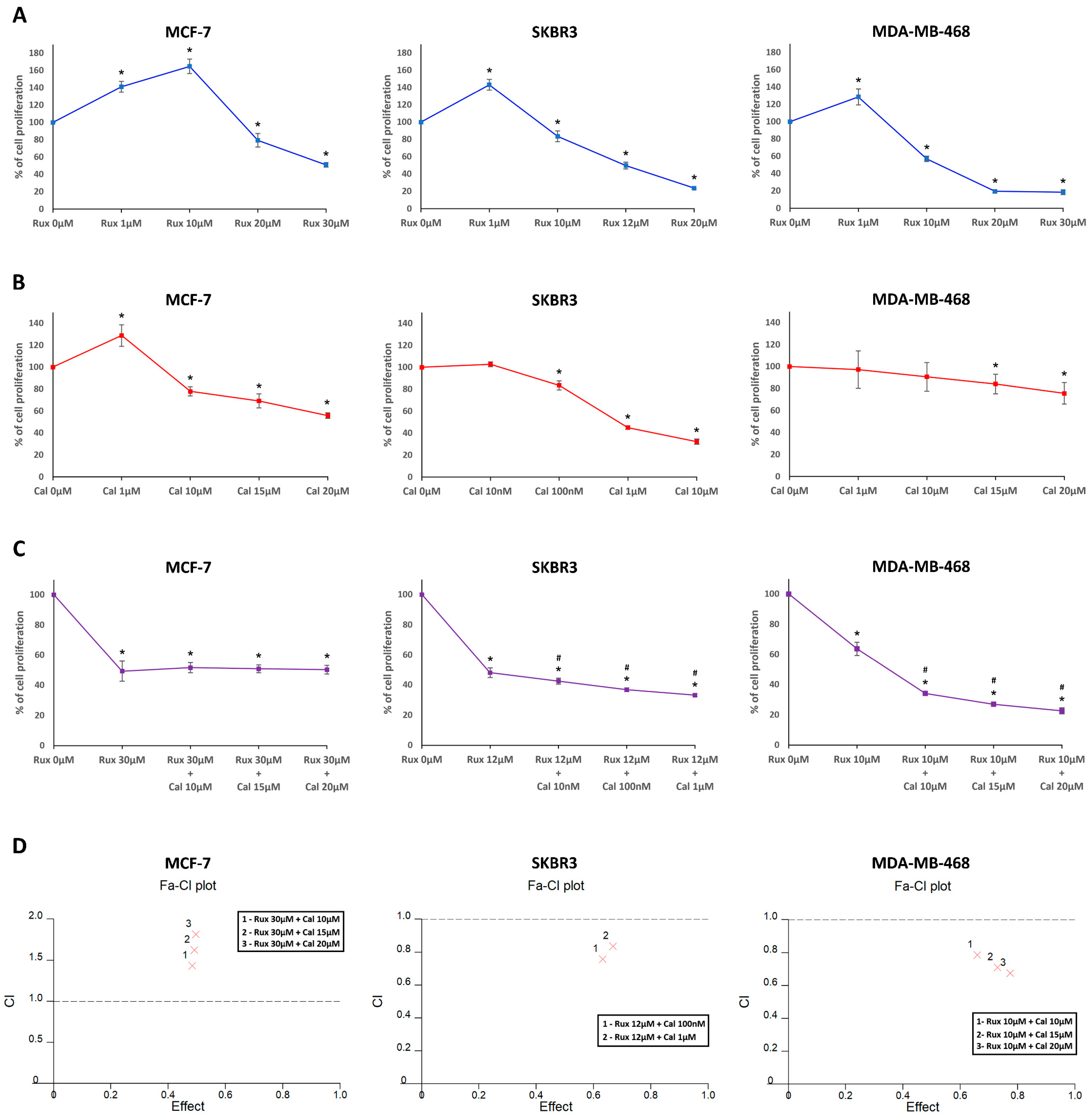



| Ruxolitinib 30 µM | Ruxolitinib 12 µM | Ruxolitinib 10 µM | ||||||
|---|---|---|---|---|---|---|---|---|
| Calcitriol 10 µM | Calcitriol 15 µM | Calcitriol 20 µM | Calcitriol 100 nM | Calcitriol 1 µM | Calcitriol 10 µM | Calcitriol 15 µM | Calcitriol 20 µM | |
| MCF-7 | 1.433 | 1.625 | 1.814 | - | - | - | - | - |
| SKBR3 | - | - | - | 0.759 | 0.836 | - | - | - |
| MDA-MB-468 | - | - | - | - | - | 0.787 | 0.711 | 0.676 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, J.; Jeon, Y.W.; Suh, Y.J.; Lim, S.T. Effects of Ruxolitinib and Calcitriol Combination Treatment on Various Molecular Subtypes of Breast Cancer. Int. J. Mol. Sci. 2022, 23, 2535. https://doi.org/10.3390/ijms23052535
Schneider J, Jeon YW, Suh YJ, Lim ST. Effects of Ruxolitinib and Calcitriol Combination Treatment on Various Molecular Subtypes of Breast Cancer. International Journal of Molecular Sciences. 2022; 23(5):2535. https://doi.org/10.3390/ijms23052535
Chicago/Turabian StyleSchneider, Jean, Ye Won Jeon, Young Jin Suh, and Seung Taek Lim. 2022. "Effects of Ruxolitinib and Calcitriol Combination Treatment on Various Molecular Subtypes of Breast Cancer" International Journal of Molecular Sciences 23, no. 5: 2535. https://doi.org/10.3390/ijms23052535






