Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism
Abstract
:1. Introduction
2. Diets and Male Fertility
2.1. Western Diet as a Risk Factor for Male Infertility
2.2. Mediterranean Diet as a Protection Factor against Male Infertility
2.3. Vegetarian Diet as a Controversial Factor for Male Infertility
3. Nutrients Impacts on Molecular Aspects Related to Sperm Quality
3.1. Dietary Fats
3.1.1. Fatty Acids
3.1.2. Dietary Cholesterol
3.2. Dietary Carbohydrates
3.3. Dietary Proteins
3.4. General Aspects concerning Caloric Nutrients and Sperm Metabolism
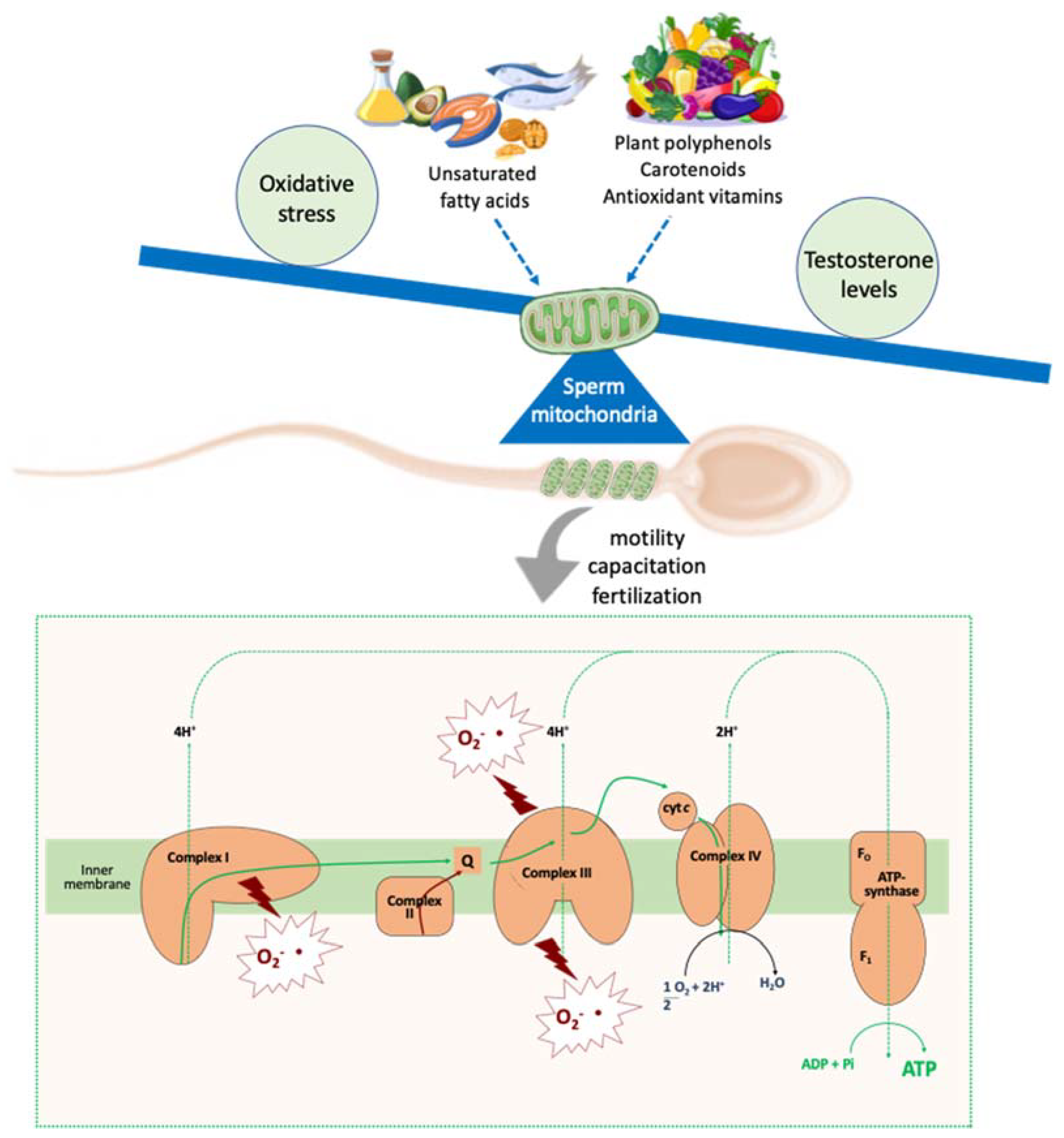
4. Antioxidants Impacts on Molecular Aspects Related to Sperm Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Arab, A.; Rafie, N.; Mansourian, M.; Miraghajani, M.; Hajianfar, H. Dietary patterns and semen quality: A systematic review and meta-analysis of observational studies. Andrology 2018, 6, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassan, F.L.; Chavarro, J.E.; Tanrikut, C. Diet and men’s fertility: Does diet affect sperm quality? Fertil. Steril. 2018, 110, 570–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoracka, K.; Eder, P.; Łykowska-Szuber, L.; Dobrowolska, A.; Krela-Kaźmierczak, I. Diet and nutritional factors in male (in)fertility—Underestimated factors. J. Clin. Med. 2020, 9, 1400. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Conte, A.; Moscatelli, N.; Zara, V. A high-fat diet negatively affects rat sperm mitochondrial respiration. Andrology 2016, 4, 520–525. [Google Scholar] [CrossRef] [Green Version]
- Ferramosca, A.; Moscatelli, N.; Di Giacomo, M.; Zara, V. Dietary fatty acids influence sperm quality and function. Andrology 2017, 5, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Varani, J. Healthful eating, the western style diet and chronic disease. Approaches Poult. Dairy Vet. Sci. 2017, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Ferramosca, A.; Di Giacomo, M.; Moscatelli, N.; Zara, V. Obesity and male infertility: Role of fatty acids in the modulation of sperm energetic metabolism. Eur. J. Lipid Sci. Technol. 2018, 120, 1700451. [Google Scholar] [CrossRef]
- Danielewicz, A.; Przybyłowicz, K.E.; Przybyłowicz, M. Dietary patterns and poor semen quality risk in men: A cross-sectional study. Nutrients 2018, 10, 1162. [Google Scholar] [CrossRef] [Green Version]
- Molaie, S.; Shahverdi, A.; Sharafi, M.; Shahhoseini, M.; Ghaleno, L.R.; Esmaeili, V.; Abed-Heydari, E.; Bucak, M.N.; Alizadeh, A. Dietary trans and saturated fatty acids effects on semen quality, hormonal levels and expression of genes related to steroid metabolism in mouse adipose tissue. Andrologia 2019, 51, e13259. [Google Scholar] [CrossRef]
- Merino, O.; Sánchez, R.; Gregorio, M.B.; Sampaio, F.; Risopatrón, J. Effect of high-fat and vitamin D deficient diet on rat sperm quality and fertility. Theriogenology 2019, 125, 6–11. [Google Scholar] [CrossRef]
- Suliga, E.; Gluszek, S. The relationship between diet, energy balance and fertility in men. Int. J. Vitam. Nutr. Res. 2020, 90, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Giahi, L.; Mohammadmoradi, S.; Javidan, A.; Sadeghi, M.R. Nutritional modifications in male infertility: A systematic review covering 2 decades. Nutr. Rev. 2015, 74, 118–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Huetosk, A.; Bullo, M.; Salas-Salvado, J. Dietary patterns, foods and nutrients in male fertility parameters and fecunda-bility: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.; Al-Beitawi, S.; Cipriani, S.; Alteri, A.; Chiaffarino, F.; Candiani, M.; Gerli, S.; Viganó, P.; Parazzini, F. Dietary habits and semen parameters: A systematic narrative review. Andrology 2018, 6, 104–116. [Google Scholar] [CrossRef] [Green Version]
- De Cosmi, V.; Parazzini, F.; Agostoni, C.; Noli, S.; Cipriani, S.; La Vecchia, I.; Ferrari, S.; Esposito, G.; Bravi, F.; Ricci, E. Antioxidant vitamins and carotenoids intake and the association with poor semen quality: A cross-sectional analysis of men referring to an Italian fertility clinic. Front Nutr. 2021, 8, 737077. [Google Scholar] [CrossRef]
- Ferramosca, A.; Lorenzetti, S.; Di Giacomo, M.; Lunetti, P.; Murrieri, F.; Capobianco, L.; Dolce, V.; Coppola, L.; Zara, V. Modulation of human sperm mitochondrial respiration efficiency by plant polyphenols. Antioxidants 2021, 10, 217. [Google Scholar] [CrossRef]
- Talebi, S.; Arab, A.; Sorraya, N. The association between dietary antioxidants and semen parameters: A cross-sectional study among Iranian infertile men. Biol. Trace Element Res. 2021, 1–8. [Google Scholar] [CrossRef]
- Jurewicz, J.; Radwan, M.; Sobala, W.; Radwan, P.; Bochenek, M.; Hanke, W. Dietary patterns and their relationship with semen quality. Am. J. Men Health 2018, 12, 575–583. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Douka, L.; Mastrominas, M.; Yiannakouris, N. Association between ad-herence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum. Reprod. 2017, 32, 215–222. [Google Scholar]
- Ricci, E.; Bravi, F.; Noli, S.; Ferrari, S.; De Cosmi, V.; La Vecchia, I.; Cavadini, M.; La Vecchia, C.; Parazzini, F. Mediterranean diet and the risk of poor semen quality: Cross-sectional analysis of men referring to an Italian fertility clinic. Andrology 2019, 7, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Caruso, P.; Caputo, M.; Cirillo, P.; Scappaticcio, L.; Longo, M.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Effects of Mediter-ranean diet on semen parameters in healthy young adults: A randomized controlled trial. Minerva Endocrinol. 2020, 45, 280–287. [Google Scholar] [PubMed]
- West, M.C.L.; Anderson, L.; Mcclure, N.; Lewis, S.E.M. Dietary oestrogens and male fertility potential. Hum. Fertil. 2005, 8, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Kanjo, Y.; Mizutani, S. A review of phytoestrogens: Their occurrence and fate in the environment. Water Res. 2010, 44, 567–577. [Google Scholar] [CrossRef]
- Orzylowska, E.M.; Jacobson, J.D.; Bareh, G.M.; Ko, E.Y.; Corselli, J.U.; Chan, P.J. Food intake diet and sperm characteristics in a blue zone: A Loma Linda study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 112–115. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Mihalca, R.; Fica, S. The impact of obesity on the male reproductive axis. J. Med. Life 2014, 7, 296–300. [Google Scholar]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef]
- Bachir, B.G.; Jarvi, K. Infectious, inflammatory, and immunologic conditions resulting in male infertility. Urol. Clin. N. Am. 2014, 41, 67–81. [Google Scholar] [CrossRef]
- Morielli, T.; O’Flaherty, C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015, 149, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Dias, T.R.; Alves, M.G.; Silva, B.M.; Oliveira, P.F. Sperm glucose transport and metabolism in diabetic individuals. Mol. Cell. Endocrinol. 2014, 396, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferramosca, A.; Zara, V. Bioenergetics of mammalian sperm capacitation. BioMed Res. Int. 2014, 2014, 902953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seethalakshmi, L.; Menon, M.; Diamond, D. The effect of streptozotocin-induced diabetes on the neuroendocrine-male reproductive tract axis of the adult rat. J. Urol. 1987, 138, 190–194. [Google Scholar] [CrossRef]
- Hassan, A.A.; Hassouna, M.M.; Taketo, T.; Gagnon, C.; Elhilali, M.M. The effect of diabetes on sexual behavior and repro-ductive tract function in male rats. J. Urol. 1993, 149, 148–154. [Google Scholar] [CrossRef]
- Leisegang, K.; Bouic, P.J.; Menkveld, R.; Henkel, R.R. Obesity is associated with increased seminal insulin and leptin alongside reduced fertility parameters in a controlled male cohort. Reprod. Biol. Endocrinol. 2014, 12, 34. [Google Scholar] [CrossRef] [Green Version]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) production alters sperm quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef]
- Zhao, J.; Zhai, L.; Liu, Z.; Wu, S.; Xu, L. Leptin level and oxidative stress contribute to obesity-induced low testosterone in murine testicular tissue. Oxid. Med. Cell. Longev. 2014, 2014, 190945. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Bañuls, C.; de Marañon, A.M.; Diaz-Morales, N.; Jover, A.; Garzon, S.; Rocha, M.; Victor, V.M.; Hernandez-Mijares, A. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic. Biol. Med. 2017, 108, 155–162. [Google Scholar] [CrossRef]
- Lunetti, P.; Capobianco, L.; Zara, V.; Ferramosca, A. Physical activity and male reproductive function: A new role for gamete mitochondria. Exerc. Sport Sci. Rev. 2021, 49, 99–106. [Google Scholar] [CrossRef]
- Ferramosca, A.; Provenzano, S.P.; Montagna, D.D.; Coppola, L.; Zara, V. Oxidative stress negatively affects human sperm mitochondrial respiration. Urology 2013, 82, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Saez Lancellotti, T.E.; Boarelli, P.V.; Monclus, M.A.; Cabrillana, M.E.; Clementi, M.A.; Espínola, L.S.; Cid Barría, J.L.; Vincenti, A.E.; Santi, A.G.; Fornés, M.W. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS ONE 2010, 5, e13457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobjer, J.; Naumovska, M.; Giwercman, Y.L.; Giwercman, A. High prevalence of androgen deficiency and abnormal lipid profile in infertile men with non-obstructive azoospermia. Int. J. Androl. 2012, 35, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Hagiuda, J.; Ishikawa, H.; Furuuchi, T.; Hanawa, Y.; Marumo, K. Relationship between dyslipidaemia and semen quality and serum sex hormone levels: An infertility study of 167 Japanese patients. Andrologia 2014, 46, 131–135. [Google Scholar] [CrossRef]
- Cutillas-Tolín, A.; Mínguez-Alarcón, L.; Mendiola, J.; López-Espín, J.J.; Jørgensen, N.; Navarrete-Muñoz, E.M.; Torres-Cantero, A.M.; Chavarro, J.E. Mediterranean and western dietary patterns are related to markers of testicular function among healthy men. Hum. Reprod. 2015, 30, 2945–2955. [Google Scholar] [CrossRef] [Green Version]
- Mínguez-Alarcón, L.; Mendiola, J.; López-Espín, J.J.; Sarabia-Cos, L.; Vivero-Salmerón, G.; Vioque, J.; Navarrete-Muñoz, E.M.; Torres-Cantero, A.M. Dietary intake of antioxidant nutrients is associated with semen quality in young university students. Hum. Reprod. 2012, 27, 2807–2814. [Google Scholar] [CrossRef] [Green Version]
- Braga, D.P.; Halpern, G.; Rita de Cássia, S.F.; Setti, A.S.; Iaconelli, A., Jr.; Borges, E., Jr. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil. Steril. 2012, 97, 53–59. [Google Scholar] [CrossRef]
- Eslamian, G.; Amirjannati, N.; Rashidkhani, B.; Sadeghi, M.R.; Hekmatdoost, A. Intake of food groups and idiopathic asthenozoospermia: A case-control study. Hum. Reprod. 2012, 27, 3328–3336. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Di Giacomo, M.; Zara, V.; Bergamo, P.; Ferramosca, A. Crosstalk between mitochondrial metabolism and oxidoreductive homeostasis: A new perspective for understanding the effects of bioactive dietary compounds. Nutr. Res. Rev. 2020, 33, 90–101. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.T.; Temple, N.; Woodside, J.V. Vegetarian diets, low-meat diets and health: A review. Public Health Nutr. 2012, 15, 2287–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozati, R.; Reddy, P.P.; Reddanna, P.; Mujtaba, R. Role of environmental estrogens in the deterioration of male factor fertility. Fertil. Steril. 2002, 78, 1187–1194. [Google Scholar] [CrossRef]
- Ferramosca, A.; Lorenzetti, S.; Di Giacomo, M.; Murrieri, F.; Coppola, L.; Zara, V. Herbicides glyphosate and glufosinate ammonium negatively affect human sperm mitochondria respiration efficiency. Reprod. Toxicol. 2020, 99, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Saez, F.; Drevet, J.R. Dietary cholesterol and lipid overload: Impact on male fertility. Oxid. Med. Cell. Longev. 2019, 2019, 4521786. [Google Scholar] [CrossRef]
- Amaral, A.; Castillo, J.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol. Cell. Proteom. 2013, 12, 330–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collodel, G.; Castellini, C.; Lee, J.C.-Y.; Signorini, C. Relevance of fatty acids to sperm maturation and quality. Oxid. Med. Cell. Longev. 2020, 2020, 7038124. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Burgos, C.; Maldonado, C.; Gerez de Burgos, N.M.; Aoki, A.; Blanco, A.; Si, Y.; Okuno, M. Intracellular localization of the testicular and sperm-specific lactate dehydrogenase isozyme C4 in mice. Biol. Reprod. 1995, 53, 84–92. [Google Scholar] [CrossRef]
- Ferramosca, A.; Conte, A.; Guerra, F.; Felline, S.; Rimoli, M.G.; Mollo, E.; Zara, V.; Terlizzi, A. Metabolites from invasive pests inhibit mitochondrial complex II: A potential strategy for the treatment of human ovarian carcinoma? Biochem. Biophys. Res. Commun. 2016, 473, 1133–1138. [Google Scholar] [CrossRef]
- Mansour, S.W.; Sangi, S.; Harsha, S.; Khaleel, M.A.; Ibrahim, A.R. Sensibility of male rats fertility against olive oil, Nigella sativa oil and pomegranate extract. Asian Pac. J. Trop. Biomed. 2013, 3, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Al-Okbi, S.Y.; Mohamed, D.A.; Hamed, T.E.; Esmail, R.S.; Donya, S.M. Prevention of renal dysfunction by nutraceuticals prepared from oil rich plant foods. Asian Pac. J. Trop. Biomed. 2014, 4, 618–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Fontdevila, M.; Bustos-Obregon, E. Cholesterol and polyunsaturated acid enriched diet: Effect on kinetics of the acrosome reaction in rabbit spermatozoa. Mol. Reprod. Dev. 1993, 35, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Kolodgie, F.D.; Katocs, A.S., Jr.; Largis, E.E.; Wrenn, S.M.; Cornhill, J.F.; Herderick, E.E.; Lee, S.J.; Virmani, R. Hypercholes-terolemia in the rabbit induced by feeding graded amounts of low-level cholesterol. Methodological considerations regarding individual variability in response to dietary cholesterol and development of lesion type. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Fontdevila, M.; Peña, W.; Bustos-Obregón, E. Experimental hypercholesterolaemia in rabbits. Effect on lipid domains in homologous spermatozoa. Andrologia 1998, 30, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Kitajima, S.; Watanabe, T.; Xu, J.; Zhang, J.; Liu, E.; Chen, Y.E. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 2015, 146, 104–119. [Google Scholar] [CrossRef] [Green Version]
- Simón, L.; Funes, A.K.; Yapur, M.A.; Cabrillana, M.E.; Monclus, M.A.; Boarelli, P.V.; Vincenti, A.E.; Saez Lancellotti, T.E.; Fornés, M.W. Manchette-acrosome disorders during spermiogenesis and low efficiency of seminiferous tubules in hypercholesterolemic rabbit model. PLoS ONE. 2017, 12, e0172994. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Liu, Y.; Xue, K.; Gu, G.; Fan, W.; Xu, Y.; Ding, Z. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS ONE 2015, 10, e0120775. [Google Scholar] [CrossRef]
- Borges, B.C.; Garcia-Galiano, D.; da Silveira Cruz-Machado, S.; Han, X.; Gavrilina, G.B.; Saunders, T.L.; Auchus, R.J.; Hammoud, S.S.; Smith, G.D.; Elias, C.F. Obesity-induced infertility in male mice is associated with disruption of Crisp4 expression and sperm fertilization capacity. Endocrinology 2017, 158, 2930–2943. [Google Scholar] [CrossRef]
- Whitfield, M.; Guiton, R.; Rispal, J.; Acar, N.; Kocer, A.; Drevet, J.R.; Saez, F. Dyslipidemia alters sperm maturation and ca-pacitation in LXR-null mice. Reproduction 2017, 154, 827–842. [Google Scholar] [CrossRef]
- Yu, C.; Jiang, F.; Zhang, M.; Luo, D.; Shao, S.; Zhao, J.; Gao, L.; Zuo, C.; Guan, Q. HC diet inhibited testosterone synthesis by activating endoplasmic reticulum stress in testicular Leydig cells. J. Cell. Mol. Med. 2019, 23, 3140–3150. [Google Scholar] [CrossRef] [PubMed]
- Funes, A.K.; Simón, L.; Colombo, R.; Avena, M.V.; Monclús, M.; Crescitelli, J.; Cabrillana, M.E.; Conte, M.I.; Cayado, N.; Boarelli, P.; et al. Impact of high fat diet on the sterol regulatory element-binding protein 2 cholesterol pathway in the testicle. Mol. Hum. Reprod. 2021, 27, gaab023. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Afeiche, M.C.; Gaskins, A.J.; Williams, P.L.; Mendiola, J.; Jørgensen, N.; Swan, S.H.; Chavarro, J.E. Sugar-sweetened beverage intake in relation to semen quality and reproductive hormone levels in young men. Hum. Reprod. 2014, 29, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Vignera, S.; Condorelli, R.A.; Vicari, E.S.D.; D’Agata, R.; Calogero, A.E. Diabetes mellitus and sperm parameters. J. Androl. 2012, 33, 145–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q. Human sperm RNA code senses dietary sugar. Nat. Rev. Endocrinol. 2020, 16, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Trigg, N.A.; Eamens, A.L.; Nixon, B. The contribution of epididymosomes to the sperm small RNA profile. Reproduction 2019, 157, R209–R223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Kirby, J.A.; Chu, C.; Gou, L.-T. Small noncoding RNAs in reproduction and infertility. Biomedicines 2021, 9, 1884. [Google Scholar] [CrossRef]
- Conine, C.C.; Sun, F.; Song, L.; Rivera-Pérez, J.A.; Rando, O.J. Small RNAs gained during epididymal transit of sperm are essential for embryonic development in mice. Dev. Cell 2018, 46, 470–480.e3. [Google Scholar] [CrossRef] [Green Version]
- Medaglia, D.S.A.; Vieira, H.R.; da Silva Silveira, S.; Siervo, G.E.M.D.L.; da Silva Marcon, M.S.; de Freitas Mathias, P.C.; Fernandes, G.S. High-fructose diet during puberty alters the sperm parameters, testosterone concentration, and histopathology of testes and epididymis in adult Wistar rats. J. Dev. Orig. Health Dis. 2021, 13, 20–27. [Google Scholar] [CrossRef]
- Anbara, H.; Sheibani, M.T.; Razi, M. Long-term effect of aspartame on male reproductive system: Evidence for testicular histomorphometrics, Hsp70-2 protein expression and biochemical status. Int. J. Fertil. Steril. 2020, 14, 91–101. [Google Scholar] [CrossRef]
- Melis, M.S. Effects of chronic administration of Stevia rebaudiana on fertility in rats. J. Ethnopharmacol. 1999, 67, 157–161. [Google Scholar] [CrossRef]
- Kille, J.W.; Ford, W.C.; McAnulty, P.; Tesh, J.M.; Ross, F.W.; Willoughby, C.R. Sucralose: Lack of effects on sperm glycolysis and reproduction in the rat. Food Chem. Toxicol. 2000, 38, S19–S29. [Google Scholar] [CrossRef]
- Yu, Z.; Nan, F.; Wang, L.Y.; Jiang, H.; Chen, W.; Jiang, Y. Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Ajuogu, P.K.; Al-Aqbi, M.A.; Hart, R.A.; Wolden, M.; Smart, N.A.; McFarlane, J.R. The effect of dietary protein intake on factors associated with male infertility: A systematic literature review and meta-analysis of animal clinical trials in rats. Nutr. Health 2020, 26, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.; Grooten, H.J.G.; Den Hartog, L.A.; Luiting, P.; Verstegen, M.W.A. The effect of a high protein intake on sperm production in boars at two semen collection frequencies. Anim. Reprod. Sci. 1988, 17, 103–113. [Google Scholar] [CrossRef]
- Lapointe, S.; Sirard, M.A. Catalase and oviductal fluid reverse the decreased motility of bovine sperm in culture medium containing specific amino acids. J. Androl. 1998, 19, 31–36. [Google Scholar]
- Johnson, Q.; Veith, W. Effect of dietary plant and animal protein intake on sperm quality in monkeys. Arch. Androl. 2001, 46, 145–151. [Google Scholar] [CrossRef]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef] [Green Version]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of sperm mitochondrial function: A key organelle for sperm motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Amaral, S.; Tavares, R.S.; Baptista, M.; Sousa, M.I.; Silva, A.; Escada-Rebelo, S.; Paiva, C.P.; Ramalho-Santos, J. Mitochondrial functionality and chemical compound action on sperm function. Curr. Med. Chem. 2016, 23, 3575–3606. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Lujan-Barroso, L.; Kuhnle, G.G.; Mulligan, A.A.; Touillaud, M.; Slimani, N.; Romieu, I.; Powell, N.; Tumino, R.; et al. Dietary intakes and food sources of phytoestrogens in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24-hour dietary recall cohort. Eur. J. Clin. Nutr. 2012, 66, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, P.; Pathak, C.M.; Khanduja, K.L. A new perspective on the quercetin paradox in male reproductive dysfunction. Phytother. Res. 2013, 27, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Acuña, C.; Lopez-Alarcón, C.; Aliaga, M.E.; Speisky, H. Inhibition of mitochondrial complex I by various non-steroidal anti-inflammatory drugs and its protection by quercetin via a coenzyme Q-like action. Chem. Biol. Interact. 2012, 199, 18–28. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Nabavi, S.M.; Braidy, N.; Setzer, W.N.; Ahmed, T.; Nabavi, S.F. Quercetin and the mitochondria: A mechanistic view. Biotechnol. Adv. 2016, 34, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Houghton, M.J.; Kerimi, A.; Tumova, S.; Boyle, J.P.; Williamson, G. Quercetin preserves redox status and stimulates mitochondrial function in metabolically-stressed HepG2 cells. Free Radic. Biol. Med. 2018, 129, 296–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Juan, M.E.; González-Pons, E.; Munuera, T.; Ballester, J.; Rodríguez-Gil, J.E.; Planas, J.M. Trans-resveratrol, a natural antioxidant from grapes, increases sperm output in healthy rats. J. Nutr. 2005, 135, 757–760. [Google Scholar] [CrossRef]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The role of resveratrol in mammalian reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.; Hajheidari, Z.; Nabavi, S.M. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 727–745. [Google Scholar] [CrossRef]
- Abdelali, A.; Al-Bader, M.; Kilarkaje, N. Effects of trans-resveratrol on hyperglycemia-induced abnormal spermatogenesis, DNA damage and alterations in poly (ADP-ribose) polymerase signaling in rat testis. Toxicol. Appl. Pharmacol. 2016, 311, 61–73. [Google Scholar] [CrossRef]
- Nordeen, S.K.; Bona, B.J.; Jones, D.N.; Lambert, J.R.; Jackson, T.A. Endocrine disrupting activities of the flavonoid nutraceuticals luteolin and quercetin. Horm. Cancer 2013, 4, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Auger, J.; Zimmermann, C.; Eustache, F.; Nef, S. Soy, phyto-oestrogens and male reproductive function: A review. Int. J. Androl. 2010, 33, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, H.A.; El-Beshbishy, H.A.; Banjar, Z.M. Mitochondrial dysfunction induced impairment of spermatogenesis in LPS-treated rats: Modulatory role of lycopene. Eur. J. Pharmacol. 2012, 677, 31–38. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Comhaire, F.H.; Mahmoud, A. The role of food supplements in the treatment of the infertile man. Reprod. Biomed. Online 2003, 7, 385–391. [Google Scholar] [CrossRef]
- Comhaire, F.H.; El Garem, Y.; Mahmoud, A.; Eertmans, F.; Schoonjans, F. Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: A double blind, randomized trial. Asian J. Androl. 2005, 7, 257–262. [Google Scholar] [CrossRef]
- Dona, G.; Kožuh, I.; Brunati, A.M.; Andrisani, A.; Ambrosini, G.; Bonanni, G.; Ragazzi, E.; Armanini, D.; Clari, G.; Bordin, L. Effect of astaxanthin on human sperm capacitation. Mar. Drugs 2013, 11, 1909–1919. [Google Scholar] [CrossRef] [Green Version]
- Wolf, A.M.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef]
- Sheweita, S.A.; Tilmisany, A.M.; Al-Sawaf, H. Mechanisms of male infertility: Role of antioxidants. Curr. Drug Metab. 2005, 6, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Yue, D.; Yan, L.; Luo, H.; Xu, X.; Jin, X. Effect of vitamin E supplementation on semen quality and the testicular cell membranal and mitochondrial antioxidant abilities in Aohan fine-wool sheep. Anim. Reprod. Sci. 2010, 118, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Abdallah, G.A.; Kamel, K.I. Effect of ascorbic acid and vitamin E supplementation on semen quality and bio-chemical parameters of male rabbits. Anim. Reprod. Sci. 2003, 76, 99–111. [Google Scholar] [CrossRef]
- Cyrus, A.; Kabir, A.; Goodarzi, D.; Moghimi, M. The effect of adjuvant vitamin C after varicocele surgery on sperm quality and quantity in infertile men: A double blind placebo controlled clinical trial. Int. Braz. J. Urol. 2015, 41, 230–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabanian, S.; Farahbod, F.; Rafieian, M.; Ganji, F.; Adib, A. The effects of vitamin C on sperm quality parameters in laboratory rats following long-term exposure to cyclophosphamide. J. Adv. Pharm. Technol. Res. 2017, 8, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Harikrishnan, R.; Abhilash, P.A.; Das, S.S.; Prathibha, P.; Rejitha, S.; John, F.; Kavitha, S.; Indira, M. Protective effect of ascorbic acid against ethanol-induced reproductive toxicity in male guinea pigs. Br. J. Nutr. 2013, 110, 689–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]

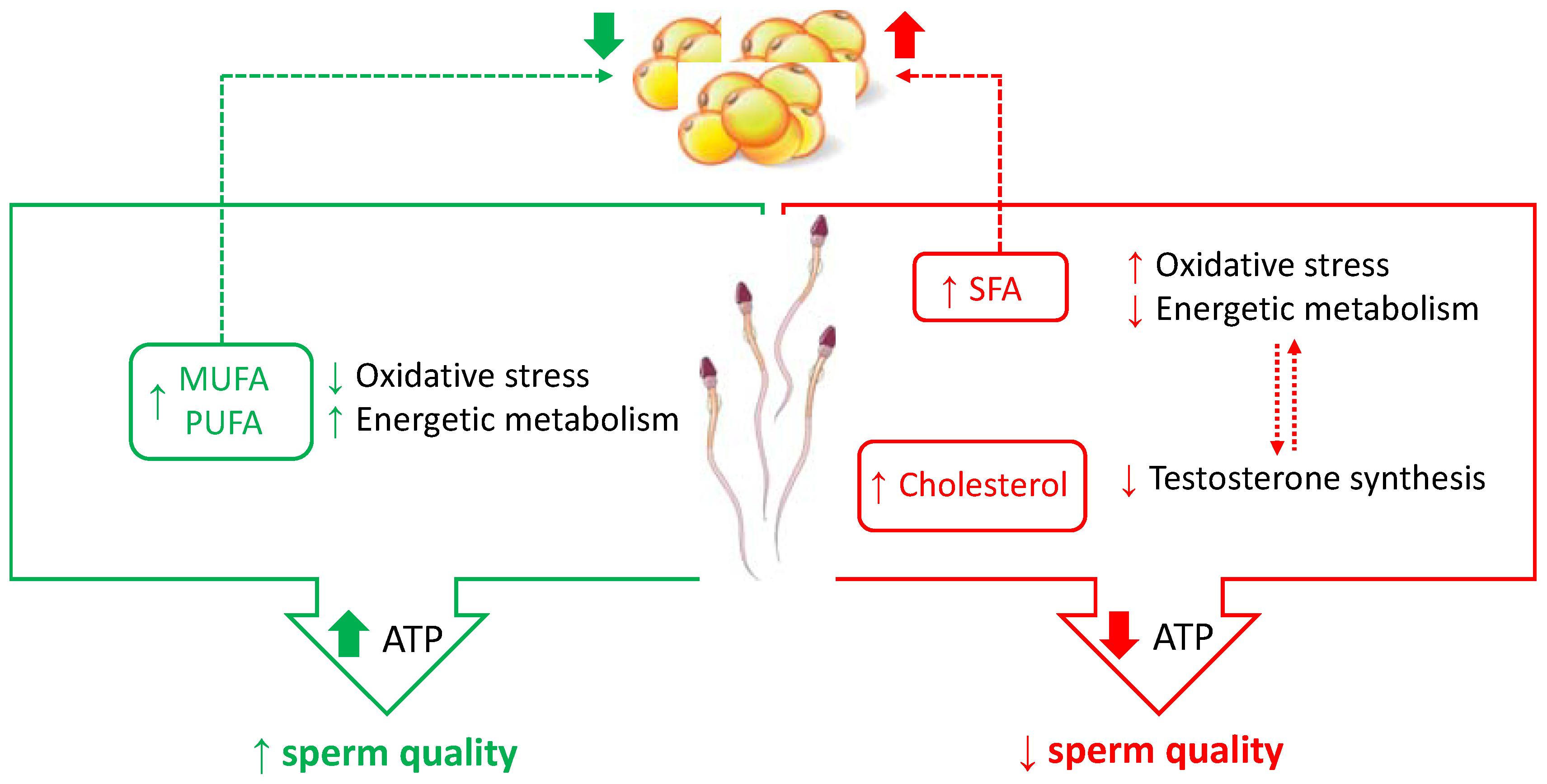
| Sperm Quality | Molecular Mechanism | References | |
|---|---|---|---|
| Cholesterol | ↓ | ↓ membrane fluidity ↓ testosterone synthesis ↑ oxidative stress | [55,71] |
| SFA | ↓ | ↑ insulin resistance ↓ sperm mitochondrial function ↑ oxidative stress | [4] |
| MUFA | ↑ | ↑ membrane fluidity ↑ sperm mitochondrial function ↓ oxidative stress | [5,7,61] |
| PUFA | ↑ | ↓ insulin resistance ↓ lipogenesis ↑ membrane fluidity ↑ sperm mitochondrial function ↓ oxidative stress | [5,57] |
| Carbohydrates | ↓ (high sugar intake) | ↑ insulin resistance ↓ testosterone synthesis ↑ oxidative stress small RNA profiles | [74,75,79] |
| Proteins | ↓ (low protein intake) | ↓ testosterone synthesis | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferramosca, A.; Zara, V. Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. Int. J. Mol. Sci. 2022, 23, 2542. https://doi.org/10.3390/ijms23052542
Ferramosca A, Zara V. Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. International Journal of Molecular Sciences. 2022; 23(5):2542. https://doi.org/10.3390/ijms23052542
Chicago/Turabian StyleFerramosca, Alessandra, and Vincenzo Zara. 2022. "Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism" International Journal of Molecular Sciences 23, no. 5: 2542. https://doi.org/10.3390/ijms23052542
APA StyleFerramosca, A., & Zara, V. (2022). Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. International Journal of Molecular Sciences, 23(5), 2542. https://doi.org/10.3390/ijms23052542







