Biocompatible Materials in Otorhinolaryngology and Their Antibacterial Properties
Abstract
:1. Introduction
1.1. Polymers
1.2. Metals and Metals Alloys
1.3. Ceramics
1.4. Composites
2. Methods
3. Biomaterials Used in Otorhinolaryngology
3.1. Cochlear Implants
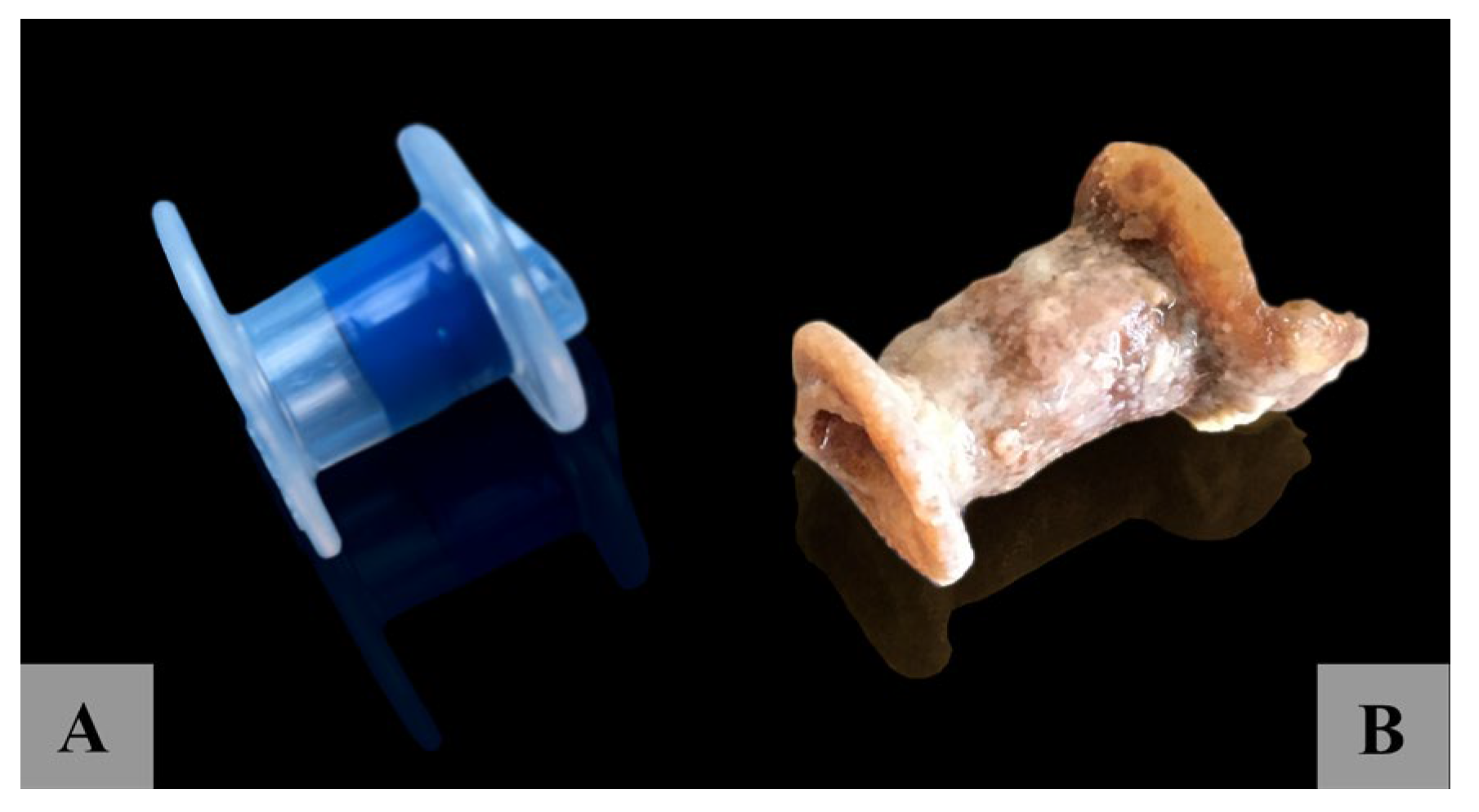
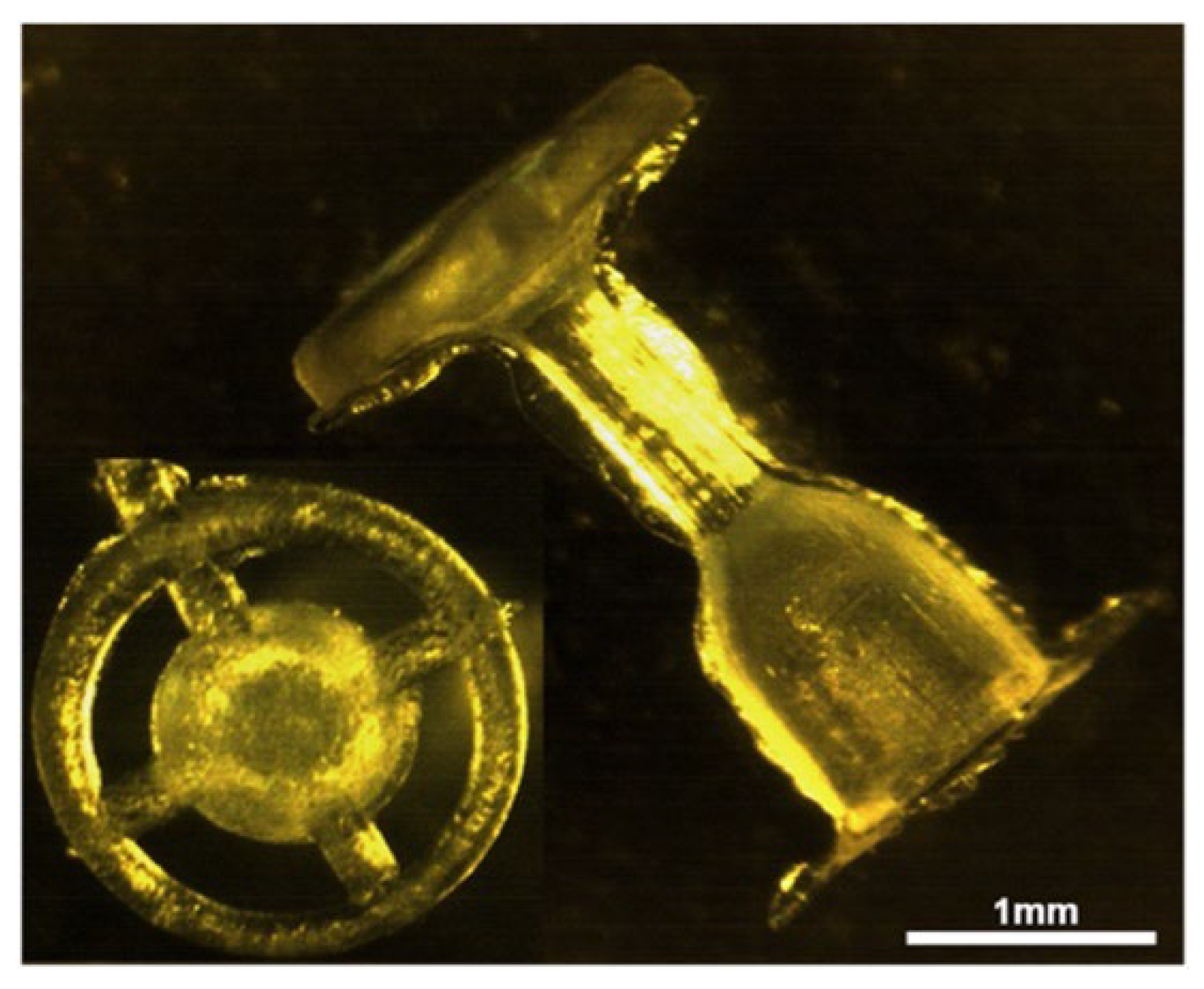
3.2. Tympanostomy Tube
3.3. Middle-Ear Prosthesis
3.4. Nasal Packing Materials
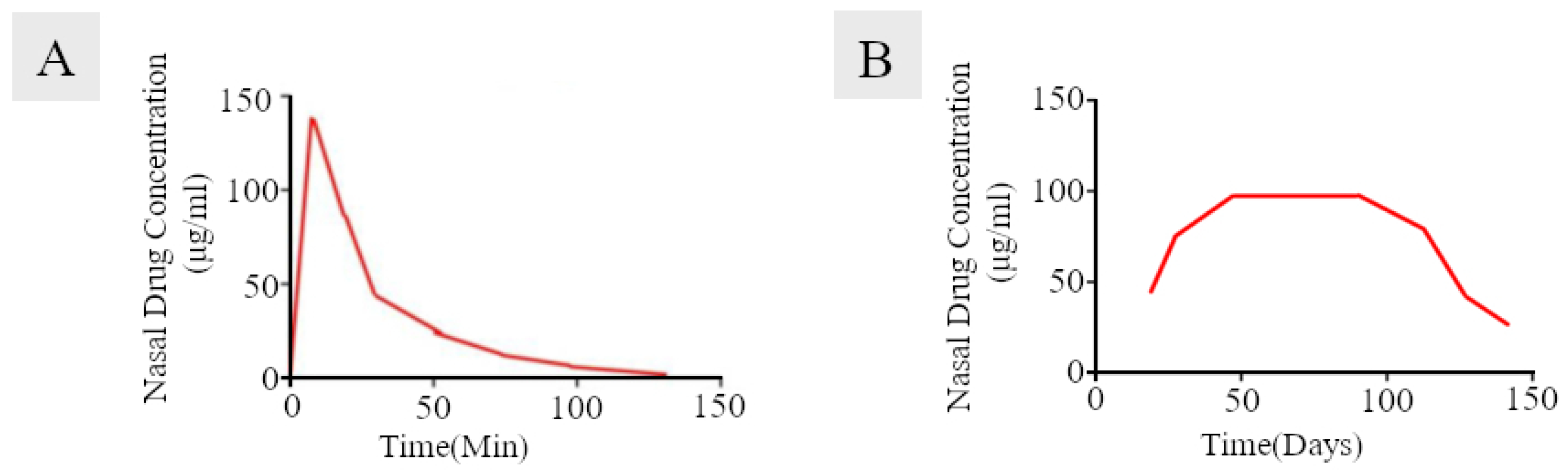
3.5. Corticosteroid-Eluting Sinus Stents
3.6. Materials for Osteosynthesis
3.7. Bone Substitution Materials
3.8. Voice Prosthesis
3.9. Tissue Engineering
4. Improving the Safety of Biomaterials by Preventing Their Microbial Colonization and Host Immune Response
4.1. Methods of Biomaterial Modifications to Increase Their Antimicrobial Properties
4.2. Selected Biomaterials with Antimicrobial Modifications for Use in Otorhinolaryngology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergman, C.P.; Aisha, S. Dental Ceramics: Microstructure, Properties and Degradation; Springer: Berlin/Heidelberg, Germany, 2013; 84p. [Google Scholar]
- Sternberg, K. Current requirements for polymeric biomaterials in otolaryngology. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2009, 8, Doc11. [Google Scholar] [CrossRef]
- Jones, J.R.; Hench, L.L. Biomedical materials for new millennium: Perspective on the future. Mater. Sci. Technol. 2001, 17, 891–900. [Google Scholar] [CrossRef]
- Hermawan, H.; Mantovani, D. Degradable metallic biomaterials: The concept, current developments and future directions. Minerva Biotecnol. 2009, 21, 207–216. [Google Scholar]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Mathur, A.B.; Collier, T.O.; Kao, W.J.; Wiggins, M.; Schubert, M.A.; Hiltner, A.; Anderson, J.M. In vivo biocompatibility and biostability of modified polyurethanes. J. Biomed. Mater. Res. 1997, 36, 246–257. [Google Scholar] [CrossRef]
- Heumann, S.; Eberl, A.; Pobeheim, H.; Liebminger, S.; Fischer-Colbrie, G.; Almansa, E.; Cavaco-Paulo, A.; Gubitz, G.M. New model substrates for enzymes hydrolysing polyethyleneterephthalate and polyamide fibres. J. Biochem. Biophys. Methods 2006, 69, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, R.N.; Lyman, D.J. Polymers in contact with the body. Environ. Health Perspect. 1975, 11, 71–74. [Google Scholar] [CrossRef]
- Galli, J.; Calo, L.; Meucci, D.; Giuliani, M.; Lucidi, D.; Paludetti, G.; Torelli, R.; Sanguinetti, M.; Parrilla, C. Biofilm in voice prosthesis: A prospective cohort study and laboratory tests using sonication and SEM analysis. Clin. Otolaryngol. 2018, 10, 1260–1265. [Google Scholar] [CrossRef]
- Spałek, J.; Deptuła, P.; Cieśluk, M.; Strzelecka, A.; Łysik, D.; Mystkowska, J.; Daniluk, T.; Król, G.; Góźdź, S.; Bucki, R.; et al. Biofilm Growth Causes Damage to Silicone Voice Prostheses in Patients after Surgical Treatment of Locally Advanced Laryngeal Cancer. Pathogens 2020, 9, 793. [Google Scholar] [CrossRef]
- Lendlein, A. Polymere als Implantatwerkstoffe. Chem. Unserer Zeit 1999, 33, 279–295. [Google Scholar] [CrossRef]
- Unverdorben, M.; Spielberger, A.; Schywalsky, M.; Labahn, D.; Hartwig, S.; Schneider, M.; Lootz, D.; Behrend, D.; Schmitz, K.; Degenhardt, R.; et al. A polyhydroxybutyrate biodegradable stent: Preliminary experience in the rabbit. Cardiovasc. Interv. Radiol. 2002, 25, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Sodian, R.; Hoerstrup, S.P.; Sperling, J.S.; Martin, D.P.; Daebritz, S.; Mayer, J.E., Jr.; Vacanti, J.P. Evaluation of biodegradable, three-dimensional matrices for tissue engineering of heart valves. ASAIO J. 2000, 46, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Kalia, V.C. Biomedical Applications of Polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261–269. [Google Scholar] [CrossRef]
- Parikh, A.; Anand, U.; Ugwu, M.C.; Feridooni, T.; Massoud, E.; Agu, R.U. Drug-eluting nasal implants: Formulation, characterization, clinical applications and challenges. Pharmaceutics 2014, 6, 249–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasi, P. Poly(lactic acid)/poly(lactic-co-glycolic acid)-based microparticles: An overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Schoubben, A.; Ricci, M.; Giovagnoli, S. Meeting the unmet: From traditional to cutting-edge techniques for poly lactide and poly lactide-co-glycolide microparticle manufacturing. J. Pharm. Investig. 2019, 49, 381–404. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 2018, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Razavi, M.; Vashaee, D.; Moharamzadeh, K.; Boccaccini, A.R.; Tayebi, L. Porous magnesium-based scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1253–1266. [Google Scholar] [CrossRef] [Green Version]
- Marti, A. Cobalt-base alloys used in bone surgery. Injury 2000, 31, D18–D21. [Google Scholar] [CrossRef]
- Ortiz, A.J.; Fernandez, E.; Vicente, A.; Calvo, J.L.; Ortiz, C. Metallic ions released from stainless steel, nickel-free, and titanium orthodontic alloys: Toxicity and DNA damage. Am. J. Orthod. Dentofac. Orthop. 2011, 140, e115–e122. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.C.; Moreira, L.M.; Santos, V.J.; Ramos, A.S.; Lyon, J.P.; Soares, C.P.; Santos, F.V. Assessment of the genetic risks of a metallic alloy used in medical implants. Genet. Mol. Biol. 2011, 34, 116–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Lourenço, M.L.; Cardoso, G.C.; dos Santos Jorge Sousa, K.; Donato, T.A.G.; Pontes, F.M.L.; Grandini, C.R. Development of novel Ti-Mo-Mn alloys for biomedical applications. Sci. Rep. 2020, 10, 6298. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz, J. Special Issue: Ti-Based Biomaterials: Synthesis, Properties and Applications. Materials 2020, 13, 1696. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Ren, Y. Nickel-free austenitic stainless steels for medical applications. Sci. Technol. Adv. Mater. 2010, 11, 014105. [Google Scholar] [CrossRef]
- Aldini, N.N.; Fini, M.; Giavaresi, G.; Torricelli, P.; Martini, L.; Giardino, R.; Ravaglioli, A.; Krajewski, A.; Mazzocchi, M.; Dubini, B.; et al. Improvement in zirconia osseointegration by means of a biological glass coating: An in vitro and in vivo investigation. J. Biomed. Mater. Res. 2002, 61, 282–289. [Google Scholar] [CrossRef]
- Saenz, A.; Brostow, W.; Rivera-Muñoz, E. Ceramic biomaterials: An introductory overview. J. Mater. Educ. 1999, 21, 297–306. [Google Scholar]
- Cranin, A.N.; Schnitman, P.A.; Rabkin, S.M.; Onesto, E.J. Alumina and zirconia coated vitallium oral endosteal implants in beagles. J. Biomed. Mater. Res. 1975, 9, 257–262. [Google Scholar] [CrossRef]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.P.; Kiattavorncharoen, S.; Lauer, H.C.; Meyer, U.; Kübler, N.R.; Handschel, J. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head Face Med. 2008, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Scholz, M.S.; Blanchfield, J.P.; Bloom, L.D.; Coburn, B.H.; Elkington, M.; Fuller, J.D.; Gilbert, M.E.; Muflahi, S.A.; Pernice, M.F.; Rae, S.I.; et al. The use of composite materials in modern orthopaedic medicine and prosthetic devices: A review. Compos. Sci. Technol. 2011, 71, 1791–1803. [Google Scholar] [CrossRef]
- Lenarz, T.; Lesinski-Schiedat, A.; Weber, B.P.; Frohne, C.; Buchner, A.; Battmer, R.D.; Parker, J.; von Wallenberg, E. The Nucleus Double Array Cochlear Implant: A new concept in obliterated cochlea. Laryngorhinootologie 1999, 78, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Scheper, V.; Hessler, R.; Hutten, M.; Wilk, M.; Jolly, C.; Lenarz, T.; Paasche, G. Local inner ear application of dexamethasone in cochlear implant models is safe for auditory neurons and increases the neuroprotective effect of chronic electrical stimulation. PLoS ONE 2017, 12, e0183820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilk, M.; Hessler, R.; Mugridge, K.; Jolly, C.; Fehr, M.; Lenarz, T.; Scheper, V. Impedance Changes and Fibrous Tissue Growth after Cochlear Implantation Are Correlated and Can Be Reduced Using a Dexamethasone Eluting Electrode. PLoS ONE 2016, 11, e0147552. [Google Scholar] [CrossRef] [PubMed]
- Lehner, E.; Gundel, D.; Liebau, A.; Plontke, S.; Mader, K. Intracochlear PLGA based implants for dexamethasone release: Challenges and solutions. Int. J. Pharm. X 2019, 1, 100015. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Plontke, S.K.; Glien, A.; Rahne, T.; Mader, K.; Salt, A.N. Controlled release dexamethasone implants in the round window niche for salvage treatment of idiopathic sudden sensorineural hearing loss. Otol. Neurotol. 2014, 35, 1168–1171. [Google Scholar] [CrossRef] [Green Version]
- Jang, C.H.; Park, H.; Cho, Y.B.; Choi, C.H.; Park, I.Y. The use of piperacillin-tazobactam coated tympanostomy tubes against ciprofloxacin-resistant Pseudomonas biofilm formation: An in vitro study. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 295–299. [Google Scholar] [CrossRef]
- Jang, C.H.; Park, H.; Cho, Y.B.; Choi, C.H. Effect of vancomycin-coated tympanostomy tubes on methicillin-resistant Staphylococcus aureus biofilm formation: In vitro study. J. Laryngol. Otol. 2010, 124, 594–598. [Google Scholar] [CrossRef]
- Saidi, I.S.; Biedlingmaier, J.F.; Whelan, P. In vivo resistance to bacterial biofilm formation on tympanostomy tubes as a function of tube material. Otolaryngol. Head Neck Surg. 1999, 120, 621–627. [Google Scholar] [CrossRef]
- Jang, C.H.; Cho, Y.B.; Choi, C.H. Effect of ion-bombarded silicone tympanostomy tube on ciprofloxacin-resistant Pseudomonas aeruginosa biofilm formation. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 1471–1473. [Google Scholar] [CrossRef]
- Joe, H.; Seo, Y.J. A newly designed tympanostomy stent with TiO2 coating to reduce Pseudomonas aeruginosa biofilm formation. J. Biomater. Appl. 2018, 33, 599–605. [Google Scholar] [CrossRef]
- de Abajo, J.; Sanhueza, I.; Giron, L.; Manrique, M. Experience with the active middle ear implant in patients with moderate-to-severe mixed hearing loss: Indications and results. Otol. Neurotol. 2013, 34, 1373–1379. [Google Scholar] [CrossRef]
- Stupp, C.H.; Stupp, H.F.; Grün, D. Replacement of ear ossicles with titanium prostheses. Laryngorhinootologie 1996, 75, 335–337. [Google Scholar] [CrossRef]
- Maassen, M.M.; Löwenheim, H.; Pfister, M.; Herberhold, S.; Jorge, J.R.; Baumann, I.; Nüsser, A.; Zimmermann, R.; Brosch, S.; Zenner, H.P. Surgical-handling properties of the titanium prosthesis in ossiculoplasty. Ear Nose Throat J. 2005, 84, 142–144. [Google Scholar] [CrossRef]
- Gostian, A.O.; Kouame, J.M.; Bremke, M.; Ortmann, M.; Hüttenbrink, K.B.; Beutner, D. Long term results of the titanium clip prosthesis. Eur. Arch. Otorhinolaryngol. 2016, 273, 4257–4266. [Google Scholar] [CrossRef]
- Wongwiwat, P.; Boonma, A.; Lee, Y.S.; Narayan, R.J. Bioceramics in ossicular replacement prostheses: A review. J. Long Term. Eff. Med. Implant. 2011, 21, 169–183. [Google Scholar] [CrossRef]
- Shinohara, T.; Gyo, K.; Saiki, T.; Yanagihara, N. Ossiculoplasty using hydroxyapatite prostheses: Long-term results. Clin. Otolaryngol. Allied Sci. 2000, 25, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Ocak, E.; Beton, S.; Meço, C.; Dursun, G. Titanium versus Hydroxyapatite Prostheses: Comparison of Hearing and Anatomical Outcomes after Ossicular Chain Reconstruction. Turk. Arch. Otorhinolaryngol. 2015, 53, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Meijer, A.G.; Segenhout, H.M.; Albers, F.W.; van de Want, H.J. Histopathology of biocompatible hydroxylapatite-polyethylene composite in ossiculoplasty. ORL J. Otorhinolaryngol. Relat. Spec. 2002, 64, 173–179. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Chichkov, B.; Adunka, O.; Pillsbury, H.; Doraiswamy, A.; Narayan, R.J. Rapid prototyping of ossicular replacement prostheses. Appl. Surf. Sci. 2007, 253, 6603–6607. [Google Scholar] [CrossRef]
- Ziabka, M.; Menaszek, E.; Tarasiuk, J.; Wronski, S. Biocompatible Nanocomposite Implant with Silver Nanoparticles for Otology-In Vivo Evaluation. Nanomaterials 2018, 8, 764. [Google Scholar] [CrossRef] [Green Version]
- Krings, J.G.; Kallogjeri, D.; Wineland, A.; Nepple, K.G.; Piccirillo, J.F.; Getz, A.E. Complications of primary and revision functional endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope 2014, 124, 838–845. [Google Scholar] [CrossRef] [Green Version]
- Okushi, T.; Yoshikawa, M.; Otori, N.; Matsuwaki, Y.; Asaka, D.; Nakayama, T.; Morimoto, T.; Moriyama, H. Evaluation of symptoms and QOL with calcium alginate versus chitin-coated gauze for middle meatus packing after endoscopic sinus surgery. Auris Nasus Larynx 2012, 39, 31–37. [Google Scholar] [CrossRef]
- Verim, A.; Seneldir, L.; Naiboglu, B.; Karaca, C.T.; Kulekci, S.; Toros, S.Z.; Oysu, C. Role of nasal packing in surgical outcome for chronic rhinosinusitis with polyposis. Laryngoscope 2014, 124, 1529–1535. [Google Scholar] [CrossRef]
- Wang, J.; Cai, C.; Wang, S. Merocel versus Nasopore for nasal packing: A meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e93959. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J. Executive summary of EPOS 2020 including integrated care pathways. Rhinology 2020, 58, 82–111. [Google Scholar] [CrossRef]
- Luong, A.; Ow, R.A.; Singh, A.; Weiss, R.L.; Han, J.K.; Gerencer, R.; Stolovitzky, J.P.; Stambaugh, J.W.; Raman, A. Safety and Effectiveness of a Bioabsorbable Steroid-Releasing Implant for the Paranasal Sinus Ostia: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg 2018, 144, 28–35. [Google Scholar] [CrossRef] [Green Version]
- US Food and Drug Administration (FDA). Premarket Approval (PMA). 2018. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm (accessed on 10 January 2022).
- Han, J.K.; Kern, R.C. Topical therapies for management of chronic rhinosinusitis: Steroid implants. Int. Forum Allergy Rhinol. 2019, 9, S22–S26. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.L.; Singh, A.; Luong, A.; Ow, R.A.; Shotts, S.D.; Sautter, N.B.; Han, J.K.; Stambaugh, J.; Raman, A. Randomized controlled trial of a bioabsorbable steroid-releasing implant in the frontal sinus opening. Laryngoscope 2016, 126, 2659–2664. [Google Scholar] [CrossRef]
- Forwith, K.D.; Han, J.K.; Stolovitzky, J.P.; Yen, D.M.; Chandra, R.K.; Karanfilov, B.; Matheny, K.E.; Stambaugh, J.W.; Gawlicka, A.K. RESOLVE: Bioabsorbable steroid-eluting sinus implants for in-office treatment of recurrent sinonasal polyposis after sinus surgery: 6-month outcomes from a randomized, controlled, blinded study. Int. Forum Allergy Rhinol. 2016, 6, 573–581. [Google Scholar] [CrossRef]
- Adriaensen, G.; Lim, K.H.; Fokkens, W.J. Safety and efficacy of a bioabsorbable fluticasone propionate-eluting sinus dressing in postoperative management of endoscopic sinus surgery: A randomized clinical trial. Int. Forum Allergy Rhinol. 2017, 7, 813–820. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Margolis, J.R. The excel stent: A good DES, but can we really stop clopidogrel after 6 months? JACC Cardiovasc. Interv. 2009, 2, 310–311. [Google Scholar] [CrossRef] [Green Version]
- Kanno, T.; Sukegawa, S.; Furuki, Y.; Nariai, Y.; Sekine, J. Overview of innovative advances in bioresorbable plate systems for oral and maxillofacial surgery. Jpn. Dent. Sci. Rev. 2018, 54, 127–138. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Nagano, D.; Shibata, A.; Sukegawa-Takahashi, Y.; Furuki, Y. The Clinical Feasibility of Newly Developed Thin Flat-Type Bioresorbable Osteosynthesis Devices for the Internal Fixation of Zygomatic Fractures: Is There a Difference in Healing Between Bioresorbable Materials and Titanium Osteosynthesis? J. Craniofac. Surg. 2016, 27, 2124–2129. [Google Scholar] [CrossRef] [Green Version]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Pinto, C.M.; Asprino, L.; de Moraes, M. Chemical and structural analyses of titanium plates retrieved from patients. Int. J. Oral Maxillofac. Surg. 2015, 44, 1005–1009. [Google Scholar] [CrossRef]
- Acero, J.; Calderon, J.; Salmeron, J.I.; Verdaguer, J.J.; Concejo, C.; Somacarrera, M.L. The behaviour of titanium as a biomaterial: Microscopy study of plates and surrounding tissues in facial osteosynthesis. J. Cranio-Maxillofac. Surg. 1999, 27, 117–123. [Google Scholar] [CrossRef]
- Lin, K.Y.; Bartlett, S.P.; Yaremchuk, M.J.; Grossman, R.F.; Udupa, J.K.; Whitaker, L.A. An experimental study on the effect of rigid fixation on the developing craniofacial skeleton. Plast. Reconstr. Surg. 1991, 87, 229–235. [Google Scholar] [CrossRef]
- Cutright, D.E.; Hunsuck, E.E.; Beasley, J.D. Fracture reduction using a biodegradable material, polylactic acid. J. Oral Surg. 1971, 29, 393–397. [Google Scholar]
- Park, Y.-W. Bioabsorbable osteofixation for orthognathic surgery. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 6. [Google Scholar] [CrossRef] [Green Version]
- Suuronen, R.; Kallela, I.; Lindqvist, C. Bioabsorbable plates and screws: Current state of the art in facial fracture repair. J. Craniomaxillofac. Trauma 2000, 6, 19–27. [Google Scholar]
- Cural, Ü.; Atalay, B.; Yildirim, M.S. Comparison of Mechanical Stabilization of the Mandibular Angulus Fracture Fixation, With Titanium Plates and Screws, Resorbable Plates and Screws, and Bone Adhesives. J. Craniofac. Surg. 2018, 29, 1780–1787. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kanno, T.; Matsumoto, K.; Sukegawa-Takahashi, Y.; Masui, M.; Furuki, Y. Complications of a poly-L-lactic acid and polyglycolic acid osteosynthesis device for internal fixation in maxillofacial surgery. Odontology 2018, 106, 360–368. [Google Scholar] [CrossRef]
- On, S.-W.; Cho, S.-W.; Byun, S.-H.; Yang, B.-E. Bioabsorbable Osteofixation Materials for Maxillofacial Bone Surgery: A Review on Polymers and Magnesium-Based Materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef]
- Sukegawa, S.; Kawai, H.; Nakano, K.; Kanno, T.; Takabatake, K.; Nagatsuka, H.; Furuki, Y. Feasible Advantage of Bioactive/Bioresorbable Devices Made of Forged Composites of Hydroxyapatite Particles and Poly-L-lactide in Alveolar Bone Augmentation: A Preliminary Study. Int. J. Med. Sci. 2019, 16, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Schumann, P.; Lindhorst, D.; Wagner, M.E.H.; Schramm, A.; Gellrich, N.C.; Rücker, M. Perspectives on Resorbable Osteosynthesis Materials in Craniomaxillofacial Surgery. Pathobiology 2013, 80, 211–217. [Google Scholar] [CrossRef]
- Lambotte, A. L’utilisation du magnesium comme materiel perdu dans l’osteosynthèse. Bull. Mem. Soc. Nat. Chir. 1932, 28, 1325–1334. [Google Scholar]
- Leonhardt, H.; Franke, A.; McLeod, N.M.H.; Lauer, G.; Nowak, A. Fixation of fractures of the condylar head of the mandible with a new magnesium-alloy biodegradable cannulated headless bone screw. Br. J. Oral Maxillofac. Surg. 2017, 55, 623–625. [Google Scholar] [CrossRef]
- Shegarfi, H.; Reikeras, O. Review article: Bone transplantation and immune response. J. Orthop. Surg. 2009, 17, 206–211. [Google Scholar] [CrossRef]
- Kloss, F.R.; Offermanns, V.; Kloss-Brandstätter, A. Comparison of allogeneic and autogenous bone grafts for augmentation of alveolar ridge defects-A 12-month retrospective radiographic evaluation. Clin. Oral Implant. Res. 2018, 29, 1163–1175. [Google Scholar] [CrossRef] [Green Version]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef] [Green Version]
- Caballé-Serrano, J.; Fujioka-Kobayashi, M.; Bosshardt, D.D.; Gruber, R.; Buser, D.; Miron, R.J. Pre-coating deproteinized bovine bone mineral (DBBM) with bone-conditioned medium (BCM) improves osteoblast migration, adhesion, and differentiation in vitro. Clin. Oral Investig. 2016, 20, 2507–2513. [Google Scholar] [CrossRef]
- Katz, J.; Mukherjee, N.; Cobb, R.R.; Bursac, P.; York-Ely, A. Incorporation and immunogenicity of cleaned bovine bone in a sheep model. J. Biomater Appl. 2009, 24, 159–174. [Google Scholar] [CrossRef]
- Cho, J.S.; Kim, H.-S.; Um, S.-H.; Rhee, S.-H. Preparation of a novel anorganic bovine bone xenograft with enhanced bioactivity and osteoconductivity. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2013, 101B, 855–869. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 82–91. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [Green Version]
- Yahav, A.; Kurtzman, G.M.; Katzap, M.; Dudek, D.; Baranes, D. Bone Regeneration: Properties and Clinical Applications of Biphasic Calcium Sulfate. Dent. Clin. N. Am. 2020, 64, 453–472. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Q.; Sculean, A.; Buser, D.; Pippenger, B.E.; Dard, M.; Shirakata, Y.; Chandad, F.; Zhang, Y. Osteoinductive potential of 4 commonly employed bone grafts. Clin. Oral Investig. 2016, 20, 2259–2265. [Google Scholar] [CrossRef]
- Donos, N.; Kostopoulos, L.; Tonetti, M.; Karring, T.; Lang, N.P. The effect of enamel matrix proteins and deproteinized bovine bone mineral on heterotopic bone formation. Clin. Oral Implant. Res. 2006, 17, 434–438. [Google Scholar] [CrossRef]
- Guillaume, B. Filling bone defects with beta-TCP in maxillofacial surgery: A review. Morphologie 2017, 101, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Friedmann, A.; Dehnhardt, J.; Kleber, B.M.; Bernimoulin, J.P. Cytobiocompatibility of collagen and ePTFE membranes on osteoblast-like cells in vitro. J. Biomed. Mater. Res. A 2008, 86, 935–941. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Labanca, M. Biomaterials in maxillofacial surgery: Membranes and grafts. Int J. Biomed. Sci 2011, 7, 81–88. [Google Scholar]
- Urban, I.A.; Monje, A. Guided Bone Regeneration in Alveolar Bone Reconstruction. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 331–338. [Google Scholar] [CrossRef]
- Jiménez Garcia, J.; Berghezan, S.; Caramês, J.M.M.; Dard, M.M.; Marques, D.N.S. Effect of cross-linked vs non-cross-linked collagen membranes on bone: A systematic review. J. Periodontal. Res. 2017, 52, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef] [Green Version]
- Martin-Thomé, H.; Bourdin, D.; Strube, N.; Saffarzadeh, A.; Morlock, J.F.; Campard, G.; Evanno, C.; Hoornaert, A.; Layrolle, P. Clinical Safety of a New Synthetic Resorbable Dental Membrane: A Case Series Study. J. Oral Implant. 2018, 44, 138–145. [Google Scholar] [CrossRef]
- Souza, F.G.R.; Santos, I.C.; Bergmann, A.; Thuler, L.C.S.; Freitas, A.S.; Freitas, E.Q.; Dias, F.L. Quality of life after total laryngectomy: Impact of different vocal rehabilitation methods in a middle income country. Health Qual. Life Outcomes 2020, 18, 92. [Google Scholar] [CrossRef]
- Bertl, K.; Zatorska, B.; Leonhard, M.; Matejka, M.; Schneider-Stickler, B. Anaerobic and microaerophilic pathogens in the biofilm formation on voice prostheses: A pilot study. Laryngoscope 2012, 122, 1035–1039. [Google Scholar] [CrossRef]
- Bucki, R.; Niemirowicz-Laskowska, K.; Deptuła, P.; Wilczewska, A.Z.; Misiak, P.; Durnaś, B.; Fiedoruk, K.; Piktel, E.; Mystkowska, J.; Janmey, P.A. Susceptibility of microbial cells to the modified PIP(2)-binding sequence of gelsolin anchored on the surface of magnetic nanoparticles. J. Nanobiotechnol. 2019, 17, 81. [Google Scholar] [CrossRef] [Green Version]
- Durnaś, B.; Wnorowska, U.; Pogoda, K.; Deptuła, P.; Wątek, M.; Piktel, E.; Głuszek, S.; Gu, X.; Savage, P.B.; Niemirowicz, K.; et al. Candidacidal Activity of Selected Ceragenins and Human Cathelicidin LL-37 in Experimental Settings Mimicking Infection Sites. PLoS ONE 2016, 11, e0157242. [Google Scholar] [CrossRef]
- Durnaś, B.; Piktel, E.; Wątek, M.; Wollny, T.; Góźdź, S.; Smok-Kalwat, J.; Niemirowicz, K.; Savage, P.B.; Bucki, R. Anaerobic bacteria growth in the presence of cathelicidin LL-37 and selected ceragenins delivered as magnetic nanoparticles cargo. BMC Microbiol. 2017, 17, 167. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Durnaś, B.; Tokajuk, G.; Piktel, E.; Michalak, G.; Gu, X.; Kułakowska, A.; Savage, P.B.; Bucki, R. Formulation and candidacidal activity of magnetic nanoparticles coated with cathelicidin LL-37 and ceragenin CSA-13. Sci. Rep. 2017, 7, 4610. [Google Scholar] [CrossRef]
- Niemirowicz-Laskowska, K.; Mystkowska, J.; Łysik, D.; Chmielewska, S.; Tokajuk, G.; Misztalewska-Turkowicz, I.; Wilczewska, A.Z.; Bucki, R. Antimicrobial and Physicochemical Properties of Artificial Saliva Formulations Supplemented with Core-Shell Magnetic Nanoparticles. Int. J. Mol. Sci. 2020, 21, 1979. [Google Scholar] [CrossRef] [Green Version]
- Vallieres, C.; Hook, A.L.; He, Y.; Crucitti, V.C.; Figueredo, G.; Davies, C.R.; Burroughs, L.; Winkler, D.A.; Wildman, R.D.; Irvine, D.J.; et al. Discovery of (meth)acrylate polymers that resist colonization by fungi associated with pathogenesis and biodeterioration. Sci. Adv. 2020, 6, eaba6574. [Google Scholar] [CrossRef]
- Niermeyer, W.L.; Rodman, C.; Li, M.M.; Chiang, T. Tissue engineering applications in otolaryngology-The state of translation. Laryngoscope Investig. Otolaryngol. 2020, 5, 630–648. [Google Scholar] [CrossRef]
- Cao, Y.; Vacanti, J.P.; Paige, K.T.; Upton, J.; Vacanti, C.A. Transplantation of Chondrocytes Utilizing a Polymer-Cell Construct to Produce Tissue-Engineered Cartilage in the Shape of a Human Ear. Plast. Reconstr. Surg. 1997, 100, 297–302. [Google Scholar] [CrossRef]
- Fulco, I.; Miot, S.; Haug, M.D.; Barbero, A.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Jundt, G.; Marsano, A.; Farhadi, J.; et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: An observational first-in-human trial. Lancet 2014, 384, 337–346. [Google Scholar] [CrossRef]
- Hoshi, K.; Fujihara, Y.; Saijo, H.; Kurabayashi, K.; Suenaga, H.; Asawa, Y.; Nishizawa, S.; Kanazawa, S.; Uto, S.; Inaki, R.; et al. Three-dimensional changes of noses after transplantation of implant-type tissue-engineered cartilage for secondary correction of cleft lip–nose patients. Regen. Ther. 2017, 7, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Jiang, H.; Yin, Z.; Liu, Y.; Zhang, Q.; Zhang, C.; Pan, B.; Zhou, J.; Zhou, X.; Sun, H.; et al. In Vitro Regeneration of Patient-specific Ear-shaped Cartilage and Its First Clinical Application for Auricular Reconstruction. EBioMedicine 2018, 28, 287–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimeskern, L.; Martínez Ávila, H.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.C.; Seonwoo, H.; Garg, P.; Jang, K.J.; Pandey, S.; Park, S.B.; Kim, H.B.; Lim, J.; Choung, Y.H.; Chung, J.H. Chitosan/PEI patch releasing EGF and the EGFR gene for the regeneration of the tympanic membrane after perforation. Biomater. Sci. 2018, 6, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Mellott, A.J.; Shinogle, H.E.; Nelson-Brantley, J.G.; Detamore, M.S.; Staecker, H. Exploiting decellularized cochleae as scaffolds for inner ear tissue engineering. Stem Cell Res. Ther. 2017, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Maughan, E.F.; Butler, C.R.; Crowley, C.; Teoh, G.Z.; den Hondt, M.; Hamilton, N.J.; Hynds, R.E.; Lange, P.; Ansari, T.; Urbani, L.; et al. A comparison of tracheal scaffold strategies for pediatric transplantation in a rabbit model. Laryngoscope 2017, 127, E449–E457. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Sundaram, S.; Le, A.V.; Huang, A.H.; Zhang, J.; Hatachi, G.; Beloiartsev, A.; Caty, M.G.; Yi, T.; Leiby, K.; et al. Engineered Tissue-Stent Biocomposites as Tracheal Replacements. Tissue Eng. Part A 2016, 22, 1086–1097. [Google Scholar] [CrossRef] [Green Version]
- Dharmadhikari, S.; Liu, L.; Shontz, K.; Wiet, M.; White, A.; Goins, A.; Akula, H.; Johnson, J.; Reynolds, S.D.; Breuer, C.K.; et al. Deconstructing tissue engineered trachea: Assessing the role of synthetic scaffolds, segmental replacement and cell seeding on graft performance. Acta Biomate.r 2020, 102, 181–191. [Google Scholar] [CrossRef]
- Best, C.A.; Pepper, V.K.; Ohst, D.; Bodnyk, K.; Heuer, E.; Onwuka, E.A.; King, N.; Strouse, R.; Grischkan, J.; Breuer, C.K.; et al. Designing a tissue-engineered tracheal scaffold for preclinical evaluation. Int. J. Pediatr. Otorhinolaryngol. 2018, 104, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Brookes, S.; Voytik-Harbin, S.; Zhang, H.; Zhang, L.; Halum, S. Motor endplate-expressing cartilage-muscle implants for reconstruction of a denervated hemilarynx. Laryngoscope 2019, 129, 1293–1300. [Google Scholar] [CrossRef]
- Herrmann, P.; Ansari, T.; Southgate, A.; Varanou Jenkins, A.; Partington, L.; Carvalho, C.; Janes, S.; Lowdell, M.; Sibbons, P.D.; Birchall, M.A. In vivo implantation of a tissue engineered stem cell seeded hemi-laryngeal replacement maintains airway, phonation, and swallowing in pigs. J. Tissue Eng. Regen. Med. 2019, 13, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.B.; Larsen, S.; Wanscher, J.H.; Faber, C.E.; Jeppesen, J. Postoperative infections following cochlear implant surgery. Acta Otolaryngol. 2018, 138, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Mikulskis, P.; Hook, A.; Dundas, A.A.; Irvine, D.; Sanni, O.; Anderson, D.; Langer, R.; Alexander, M.R.; Williams, P.; Winkler, D.A. Prediction of Broad-Spectrum Pathogen Attachment to Coating Materials for Biomedical Devices. ACS Appl. Mater. Interfaces 2018, 10, 139–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.P.; Shepherd, R.K.; Robins-Browne, R.M.; Clark, G.M.; O’Leary, S.J. Pneumococcal meningitis post-cochlear implantation: Potential routes of infection and pathophysiology. Otolaryngol. Head Neck Surg. 2010, 143, S15–S23. [Google Scholar] [CrossRef]
- Kirchhoff, L.; Arweiler-Harbeck, D.; Arnolds, J.; Hussain, T.; Hansen, S.; Bertram, R.; Buer, J.; Lang, S.; Steinmann, J.; Höing, B. Imaging studies of bacterial biofilms on cochlear implants-Bioactive glass (BAG) inhibits mature biofilm. PLoS ONE 2020, 15, e0229198. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Blanco, D.; Lynn, A.; Rosch, J.; Noreldin, R.; Salerni, A.; Lambert, C.; Rao, R.P. A pre-therapeutic coating for medical devices that prevents the attachment of Candida albicans. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Kao, W.K.; Gagnon, P.M.; Vogel, J.P.; Chole, R.A. Surface charge modification decreases Pseudomonas aeruginosa adherence in vitro and bacterial persistence in an in vivo implant model. Laryngoscope 2017, 127, 1655–1661. [Google Scholar] [CrossRef]
- Chen, R.; Willcox, M.D.; Ho, K.K.; Smyth, D.; Kumar, N. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 2016, 85, 142–151. [Google Scholar] [CrossRef]
- Höing, B.; Kirchhoff, L.; Arnolds, J.; Hussain, T.; Buer, J.; Lang, S.; Arweiler-Harbeck, D.; Steinmann, J. Bioactive Glass Granules Inhibit Mature Bacterial Biofilms on the Surfaces of Cochlear Implants. Otol. Neurotol. 2018, 39, e985–e991. [Google Scholar] [CrossRef]
- Parent, M.; Magnaudeix, A.; Delebassée, S.; Sarre, E.; Champion, E.; Viana Trecant, M.; Damia, C. Hydroxyapatite microporous bioceramics as vancomycin reservoir: Antibacterial efficiency and biocompatibility investigation. J. Biomater. Appl. 2016, 31, 488–498. [Google Scholar] [CrossRef]
- Lim, D.J.; Skinner, D.; Mclemore, J.; Rivers, N.; Elder, J.B.; Allen, M.; Koch, C.; West, J.; Zhang, S.; Thompson, H.M.; et al. In-vitro evaluation of a ciprofloxacin and azithromycin sinus stent for Pseudomonas aeruginosa biofilms. Int Forum Allergy Rhinol. 2020, 10, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Şevik Eliçora, S.; Erdem, D.; Dinç, A.E.; Altunordu Kalaycı, Ö.; Hazer, B.; Yurdakan, G.; Külah, C. Effects of polymer-based, silver nanoparticle-coated silicone splints on the nasal mucosa of rats. Eur. Arch. Otorhinolaryngol. 2017, 274, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Danti, S.; Azimi, B.; Candito, M.; Fusco, A.; Sorayani Bafqi, M.S.; Ricci, C.; Milazzo, M.; Cristallini, C.; Latifi, M.; Donnarumma, G.; et al. Lithium niobate nanoparticles as biofunctional interface material for inner ear devices. Biointerphases 2020, 15, 031004. [Google Scholar] [CrossRef]
- Edmondson, S.; Osborne, V.L.; Huck, W.T.S. Polymer brushes via surface-initiated polymerizations. Chem. Soc. Rev. 2004, 33, 14–22. [Google Scholar] [CrossRef]
- Kabirian, F.; Ditkowski, B.; Zamanian, A.; Hoylaerts, M.F.; Mozafari, M.; Heying, R. Controlled NO-Release from 3D-Printed Small-Diameter Vascular Grafts Prevents Platelet Activation and Bacterial Infectivity. ACS Biomater. Sci. Eng. 2019, 5, 2284–2296. [Google Scholar] [CrossRef]
- Gao, Q.; Yu, M.; Su, Y.; Xie, M.; Zhao, X.; Li, P.; Ma, P.X. Rationally designed dual functional block copolymers for bottlebrush-like coatings: In vitro and in vivo antimicrobial, antibiofilm, and antifouling properties. Acta Biomater. 2017, 51, 112–124. [Google Scholar] [CrossRef]
- Cheng, Q.; Asha, A.B.; Liu, Y.; Peng, Y.-Y.; Diaz-Dussan, D.; Shi, Z.; Cui, Z.; Narain, R. Antifouling and Antibacterial Polymer-Coated Surfaces Based on the Combined Effect of Zwitterions and the Natural Borneol. ACS Appl. Mater. Interfaces 2021, 13, 9006–9014. [Google Scholar] [CrossRef]
- Kurowska, M.; Eickenscheidt, A.; Guevara-Solarte, D.-L.; Widyaya, V.T.; Marx, F.; Al-Ahmad, A.; Lienkamp, K. A Simultaneously Antimicrobial, Protein-Repellent, and Cell-Compatible Polyzwitterion Network. Biomacromolecules 2017, 18, 1373–1386. [Google Scholar] [CrossRef]
- Qiu, H.; Si, Z.; Luo, Y.; Feng, P.; Wu, X.; Hou, W.; Zhu, Y.; Chan-Park, M.B.; Xu, L.; Huang, D. The Mechanisms and the Applications of Antibacterial Polymers in Surface Modification on Medical Devices. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Wong, S.Y.; Han, L.; Timachova, K.; Veselinovic, J.; Hyder, M.N.; Ortiz, C.; Klibanov, A.M.; Hammond, P.T. Drastically Lowered Protein Adsorption on Microbicidal Hydrophobic/Hydrophilic Polyelectrolyte Multilayers. Biomacromolecules 2012, 13, 719–726. [Google Scholar] [CrossRef]
- Meng, S.; Liu, Z.; Shen, L.; Guo, Z.; Chou, L.L.; Zhong, W.; Du, Q.; Ge, J. The effect of a layer-by-layer chitosan–heparin coating on the endothelialization and coagulation properties of a coronary stent system. Biomaterials 2009, 30, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Dai, F.; Li, H.; Wang, C.; Shi, X.; Cheng, Y.; Deng, H. Chitosan and collagen layer-by-layer assembly modified oriented nanofibers and their biological properties. Carbohydr. Polym. 2021, 254, 117438. [Google Scholar] [CrossRef] [PubMed]
- Vaterrodt, A.; Thallinger, B.; Daumann, K.; Koch, D.; Guebitz, G.M.; Ulbricht, M. Antifouling and Antibacterial Multifunctional Polyzwitterion/Enzyme Coating on Silicone Catheter Material Prepared by Electrostatic Layer-by-Layer Assembly. Langmuir 2016, 32, 1347–1359. [Google Scholar] [CrossRef]
- Ghamrawi, S.; Bouchara, J.-P.; Tarasyuk, O.; Rogalsky, S.; Lyoshina, L.; Bulko, O.; Bardeau, J.-F. Promising silicones modified with cationic biocides for the development of antimicrobial medical devices. Mater. Sci. Eng. C 2017, 75, 969–979. [Google Scholar] [CrossRef]
- Dirain, C.O.; Silva, R.C.; Antonelli, P.J. Prevention of biofilm formation by polyquaternary polymer. Int. J. Pediatric Otorhinolaryngol. 2016, 88, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Azghani, A.O.; Omri, A. Antimicrobial efficacy of a new antibiotic-loaded poly(hydroxybutyric-co-hydroxyvaleric acid) controlled release system. J. Antimicrob. Chemother. 2004, 54, 1013–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, E.M.; Valentin, T.; Panilaitis, B.; Omenetto, F.; Kaplan, D.L. Antibiotic-Releasing Silk Biomaterials for Infection Prevention and Treatment. Adv. Funct. Mater. 2013, 23, 854–861. [Google Scholar] [CrossRef]
- Marsili, L.; Dal Bo, M.; Berti, F.; Toffoli, G. Chitosan-Based Biocompatible Copolymers for Thermoresponsive Drug Delivery Systems: On the Development of a Standardization System. Pharmaceutics 2021, 13, 1876. [Google Scholar] [CrossRef]
- Divya, K.P.; Miroshnikov, M.; Dutta, D.; Vemula, P.K.; Ajayan, P.M.; John, G. In Situ Synthesis of Metal Nanoparticle Embedded Hybrid Soft Nanomaterials. Acc. Chem. Res. 2016, 49, 1671–1680. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Ma, L.; Li, K.; Xia, J.; Chen, C.; Liu, Y.; Lang, S.; Yu, L.; Liu, G. Commercial soft contact lenses engineered with zwitterionic silver nanoparticles for effectively treating microbial keratitis. J. Colloid Interface Sci. 2022, 610, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Sang, T.; Li, K.; Fischer, N.G.; Mutreja, I.; Echeverría, C.; Kumar, D.; Tang, Z.; Aparicio, C. Hybrid nanocoatings of self-assembled organic-inorganic amphiphiles for prevention of implant infections. Acta Biomater. 2022, 140, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Duda, F.; Bradel, S.; Bleich, A.; Abendroth, P.; Heemeier, T.; Ehlert, N.; Behrens, P.; Esser, K.H.; Lenarz, T.; Brandes, G.; et al. Biocompatibility of silver containing silica films on Bioverit® II middle ear prostheses in rabbits. J. Biomater. Appl. 2015, 30, 17–29. [Google Scholar] [CrossRef]
- Jang, C.H.; Cho, Y.B.; Jang, Y.S.; Kim, M.S.; Kim, G.H. Antibacterial effect of electrospun polycaprolactone/polyethylene oxide/vancomycin nanofiber mat for prevention of periprosthetic infection and biofilm formation. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Ziabka, M.; Dziadek, M.; Krolicka, A. Biological and Physicochemical Assessment of Middle Ear Prosthesis. Polymers 2019, 11, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziabka, M.; Dziadek, M.; Menaszek, E.; Banasiuk, R.; Krolicka, A. Middle Ear Prosthesis with Bactericidal Efficacy-In Vitro Investigation. Molecules 2017, 22, 681. [Google Scholar] [CrossRef] [Green Version]
- Barros, J.; Grenho, L.; Fernandes, M.H.; Manuel, C.M.; Melo, L.F.; Nunes, O.C.; Monteiro, F.J.; Ferraz, M.P. Anti-sessile bacterial and cytocompatibility properties of CHX-loaded nanohydroxyapatite. Colloids Surf. B Biointerfaces 2015, 130, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Weng, W.; Li, X.; Nie, W.; Liu, H.; Liu, S.; Huang, J.; Zhou, Q.; He, J.; Su, J.; Dong, Z.; et al. One-Step Preparation of an AgNP-nHA@RGO Three-Dimensional Porous Scaffold and Its Application in Infected Bone Defect Treatment. Int. J. Nanomed. 2020, 15, 5027–5042. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Ke, Q.; Yin, W.; Zhang, C.; Guo, Y.; Guan, J. Magnetic lanthanum-doped hydroxyapatite/chitosan scaffolds with endogenous stem cell-recruiting and immunomodulatory properties for bone regeneration. J. Mater. Chem. B 2020, 8, 5280–5292. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Guo, G.; Tan, J.; Wang, Q.; Tang, J.; Liu, W.; Shen, H.; Li, J.; Zhang, X. Enhanced Anti-Infective Efficacy of ZnO Nanoreservoirs through a Combination of Intrinsic Anti-Biofilm Activity and Reinforced Innate Defense. ACS Appl. Mater. Interfaces 2017, 9, 33609–33623. [Google Scholar] [CrossRef]
- Chen, B.; You, Y.; Ma, A.; Song, Y.; Jiao, J.; Song, L.; Shi, E.; Zhong, X.; Li, Y.; Li, C. Zn-Incorporated TiO. Int. J. Nanomed. 2020, 15, 2095–2118. [Google Scholar] [CrossRef] [PubMed] [Green Version]

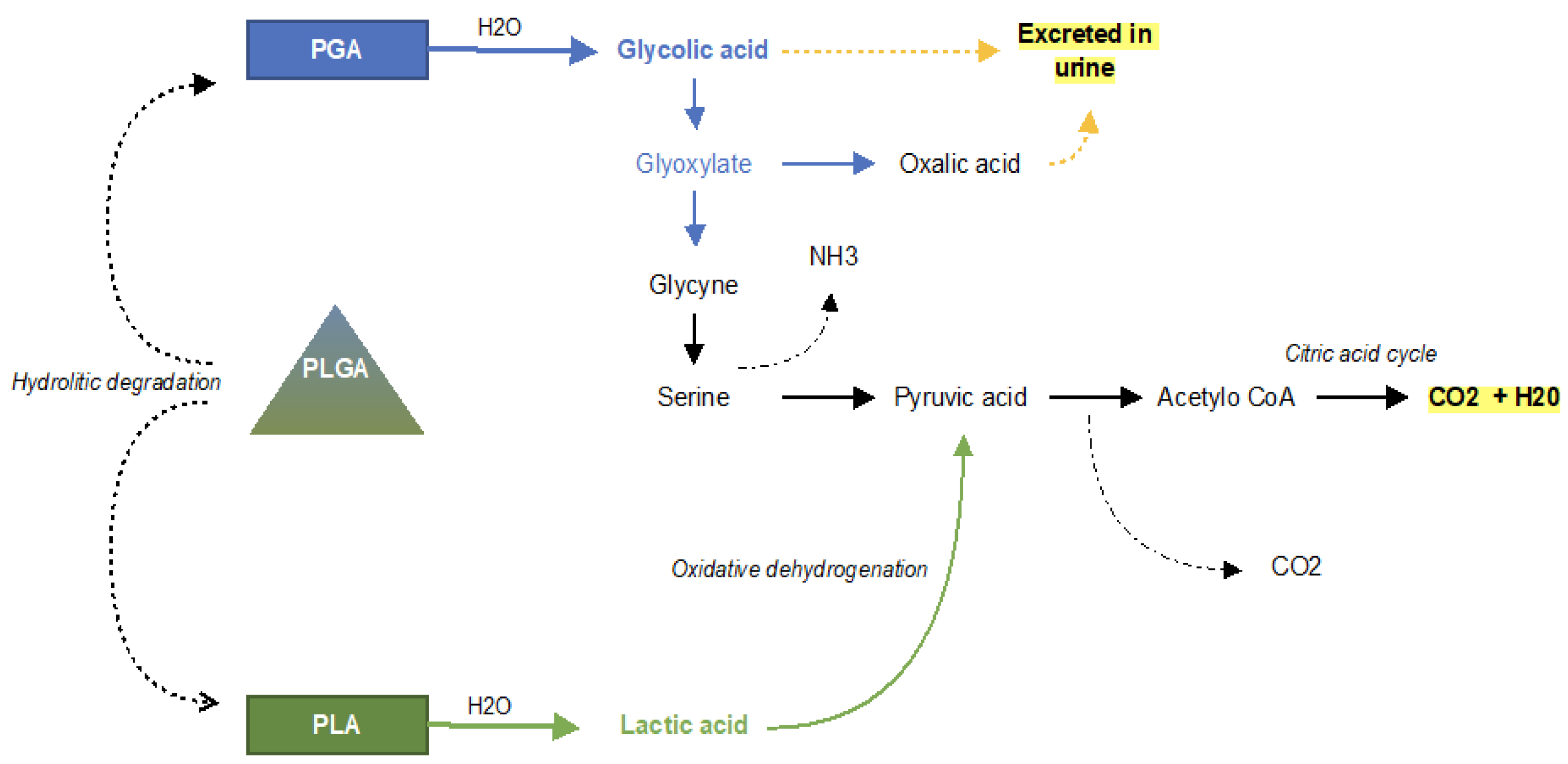
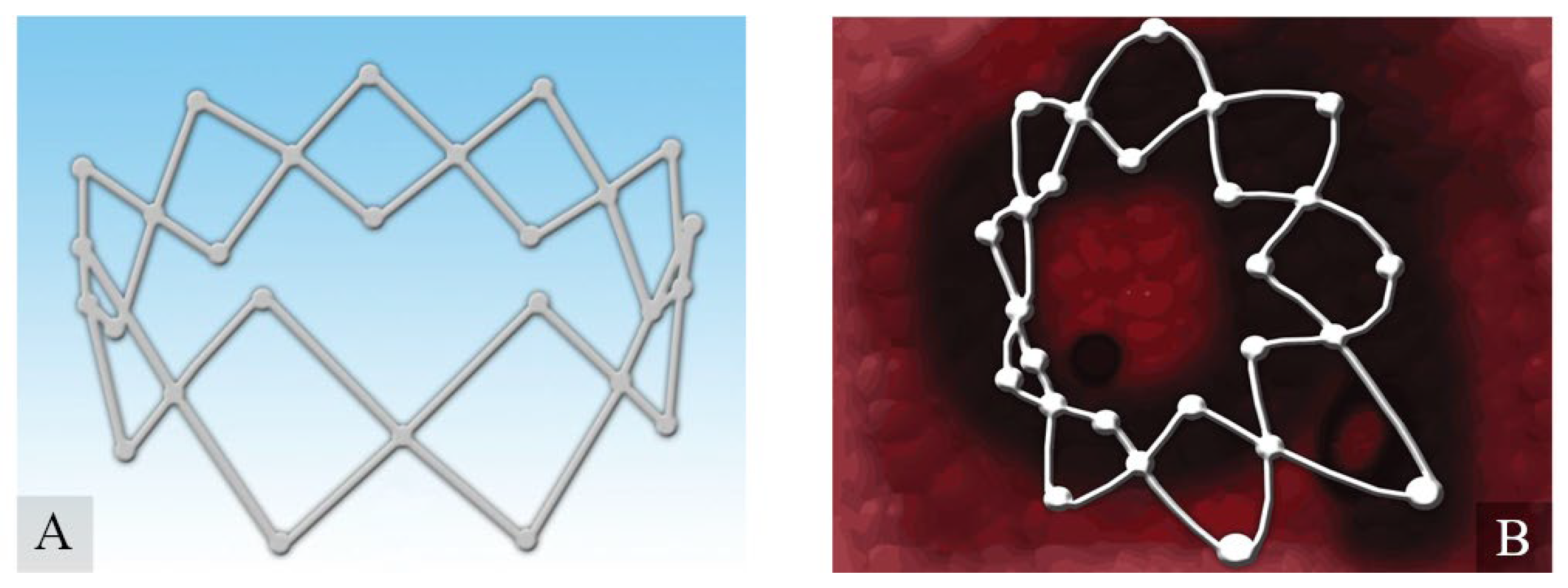

| Material | PGA Polyglycolic Acid | PLA Polylactic Acid | Copolymers of PGA, PLLA, PDLA | uHA/PLLA Composites of Unsintered Hydroxyapatite and Poly-L-Lactide | |
|---|---|---|---|---|---|
| PLLA Poly-L-Lactide | PDLA Poly-D-Lactide | ||||
| 1st Generation | 2nd Generation | 3rd Generation | |||
| Application | High molecular weight; highly crystalline; rapidly degradable; radiotransparency; first bioresorbable polymer clinically used. | High molecular weight due to its crystallinity and hydrophobicity; resistant to hydrolysis; radiotransparency. | High molecular weight; lower crystallinity; less resistant to hydrolysis; highly biocompatible compared to PLLA radiotransparency. | Their properties can be controlled by varying the ratio of glycolide to lactide for different compositions. Radiotransparency. | Contains 30–40% weight fractions of raw hydroxyapatite, neither calcined nor sintered material. Osteoconductive capacity (can be complete replacement by bone tissue); radiopacity. |
| Early loss of mechanical strength after 4–7 weeks, clearance time is 6–12 months [79] | Total resorption is over 3.5 years in vivo, in vitro about 2 years [79] | - | Resorption time of 12–18 months. In general, a higher glycolide content leads to a faster rate of degradation. | The PLLA matrix is completely absent from the composites after 4 years and almost all u-HA particles are replaced after 5.5 years [77] | |
| Pure PGA, due to its durability, which is insufficient to allow for complete bone healing, has rather minimal usefulness in maxillofacial surgery [68]. Biofix® SR-PGA (self-reinforced PGA). | GrandFix® FixSorb-MX® | There is no study using pure PDLA for osteofixation in the maxillofacial surgery. | SonicWeld Rx® (PDDLA 100%) LactoSorb® (PLLA (82%) + PGA (18%)) RapidSorb® (PLLA 85% + PGA 15%) Delta® PLLA (85%) + PGA (10%) + PDLA (5%) PolyMax® (PLLA70% + PLDLA (30%) | Osteotrans MX® (plate: PLLA 60 wt% + u-HA 40 wt%; screw: PLLA (70 wt%) + u-HA (30 wt%)) | |
| Method of Material Antimicrobial Functionalization | Application |
|---|---|
| anti-fouling | cochlear implants [127,131] |
| anti-adhesive | dental resin and polydi-methylsiloxane elastomer (PDMS) [128] |
| surface–charge modification | titanium micro-screws [129] |
| coating with antibiotics | bone implants [132], tympanostomy tubes [41], paranasal sinus stents [133], |
| coating with antimicrobial agent such as nanoparticles | nasal mucosa splints [134], inner ear implants [135], middle ear implants [54], bone tissue scaffolds [86,90,91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spałek, J.; Ociepa, P.; Deptuła, P.; Piktel, E.; Daniluk, T.; Król, G.; Góźdź, S.; Bucki, R.; Okła, S. Biocompatible Materials in Otorhinolaryngology and Their Antibacterial Properties. Int. J. Mol. Sci. 2022, 23, 2575. https://doi.org/10.3390/ijms23052575
Spałek J, Ociepa P, Deptuła P, Piktel E, Daniluk T, Król G, Góźdź S, Bucki R, Okła S. Biocompatible Materials in Otorhinolaryngology and Their Antibacterial Properties. International Journal of Molecular Sciences. 2022; 23(5):2575. https://doi.org/10.3390/ijms23052575
Chicago/Turabian StyleSpałek, Jakub, Przemysław Ociepa, Piotr Deptuła, Ewelina Piktel, Tamara Daniluk, Grzegorz Król, Stanisław Góźdź, Robert Bucki, and Sławomir Okła. 2022. "Biocompatible Materials in Otorhinolaryngology and Their Antibacterial Properties" International Journal of Molecular Sciences 23, no. 5: 2575. https://doi.org/10.3390/ijms23052575







