Distinct Firing Activities of the Hypothalamic Arcuate Nucleus Neurons to Appetite Hormones
Abstract
:1. Introduction
2. Results
2.1. Three-Dimensional Distribution of AgRP, POMC, and TH-Positive Neurons in the Hypothalamic Arc
2.2. Distinct Electrophysiological Properties of Each Arc Neuron
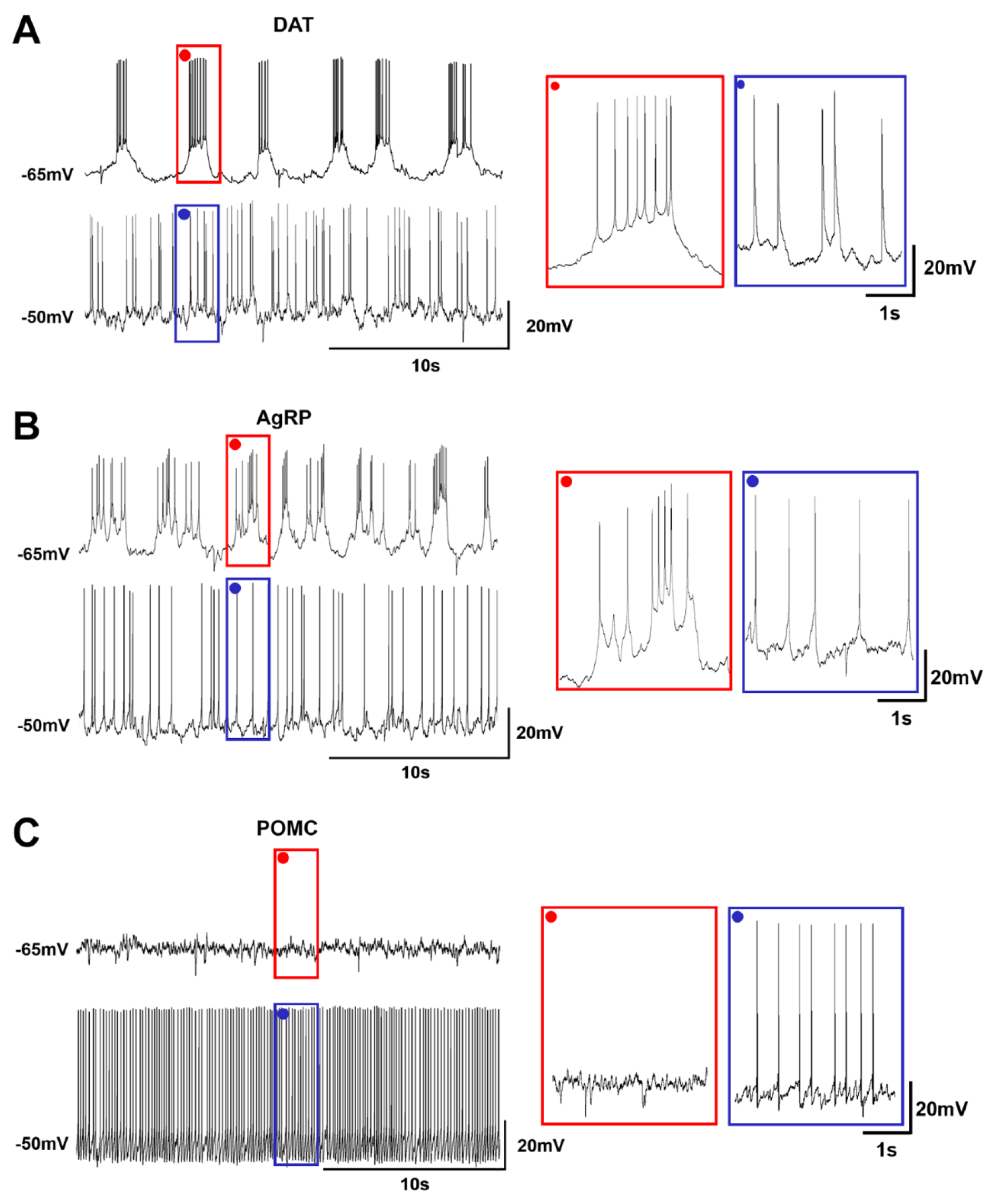
2.3. Heterogeneous Firing Patterns of Arc Neurons Depending on the RMP
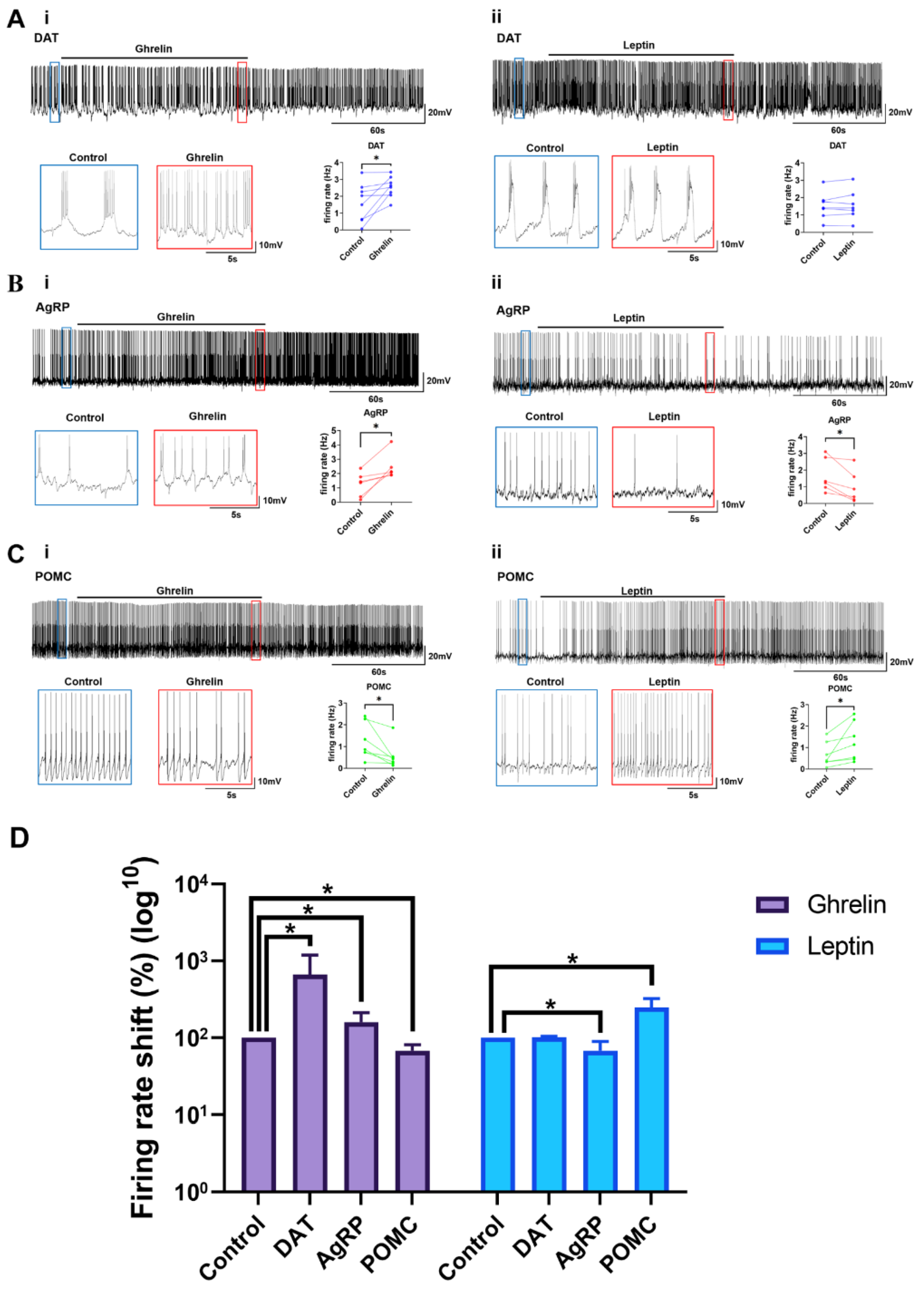
2.4. Ghrelin and Leptin Reactivity of the Arc Neurons
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.2. Sample Preparation of Hypothalamic Region for Deep Tissue Immunostaining
4.3. Slice Preparation
4.4. Patch-Clamp Recording
4.5. Pharmacology Application
4.6. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saper, C.B.; Lowell, B.B. The hypothalamus. Curr. Biol. 2014, 24, R1111–R1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Top, M.; Lee, K.; Whyment, A.D.; Blanks, A.M.; Spanswick, D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat. Neurosci. 2004, 7, 493–494. [Google Scholar] [CrossRef] [PubMed]
- Millington, G.W. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr. Metab. 2007, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olofsson, L.E.; Pierce, A.A.; Xu, A.W. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15932–15937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Gao, Y.; Yao, T.; Huang, Y.; He, Z.; Kong, X.; Yu, K.J.; Wang, R.T.; Guo, H.; Yan, J.; et al. Adiponectin potentiates the acute effects of leptin in arcuate Pomc neurons. Mol. Metab. 2016, 5, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschop, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Baver, S.B.; Hope, K.; Guyot, S.; Bjorbaek, C.; Kaczorowski, C.; O’Connell, K.M. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 2014, 34, 5486–5496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, H.; Daikoku, S. Functional topography of the rat hypothalamic dopamine neuron systems: Retrograde tracing and immunohistochemical study. J. Comp. Neurol. 1987, 265, 242–253. [Google Scholar] [CrossRef]
- Zhang, X.; van den Pol, A.N. Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat. Neurosci. 2016, 19, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Könner, A.C.; Janoschek, R.; Plum, L.; Jordan, S.D.; Rother, E.; Ma, X.; Xu, C.; Enriori, P.; Hampel, B.; Barsh, G.S.; et al. Insulin Action in AgRP-Expressing Neurons Is Required for Suppression of Hepatic Glucose Production. Cell Metab. 2007, 5, 438–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.Y.; Gao, Y.; Alhadeff, A.L.; Castorena, C.M.; Huang, Y.R.; Lieu, L.; Afrin, S.; Sun, J.; Betley, J.N.; Guo, H.B.; et al. Cellular and synaptic reorganization of arcuate NPY/AgRP and POMC neurons after exercise. Mol. Metab. 2018, 18, 107–119. [Google Scholar] [CrossRef]
- Kim, J.D.; Leyva, S.; Diano, S. Hormonal regulation of the hypothalamic melanocortin system. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef]
- Deem, J.D.; Faber, C.L.; Morton, G.J. AgRP neurons: Regulators of feeding, energy expenditure, and behavior. FEBS J. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Isokawa, M. Membrane time constant as a tool to assess cell degeneration. Brain Res. Protoc. 1997, 1, 114–116. [Google Scholar] [CrossRef]
- Tschuluun, N.; Hall, W.M.; Mulloney, B. State-changes in the swimmeret system: A neural circuit that drives locomotion. J. Exp. Biol. 2009, 212, 3605–3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Torrelo, J.; Torres, B.; Carrascal, L. Modulation of the input-output function by GABAA receptor-mediated currents in rat oculomotor nucleus motoneurons. J. Physiol. 2014, 592, 5047–5064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rau, A.R.; Hentges, S.T. GABAergic Inputs to POMC Neurons Originating from the Dorsomedial Hypothalamus Are Regulated by Energy State. J. Neurosci. 2019, 39, 6449–6459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rau, A.R.; Hentges, S.T. The Relevance of AgRP Neuron-Derived GABA Inputs to POMC Neurons Differs for Spontaneous and Evoked Release. J. Neurosci. 2017, 37, 7362–7372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, A.; Takeuchi, N. A study of the action of picrotoxin on the inhibitory neuromuscular junction of the crayfish. J. Physiol. 1969, 205, 377–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cope, D.W. GABAA Receptor-Mediated Tonic Inhibition in Thalamic Neurons. J. Neurosci. 2005, 25, 11553–11563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Zhang, J.J.; Hu, G.Y.; Wu, C.P. Different mechanisms underlying the repolarization of narrow and wide action potentials in pyramidal cells and interneurons of cat motor cortex. Neuroscience 1996, 73, 57–68. [Google Scholar] [CrossRef]
- Kasten, M.R.; Rudy, B.; Anderson, M.P. Differential regulation of action potential firing in adult murine thalamocortical neurons by Kv3.2, Kv1, and SK potassium and N-type calcium channels. J. Physiol. 2007, 584, 565–582. [Google Scholar] [CrossRef]
- Payeur, A.; Guerguiev, J.; Zenke, F.; Richards, B.A.; Naud, R. Burst-dependent synaptic plasticity can coordinate learning in hierarchical circuits. Nat. Neurosci. 2021, 24, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Izhikevich, E.M. Neural Excitability, Spiking and Bursting. Int. J. Bifurc. Chaos 2000, 10, 1171–1266. [Google Scholar] [CrossRef] [Green Version]
- Belykh, I.; de Lange, E.; Hasler, M. Synchronization of Bursting Neurons: What Matters in the Network Topology. Phys. Rev. Lett. 2005, 94, 188101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bright, D.P.; Aller, M.I.; Brickley, S.G. Synaptic Release Generates a Tonic GABAA Receptor-Mediated Conductance That Modulates Burst Precision in Thalamic Relay Neurons. J. Neurosci. 2007, 27, 2560–2569. [Google Scholar] [CrossRef] [Green Version]
- Edelmann, E.; Cepeda-Prado, E.; Franck, M.; Lichtenecker, P.; Brigadski, T.; Leßmann, V. Theta Burst Firing Recruits BDNF Release and Signaling in Postsynaptic CA1 Neurons in Spike-Timing-Dependent LTP. Neuron 2015, 86, 1041–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huguenard, J.; Prince, D. A novel T-type current underlies prolonged Ca(2+)-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J. Neurosci. 1992, 12, 3804–3817. [Google Scholar] [CrossRef] [PubMed]
- Chergui, K.; Akaoka, H.; Charléty, P.J.; Saunier, C.F.; Buda, M.; Chouvet, G. Subthalamic nucleus modulates burst firing of nigral dopamine neurones via NMDA receptors. Neuroreport 1994, 5, 1185–1188. [Google Scholar] [CrossRef]
- Liu, J.; McDaid, L.; Araque, A.; Wade, J.; Harkin, J.; Karim, S.; Henshall, D.C.; Connolly, N.M.C.; Johnson, A.P.; Tyrrell, A.M.; et al. GABA Regulation of Burst Firing in Hippocampal Astrocyte Neural Circuit: A Biophysical Model. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hommel, J.D.; Trinko, R.; Sears, R.M.; Georgescu, D.; Liu, Z.W.; Gao, X.B.; Thurmon, J.J.; Marinelli, M.; DiLeone, R.J. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006, 51, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]

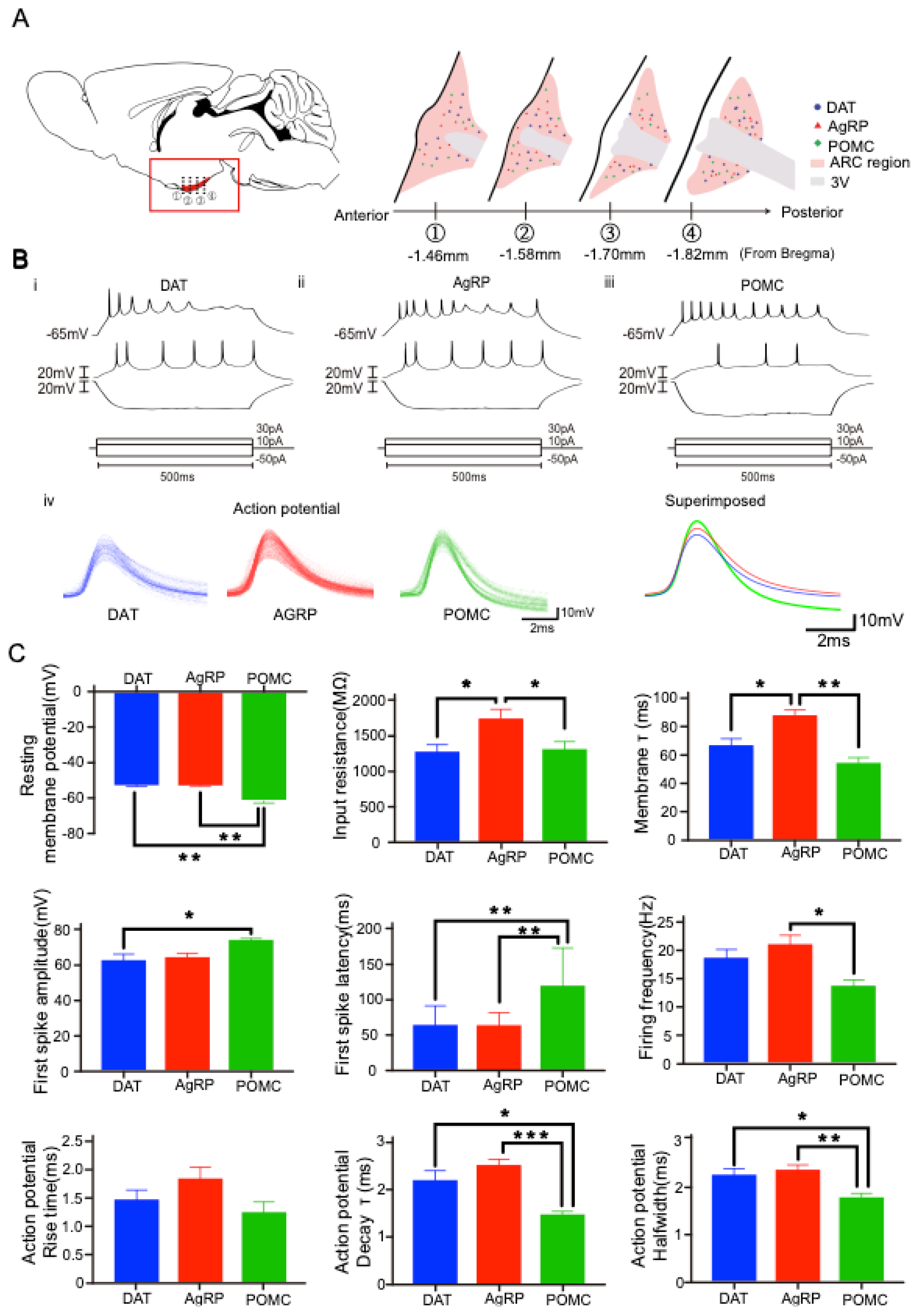
| Intrinsic Properties | DAT | AgRP | POMC |
|---|---|---|---|
| RMP (mV) | −52.24 ± 4.18 + | −52.38 ± 3.80 # | −60.54 ± 7.06 *,+ |
| Input resistance (MΩ) | 1286.88 ± 352.32 * | 1751.73 ± 584.75 *,# | 1321.86 ± 418.18 # |
| Membrane τ (ms) | 66.06 ± 22.45 * | 87.22 ± 22.03 *,# | 54.06 ± 18.43 # |
| First spike amplitude (mV) | 62.38 ± 16.68 + | 64.44 ± 12.16 | 73.48 ± 7.78+ |
| First spike latency (ms) | 81.80 ± 54.24 * | 60.12 ± 18.36 # | 119.74 ± 53.73 *,# |
| Firing Frequency (Hz) | 17.53 ± 7.03 | 19.56 ± 8.64 # | 13.57 ± 5.15 # |
| Rising time (ms) | 1.46 ± 0.59 | 1.83 ± 0.76 | 1.23 ± 0.60 |
| Halfwidth (ms) | 2.27 ± 0.42 + | 2.37 ± 0.38 # | 1.80 ± 0.32 #,+ |
| Decay τ (ms) | 2.21 ± 0.69 + | 2.53 ± 0.50 # | 1.47 ± 0.35 #,+ |
| Firing pattern | burst | irregular burst | tonic |
| Firing mode | regular | adapting | adapting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, J.; Park, B.S.; Jang, D.; Kim, D.; Tu, T.H.; Ryu, Y.; Ha, C.M.; Koch, M.; Yang, S.; Kim, J.G.; et al. Distinct Firing Activities of the Hypothalamic Arcuate Nucleus Neurons to Appetite Hormones. Int. J. Mol. Sci. 2022, 23, 2609. https://doi.org/10.3390/ijms23052609
Na J, Park BS, Jang D, Kim D, Tu TH, Ryu Y, Ha CM, Koch M, Yang S, Kim JG, et al. Distinct Firing Activities of the Hypothalamic Arcuate Nucleus Neurons to Appetite Hormones. International Journal of Molecular Sciences. 2022; 23(5):2609. https://doi.org/10.3390/ijms23052609
Chicago/Turabian StyleNa, Junewoo, Byong Seo Park, Doohyeong Jang, Donggue Kim, Thai Hien Tu, Youngjae Ryu, Chang Man Ha, Marco Koch, Sungchil Yang, Jae Geun Kim, and et al. 2022. "Distinct Firing Activities of the Hypothalamic Arcuate Nucleus Neurons to Appetite Hormones" International Journal of Molecular Sciences 23, no. 5: 2609. https://doi.org/10.3390/ijms23052609






