Oxygen-Ozone Therapy for Reducing Pro-Inflammatory Cytokines Serum Levels in Musculoskeletal and Temporomandibular Disorders: A Comprehensive Review
Abstract
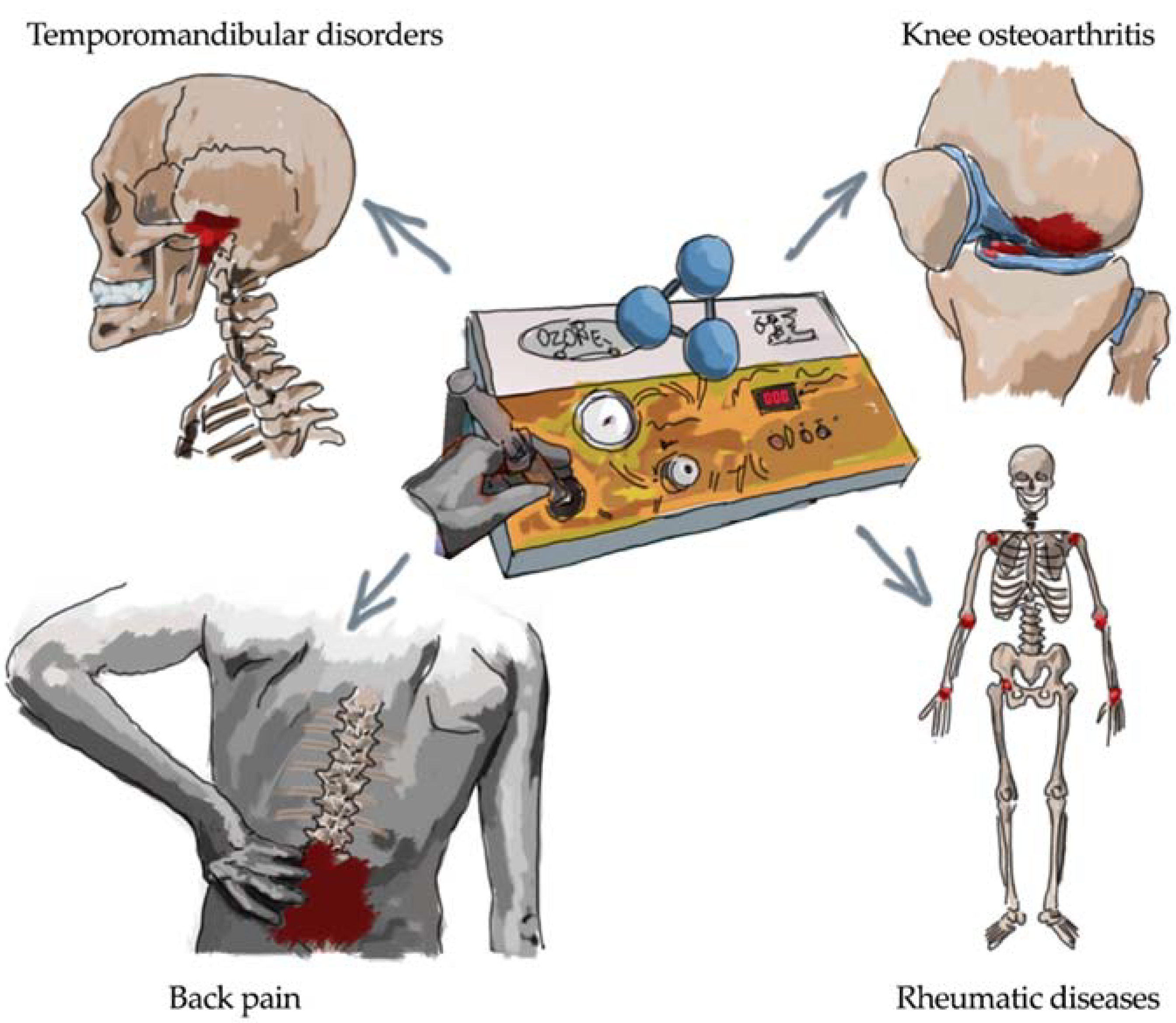
1. Introduction
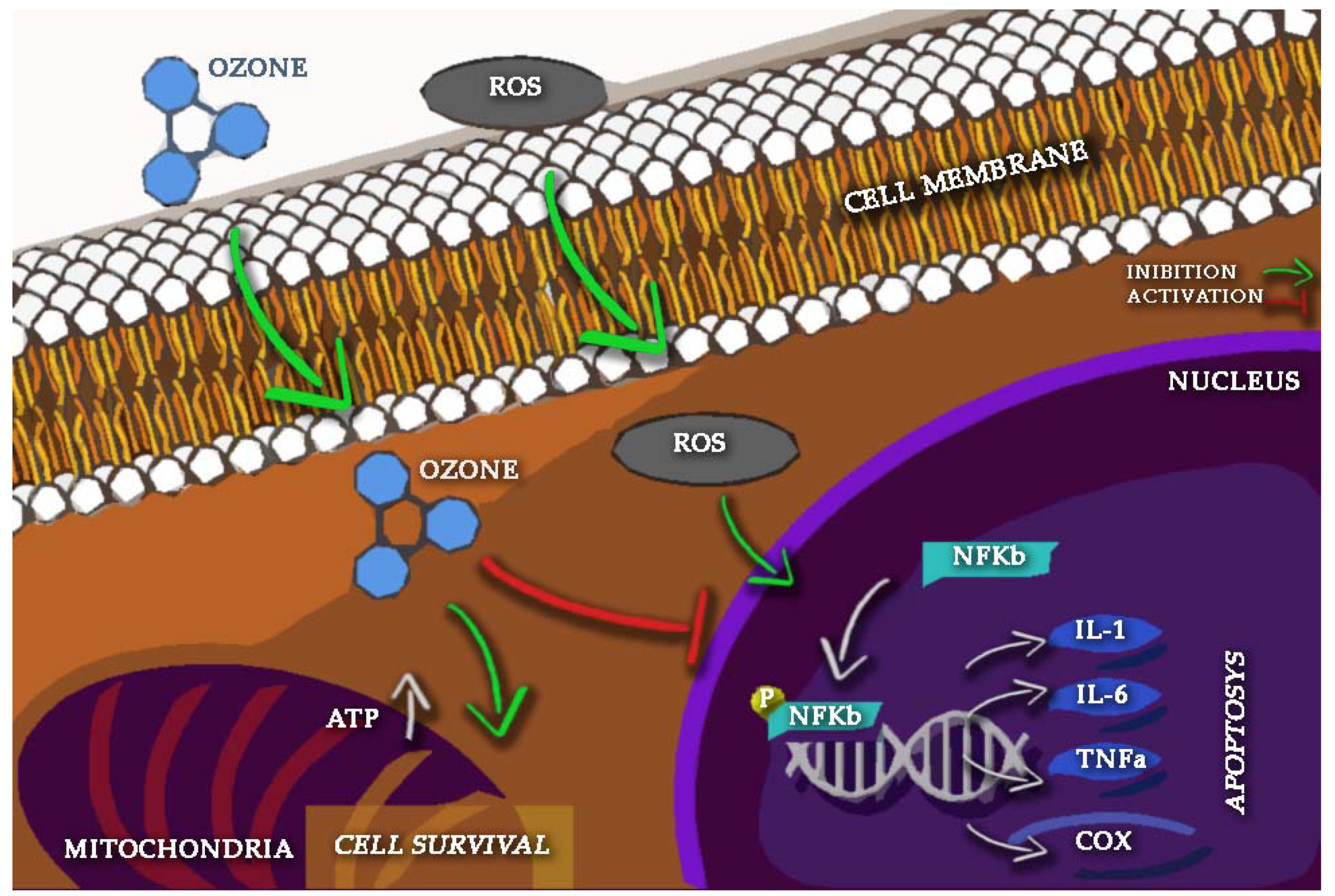
2. Oxygen-Ozone as Anti-Inflammatory Therapy
3. Oxygen-Ozone and Back Pain
4. Oxygen-Ozone and Osteoarthritis
5. Oxygen-Ozone and Rheumatic Diseases
6. Oxygen-Ozone and Temporomandibular Disorders
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxid. Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef] [PubMed]
- Akkawi, I. Ozone therapy for musculoskeletal disorders: Current concepts. Acta Biomed. 2020, 91, e2020191. [Google Scholar] [CrossRef][Green Version]
- de Sire, A.; Stagno, D.; Minetto, M.A.; Cisari, C.; Baricich, A.; Invernizzi, M. Long-term effects of intra-articular oxygen-ozone therapy versus hyaluronic acid in older people affected by knee osteoarthritis: A randomized single-blind extension study. J. Back Musculoskelet. Rehabil. 2020, 33, 347–354. [Google Scholar] [CrossRef]
- Apuzzo, D.; Giotti, C.; Pasqualetti, P.; Ferrazza, P.; Soldati, P.; Zucco, G.M. An observational retrospective/horizontal study to compare oxygen-ozone therapy and/or global postural re-education in complicated chronic low back pain. Funct. Neurol. 2014, 29, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Galiè, M.; Covi, V.; Tabaracci, G.; Malatesta, M. The role of Nrf2 in the antioxidant cellular response to medical ozone exposure. Int. J. Mol. Sci. 2019, 20, 4009. [Google Scholar] [CrossRef]
- Paoloni, M.; Di Sante, L.; Cacchio, A.; Apuzzo, D.; Marotta, S.; Razzano, M.; Franzini, M.; Santilli, V. Intramuscular oxygen-ozone therapy in the treatment of acute back pain with lumbar disc herniation: A multicenter, randomized, double-blind, clinical trial of active and simulated lumbar paravertebral injection. Spine 2009, 34, 1337–1344. [Google Scholar] [CrossRef]
- Baranova, I.V.; Bezsmertnyi, Y.A.; Bezsmertnaya, H.V.; Postovitenko, K.P.; Iliuk, I.A.; Gumeniuk, A.F. Analgetic effect of ozone therapy: Myths of reality? Pol. Ann. Med. 2020, 27, 62–67. [Google Scholar] [CrossRef]
- Rajkumar, K.V.; Jeevitha, M.; Sangeetha, S. Knowledge and awareness of ozone therapy among dental professionals. Int. J. Res. Pharm. Sci. 2020, 11, 303–307. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Holland, O.J.; Vanderlelie, J.J. Ozone therapy for the treatment of chronic wounds: A systematic review. Int. Wound J. 2018, 15, 633–644. [Google Scholar] [CrossRef]
- Sanjeevi, J.; Santhosh Kumar, M.P. Ozone therapy in dentistry. Drug Invent. Today 2019, 12, 154–157. [Google Scholar] [CrossRef]
- Masan, J.; Sramka, M.; Rabarova, D. The possibilities of using the effects of ozone therapy in neurology. Neuroendocrinol. Lett. 2021, 42, 13–21. [Google Scholar] [PubMed]
- Smith, N.; Wilson, A.; Gandhi, J.; Vatsia, S.; Khan, S. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med. Gas Res. 2017, 7, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Knölker, H.J.; Simons, K. Subcellular targeting strategies for drug design and delivery. Nat. Rev. Drug Discov. 2010, 9, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Trush, M. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9–21. [Google Scholar] [CrossRef]
- Dumas, A.; Knaus, U.G. Raising the ‘Good’ Oxidants for Immune Protection. Front. Immunol. 2021, 12, 42. [Google Scholar] [CrossRef]
- Liu, M.; Xie, Z.; Sun, G.; Chen, L.; Qi, D.; Zhang, H.; Xiong, J.; Furey, A.; Rahman, P.; Lei, G.; et al. Macrophage migration inhibitory factor may play a protective role in osteoarthritis. Arthritis Res. Ther. 2021, 23, 59. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Marques, R.E.; Guabiraba, R.; Russo, R.C.; Teixeira, M.M. Targeting CCL5 in inflammation. Expert Opin. Ther. Targets 2013, 17, 1439–1460. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Namakanova, O.A.; Gorshkova, E.A.; Medvedovskaya, A.D.; Nedospasov, S.A.; Drutskaya, M.S. Novel Anti-Cytokine Strategies for Prevention and Treatment of Respiratory Allergic Diseases. Front. Immunol. 2021, 12, 1842. [Google Scholar] [CrossRef]
- Malferrari, M.; Becconi, M.; Rapino, S. Electrochemical monitoring of reactive oxygen/nitrogen species and redox balance in living cells. Anal. Bioanal. Chem. 2019, 411, 4365–4374. [Google Scholar] [CrossRef]
- Vaneev, A.N.; Gorelkin, P.V.; Garanina, A.S.; Lopatukhina, H.V.; Vodopyanov, S.S.; Alova, A.V.; Ryabaya, O.O.; Akasov, R.A.; Zhang, Y.; Novak, P.; et al. In Vitro and in Vivo Electrochemical Measurement of Reactive Oxygen Species after Treatment with Anticancer Drugs. Anal. Chem. 2020, 92, 8010–8014. [Google Scholar] [CrossRef] [PubMed]
- de lo Erario, M.Á.; Croce, E.; Moviglia Brandolino, M.T.; Moviglia, G.; Grangeat, A.M. Ozone as modulator of resorption and inflammatory response in extruded nucleus pulposus herniation. Revising concepts. Int. J. Mol. Sci. 2021, 22, 9946. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.M.; Pacheco, R.L.; Bussadori, S.K.; Santos, E.M.; Riera, R.; de Oliveira Cruz Latorraca, C.; Mota, P.; Benavent Caldas Bellotto, E.F.; Martimbianco, A.L.C. Effectiveness and Safety of Ozone Therapy in Dental Caries Treatment: Systematic Review and Meta-analysis. J. Evid. Based. Dent. Pract. 2020, 20, 101472. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.G.; Guerra, M.M.D.; Carballo-Reyes, A.L.; Márquez, J.A.S.; Fernández, O.E.L.; Betancourt, F.F.; Zamora-Rodríguez, Z.; García, L.A.F.; Iznaga, R.S.; Casanueva, R.M.; et al. Observance of adverse reactions and analysis of biosafety compliance in the rectal application of ozone therapy in COVID-19 Cuban patients with acute infection or convalescent. J. Pharm. Pharmacogn. Res. 2021, 9, 465–473. [Google Scholar]
- Bocci, V. The Clinical Application of Ozonetherapy. In OZONE; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Franzini, M.; Valdenassi, L.; Ricevuti, G.; Chirumbolo, S.; Depfenhart, M.; Bertossi, D.; Tirelli, U. Oxygen-ozone (O2-O3) immunoceutical therapy for patients with COVID-19. Preliminary evidence reported. Int. Immunopharmacol. 2020, 88, 106879. [Google Scholar] [CrossRef]
- Ruiz-Fernández, C.; Francisco, V.; Pino, J.; Mera, A.; González-Gay, M.A.; Gómez, R.; Lago, F.; Gualillo, O. Molecular relationships among obesity, inflammation and intervertebral disc degeneration: Are adipokines the common link? Int. J. Mol. Sci. 2019, 20, 2030. [Google Scholar] [CrossRef]
- Farrell, S.F.; de Zoete, R.M.J.; Cabot, P.J.; Sterling, M. Systemic inflammatory markers in neck pain: A systematic review with meta-analysis. Eur. J. Pain 2020, 24, 1666–1686. [Google Scholar] [CrossRef]
- Blüher, M. Inflammation: Between obesity, diabetes and exercise. Diabetologe 2021, 17, 141–148. [Google Scholar] [CrossRef]
- Paradis, M.E.; Couture, P.; Gigleux, I.; Marin, J.; Vohl, M.C.; Lamarche, B. Impact of systemic enzyme supplementation on low-grade inflammation in humans. PharmaNutrition 2015, 3, 83–88. [Google Scholar] [CrossRef]
- Mobasheri, A.; Henrotin, Y. Biomarkers of (osteo)arthritis. Biomarkers 2015, 20, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Suzuki, M.; Sakuma, Y.; Yamauchi, K.; Orita, S.; Miyagi, M.; Ishikawa, T.; Kamoda, H.; Oikawa, Y.; Kanisawa, I.; et al. Differences in levels of inflammatory mediators in meniscal and synovial tissue of patients with meniscal lesions. J. Exp. Orthop. 2016, 3, 7. [Google Scholar] [CrossRef]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef]
- Yucel-Lindberg, T.; Båge, T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 2013, 15, e7. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Huang, J.; Zhao, K.; Chen, X.; Yin, Z.; Heng, B.C.; Chen, W.; Shen, W. The roles of inflammatory mediators and immunocytes in tendinopathy. J. Orthop. Transl. 2018, 14, 23–33. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef]
- Seyam, O.; Smith, N.L.; Reid, I.; Gandhi, J.; Jiang, W.; Khan, S.A. Clinical utility of ozone therapy for musculoskeletal disorders. Med. Gas Res. 2018, 8, 103–110. [Google Scholar]
- Bonetti, M.; Zambello, A.; Princiotta, C.; Pellicanò, G.; Della Gatta, L.; Muto, M. Non-discogenic low back pain treated with oxygen-ozone: Outcome in selected applications. J. Biol. Regul. Homeost. Agents 2020, 34, 21–30. [Google Scholar]
- Han, H.J.; Kim, J.Y.; Jang, H.Y.; Lee, B.; Yoon, J.H.; Jang, S.K.; Choi, S.H.; Jeong, S.W. Fluoroscopic-guided intradiscal oxygen-ozone injection therapy for thoracolumbar intervertebral disc herniations in dogs. In Vivo 2007, 21, 609–613. [Google Scholar] [PubMed]
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Bestch, M.; Tingart, M.; Baroncini, A. Ozone injection therapy for intervertebral disc herniation. Br. Med. Bull. 2020, 136, 88–106. [Google Scholar] [CrossRef]
- Melchionda, D.; Milillo, P.; Manente, G.; Stoppino, L.; Macarini, L. Treatment of radiculopathies: A study of efficacy and tollerability of paravertebral oxygen-ozone injections compared with pharmacological anti-inflammatory treatment. J. Biol. Regul. Homeost. Agents 2012, 26, 467–474. [Google Scholar] [PubMed]
- Dal Fior, S.; Gaido, C.; Carnino, I.; Gamna, F.; Busso, C.; Massazza, G.; Minetto, M.A. Clinical predictors of response to ozone therapy for treatment of discogenic and non-discogenic low back pain. J. Biol. Regul. Homeost. Agents 2020, 34, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães, F.N.O.; Soares, S.C.; Torres, J.M.; Ungaretti, A.; Cacciacarro, M.F.; Teixeira, M.J.; Fonoff, E.T. Effects of ozone applied by spinal endoscopy in patients with chronic pain related to failed back surgery syndrome: A pilot study. Neuropsychiatr. Dis. Treat. 2013, 9, 1759–1766. [Google Scholar] [CrossRef]
- Barbosa, D.C.; Ângelos, J.S.; De Macena, G.M.J.; De Oliveira Magalhães, F.N.; Fonoff, E.T. Effects of ozone on the pain and disability in patients with failed back surgery syndrome. Rev. Assoc. Med. Bras. 2017, 63, 355–360. [Google Scholar] [CrossRef]
- Sconza, C.; Respizzi, S.; Virelli, L.; Vandenbulcke, F.; Iacono, F.; Kon, E.; Di Matteo, B. Oxygen–Ozone Therapy for the Treatment of Knee Osteoarthritis: A Systematic Review of Randomized Controlled Trials. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 277–286. [Google Scholar] [CrossRef]
- Travagli, V.; Zanardi, I.; Bernini, P.; Nepi, S.; Tenori, L.; Bocci, V. Effects of ozone blood treatment on the metabolite profile of human blood. Int. J. Toxicol. 2010, 29, 165–174. [Google Scholar] [CrossRef]
- Gökhan Ulusoy, R.; Bilge, A.; Öztürk, Ö. Comparison of corticosteroid injection and ozone injection for relief of pain in chronic lateral epicondylitis. Acta Orthop. Belg. 2019, 85, 317–324. [Google Scholar]
- Moreno-Fernández, A.M.; Macías-García, L.; Valverde-Moreno, R.; Ortiz, T.; Fernández-Rodríguez, A.; Moliní-Estrada, A.; De-Miguel, M. Autohemotherapy with ozone as a possible effective treatment for Fibromyalgia. Acta Reumatol. Port. 2019, 44, 244–249. [Google Scholar]
- Storheim, K.; Zwart, J.A. Musculoskeletal disorders and the Global Burden of Disease study. Ann. Rheum. Dis. 2014, 73, 949–950. [Google Scholar] [CrossRef] [PubMed]
- Minetto, M.A.; Giannini, A.; McConnell, R.; Busso, C.; Torre, G.; Massazza, G. Common musculoskeletal disorders in the elderly: The star triad. J. Clin. Med. 2020, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.F.; Cruz, M.T.; Gualillo, O. Editorial: The Physiology of Inflammation—The Final Common Pathway to Disease. Front. Physiol. 2018, 9, 1741. [Google Scholar] [CrossRef]
- Berenbaum, F.; Walker, C. Osteoarthritis and inflammation: A serious disease with overlapping phenotypic patterns. Postgrad. Med. 2020, 132, 377–384. [Google Scholar] [CrossRef]
- Bocci, V.A. Scientific and medical aspects of ozone therapy. State of the art. Arch. Med. Res. 2006, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Agostini, F.; Lippi, L.; Mangone, M.; Marchese, S.; Cisari, C.; Bernetti, A.; Invernizzi, M. Oxygen–ozone therapy in the rehabilitation field: State of the art on mechanisms of action, safety and effectiveness in patients with musculoskeletal disorders. Biomolecules 2021, 11, 356. [Google Scholar] [CrossRef]
- Bernetti, A.; Agostini, F.; de Sire, A.; Mangone, M.; Tognolo, L.; Di Cesare, A.; Ruiu, P.; Paolucci, T.; Invernizzi, M.; Paoloni, M. Neuropathic pain and rehabilitation: A systematic review of international guidelines. Diagnostics 2021, 11, 74. [Google Scholar] [CrossRef]
- de Sire, A.; Baricich, A.; Minetto, M.A.; Cisari, C.; Invernizzi, M. Low back pain related to a sacral insufficiency fracture: Role of paravertebral oxygen-ozone therapy in a paradigmatic case of nociplastic pain. Funct. Neurol. 2019, 34, 119–122. [Google Scholar]
- İnal, M.; Dokumacioglu, A.; Özcelik, E.; Ucar, O. The effects of ozone therapy and coenzyme Q 10 combination on oxidative stress markers in healthy subjects. Ir. J. Med. Sci. 2011, 180, 703–707. [Google Scholar] [CrossRef]
- Manoto, S.L.; Maepa, M.J.; Motaung, S.K. Medical ozone therapy as a potential treatment modality for regeneration of damaged articular cartilage in osteoarthritis. Saudi J. Biol. Sci. 2018, 25, 672–679. [Google Scholar] [CrossRef]
- Sagai, M.; Bocci, V. Mechanisms of action involved in ozone therapy: Is healing induced via a mild oxidative stress? Med. Gas Res. 2011, 1, 1–18. [Google Scholar] [CrossRef]
- Thi Xuan, N.; Hai Ha, N.; Thanh Chung, D. Vitamin E Attenuates FasL-Induced Apoptotic Death of Dendritic Cells Through PI3K Signalling. VNU J. Sci. Med. Pharm. Sci. 2021, 37, 4268. [Google Scholar] [CrossRef]
- Mourkioti, F.; Kratsios, P.; Luedde, T.; Song, Y.H.; Delafontaine, P.; Adami, R.; Parente, V.; Bottinelli, R.; Pasparakis, M.; Rosenthal, N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J. Clin. Investig. 2006, 116, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Yan, W.; Lou, J.; Chen, X. Effect of ozone on vascular endothelial growth factor (VEGF) and related inflammatory cytokines in rats with diabetic retinopathy. Genet. Mol. Res. 2016, 15, 15027558. [Google Scholar] [CrossRef]
- Peralta, C.; León, O.; Xaus, C.; Prats, N.; Jalil, E.; Planell, E.S.; Puig-Parellada, P.; Gelpí, E.; Roselló-Catafau, J. Protective effect of ozone treatment on the injury associated with hepatic ischemia-reperfusion: Antioxidant-prooxidant balance. Free Radic. Res. 1999, 31, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Xaus, C.; Bartrons, R.; Leon, O.S.; Gelpi, E.; Rosello-Catafau, J. Effect of ozone treatment on reactive oxygen species and adenosine production during hepatic ischemia-reperfusion. Free Radic. Res. 2000, 33, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, T.; Cardarola, A.; Colonnelli, P.; Ferracuti, G.; Gonnella, R.; Murgia, M.; Santilli, V.; Paoloni, M.; Bernetti, A.; Agostini, F.; et al. Give me a kiss! An integrative rehabilitative training program with motor imagery and mirror therapy for recovery of facial palsy. Eur. J. Phys. Rehabil. Med. 2020, 56, 58–67. [Google Scholar] [CrossRef]
- Longas Vélez, B.P. Ozone therapy, a supplement for patients with fibromyalgia. Rev. Esp. Ozonoter. 2014, 4, 39–49. [Google Scholar]
- Paolucci, T.; Agostini, F.; Bernetti, A.; Paoloni, M.; Mangone, M.; Santilli, V.; Pezzi, L.; Bellomo, R.G.; Saggini, R. Integration of focal vibration and intra-articular oxygen–ozone therapy in rehabilitation of painful knee osteoarthritis. J. Int. Med. Res. 2021, 49, 6705. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Bernetti, A.; Di Giacomo, G.; Viva, M.G.; Paoloni, M.; Mangone, M.; Santilli, V.; Masiero, S. Rehabilitative Good Practices in the Treatment of Sarcopenia. Am. J. Phys. Med. Rehabil. 2020, 100, 280–287. [Google Scholar] [CrossRef]
- Biazzo, A.; Corriero, A.S.; Confalonieri, N. Intramuscular oxygen-ozone therapy in the treatment of low back pain. Acta Biomed 2018, 89, 41–46. [Google Scholar] [CrossRef]
- Cantele, F.; Tognolo, L.; Caneva, F.; Formaggio, E.; Copetti, V.; Venturin, A.; Caregnato, A.; Masiero, S. Influence of pain-related psychological factors on therapeutic outcomes in patients with chronic low back pain after oxygen-ozone treatment: A case-series. Eur. J. Transl. Myol. 2021, 31, 9906. [Google Scholar] [CrossRef] [PubMed]
- Wong, O.; Zhang, G.; Matthews, H.; Skalski, M.; Asadi, H.; Lalloo, S.; Kurda, D. Image-guided spinal injection for pain management. J. Med. Imaging Radiat. Oncol. 2021, 66, 79–91. [Google Scholar] [CrossRef]
- Alyan, S.; Zaghlol, R.; Mustafa, S.A. Efficacy of combined paravertebral ozone (O2O3) therapy with physiotherapy in patients with chronic mechanical low back pain. Egypt. Rheumatol. Rehabil. 2018, 45, 106–111. [Google Scholar] [CrossRef]
- Sucuoğlu, H.; Soydaş, N. Does paravertebral ozone injection have efficacy as an additional treatment for acute lumbar disc herniation? A randomized, double-blind, placebo-controlled study. J. Back Musculoskelet. Rehabil. 2021, 34, 725–733. [Google Scholar] [CrossRef]
- Cunha, C.; Teixeira, G.Q.; Machado, C.; Pereira, C.L.; Ferreira, J.R.; Molinos, M.; Santos, S.G.; Barbosa, M.A.; Goncalves, R.M. Modulation of the In Vivo Inflammatory Response by Pro- Versus Anti-Inflammatory Intervertebral Disc Treatments. Int. J. Mol. Sci. 2020, 21, 1730. [Google Scholar] [CrossRef]
- Andreula, C.F.; Simonetti, L.; de Santis, F.; Agati, R.; Ricci, R.; Leonardi, M. Minimally Invasive Oxygen-Ozone Therapy for Lumbar Disk Herniation. Am. J. Neuroradiol. 2003, 24, 996–1000. [Google Scholar]
- De Geer, C.M. Cytokine Involvement in Biological Inflammation Related to Degenerative Disorders of the Intervertebral Disk: A Narrative Review. J. Chiropr. Med. 2018, 17, 54–62. [Google Scholar] [CrossRef]
- Li, J.-K.; Nie, L.; Zhao, Y.-P.; Zhang, Y.-Q.; Wang, X.; Wang, S.-S.; Liu, Y.; Zhao, H.; Cheng, L. IL-17 mediates inflammatory reactions via p38/c-Fos and JNK/c-Jun activation in an AP-1-dependent manner in human nucleus pulposus cells. J. Transl. Med. 2016, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Brisby, H. Pathology and possible mechanisms of nervous system response to disc degeneration. J. Bone Jt. Surg. Ser. A 2006, 88, 68–71. [Google Scholar]
- Perolat, R.; Kastler, A.; Nicot, B.; Pellat, J.-M.; Tahon, F.; Attye, A.; Heck, O.; Boubagra, K.; Grand, S.; Krainik, A. Facet joint syndrome: From diagnosis to interventional management. Insights Imaging 2018, 9, 773–789. [Google Scholar] [CrossRef]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-κB: At the Borders of Autoimmunity and Inflammation. Front. Immunol. 2021, 2021, 3169. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Niu, T.; Lv, C.; Yi, G.; Tang, H.; Gong, C.; Niu, S. Therapeutic Effect of Medical Ozone on Lumbar Disc Herniation. Med. Sci. Monit. 2018, 24, 1962–1969. [Google Scholar] [CrossRef]
- O’Neill, T.W.; McCabe, P.S.; McBeth, J. Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 312–326. [Google Scholar] [CrossRef]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Liang, X.; Kawashima, T.; Coggeshall, M. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta—Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperon 2020, 25, 395–406. [Google Scholar] [CrossRef]
- de Sire, A.; Marotta, N.; Marinaro, C.; Curci, C.; Invernizzi, M.; Ammendolia, A. Role of Physical Exercise and Nutraceuticals in Modulating Molecular Pathways of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5722. [Google Scholar] [CrossRef]
- Fernández-Cuadros, M.E.; Pérez-Moro, O.S.; Albaladejo-Florin, M.J.; Alava-Rabasa, S. El ozono intraarticular modula la inflamación, mejora el dolor, la rigidez, la función y tiene un efecto anabólico sobre la artrosis de rodilla: Estudio cuasi-experimental prospectivo tipo antes-después, 115 pacientes. Rev. Soc. Esp. Dolor 2020, 27, 78–88. [Google Scholar] [CrossRef]
- Rankothgedera, S.; Atukorala, I.; Fernando, C.; Munidasa, D.; Wijayaratne, L.; Udagama, P. A potential diagnostic serum immunological marker panel to differentiate between primary and secondary knee osteoarthritis. PLoS ONE 2021, 16, e0257507. [Google Scholar] [CrossRef]
- Chow, Y.Y.; Chin, K.-Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef]
- Vincent, T.L. Mechanoflammation in osteoarthritis pathogenesis. Semin. Arthritis Rheum. 2019, 49, S36–S38. [Google Scholar] [CrossRef]
- Kraus, V.B.; Karsdal, M.A. Osteoarthritis: Current Molecular Biomarkers and the Way Forward. Calcif. Tissue Res. 2021, 109, 329–338. [Google Scholar] [CrossRef]
- Bar-Or, D.; Rael, L.; Thomas, G.W.; Brody, E.N. Inflammatory Pathways in Knee Osteoarthritis: Potential Targets for Treatment. Curr. Rheumatol. Rev. 2015, 11, 50–58. [Google Scholar] [CrossRef]
- Wang, M.-N.; Liu, L.; Zhao, L.-P.; Yuan, F.; Fu, Y.-B.; Xu, X.-B.; Li, B. Research of inflammatory factors and signaling pathways in knee osteoarthritis. Zhongguo Gu Shang 2020, 33, 388–392. [Google Scholar] [PubMed]
- Costa, T.; Rodrigues-Manica, S.; Lopes, C.; Gomes, J.; Marona, J.; Falcao, S.; Branco, J. Ozonoterapia na Osteoartrose do Joelho: Revisão Sistemática. Acta Méd. Port. 2018, 31, 576–580. [Google Scholar] [CrossRef]
- Sconza, C.; Leonardi, G.; Kon, E.; Respizzi, S.; Massazza, G.; Marcacci, M.; Di Matteo, B. Oxygen-ozone therapy for the treatment of low back pain: A systematic review of randomized controlled trials. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6034–6046. [Google Scholar] [CrossRef]
- Barbosa, L.T.; Rodrigues, C.F.D.S.; De Andrade, R.R.; Barbosa, F.T. The effectiveness of percutaneous injections of ozonotherapy in low back pain. Rev. Assoc. Méd. Bras. 2020, 66, 1146–1151. [Google Scholar] [CrossRef]
- Hashemi, M.; Khameneh, S.M.H.; Dadkhah, P.; Mohajerani, S.A. Effect of Intraarticular injection of ozone on inflammatory cytokines in knee osteoarthritis. J. Cell. Mol. Anesth. 2017, 2, 37–42. [Google Scholar]
- Sconza, C.; Braghetto, G.; Respizzi, S.; Morenghi, E.; Kon, E.; Di Matteo, B. Ultrasound-guided periradicular oxygen-ozone injections as a treatment option for low back pain associated with sciatica. Int. Orthop. 2021, 45, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Iorio, G.C.; Ammendolia, A.; Marotta, N.; Ricardi, U.; de Sire, A. A bond between rheumatic diseases and cancer in the elderly: The interleukin-6 pathway. Int. J. Rheum. Dis. 2021, 24, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Sukhanov, S.; Anwar, A.; Shai, S.-Y.; Delafontaine, P. Aging, Atherosclerosis, and IGF-1. J. Gerontol. Ser. A 2012, 67, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Tumolo, M.R.; Panico, A.; De Donno, A.; Mincarone, P.; Leo, C.G.; Guarino, R.; Bagordo, F.; Serio, F.; Idolo, A.; Grassi, T.; et al. The expression of microRNAs and exposure to environmental contaminants related to human health: A review. Int. J. Environ. Health Res. 2020, 32, 332–354. [Google Scholar] [CrossRef]
- Bromberg, P.A. Mechanisms of the acute effects of inhaled ozone in humans. Biochim. Biophys. Acta—Gen. Subj. 2016, 1860, 2771–2781. [Google Scholar] [CrossRef]
- Arancibia, S.E.; Zimbron, L.F.H.; Martinez, A.E.R.; Maldonado, P.D.; Perez, G.E.; Parada, M.E. Oxidative stress-dependent changes in immune responses and cell death in the substantia nigra after ozone exposure in rat. Front. Aging Neurosci. 2015, 7, 65. [Google Scholar] [CrossRef]
- Huth, K.; Saugel, B.; Jakob, F.; Cappello, C.; Quirling, M.; Paschos, E.; Ern, K.; Hickel, R.; Brand, K. Effect of Aqueous Ozone on the NF-κB System. J. Dent. Res. 2007, 86, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Renate, V.H. Chronic inflammatory processes and the low-dose ozone concept based on the International guidelines of medical ozone: Signal transduction and Bioregulation through «Ozone peroxides» as second Messenger molecules. Медицинский Альманах 2013, 3, 33. [Google Scholar]
- Zhang, C.; Ma, S.; Zhao, X.; Wen, B.; Sun, P.; Fu, Z. Upregulation of antioxidant and autophagy pathways via NRF2 activation protects spinal cord neurons from ozone damage. Mol. Med. Rep. 2021, 23, 428. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; Riera-Romo, M.; Mesta, F.; Hernández-Matos, Y.; Barrios, J.M.; Martínez-Sánchez, G.; Al-Dalaien, S.M. Medical ozone promotes Nrf2 phosphorylation reducing oxidative stress and pro-inflammatory cytokines in multiple sclerosis patients. Eur. J. Pharmacol. 2017, 811, 148–154. [Google Scholar] [CrossRef]
- Zeng, J.; Lei, L.; Zeng, Q.; Yao, Y.; Wu, Y.; Li, Q.; Gao, L.; Du, H.; Xie, Y.; Huang, J.; et al. Ozone Therapy Attenuates NF-κB-Mediated Local Inflammatory Response and Activation of Th17 Cells in Treatment for Psoriasis. Int. J. Biol. Sci. 2020, 16, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, F.; Zhang, L.; Sun, T.; Fu, Z. Intrathecal injection of ozone alleviates CCI-induced neuropathic pain via the GluR6-NF-κB/p65 signalling pathway in rats. Mol. Med. Rep. 2021, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Babaei-Ghazani, A.; Najarzadeh, S.; Mansoori, K.; Forogh, B.; Madani, S.P.; Ebadi, S.; Fadavi, H.R.; Eftekharsadat, B. The effects of ultrasound-guided corticosteroid injection compared to oxygen–ozone (O2–O3) injection in patients with knee osteoarthritis: A randomized controlled trial. Clin. Rheumatol. 2018, 37, 2517–2527. [Google Scholar] [CrossRef] [PubMed]
- Hedayatabad, J.J.; Kachooei, A.R.; Chaharjouy, N.T.; Vaziri, N.; Mehrad-Majd, H.; Emadzadeh, M.; Abolghasemian, M.; Ebrahimzadeh, M.H. The Effect of Ozone (O3) versus Hyaluronic Acid on Pain and Function in Patients with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Arch. Bone Jt. Surg 2020, 8, 343–354. [Google Scholar]
- Hashemi, M.; Taheri, M.; Dadkhah, P.; Hassani, H.; Ataie, M.; Ghasemi, M.; Pourhoseingholi, M.A.; Solhpour, A. Comparison of Two Different Ozone Injection Sites for Knee Osteoarthritis, Tibio-femoral Joint versus Supra-patellar Recess: An Open Randomized Clinical Trial. J. Pharm. Res. Int. 2020, 32, 37–49. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Hosseini, P.G.; Bahrami, M.H.; Roghani, R.S.; Fathi, M.; Ahangar, A.G.; Darvish, M. The comparison effects of intra-articular injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and ozone in knee osteoarthritis: A one year randomized clinical trial. BMC Musculoskelet. Disord. 2021, 22, 134. [Google Scholar] [CrossRef]
- Duymus, T.M.; Mutlu, D.T.; Dernek, B.; Komur, B.; Aydogmus, S.; Kesiktas, F.N. Choice of intra-articular injection in treatment of knee osteoarthritis: Platelet-rich plasma, hyaluronic acid or ozone options. Knee Surg. Sports Traumatol. Arthrosc. 2016, 25, 485–492. [Google Scholar] [CrossRef]
- Haydt, R.; Boyle, B.; Meyers, M.; Weissberg, S.; Dyrli, K. Comparison of Platelet Rich Plasma and Oxygen Ozone Injections for Knee Osteoarthritis: A Systematic Review. Arch. Phys. Med. Rehabil. 2019, 100, e149. [Google Scholar] [CrossRef]
- Raeissadat, S.A.; Tabibian, E.; Rayegani, S.M.; Rahimi-Dehgolan, S.; Babaei-Ghazani, A. An investigation into the efficacy of intra-articular ozone (O2–O3) injection in patients with knee osteoarthritis: A systematic review and meta-analysis. J. Pain Res. 2018, 11, 2537–2550. [Google Scholar] [CrossRef]
- Cardelli, R.; de Santis, F.; Dall’Olio, M.; Leonardi, M. Osteoarthritis of the hip treated by intra-articular infiltration of oxygen-ozone and hyaluronic acid (Hyalubrix®). Preliminary results. Int. J. Ozone Ther. 2008, 7, 66–69. [Google Scholar]
- Tartari, A.P.S.; Moreira, F.F.; Pereira, M.C.D.S.; Carraro, E.; Cidral-Filho, F.J.; Salgado, A.I.; Kerppers, I.I. Anti-inflammatory Effect of Ozone Therapy in an Experimental Model of Rheumatoid Arthritis. Inflammation 2020, 43, 985–993. [Google Scholar] [CrossRef]
- Saraiva, L.; Konzen, V.D.M.; Batista, J.S.; Jorge, M.S.G.; Garcia, G.S.; Wibelinger, L.M. Treatment of Rheumatoid Arthritis with Ozone Therapy: Systematic Review. Temas Saúde 2020, 20, 4–8. [Google Scholar] [CrossRef]
- Rajaiah, R.; Puttabyatappa, M.; Polumuri, S.K.; Moudgil, K.D. Interleukin-27 and Interferon-γ Are Involved in Regulation of Autoimmune Arthritis. J. Biol. Chem. 2011, 286, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Fernández, O.S.L.; Viebahn-Haensler, R.; Cabreja, G.L.; Espinosa, I.S.; Matos, Y.H.; Roche, L.D.; Santos, B.T.; Oru, G.T.; Vega, J.C.P. Medical ozone increases methotrexate clinical response and improves cellular redox balance in patients with rheumatoid arthritis. Eur. J. Pharmacol. 2016, 789, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Scassellati, C.; Galoforo, A.C.; Bonvicini, C.; Esposito, C.; Ricevuti, G. Ozone: A natural bioactive molecule with antioxidant property as potential new strategy in aging and in neurodegenerative disorders. Ageing Res. Rev. 2020, 63, 101138. [Google Scholar] [CrossRef]
- Zeng, J.; Tang, Z.; Zhang, Y.; Tong, X.; Dou, J.; Gao, L.; Ding, S.; Lu, J. Ozonated autohemotherapy elevates PPAR-γ expression in CD4+ T cells and serum HDL-C levels, a potential immunomodulatory mechanism for treatment of psoriasis. Am. J. Transl. Res. 2021, 13, 349–359. [Google Scholar]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. The Purinergic System as a Pharmacological Target for the Treatment of Immune-Mediated Inflammatory Diseases. Pharmacol. Rev. 2019, 71, 345–382. [Google Scholar] [CrossRef]
- Ayers, N.B.; Sun, C.-M.; Chen, S.-Y. Transforming growth factor-β signaling in systemic sclerosis. J. Biomed. Res. 2017, 32, 3–12. [Google Scholar]
- Yagci, I.; Kenis-Coskun, O.; Ozsoy, T.; Ozen, G.; Direskeneli, H. Increased stiffness of median nerve in systemic sclerosis. BMC Musculoskelet. Disord. 2017, 18, 434. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Curci, C.; Ferrara, M.; Losco, L.; Spalek, R.; Cisari, C.; Invernizzi, M.; Solaro, C. Efficacy of kinesio taping on hand functioning in patients with mild carpal tunnel syndrome. A double-blind randomized controlled trial. J. Hand Ther. 2021, in press. [Google Scholar] [CrossRef]
- Elawamy, A.; Hassanien, M.; Talaat, E.A.; Ali, A.M.; Roushdy, A.S.I.; Kamel, E.Z. Intra-Carpal Injection of Ozone versus Methylprednisolone in Carpal Tunnel Syndrome of Systemic Sclerosis Patients: A Randomized Single-Blind Clinical Trial. Pain Phys. 2021, 24, E453–E458. [Google Scholar] [CrossRef]
- Rascaroli, M.W.; Borghi, B.; Rascaroli, A.; Travagli, V. Ozone therapy in idiopathic carpal tunnel syndrome. Biochemical, neurophysiological and clinical aspects. J. Ozone Ther. 2018, 2, 11286. [Google Scholar] [CrossRef]
- Rascaroli, M.; Borghi, B. Ozone Therapy in Idiopathic Carpal Tunnel Syndrome. Biochemical, Neurophysiological and Clinical Aspects. J. Ozone Ther. 2019, 3, 49–50. [Google Scholar] [CrossRef]
- Bahrami, M.H.; Raeissadat, S.A.; Nezamabadi, M.; Hojjati, F.; Rahimi-Dehgolan, S. Interesting effectiveness of ozone injection for carpal tunnel syndrome treatment: A randomized controlled trial. Orthop. Res. Rev. 2019, 11, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Marotta, N.; Demeco, A.; Marinaro, C.; Moggio, L.; Pino, I.; Barletta, M.; Petraroli, A.; Ammendolia, A. Comparative Effectiveness of Orthoses for Thumb Osteoarthritis: A Systematic Review and Network Meta-analysis. Arch. Phys. Med. Rehabil. 2021, 102, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.; Gingery, A.; Oki, G.; Zhao, C.; Amadio, P.C.; Yang, T.-H. Blocking fibrotic signaling in fibroblasts from patients with carpal tunnel syndrome. J. Cell. Physiol. 2017, 233, 2067–2074. [Google Scholar] [CrossRef]
- Yang, T.-H.; Gingery, A.; Thoreson, A.R.; Larson, D.R.; Zhao, C.; Amadio, P.C. Triamcinolone Acetonide affects TGF-β signaling regulation of fibrosis in idiopathic carpal tunnel syndrome. BMC Musculoskelet. Disord. 2018, 19, 342. [Google Scholar] [CrossRef]
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef]
- Guillén, A.G.; Karu, L.T.; Singh, J.A.; Dalbeth, N. Gender and Ethnic Inequities in Gout Burden and Management. Rheum. Dis. Clin. N. Am. 2020, 46, 693–703. [Google Scholar] [CrossRef]
- Bilge, A.; Tüysüz, M.; Öztürk, Ö.; Adali, Y.; Eroğlu, H.A.; Makav, M.; Uslu, G.A.; Tiskaoglu, R. Deneysel Olarak Gut Oluşturulmuş Ratlarda Ozon Terapinin Etkisi. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 245–249. [Google Scholar] [CrossRef]
- Abdullah, D.M.; Kabil, S.L. Ozone Therapy Alleviates Monosodium Urate Induced Acute Gouty Arthritis in Rats Through Inhibition of NLRP3 Inflammasome. Curr. Drug Ther. 2021, 16, 345–353. [Google Scholar] [CrossRef]
- Saruhanoğlu, A.; Gökçen-Röhlig, B.; Saruhanoğlu, C.; Öngül, D.; Koray, M. Frequency of temporomandibular disorder signs and symptoms among call center employees. CRANIO J. Craniomandib. Pract. 2017, 35, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Rongo, R.; Ekberg, E.; Nilsson, I.; Al-Khotani, A.; Alstergren, P.; Conti, P.C.R.; Durham, J.; Goulet, J.; Hirsch, C.; Kalaykova, S.I.; et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for children and adolescents: An international Delphi study—Part 1-Development of Axis I. J. Oral Rehabil. 2021, 48, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Steenks, M.; Türp, J.; De Wijer, A. Reliability and Validity of the Diagnostic Criteria for Temporomandibular Disorders Axis I in Clinical and Research Settings: A Critical Appraisal. J. Oral Facial Pain Headache 2018, 32, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Gauer, R.L.; Semidey, M.J. Diagnosis and treatment of temporomandibular disorders. Am. Fam. Phys. 2015, 91, 378–386. [Google Scholar]
- Wieckiewicz, M.; Smardz, J.; Martynowicz, H.; Wojakowska, A.; Mazur, G.; Winocur, E. Distribution of temporomandibular disorders among sleep bruxers and non-bruxers—A polysomnographic study. J. Oral Rehabil. 2020, 47, 820–826. [Google Scholar] [CrossRef]
- Manfredini, D.; Guarda-Nardini, L.; Winocur, E.; Piccotti, F.; Ahlberg, J.; Lobbezoo, F. Research diagnostic criteria for temporomandibular disorders: A systematic review of axis I epidemiologic findings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 453–462. [Google Scholar] [CrossRef]
- Talaat, W.M.; Adel, O.I.; Al Bayatti, S. Prevalence of temporomandibular disorders discovered incidentally during routine dental examination using the Research Diagnostic Criteria for Temporomandibular Disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 250–259. [Google Scholar] [CrossRef]
- Slade, G.; Ohrbach, R.; Greenspan, J.; Fillingim, R.; Bair, E.; Sanders, A.; Dubner, R.; Diatchenko, L.; Meloto, C.; Smith, S.; et al. Painful Temporomandibular Disorder: Decade of Discovery from OPPERA Studies. J. Dent. Res. 2016, 95, 1084–1092. [Google Scholar] [CrossRef]
- Karlsson, O.; Karlsson, T.; Pauli, N.; Andréll, P.; Finizia, C. Jaw exercise therapy for the treatment of trismus in head and neck Cancer: A prospective three-year follow-up study. Support. Care Cancer 2021, 29, 3793–3800. [Google Scholar] [CrossRef]
- Özalp, Ö.; Yıldırımyan, N.; Sindel, A.; Altay, M.A.; Kişnişci, R.Ş. Evaluation of the Short-Term Efficacy of Transdermal Ozone Therapy in Turkish Patients with Internal Derangement of the Temporomandibular Joint. Pesqui. Bras. Odontopediatr. Clín. Integr. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Fillingim, R.; Ohrbach, R.; Greenspan, J.; Sanders, A.; Rathnayaka, N.; Maixner, W.; Slade, G. Associations of Psychologic Factors with Multiple Chronic Overlapping Pain Conditions. J. Oral Facial Pain Headache 2020, 34, s85–s100. [Google Scholar] [CrossRef]
- A Review the Relationship of Bruxism with Temporomandibular Disorders in Children. Syst. Rev. Pharm. 2020, 11, 136–142. [CrossRef]
- Seo, H.; Jung, B.; Yeo, J.; Kim, K.-W.; Cho, J.-H.; Lee, Y.J.; Ha, I.-H. Healthcare utilisation and costs for temporomandibular disorders: A descriptive, cross-sectional study. BMJ Open 2020, 10, e036768. [Google Scholar] [CrossRef] [PubMed]
- Okeson, J. Management of Temporomandibular Disorder and Occlusion, 4th ed.; Elsevier: Mosby, MO, USA, 1998; pp. 349–380. [Google Scholar]
- Glaros, A.G.; Tabacchi, K.N.; Glass, E.G. Effect of parafunctional clenching on TMD pain. J. Orofac. Pain 1998, 12, 145–152. [Google Scholar] [PubMed]
- Fillingim, R.B.; Ohrbach, R.; Greenspan, J.; Knott, C.; Diatchenko, L.; Dubner, R.; Bair, E.; Baraian, C.; Mack, N.; Slade, G.D.; et al. Psychological Factors Associated With Development of TMD: The OPPERA Prospective Cohort Study. J. Pain 2013, 14, T75–T90. [Google Scholar] [CrossRef] [PubMed]
- Dıraçoǧlu, D.; Yıldırım, N.K.; Saral, I.; Özkan, M.; Karan, A.; Özkan, S.; Aksoy, C. Temporomandibular dysfunction and risk factors for anxiety and depression. J. Back Musculoskelet. Rehabil. 2016, 29, 487–491. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Al-Kheraif, A.A.; Vohra, F.; Ghanem, A.; Malmstrom, H.; Romanos, G.E.; Javed, F. Cytokine profile in the synovial fluid of patients with temporomandibular joint disorders: A systematic review. Cytokine 2016, 77, 98–106. [Google Scholar] [CrossRef]
- de Bont, L.G.; Stegenga, B. Pathology of temporomandibular joint internal derangement and osteoarthrosis. Int. J. Oral Maxillofac. Surg. 1993, 22, 71–74. [Google Scholar] [CrossRef]
- Sandler, N.; Buckley, M.J.; Cillo, J.; Braun, T.W. Correlation of inflammatory cytokines with arthroscopic findings in patients with temporomandibular joint internal derangements. J. Oral Maxillofac. Surg. 1998, 56, 534–543. [Google Scholar] [CrossRef]
- Tanaka, A.; Kumagai, S.; Kawashiri, S.; Takatsuka, S.; Nakagawa, K.; Yamamoto, E.; Matsumoto, N. Expression of matrix metalloproteinase-2 and -9 in synovial fluid of the temporomandibular joint accompanied by anterior disc displacement. J. Oral Pathol. Med. 2001, 30, 59–64. [Google Scholar] [CrossRef]
- Yoshida, K.; Takatsuka, S.; Tanaka, A.; Hatada, E.; Nakamura, H.; Nakagawa, K.; Okada, Y. Aggrecanase analysis of synovial fluid of temporomandibular joint disorders. Oral Dis. 2005, 11, 299–302. [Google Scholar] [CrossRef]
- Lv, X.; Li, Q.; Wu, S.; Sun, J.; Zhang, M.; Chen, Y.-J. Psychological stress alters the ultrastructure and increases IL-1β and TNF-α in mandibular condylar cartilage. Braz. J. Med. Biol. Res. 2012, 45, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Ulmner, M.; Sugars, R.; Naimi-Akbar, A.; Suslu, S.; Reseland, J.E.; Kruger-Weiner, C.; Lund, B. Synovial tissue cytokine profile in disc displacement of the temporomandibular joint. J. Oral Rehabil. 2020, 47, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Son, C.; Park, Y.K.; Park, J.W. Long-term evaluation of temporomandibular disorders in association with cytokine and autoantibody status in young women. Cytokine 2021, 144, 155551. [Google Scholar] [CrossRef] [PubMed]
- Deregibus, A.; Ferrillo, M.; Grazia Piancino, M.; Chiara Domini, M.; de Sire, A.; Castroflorio, T. Are occlusal splints effective in reducing myofascial pain in patients with muscle-related temporomandibular disorders? A randomized-controlled trial. Turk. J. Phys. Med. Rehabil. 2021, 67, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Vrbanović, E.; Alajbeg, I.Z. Long-term Effectiveness of Occlusal Splint Therapy Compared to Placebo in Patients with Chronic Temporomandibular Disorders. Acta Stomatol. Croat. 2019, 53, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, A.; Iodice, G.; Vollaro, S.; Steenks, M.H.; Farella, M. Evaluation of the short-term effectiveness of education versus an occlusal splint for the treatment of myofascial pain of the jaw muscles. J. Am. Dent. Assoc. 2012, 143, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Armijo-Olivo, S.; Pitance, L.; Singh, V.; Neto, F.; Thie, N.; Michelotti, A. Effectiveness of Manual Therapy and Therapeutic Exercise for Temporomandibular Disorders: Systematic Review and Meta-Analysis. Phys. Ther. 2016, 96, 9–25. [Google Scholar] [CrossRef]
- Ketabi, M.A.; Sabzijati, M. Effect of low-level laser on controlling temporomandibular disorders. Rev. Latinoam. Hipertens. 2020, 15, 138–143. [Google Scholar]
- Fertout, A.; Manière-Ezvan, A.; Lupi, L.; Ehrmann, E. Management of temporomandibular disorders with transcutaneous electrical nerve stimulation: A systematic review. CRANIO J. Craniomandib. Pract. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yamaner, F.E.; Celakil, T.; Roehlig, B.G. Comparison of the efficiency of two alternative therapies for the management of temporomandibular disorders. CRANIO J. Craniomandib. Pract. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Oshaghi, S.; Haghighat, S. Effectiveness of ozone injection therapy in temporomandibular disorders. Adv. Biomed. Res. 2020, 9, 73. [Google Scholar] [CrossRef]
- Ávila-Curiel, B.X.; Gómez-Aguirre, J.N.; Gijón-Soriano, A.L.; Acevedo-Mascarúa, A.E.; Argueta-Figueroa, L.; Torres-Rosas, R. Intervenciones complementarias para el tratamiento de dolor en pacientes con alteraciones temporomandibulares: Una revisión sistemática. Rev. Int. Acupunt. 2020, 14, 151–159. [Google Scholar] [CrossRef]
- Ferrillo, M.; Ammendolia, A.; Paduano, S.; Calafiore, D.; Marotta, N.; Migliario, M.; Fortunato, L.; Giudice, A.; Michelotti, A.; de Sire, A. Efficacy of rehabilitation on reducing pain in muscle-related temporomandibular disorders: Systematic review and meta-analysis of randomized controlled trials. J. Back Musculoskelet. Rehabil. 2022, 1, 1–16. [Google Scholar] [CrossRef]
- Celakil, T.; Muric, A.; Roehlig, B.G.; Evlioglu, G.; Keskin, H. Effect of high-frequency bio-oxidative ozone therapy for masticatory muscle pain: A double-blind randomised clinical trial. J. Oral Rehabil. 2017, 44, 442–451. [Google Scholar] [CrossRef]
- Celakil, T.; Muric, A.; Roehlig, B.G.; Evlioglu, G. Management of pain in TMD patients: Bio-oxidative ozone therapy versus occlusal splints. CRANIO J. Craniomandib. Pract. 2019, 37, 85–93. [Google Scholar] [CrossRef]
- Daif, E.T. Role of intra-articular ozone gas injection in the management of internal derangement of the temporomandibular joint. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, e10–e14. [Google Scholar] [CrossRef]
- Doğan, M.; Doğan, D.Ö.; Duger, C.; Kol, I.Ö.; Akpınar, A.; Mutaf, B.; Akar, T. Effects of High-Frequency Bio-Oxidative Ozone Therapy in Temporomandibular Disorder-Related Pain. Med. Princ. Pract. 2014, 23, 507–510. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sire, A.; Marotta, N.; Ferrillo, M.; Agostini, F.; Sconza, C.; Lippi, L.; Respizzi, S.; Giudice, A.; Invernizzi, M.; Ammendolia, A. Oxygen-Ozone Therapy for Reducing Pro-Inflammatory Cytokines Serum Levels in Musculoskeletal and Temporomandibular Disorders: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 2528. https://doi.org/10.3390/ijms23052528
de Sire A, Marotta N, Ferrillo M, Agostini F, Sconza C, Lippi L, Respizzi S, Giudice A, Invernizzi M, Ammendolia A. Oxygen-Ozone Therapy for Reducing Pro-Inflammatory Cytokines Serum Levels in Musculoskeletal and Temporomandibular Disorders: A Comprehensive Review. International Journal of Molecular Sciences. 2022; 23(5):2528. https://doi.org/10.3390/ijms23052528
Chicago/Turabian Stylede Sire, Alessandro, Nicola Marotta, Martina Ferrillo, Francesco Agostini, Cristiano Sconza, Lorenzo Lippi, Stefano Respizzi, Amerigo Giudice, Marco Invernizzi, and Antonio Ammendolia. 2022. "Oxygen-Ozone Therapy for Reducing Pro-Inflammatory Cytokines Serum Levels in Musculoskeletal and Temporomandibular Disorders: A Comprehensive Review" International Journal of Molecular Sciences 23, no. 5: 2528. https://doi.org/10.3390/ijms23052528
APA Stylede Sire, A., Marotta, N., Ferrillo, M., Agostini, F., Sconza, C., Lippi, L., Respizzi, S., Giudice, A., Invernizzi, M., & Ammendolia, A. (2022). Oxygen-Ozone Therapy for Reducing Pro-Inflammatory Cytokines Serum Levels in Musculoskeletal and Temporomandibular Disorders: A Comprehensive Review. International Journal of Molecular Sciences, 23(5), 2528. https://doi.org/10.3390/ijms23052528













