Methyl Donors Reduce Cell Proliferation by Diminishing Erk-Signaling and NFkB Levels, While Increasing E-Cadherin Expression in Panc-1 Cell Line
Abstract
1. Introduction
- Some aspects of inflammatory processes influenced by pancreatic cancer cells;
- The expression levels of related metastatic markers in Panc-1 cancer cell line.
2. Results
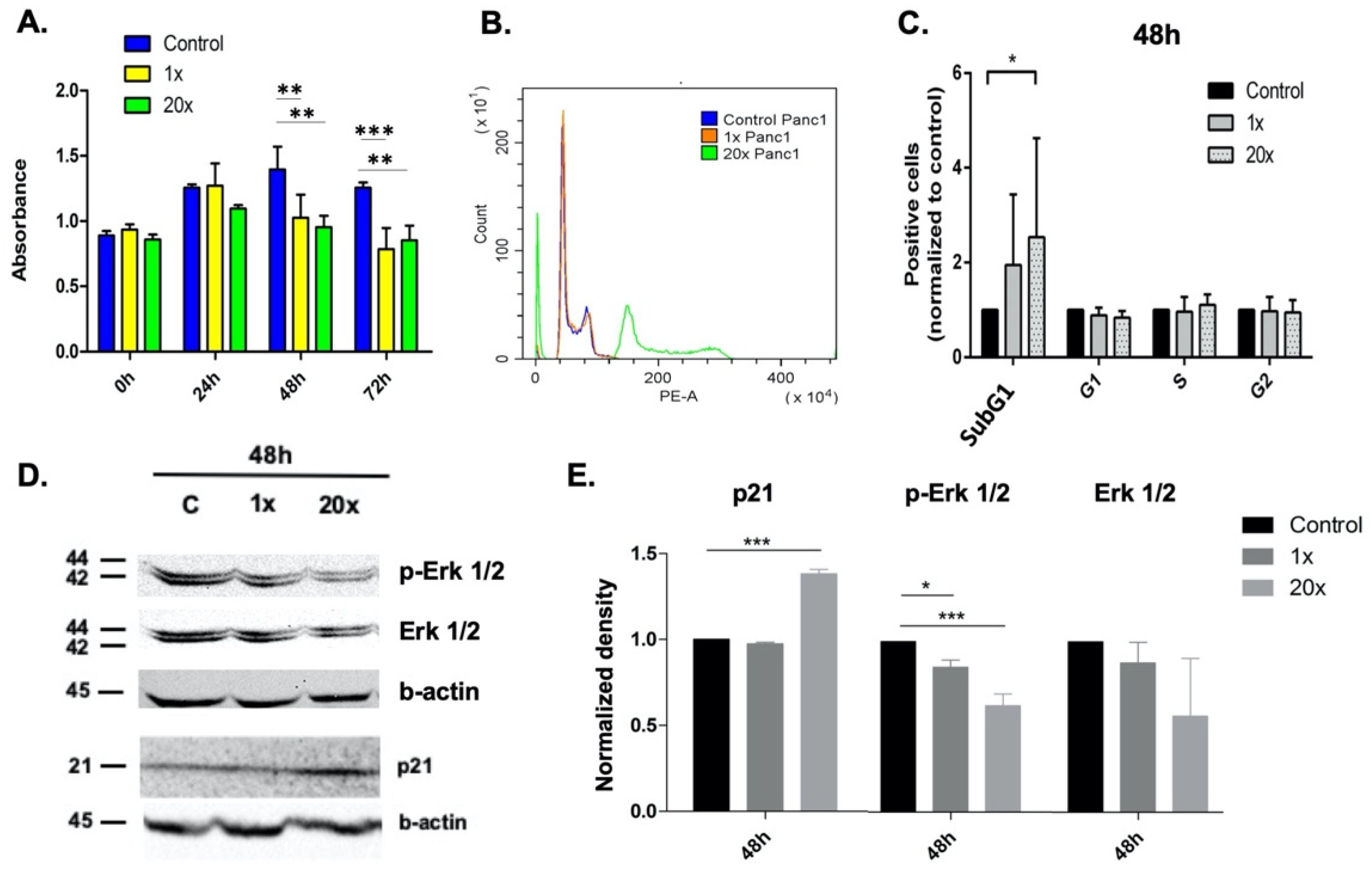
2.1. Methyl Donors Affect Tumor Cell Proliferation
2.2. The Effect of Methyl Donors on Cell Cycle
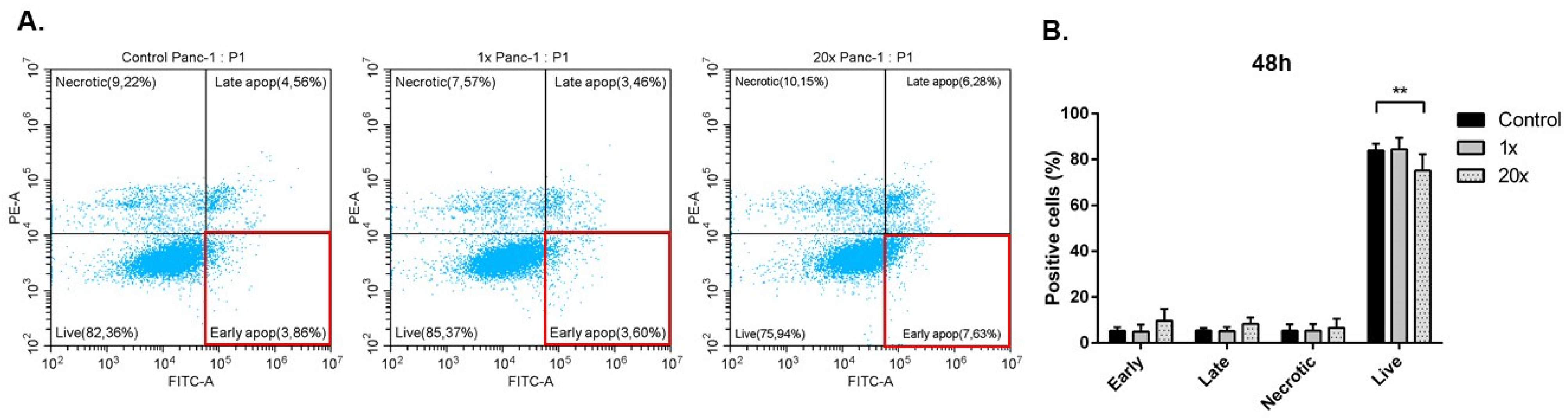
2.3. Detection of Apoptosis and Related Pathway Elements after Methyl-Donor Treatments
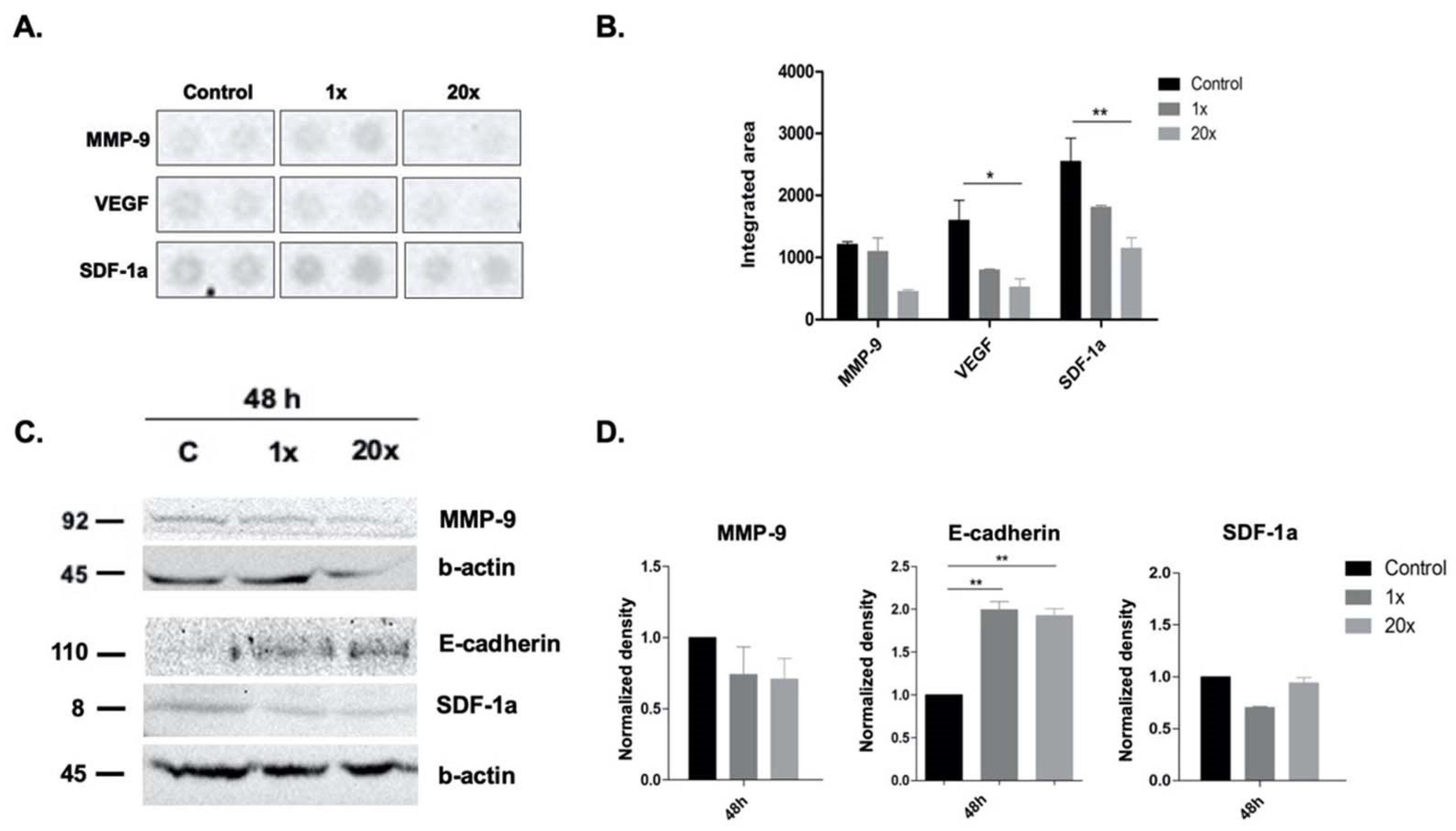
2.4. The Effects of Methyl Donors on Metastatic Potential of Panc-1 Cell Line
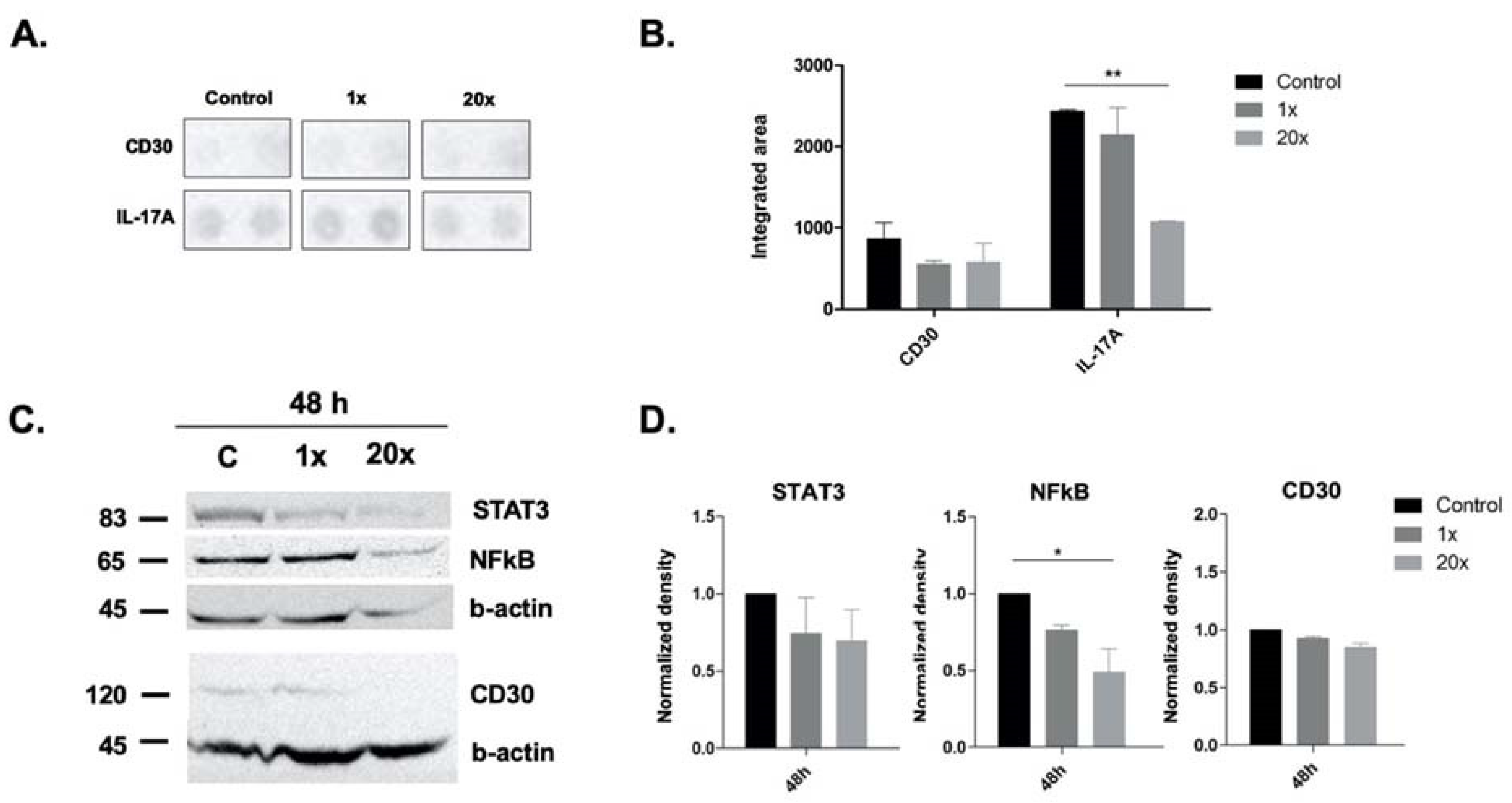
2.5. The Effect of Methyl Donors on Immune-Related Pathways and Molecules
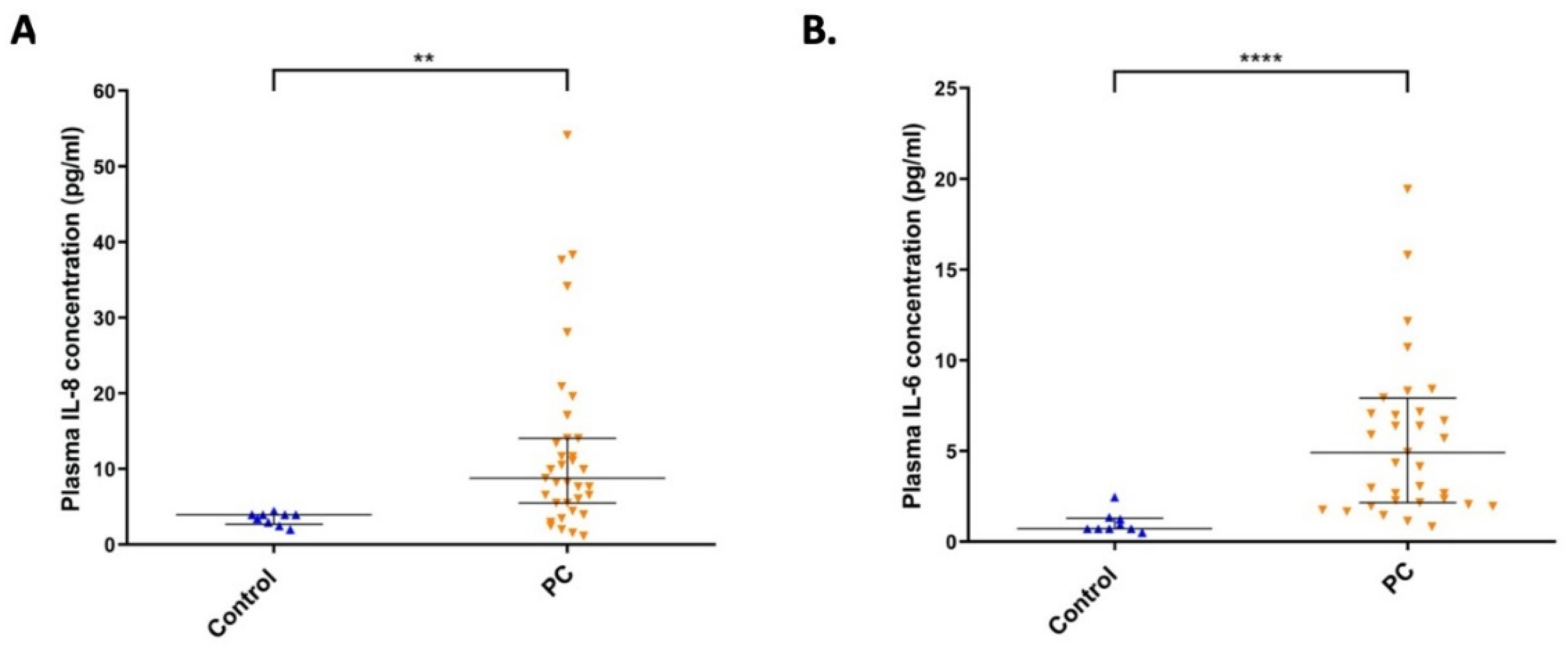
2.6. The Level of IL-8 and IL-6 Cytokines in Plasma Samples from Pancreatic Cancer Patients
3. Discussion
4. Materials and Methods
4.1. Human Samples
4.2. Cell Culture Conditions
4.3. Methyl-Donor Treatment
4.4. Cell Proliferation Assay
4.5. Cell Cycle Measurement
4.6. Detection of Apoptosis
4.7. Western Blot Analysis
4.8. Detection of Inflammatory Cytokine Levels in Panc-1 Cell Line
4.9. Quantification of IL-6 and IL-8 Cytokine Levels in Pancreatic Cancer Patients
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.P.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ali, M.M. Methyl Donor Micronutrients that Modify DNA Methylation and Cancer Outcome. Nutrients 2019, 11, 608. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Poirier, L.A. The effects of diet, genetics and chemicals on toxicity and aberrant DNA methylation: An introduction. J. Nutr. 2002, 132, 2336s–2339s. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Muhl, D.; Mohacsi, R.; Nemeth, Z.; Dank, M. B vitamin intake and the risk of colorectal cancer development: A systematic review and meta-analysis of observational studies. J. Sci. Tech. Res. 2022, 40, 13. [Google Scholar]
- Zeng, J.; Gu, Y.; Fu, H.; Liu, C.; Zou, Y.; Chang, H. Association Between One-carbon Metabolism-related Vitamins and Risk of Breast Cancer: A Systematic Review and Meta-analysis of Prospective Studies. Clin. Breast Cancer. 2020, 20, e469–e480. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.H.; Huang, X.Z.; Wang, S.B.; Li, Y.; Wang, L.Y.; Wang, H.C.; Zhang, C.H.; Zhang, C.; Liu, P.H.; Wang, Z.N. A dose-response meta-analysis reveals an association between vitamin B12 and colorectal cancer risk. Public Health Nutr. 2016, 19, 1446–1456. [Google Scholar] [CrossRef]
- Ben, S.; Du, M.; Ma, G.; Qu, J.; Zhu, L.; Chu, H.; Zhang, Z.; Wu, Y.; Gu, D.; Wang, M. Vitamin B2 intake reduces the risk for colorectal cancer: A dose-response analysis. Eur. J. Nutr. 2019, 58, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, H.; Deng, H.; Wang, Z. Association of One-Carbon Metabolism-Related Vitamins (Folate, B6, B12), Homocysteine and Methionine with the Risk of Lung Cancer: Systematic Review and Meta-Analysis. Front. Oncol. 2018, 8, 493. [Google Scholar] [CrossRef]
- Chen, P.; Li, C.; Li, X.; Li, J.; Chu, R.; Wang, H. Higher dietary folate intake reduces the breast cancer risk: A systematic review and meta-analysis. Br. J. Cancer 2014, 110, 2327–2338. [Google Scholar] [CrossRef]
- Wu, W.; Kang, S.; Zhang, D. Association of vitamin B6, vitamin B12 and methionine with risk of breast cancer: A dose-response meta-analysis. Br. J. Cancer 2013, 109, 1926–1944. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Zhu, Y.; Tao, Y.; Li, X.; Zhai, D.; Bu, Y.; Wan, Z.; Wang, Y.; Yu, Z. Association between Folate and Health Outcomes: An Umbrella Review of Meta-Analyses. Front. Public Health 2020, 8, 550753. [Google Scholar] [CrossRef]
- Fu, H.; Zeng, J.; Liu, C.; Gu, Y.; Zou, Y.; Chang, H. Folate Intake and Risk of Pancreatic Cancer: A Systematic Review and Updated Meta-Analysis of Epidemiological Studies. Dig. Dis. Sci. 2021, 66, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.H.; Mao, Q.Q. Vitamin B6, vitamin B12 and methionine and risk of pancreatic cancer: A meta-analysis. Nutr. J. 2020, 19, 111. [Google Scholar] [CrossRef] [PubMed]
- Machover, D.; Almohamad, W.; Castagné, V.; Desterke, C.; Gomez, L.; Gaston-Mathé, Y.; Boucheix, C.; Goldschmidt, E. Pharmacologic modulation of 5-fluorouracil by folinic acid and high-dose pyridoxine for treatment of patients with digestive tract carcinomas. Sci. Rep. 2021, 11, 12668. [Google Scholar] [CrossRef] [PubMed]
- Nitter, M.; Norgård, B.; De Vogel, S.; Eussen, S.J.P.M.; Meyer, K.; Ulvik, A.; Ueland, P.M.; Nygård, O.; Vollset, S.E.; Bjørge, T.; et al. Plasma methionine, choline, betaine, and dimethylglycine in relation to colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Ann. Oncol. 2014, 25, 1609–1615. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Zeisel, S.H. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J. Nutr. 2002, 132, 2333s–2335s. [Google Scholar] [CrossRef]
- Zhang, Z.; Rigas, B. NF-kappaB, inflammation and pancreatic carcinogenesis: NF-kappaB as a chemoprevention target (review). Int. J. Oncol. 2006, 29, 185–192. [Google Scholar] [PubMed]
- Nomura, A.; Majumder, K.; Giri, B.; Dauer, P.; Dudeja, V.; Roy, S.; Banerjee, S.; Saluja, A.K. Inhibition of NF-kappa B pathway leads to deregulation of epithelial-mesenchymal transition and neural invasion in pancreatic cancer. Lab. Investig. 2016, 96, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear factor-kappaB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef]
- Xie, S.; Li, J.; Wang, J.H.; Wu, Q.; Yang, P.; Hsu, H.C.; Smythies, L.E.; Mountz, J.D. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J. Immunol. 2010, 184, 2289–2296. [Google Scholar] [CrossRef]
- Mucciolo, G.; Curcio, C.; Roux, C.; Li, W.Y.; Capello, M.; Curto, R.; Chiarle, R.; Giordano, D.; Satolli, M.A.; Lawlor, R.; et al. IL17A critically shapes the transcriptional program of fibroblasts in pancreatic cancer and switches on their protumorigenic functions. Proc. Natl. Acad. Sci. USA 2021, 118, e2020395118. [Google Scholar] [CrossRef]
- Yang, J.; Liao, X.; Agarwal, M.K.; Barnes, L.; Auron, P.E.; Stark, G.R. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007, 21, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Herrmann, A.; Deng, J.H.; Kujawski, M.; Niu, G.; Li, Z.; Forman, S.; Jove, R.; Pardoll, D.M.; Yu, H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell 2009, 15, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wu, D.; Zhao, L.; Huang, L.; Shen, G.; Huang, J.; Chai, Y. Prognostic role of STAT3 in solid tumors: A systematic review and meta-analysis. Oncotarget 2016, 7, 19863–19883. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Shankar, S.; Srivastava, R.K. STAT3 as an emerging molecular target in pancreatic cancer. Gastrointest. Cancer Targets Ther. 2014, 4, 115–122. [Google Scholar]
- Kiss, E.; Forika, G.; Mohacsi, R.; Nemeth, Z.; Krenacs, T.; Dank, M. Methyl-Donors Can Induce Apoptosis and Attenuate Both the Akt and the Erk1/2 Mediated Proliferation Pathways in Breast and Lung Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 3598. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, G.; Feng, M.; Zheng, S.; Cao, Z.; Qiu, J.; You, L.; Zheng, L.; Hu, Y.; Zhang, T.; et al. NF-κB in pancreatic cancer: Its key role in chemoresistance. Cancer Lett. 2018, 421, 127–134. [Google Scholar] [CrossRef] [PubMed]
- NIDDK. Vitamin B. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]; National Institute of Diabetes and Digestive and Kidney Disease (NIDDK): Bethesda, MD, USA, 2012. [Google Scholar]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Todd, D.E.; Densham, R.M.; Molton, S.A.; Balmanno, K.; Newson, C.; Weston, C.R.; Garner, A.P.; Scott, L.; Cook, S.J. ERK1/2 and p38 cooperate to induce a p21CIP1-dependent G1 cell cycle arrest. Oncogene 2004, 23, 3284–3295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, Y.; Yang, S.; Sun, N.; Chen, J. Differential expression of STAT1 and p21 proteins predicts pancreatic cancer progression and prognosis. Pancreas 2014, 43, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Hakem, R.; Mak, T.W. Executionary pathway for apoptosis: Lessons from mutant mice. Cell Res. 2000, 10, 267–278. [Google Scholar] [CrossRef]
- Lopes, R.B.; Gangeswaran, R.; McNeish, I.A.; Wang, Y.; Lemoine, N.R. Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int. J. Cancer 2007, 120, 2344–2352. [Google Scholar] [CrossRef] [PubMed]
- Pinho, A.V.; Chantrill, L.; Rooman, I. Chronic pancreatitis: A path to pancreatic cancer. Cancer Lett. 2014, 345, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, D.; Lu, Y.; Dong, S.-C.; Ma, R.; Tang, W.-Y.; Wu, J.-Q.; Feng, J.-F. A new immunotherapy strategy targeted CD30 in peripheral T-cell lymphomas: CAR-modified T-cell therapy based on CD30 mAb. Cancer Gene Ther. 2022, 29, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Wheler, J.J.; Einhorn, L.; Dowlati, A.; Shapiro, G.I.; Hilton, J.; Burke, J.M.; Siddiqi, T.; Whiting, N.; Jalal, S.I. A phase 2, open-label study of brentuximab vedotin in patients with CD30-expressing solid tumors. Investig. New Drugs 2019, 37, 738–747. [Google Scholar] [CrossRef] [PubMed]
- van der Weyden, C.A.; Pileri, S.A.; Feldman, A.L.; Whisstock, J.; Prince, H.M. Understanding CD30 biology and therapeutic targeting: A historical perspective providing insight into future directions. Blood Cancer J. 2017, 7, e603. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Rembrink, K.; Goepel, M.; Meyer-Schwickerath, M.; Rubben, H. E-cadherin: A marker for differentiation and invasiveness in prostatic carcinoma. Urol. Res. 1993, 21, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Koshiba, T.; Hosotani, R.; Miyamoto, Y.; Ida, J.; Tsuji, S.; Nakajima, S.; Kawaguchi, M.; Kobayashi, H.; Doi, R.; Hori, T.; et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: A possible role for tumor progression. Clin. Cancer Res. 2000, 6, 3530–3535. [Google Scholar] [PubMed]
- Gelmini, S.; Mangoni, M.; Serio, M.; Romagnani, P.; Lazzeri, E. The critical role of SDF-1/CXCR4 axis in cancer and cancer stem cells metastasis. J. Endocrinol. Investig. 2008, 31, 809–819. [Google Scholar] [CrossRef]
- Li, X.; Ma, Q.; Xu, Q.; Liu, H.; Lei, J.; Duan, W.; Bhat, K.; Wang, F.; Wu, E.; Wang, Z. SDF-1/CXCR4 signaling induces pancreatic cancer cell invasion and epithelial-mesenchymal transition in vitro through non-canonical activation of Hedgehog pathway. Cancer Lett. 2012, 322, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Salvolini, E.; Pompei, V.; Pozzi, V.; Salvucci, A.; Molinelli, E.; Brisigotti, V.; Sartini, D.; Campanati, A.; Offidani, A.; et al. Nicotinamide N-methyltransferase gene silencing enhances chemosensitivity of melanoma cell lines. Pigment Cell Melanoma Res. 2021, 34, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, V.; Salvolini, E.; Lucarini, G.; Salvucci, A.; Campagna, R.; Rubini, C.; Sartini, D.; Emanuelli, M. Cancer stem cell enrichment is associated with enhancement of nicotinamide N-methyltransferase expression. IUBMB Life 2020, 72, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; van Haren, M.J.; Buijs, N.; Innocenti, P.; Zhang, Y.; Sartini, D.; Campagna, R.; Emanuelli, M.; Parsons, R.B.; Jespers, W.; et al. Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics. J. Med. Chem. 2021, 64, 12938–12963. [Google Scholar] [CrossRef]
- Ulanovskaya, O.A.; Zuhl, A.M.; Cravatt, B.F. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat. Chem. Biol. 2013, 9, 300–306. [Google Scholar] [CrossRef]
- Uphoff, C.C.; Drexler, H.G. Detection of mycoplasma in leukemia-lymphoma cell lines using polymerase chain reaction. Leukemia 2002, 16, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Cho, K.; Bae, D.R.; Joo, N.E.; Kim, H.H.; Mabasa, L.; Fowler, A.W. Methyl-donor nutrients inhibit breast cancer cell growth. In Vitro Cell. Dev. Biol. Anim. 2008, 44, 268–272. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, E.; Forika, G.; Dank, M.; Krenacs, T.; Nemeth, Z. Methyl Donors Reduce Cell Proliferation by Diminishing Erk-Signaling and NFkB Levels, While Increasing E-Cadherin Expression in Panc-1 Cell Line. Int. J. Mol. Sci. 2022, 23, 2546. https://doi.org/10.3390/ijms23052546
Kiss E, Forika G, Dank M, Krenacs T, Nemeth Z. Methyl Donors Reduce Cell Proliferation by Diminishing Erk-Signaling and NFkB Levels, While Increasing E-Cadherin Expression in Panc-1 Cell Line. International Journal of Molecular Sciences. 2022; 23(5):2546. https://doi.org/10.3390/ijms23052546
Chicago/Turabian StyleKiss, Eva, Gertrud Forika, Magdolna Dank, Tibor Krenacs, and Zsuzsanna Nemeth. 2022. "Methyl Donors Reduce Cell Proliferation by Diminishing Erk-Signaling and NFkB Levels, While Increasing E-Cadherin Expression in Panc-1 Cell Line" International Journal of Molecular Sciences 23, no. 5: 2546. https://doi.org/10.3390/ijms23052546
APA StyleKiss, E., Forika, G., Dank, M., Krenacs, T., & Nemeth, Z. (2022). Methyl Donors Reduce Cell Proliferation by Diminishing Erk-Signaling and NFkB Levels, While Increasing E-Cadherin Expression in Panc-1 Cell Line. International Journal of Molecular Sciences, 23(5), 2546. https://doi.org/10.3390/ijms23052546






