Key Role of Astrocytes in Postnatal Brain and Retinal Angiogenesis
Abstract
1. Introduction
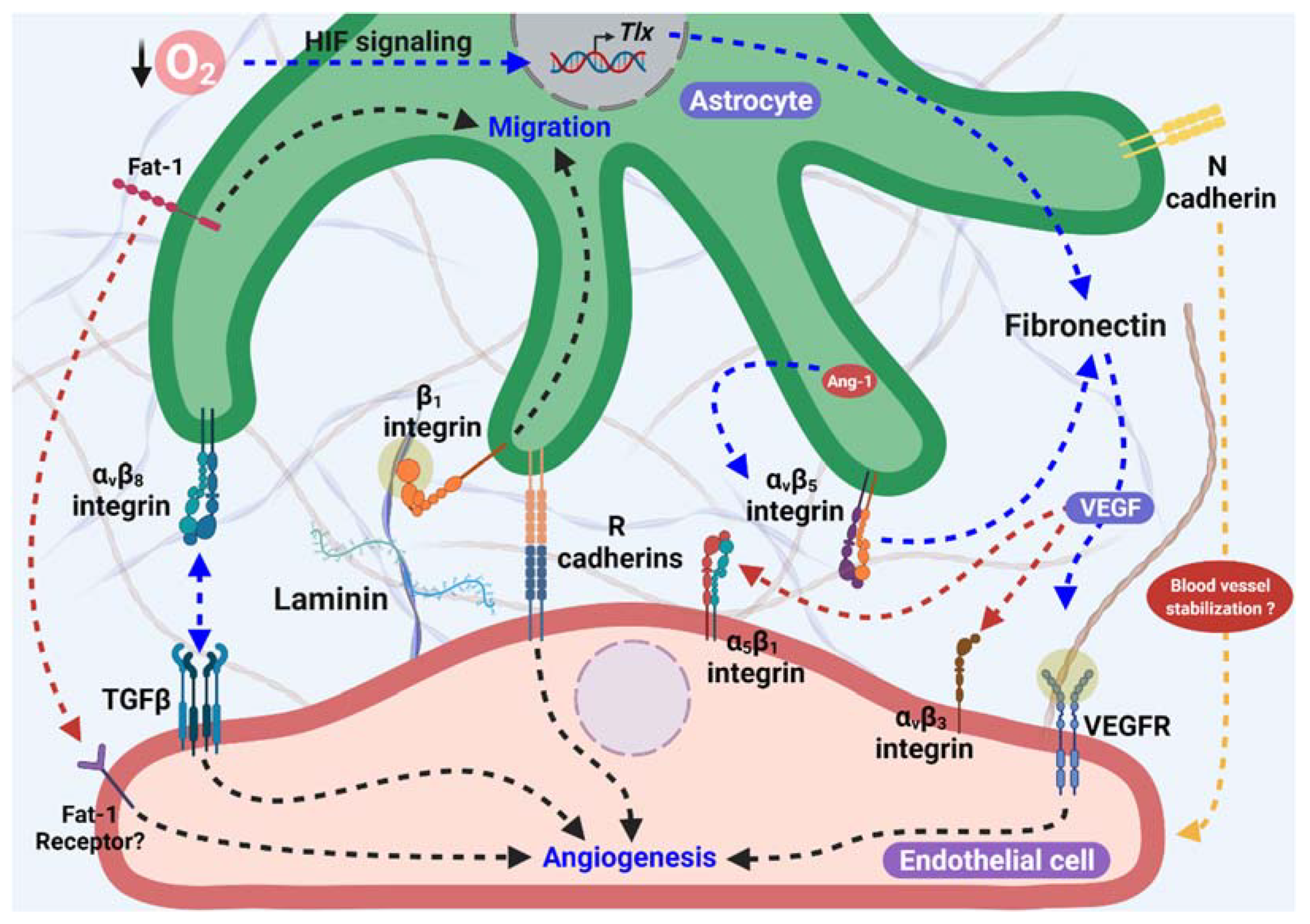
2. Astrocytes as Templates for Angiogenesis
2.1. Cadherins: A Possible Role as an Angiogenic Cue
2.2. Fibronectin and Integrins in Scaffold Formation
2.3. Laminins as a Template for Angiogenesis
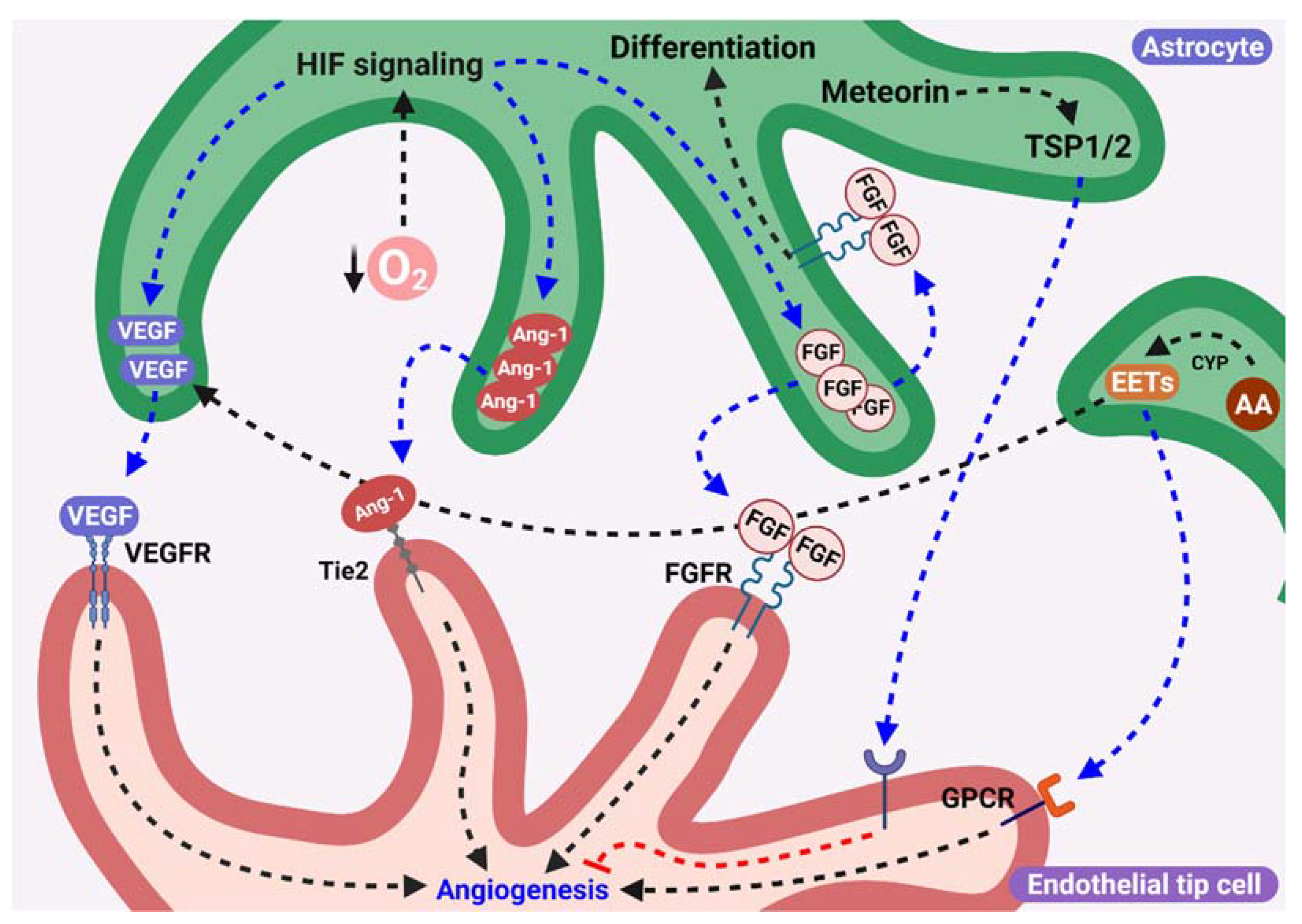
3. Pro- or Antiangiogenic Factors Released by Astrocytes
3.1. Release of VEGF from Aastrocytes as a Proangiogenic Stimulus
3.2. Role of FGF-2 from Astrocytes during Angiogenesis
3.3. Participation of Angiopoietins Secreted by Astrocytes in Angiogenesis
3.4. Astrocytic Epoxyeicosatrienoic Acids Are Proangiogenic Factors
3.5. Possible Role of Meteorin in Vascular Consolidation
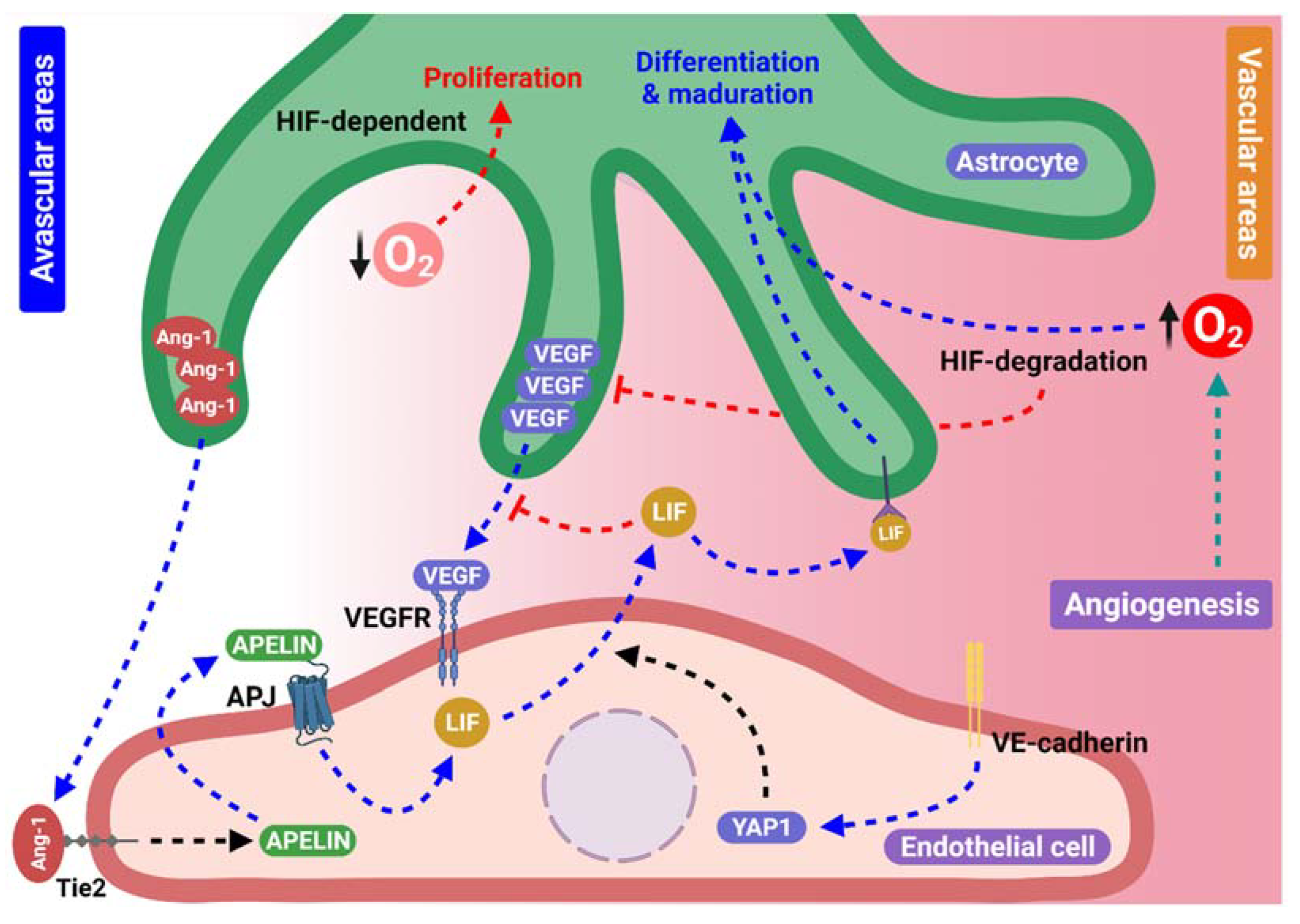
4. Feedback between Astrocytes and Blood Vessels
4.1. Participation of the Apelin/APJ System in Astrocyte Network Formation in an Endothelial-Dependent Manner
4.2. Endothelial Yes-Associated Protein 1 and Astrocyte Scaffolding Formation
4.3. Oxygen Levels and Hypoxia-Inducible Factors Provide Essential Cues for Astrocyte Behavior during Angiogenesis
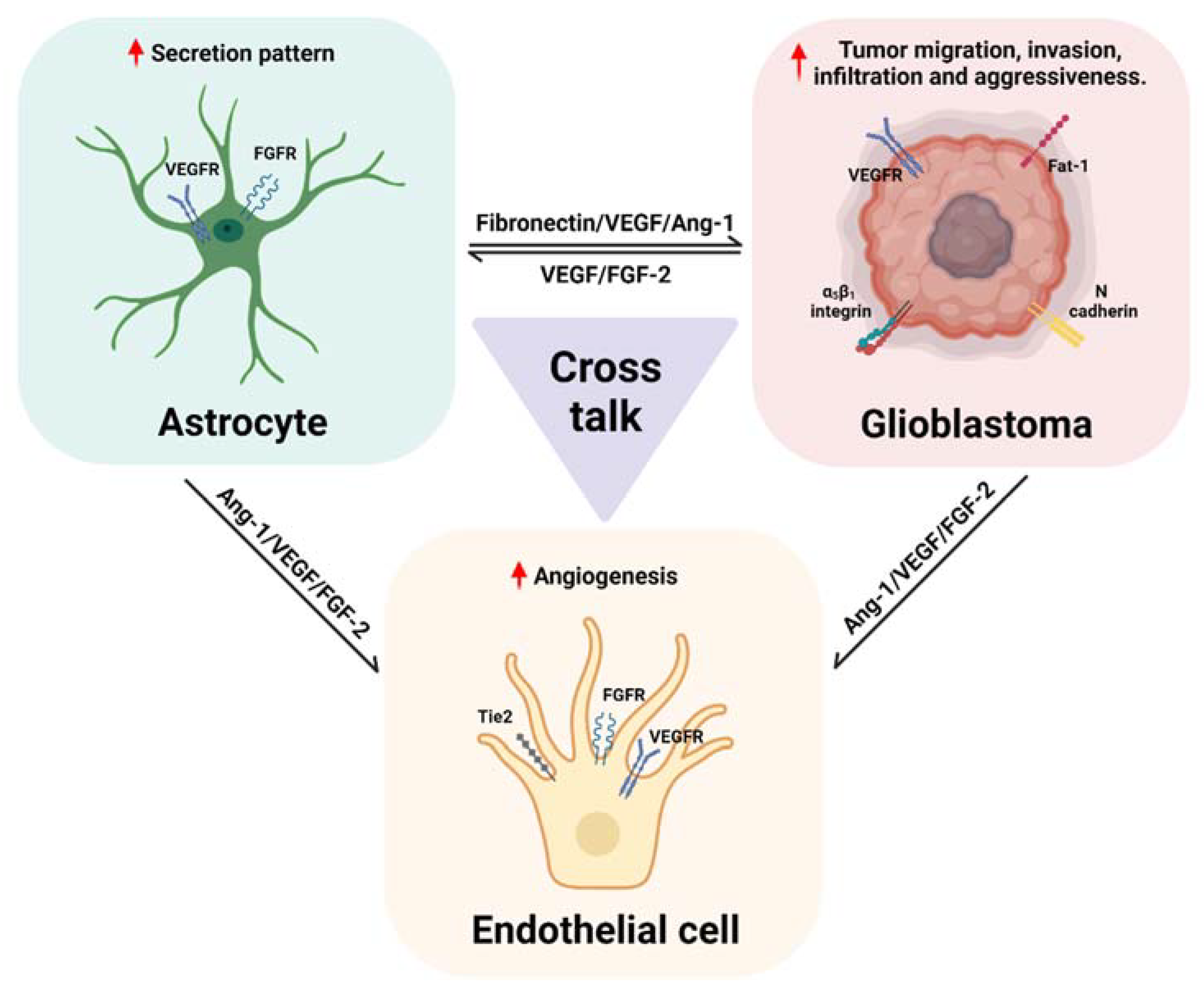
5. A Possible Role of Astrocytes in Tumoral Angiogenesis and Signaling Pathways Involved in Glioblastoma
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haydon, P.G.; Parpura, V. (Eds.) Astrocytes in (Patho) Physiology of the Nervous System; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-79491-4. [Google Scholar]
- Zerlin, M.; Goldman, J.E. Interactions between glial progenitors and blood vessels during early postnatal corticogenesis: Blood vessel contact represents an early stage of astrocyte differentiation. J. Comp. Neurol. 1997, 387, 537–546. [Google Scholar] [CrossRef]
- Mi, H.; Haeberle, H.; Barres, B.A. Induction of astrocyte differentiation by endothelial cells. J. Neurosci. 2001, 21, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Kwon, H.J.; Huang, Z. A Functional Requirement for Astroglia in Promoting Blood Vessel Development in the Early Postnatal Brain. PLoS ONE 2012, 7, e48001. [Google Scholar] [CrossRef] [PubMed]
- Gariano, R.F.; Gardner, T.W. Retinal angiogenesis in development and disease. Nature 2005, 438, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Fruttiger, M. Development of the retinal vasculature. Angiogenesis 2007, 10, 77–88. [Google Scholar] [CrossRef]
- Lee, H.S.; Han, J.; Bai, H.-J.; Kim, K.-W. Brain angiogenesis in developmental and pathological processes: Regulation, molecular and cellular communication at the neurovascular interface. FEBS J. 2009, 276, 4622–4635. [Google Scholar] [CrossRef]
- Biswas, S.; Cottarelli, A.; Agalliu, D. Neuronal and glial regulation of CNS angiogenesis and barriergenesis. Development 2020, 147, dev182279. [Google Scholar] [CrossRef]
- Reemst, K.; Noctor, S.C.; Lucassen, P.J.; Hol, E.M. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front. Hum. Neurosci. 2016, 10, 566. [Google Scholar] [CrossRef]
- Akdemir, E.S.; Huang, A.Y.-S.; Deneen, B. Astrocytogenesis: Where, when, and how. F1000Research 2020, 9, 233. [Google Scholar] [CrossRef]
- Kubota, Y.; Suda, T. Feedback Mechanism between Blood Vessels and Astrocytes in Retinal Vascular development. Trends Cardiovasc. Med. 2009, 19, 38–43. [Google Scholar] [CrossRef]
- Hirota, S.; Liu, Q.; Lee, H.S.; Hossain, M.G.; Lacy-Hulbert, A.; McCarty, J.H. The astrocyte-expressed integrin αvβ8 governs blood vessel sprouting in the developing retina. Development 2011, 138, 5157–5166. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Zhang, X. Development of astrocytes in the vertebrate eye. Dev. Dyn. 2014, 243, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.L.; Puñal, V.M.; Kerstein, P.C.; Brzezinski, J.A.; Glaser, T.; Wright, K.M.; Kay, J.N. Astrocytes follow ganglion cell axons to establish an angiogenic template during retinal development. Glia 2017, 65, 1697–1716. [Google Scholar] [CrossRef] [PubMed]
- Phng, L.-K.; Stanchi, F.; Gerhardt, H. Filopodia are dispensable for endothelial tip cell guidance. Development 2013, 140, 4031–4040. [Google Scholar] [CrossRef] [PubMed]
- Maître, J.-L.; Heisenberg, C.-P. Three Functions of Cadherins in Cell Adhesion. Curr. Biol. 2013, 23, R626–R633. [Google Scholar] [CrossRef] [PubMed]
- Halbleib, J.M.; Nelson, W.J. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006, 20, 3199–3214. [Google Scholar] [CrossRef]
- Schnädelbach, O.; Blaschuk, O.W.; Symonds, M.; Gour, B.J.; Doherty, P.; Fawcett, J.W. N-Cadherin Influences Migration of Oligodendrocytes on Astrocyte Monolayers. Mol. Cell. Neurosci. 2000, 15, 288–302. [Google Scholar] [CrossRef]
- Sabatini, P.J.B.; Zhang, M.; Silverman-Gavrila, R.; Bendeck, M.P.; Langille, B.L. Homotypic and Endothelial Cell Adhesions via N-Cadherin Determine Polarity and Regulate Migration of Vascular Smooth Muscle Cells. Circ. Res. 2008, 103, 405–412. [Google Scholar] [CrossRef]
- Inuzuka, H.; Miyatani, S.; Takeichi, M. R-cadherin: A novel Ca2+-dependent cell-cell adhesion molecule expressed in the retina. Neuron 1991, 7, 69–79. [Google Scholar] [CrossRef]
- Redies, C.; Takeichi, M. N- and R-cadherin expression in the optic nerve of the chicken embryo. Glia 1993, 8, 161–171. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Aguilar, E.; Friedlander, M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3500–3510. [Google Scholar]
- Ahmed, A.F.; de Bock, C.E.; Sontag, E.; Hondermarck, H.; Lincz, L.F.; Thorne, R.F. FAT1 cadherin controls neuritogenesis during NTera2 cell differentiation. Biochem. Biophys. Res. Commun. 2019, 514, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Takeichi, M. Mammalian Fat1 cadherin regulates actin dynamics and cell–cell contact. J. Cell Biol. 2004, 165, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Helmbacher, F. Astrocyte-intrinsic and -extrinsic Fat1 activities regulate astrocyte development and angiogenesis in the retina. Development 2022, 149, dev192047. [Google Scholar] [CrossRef]
- Caruso, N.; Herberth, B.; Bartoli, M.; Puppo, F.; Dumonceaux, J.; Zimmermann, A.; Denadai, S.; Lebossé, M.; Roche, S.; Geng, L.; et al. Deregulation of the Protocadherin Gene FAT1 Alters Muscle Shapes: Implications for the Pathogenesis of Facioscapulohumeral Dystrophy. PLoS Genet. 2013, 9, e1003550. [Google Scholar] [CrossRef] [PubMed]
- Danen, E.H.J.; Yamada, K.M. Fibronectin, integrins, and growth control. J. Cell. Physiol. 2001, 189, 1–13. [Google Scholar] [CrossRef]
- Stenzel, D.; Lundkvist, A.; Sauvaget, D.; Busse, M.; Graupera, M.; van der Flier, A.; Wijelath, E.S.; Murray, J.; Sobel, M.; Costell, M.; et al. Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis. Development 2011, 138, 4451–4463. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, L. Chapter 2 PI3K/PTEN Signaling in Angiogenesis and Tumorigenesis. Adv. Cancer Res. 2009, 102, 19–65. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Zhang, W.; Ito, Y.; Berlin, E.; Roberts, R.; Berkowitz, B.A. Role of Hypoxia during Normal Retinal Vessel Development and in Experimental Retinopathy of Prematurity. Investig. Opthalmol. Vis. Sci. 2003, 44, 3119. [Google Scholar] [CrossRef] [PubMed]
- Caprara, C.; Grimm, C. From oxygen to erythropoietin: Relevance of hypoxia for retinal development, health and disease. Prog. Retin. Eye Res. 2012, 31, 89–119. [Google Scholar] [CrossRef] [PubMed]
- Uemura, A. Tlx acts as a proangiogenic switch by regulating extracellular assembly of fibronectin matrices in retinal astrocytes. J. Clin. Investig. 2006, 116, 369–377. [Google Scholar] [CrossRef]
- Wang, T.; Xiong, J.-Q. The Orphan Nuclear Receptor TLX/NR2E1 in Neural Stem Cells and Diseases. Neurosci. Bull. 2016, 32, 108–114. [Google Scholar] [CrossRef][Green Version]
- Sun, G.; Cui, Q.; Shi, Y. Nuclear Receptor TLX in Development and Diseases. Curr. Top. Dev. Biol. 2017, 125, 257–273. [Google Scholar] [PubMed]
- Griffett, K.; Bedia-Diaz, G.; Hegazy, L.; de Vera, I.M.S.; Wanninayake, U.S.; Billon, C.; Koelblen, T.; Wilhelm, M.L.; Burris, T.P. The Orphan Nuclear Receptor TLX Is a Receptor for Synthetic and Natural Retinoids. Cell Chem. Biol. 2020, 27, 1272–1284.e4. [Google Scholar] [CrossRef] [PubMed]
- Brindle, N.P.J.; Saharinen, P.; Alitalo, K. Signaling and Functions of Angiopoietin-1 in Vascular Protection. Circ. Res. 2006, 98, 1014–1023. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.E.; Choi, D.-K.; Jang, J.Y.; Jung, J.-J.; Kiyonari, H.; Shioi, G.; Chang, W.; Suda, T.; Mochizuki, N.; et al. Angiopoietin-1 Guides Directional Angiogenesis through Integrin αvβ5 Signaling for Recovery of Ischemic Retinopathy. Sci. Transl. Med. 2013, 5, 203ra127. [Google Scholar] [CrossRef]
- Wang, J.; Milner, R. Fibronectin promotes brain capillary endothelial cell survival and proliferation through alpha5beta1 and alphavbeta3 integrins via MAP kinase signalling. J. Neurochem. 2006, 96, 148–159. [Google Scholar] [CrossRef]
- Li, L.; Liu, F.; Welser-Alves, J.V.; McCullough, L.D.; Milner, R. Upregulation of fibronectin and the α5β1 and αvβ3 integrins on blood vessels within the cerebral ischemic penumbra. Exp. Neurol. 2012, 233, 283–291. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, B.; Wang, F.; Huang, H.; Milner, R.; Li, L. The temporal expression patterns of fibronectin and its receptors-α5β1 and αvβ3 integrins on blood vessels after cerebral ischemia. Restor. Neurol. Neurosci. 2015, 33, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Gline, S.; Mu, D.; Collins, R.; Araya, J.; Dolganov, G.; Einheber, S.; Boudreau, N.; Nishimura, S.L. Integrin αvβ8-Mediated Activation of Transforming Growth Factor-β by Perivascular Astrocytes. Am. J. Pathol. 2005, 166, 1883–1894. [Google Scholar] [CrossRef]
- Gnanaguru, G.; Bachay, G.; Biswas, S.; Pinzon-Duarte, G.; Hunter, D.D.; Brunken, W.J. Laminins containing the 2 and 3 chains regulate astrocyte migration and angiogenesis in the retina. Development 2013, 140, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Bachay, G.; Chu, J.; Hunter, D.D.; Brunken, W.J. Laminin-Dependent Interaction between Astrocytes and Microglia. Am. J. Pathol. 2017, 187, 2112–2127. [Google Scholar] [CrossRef] [PubMed]
- Belkin, A.M.; Stepp, M.A. Integrins as receptors for laminins. Microsc. Res. Tech. 2000, 51, 280–301. [Google Scholar] [CrossRef]
- Tao, C.; Zhang, X. Retinal Proteoglycans Act as Cellular Receptors for Basement Membrane Assembly to Control Astrocyte Migration and Angiogenesis. Cell Rep. 2016, 17, 1832–1844. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Katayama, T.; Prat, A. Glial influence on the blood brain barrier. Glia 2013, 61, 1939–1958. [Google Scholar] [CrossRef]
- Paredes, I.; Himmels, P.; Ruiz de Almodóvar, C. Neurovascular Communication during CNS Development. Dev. Cell 2018, 45, 10–32. [Google Scholar] [CrossRef]
- Bozoyan, L.; Khlghatyan, J.; Saghatelyan, A. Astrocytes Control the Development of the Migration-Promoting Vasculature Scaffold in the Postnatal Brain via VEGF Signaling. J. Neurosci. 2012, 32, 1687–1704. [Google Scholar] [CrossRef]
- Chow, J.; Ogunshola, O.; Fan, S.-Y.; Li, Y.; Ment, L.R.; Madri, J.A. Astrocyte-derived VEGF mediates survival and tube stabilization of hypoxic brain microvascular endothelial cells in vitro. Dev. Brain Res. 2001, 130, 123–132. [Google Scholar] [CrossRef]
- Stone, J.; Itin, A.; Alon, T.; Pe’er, J.; Gnessin, H.; Chan-Ling, T.; Keshet, E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 1995, 15, 4738–4747. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Powner, M.B.; Gandhi, P.; Clarkin, C.; Gutmann, D.H.; Johnson, R.S.; Ferrara, N.; Fruttiger, M. Astrocyte-Derived Vascular Endothelial Growth Factor Stabilizes Vessels in the Developing Retinal Vasculature. PLoS ONE 2010, 5, e11863. [Google Scholar] [CrossRef]
- Weidemann, A.; Krohne, T.U.; Aguilar, E.; Kurihara, T.; Takeda, N.; Dorrell, M.I.; Simon, M.C.; Haase, V.H.; Friedlander, M.; Johnson, R.S. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia 2010, 58, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Rattner, A.; Williams, J.; Nathans, J. Roles of HIFs and VEGF in angiogenesis in the retina and brain. J. Clin. Investig. 2019, 129, 3807–3820. [Google Scholar] [CrossRef]
- Duan, L.-J.; Takeda, K.; Fong, G.-H. Hypoxia Inducible Factor-2α Regulates the Development of Retinal Astrocytic Network by Maintaining Adequate Supply of Astrocyte Progenitors. PLoS ONE 2014, 9, e84736. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, REVIEWS3005. [Google Scholar] [CrossRef]
- Terwisscha van Scheltinga, A.F.; Bakker, S.C.; Kahn, R.S.; Kas, M.J.H. Fibroblast Growth Factors in Neurodevelopment and Psychopathology. Neuroscientist 2013, 19, 479–494. [Google Scholar] [CrossRef]
- Reuss, B.; Dono, R.; Unsicker, K. Functions of Fibroblast Growth Factor (FGF)-2 and FGF-5 in Astroglial Differentiation and Blood-Brain Barrier Permeability: Evidence from Mouse Mutants. J. Neurosci. 2003, 23, 6404–6412. [Google Scholar] [CrossRef]
- Chadashvili, T.; Peterson, D.A. Cytoarchitecture of fibroblast growth factor receptor 2 (FGFR-2) immunoreactivity in astrocytes of neurogenic and non-neurogenic regions of the young adult and aged rat brain. J. Comp. Neurol. 2006, 498, 1–15. [Google Scholar] [CrossRef]
- Savchenko, E.; Teku, G.N.; Boza-Serrano, A.; Russ, K.; Berns, M.; Deierborg, T.; Lamas, N.J.; Wichterle, H.; Rothstein, J.; Henderson, C.E.; et al. FGF family members differentially regulate maturation and proliferation of stem cell-derived astrocytes. Sci. Rep. 2019, 9, 9610. [Google Scholar] [CrossRef] [PubMed]
- Oh, L.Y.S.; Denninger, A.; Colvin, J.S.; Vyas, A.; Tole, S.; Ornitz, D.M.; Bansal, R. Fibroblast Growth Factor Receptor 3 Signaling Regulates the Onset of Oligodendrocyte Terminal Differentiation. J. Neurosci. 2003, 23, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Li, J.-J.; Masuda, S.; Qi, Z.; Yamamoto, H.; Takeshita, A. Adenovirus-Mediated Expression of the Secreted Form of Basic Fibroblast Growth Factor (FGF-2) Induces Cellular Proliferation and Angiogenesis In Vivo. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Poole, T.J.; Finkelstein, E.B.; Cox, C.M. The role of FGF and VEGF in angioblast induction and migration during vascular development. Dev. Dyn. 2001, 220, 1–17. [Google Scholar] [CrossRef]
- Bahramsoltani, M.; De Spiegelaere, W.; Janczyk, P.; Hiebl, B.; Cornillie, P.; Plendl, J. Quantitation of angiogenesis in vitro induced by VEGF-A and FGF-2 in two different human endothelial cultures—An all-in-one assay. Clin. Hemorheol. Microcirc. 2010, 46, 189–202. [Google Scholar] [CrossRef]
- Sun, H.-J.; Cai, W.-W.; Gong, L.-L.; Wang, X.; Zhu, X.-X.; Wan, M.-Y.; Wang, P.-Y.; Qiu, L.-Y. FGF-2-mediated FGFR1 signaling in human microvascular endothelial cells is activated by vaccarin to promote angiogenesis. Biomed. Pharmacother. 2017, 95, 144–152. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. VEGF and FGF-2: Promising targets for the treatment of respiratory disorders. Respir. Med. 2019, 156, 33–46. [Google Scholar] [CrossRef]
- Bendfeldt, K.; Radojevic, V.; Kapfhammer, J.; Nitsch, C. Basic Fibroblast Growth Factor Modulates Density of Blood Vessels and Preserves Tight Junctions in Organotypic Cortical Cultures of Mice: A New In Vitro Model of the Blood-Brain Barrier. J. Neurosci. 2007, 27, 3260–3267. [Google Scholar] [CrossRef]
- Klint, P.; Kanda, S.; Kloog, Y.; Claesson-Welsh, L. Contribution of Src and Ras pathways in FGF-2 induced endothelial cell differentiation. Oncogene 1999, 18, 3354–3364. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F.; Lee, J.W.-K.; Cotman, C.W. Distribution of basic fibroblast growth factor in the developing rat brain. Neuroscience 1994, 61, 911–923. [Google Scholar] [CrossRef]
- Proia, P.; Schiera, G.; Mineo, M.; Ingrassia, A.; Santoro, G.; Savettieri, G.; Di Liegro, I. Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int. J. Mol. Med. 2008, 21, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Ganat, Y.; Soni, S.; Chacon, M.; Schwartz, M.; Vaccarino, F. Chronic hypoxia up-regulates fibroblast growth factor ligands in the perinatal brain and induces fibroblast growth factor-responsive radial glial cells in the sub-ependymal zone. Neuroscience 2002, 112, 977–991. [Google Scholar] [CrossRef]
- Dong, Z.; Santeford, A.; Ban, N.; Lee, T.J.; Smith, C.; Ornitz, D.M.; Apte, R.S. FGF2-induced STAT3 activation regulates pathologic neovascularization. Exp. Eye Res. 2019, 187, 107775. [Google Scholar] [CrossRef]
- Zubilewicz, A.; Hecquet, C.; Jeanny, J.-C.; Soubrane, G.; Courtois, Y.; Mascarelli, F. Two distinct signalling pathways are involved in FGF2-stimulated proliferation of choriocapillary endothelial cells: A comparative study with VEGF. Oncogene 2001, 20, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, M.I.; Aguilar, E.; Jacobson, R.; Trauger, S.A.; Friedlander, J.; Siuzdak, G.; Friedlander, M. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia 2010, 58, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Reiss, Y. Angiopoietins. In Angiogenesis Inhibition; Recent Results in Cancer Research; Liersch, R., Berdel, W.E., Kessler, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 180, pp. 97–102. ISBN 978-3-540-78280-3. [Google Scholar]
- Sato, T.N.; Tozawa, Y.; Deutsch, U.; Wolburg-Buchholz, K.; Fujiwara, Y.; Gendron-Maguire, M.; Gridley, T.; Wolburg, H.; Risau, W.; Qin, Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 1995, 376, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Koblizek, T.I.; Runttng, A.S.; Stacker, S.A.; Wilks, A.F.; Risau, W.; Deutsch, U. Tie2 Receptor Expression and Phosphorylation in Cultured Cells and Mouse Tissues. Eur. J. Biochem. 1997, 244, 774–779. [Google Scholar] [CrossRef]
- Wong, A.L.; Haroon, Z.A.; Werner, S.; Dewhirst, M.W.; Greenberg, C.S.; Peters, K.G. Tie2 Expression and Phosphorylation in Angiogenic and Quiescent Adult Tissues. Circ. Res. 1997, 81, 567–574. [Google Scholar] [CrossRef]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef]
- Acker, T.; Beck, H.; Plate, K.H. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech. Dev. 2001, 108, 45–57. [Google Scholar] [CrossRef]
- Beck, H.; Acker, T.; Wiessner, C.; Allegrini, P.R.; Plate, K.H. Expression of Angiopoietin-1, Angiopoietin-2, and Tie Receptors after Middle Cerebral Artery Occlusion in the Rat. Am. J. Pathol. 2000, 157, 1473–1483. [Google Scholar] [CrossRef]
- Cho, S.-R.; Suh, H.; Yu, J.; Kim, H.; Seo, J.; Seo, C. Astroglial Activation by an Enriched Environment after Transplantation of Mesenchymal Stem Cells Enhances Angiogenesis after Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 2016, 17, 1550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, Y.; Wang, Y.; Mao, L.; Gao, Y.; He, Q.; Huang, M.; Chen, S.; Hu, B. Sonic Hedgehog (Shh) Regulates the Expression of Angiogenic Growth Factors in Oxygen–Glucose-Deprived Astrocytes by Mediating the Nuclear Receptor NR2F2. Mol. Neurobiol. 2013, 47, 967–975. [Google Scholar] [CrossRef]
- He, Q.-W.; Xia, Y.-P.; Chen, S.-C.; Wang, Y.; Huang, M.; Huang, Y.; Li, J.-Y.; Li, Y.-N.; Gao, Y.; Mao, L.; et al. Astrocyte-Derived Sonic Hedgehog Contributes to Angiogenesis in Brain Microvascular Endothelial Cells via RhoA/ROCK Pathway After Oxygen–Glucose Deprivation. Mol. Neurobiol. 2013, 47, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog Pathway Promotes Blood-Brain Barrier Integrity and CNS Immune Quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.A.; Fu, M.; Garcia, A.D.R. Sonic hedgehog signaling in astrocytes. Cell. Mol. Life Sci. 2021, 78, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Zacharek, A.; Chen, J.; Li, A.; Cui, X.; Li, Y.; Roberts, C.; Feng, Y.; Gao, Q.; Chopp, M. Angiopoietin1/TIE2 and VEGF/FLK1 Induced by MSC Treatment Amplifies Angiogenesis and Vascular Stabilization after Stroke. J. Cereb. Blood Flow Metab. 2007, 27, 1684–1691. [Google Scholar] [CrossRef]
- Alkayed, N.J.; Narayanan, J.; Gebremedhin, D.; Medhora, M.; Roman, R.J.; Harder, D.R. Molecular Characterization of an Arachidonic Acid Epoxygenase in Rat Brain Astrocytes. Stroke 1996, 27, 971–979. [Google Scholar] [CrossRef]
- Spector, A.A.; Fang, X.; Snyder, G.D.; Weintraub, N.L. Epoxyeicosatrienoic acids (EETs): Metabolism and biochemical function. Prog. Lipid Res. 2004, 43, 55–90. [Google Scholar] [CrossRef]
- Fleming, I. Epoxyeicosatrienoic acids, cell signaling and angiogenesis. Prostaglandins Other Lipid Mediat. 2007, 82, 60–67. [Google Scholar] [CrossRef]
- Tuor, U.I.; Kurpita, G.; Simone, C. Correlation of local changes in cerebral blood flow, capillary density, and cytochrome oxidase during development. J. Comp. Neurol. 1994, 342, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Munzenmaier, D.H.; Harder, D.R. Cerebral microvascular endothelial cell tube formation: Role of astrocytic epoxyeicosatrienoic acid release. Am. J. Physiol. Circ. Physiol. 2000, 278, H1163–H1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Harder, D.R. Cerebral Capillary Endothelial Cell Mitogenesis and Morphogenesis Induced by Astrocytic Epoxyeicosatrienoic Acid. Stroke 2002, 33, 2957–2964. [Google Scholar] [CrossRef]
- Harder, D.R.; Zhang, C.; Gebremedhin, D. Astrocytes Function in Matching Blood Flow to Metabolic Activity. Physiology 2002, 17, 27–31. [Google Scholar] [CrossRef]
- Capozzi, M.E.; McCollum, G.W.; Penn, J.S. The Role of Cytochrome P450 Epoxygenases in Retinal Angiogenesis. Investig. Opthalmol. Vis. Sci. 2014, 55, 4253. [Google Scholar] [CrossRef] [PubMed]
- Nishino, J.; Yamashita, K.; Hashiguchi, H.; Fujii, H.; Shimazaki, T.; Hamada, H. Meteorin: A secreted protein that regulates glial cell differentiation and promotes axonal extension. EMBO J. 2004, 23, 1998–2008. [Google Scholar] [CrossRef]
- Lee, H.S.; Han, J.; Lee, S.-H.; Park, J.A.; Kim, K.-W. Meteorin promotes the formation of GFAP-positive glia via activation of the Jak-STAT3 pathway. J. Cell Sci. 2010, 123, 1959–1968. [Google Scholar] [CrossRef]
- Park, J.A.; Lee, H.S.; Ko, K.J.; Park, S.Y.; Kim, J.H.; Choe, G.; Kweon, H.-S.; Song, H.S.; Ahn, J.-C.; Yu, Y.S.; et al. Meteorin regulates angiogenesis at the gliovascular interface. Glia 2008, 56, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J. The functions of thrombospondin-1 and-2. Curr. Opin. Cell Biol. 2000, 12, 634–640. [Google Scholar] [CrossRef]
- Lawler, P.R.; Lawler, J. Molecular Basis for the Regulation of Angiogenesis by Thrombospondin-1 and -2. Cold Spring Harb. Perspect. Med. 2012, 2, a006627. [Google Scholar] [CrossRef]
- Sakimoto, S.; Kidoya, H.; Naito, H.; Kamei, M.; Sakaguchi, H.; Goda, N.; Fukamizu, A.; Nishida, K.; Takakura, N. A role for endothelial cells in promoting the maturation of astrocytes through the apelin/APJ system in mice. Development 2012, 139, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Koblar, S.A.; Turnley, A.M.; Classon, B.J.; Reid, K.L.; Ware, C.B.; Cheema, S.S.; Murphy, M.; Bartlett, P.F. Neural precursor differentiation into astrocytes requires signaling through the leukemia inhibitory factor receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 3178–3181. [Google Scholar] [CrossRef] [PubMed]
- Asano, H.; Aonuma, M.; Sanosaka, T.; Kohyama, J.; Namihira, M.; Nakashima, K. Astrocyte Differentiation of Neural Precursor Cells is Enhanced by Retinoic Acid Through a Change in Epigenetic Modification. Stem Cells 2009, 27, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Kidoya, H.; Ueno, M.; Yamada, Y.; Mochizuki, N.; Nakata, M.; Yano, T.; Fujii, R.; Takakura, N. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008, 27, 522–534. [Google Scholar] [CrossRef]
- Sakabe, M.; Fan, J.; Odaka, Y.; Liu, N.; Hassan, A.; Duan, X.; Stump, P.; Byerly, L.; Donaldson, M.; Hao, J.; et al. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc. Natl. Acad. Sci. USA 2017, 114, 10918–10923. [Google Scholar] [CrossRef] [PubMed]
- Hooglugt, A.; van der Stoel, M.M.; Boon, R.A.; Huveneers, S. Endothelial YAP/TAZ Signaling in Angiogenesis and Tumor Vasculature. Front. Oncol. 2021, 10, 3162. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Kwon, Y.-G. Roles of YAP in mediating endothelial cell junctional stability and vascular remodeling. BMB Rep. 2015, 48, 429–430. [Google Scholar] [CrossRef]
- Choi, H.-J.; Zhang, H.; Park, H.; Choi, K.-S.; Lee, H.-W.; Agrawal, V.; Kim, Y.-M.; Kwon, Y.-G. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat. Commun. 2015, 6, 6943. [Google Scholar] [CrossRef]
- Ai, L.-Q.-Y.; Zhu, J.-Y.; Chen, X.; Li, X.; Luo, L.-L.; Hu, Q.-M.; Lin, S.; Ye, J. Endothelial Yes-Associated Protein 1 Promotes Astrocyte Proliferation and Maturation via Cytoplasmic Leukemia Inhibitory Factor Secretion in Oxygen-Induced Retinopathy. Investig. Opthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef]
- Nakamura-Ishizu, A.; Kurihara, T.; Okuno, Y.; Ozawa, Y.; Kishi, K.; Goda, N.; Tsubota, K.; Okano, H.; Suda, T.; Kubota, Y. The formation of an angiogenic astrocyte template is regulated by the neuroretina in a HIF-1-dependent manner. Dev. Biol. 2012, 363, 106–114. [Google Scholar] [CrossRef]
- West, H. Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development 2005, 132, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Brunclik, N.; Bürgi-Taboada, C.; Antoniou, X.; Gassmann, M.; Ogunshola, O.O. Astrocyte responses to injury: VEGF simultaneously modulates cell death and proliferation. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R864–R873. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.-J.; Pan, S.J.; Sato, T.N.; Fong, G.-H. Retinal Angiogenesis Regulates Astrocytic Differentiation in Neonatal Mouse Retinas by Oxygen Dependent Mechanisms. Sci. Rep. 2017, 7, 17608. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Irvin, D.M.; McNeill, R.S.; Bash, R.E.; Miller, C.R. Intrinsic Astrocyte Heterogeneity Influences Tumor Growth in Glioma Mouse Models. Brain Pathol. 2017, 27, 36–50. [Google Scholar] [CrossRef]
- Tiwary, S.; Morales, J.E.; Kwiatkowski, S.C.; Lang, F.F.; Rao, G.; McCarty, J.H. Metastatic Brain Tumors Disrupt the Blood-Brain Barrier and Alter Lipid Metabolism by Inhibiting Expression of the Endothelial Cell Fatty Acid Transporter Mfsd2a. Sci. Rep. 2018, 8, 8267. [Google Scholar] [CrossRef]
- Richards, M.; Anderson, M.; Carter, P.; Ebert, B.L.; Mossialos, E. Focusing on brain tumours and brain metastasis. Nat. Rev. Cancer 2020, 20, 565–567. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. Genetic Pathways to Primary and Secondary Glioblastoma. Am. J. Pathol. 2007, 170, 1445–1453. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Francis, S.S.; Barnholtz-Sloan, J.S. Epidemiology of Brain and Other CNS Tumors. Curr. Neurol. Neurosci. Rep. 2021, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Cui, B.; Liu, Z.; Shen, H. Novel insights into astrocyte-mediated signaling of proliferation, invasion and tumor immune microenvironment in glioblastoma. Biomed. Pharmacother. 2020, 126, 110086. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Choi, S.; Lee, M.S.; Kurmashev, A.; Lee, H.N.; Ko, Y.; Lee, K.; Jeong, S.; Seong, J.; Kang, J.H.; et al. Retraction fibers produced by fibronectin-integrin α5β1 interaction promote motility of brain tumor cells. FASEB J. 2021, 35, e21906. [Google Scholar] [CrossRef]
- Schiffer, D.; Annovazzi, L.; Casalone, C.; Corona, C.; Mellai, M. Glioblastoma: Microenvironment and Niche Concept. Cancers 2018, 11, 5. [Google Scholar] [CrossRef]
- Biasoli, D.; Sobrinho, M.F.; da Fonseca, A.C.C.; de Matos, D.G.; Romão, L.; de Moraes Maciel, R.; Rehen, S.K.; Moura-Neto, V.; Borges, H.L.; Lima, F.R.S. Glioblastoma cells inhibit astrocytic p53-expression favoring cancer malignancy. Oncogenesis 2014, 3, e123. [Google Scholar] [CrossRef]
- Noronha, C.; Ribeiro, A.S.; Taipa, R.; Castro, D.S.; Reis, J.; Faria, C.; Paredes, J. Cadherin Expression and EMT: A Focus on Gliomas. Biomedicines 2021, 9, 1328. [Google Scholar] [CrossRef]
- Camand, E.; Peglion, F.; Osmani, N.; Sanson, M.; Etienne-Manneville, S. N-cadherin expression level modulates integrin-mediated polarity and strongly impacts on the speed and directionality of glial cell migration. J. Cell Sci. 2012, 125, 844–857. [Google Scholar] [CrossRef]
- Shinoura, N.; Paradies, N.; Warnick, R.; Chen, H.; Larson, J.; Tew, J.; Simon, M.; Lynch, R.; Kanai, Y.; Hirohashi, S.; et al. Expression of N-cadherin and α-catenin in astrocytomas and glioblastomas. Br. J. Cancer 1995, 72, 627–633. [Google Scholar] [CrossRef]
- Péglion, F.; Etienne-Manneville, S. N-cadherin expression level as a critical indicator of invasion in non-epithelial tumors. Cell Adh. Migr. 2012, 6, 327–332. [Google Scholar] [CrossRef]
- Kohutek, Z.A.; DiPierro, C.G.; Redpath, G.T.; Hussaini, I.M. ADAM-10-Mediated N-Cadherin Cleavage Is Protein Kinase C-Dependent and Promotes Glioblastoma Cell Migration. J. Neurosci. 2009, 29, 4605–4615. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Magro, G.; Cardile, V.; Coco, M.; Marzagalli, R.; Castrogiovanni, P.; Imbesi, R.; Graziano, A.C.E.; Barone, F.; Di Rosa, M.; et al. Characterization of matrix metalloproteinase-2 and -9, ADAM-10 and N-cadherin expression in human glioblastoma multiforme. Cell Tissue Res. 2015, 362, 45–60. [Google Scholar] [CrossRef]
- Osuka, S.; Zhu, D.; Zhang, Z.; Li, C.; Stackhouse, C.T.; Sampetrean, O.; Olson, J.J.; Gillespie, G.Y.; Saya, H.; Willey, C.D.; et al. N-cadherin upregulation mediates adaptive radioresistance in glioblastoma. J. Clin. Investig. 2021, 131, e136098. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Gong, Y.; Liang, X. Role of FAT1 in health and disease (Review). Oncol. Lett. 2021, 21, 398. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, C.; Irshad, K.; Gupta, Y.; Sarkar, C.; Suri, A.; Chattopadhyay, P.; Sinha, S.; Chosdol, K. NFκB is a critical transcriptional regulator of atypical cadherin FAT1 in glioma. BMC Cancer 2020, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, B.; Irshad, K.; Madan, E.; Aggarwal, N.; Sarkar, C.; Chandra, P.S.; Gupta, D.K.; Chattopadhyay, P.; Sinha, S.; Chosdol, K. FAT1 acts as an upstream regulator of oncogenic and inflammatory pathways, via PDCD4, in glioma cells. Oncogene 2013, 32, 3798–3808. [Google Scholar] [CrossRef]
- Madan, E.; Dikshit, B.; Gowda, S.H.; Srivastava, C.; Sarkar, C.; Chattopadhyay, P.; Sinha, S.; Chosdol, K. FAT1 is a novel upstream regulator of HIF1α and invasion of high grade glioma. Int. J. Cancer 2016, 139, 2570–2582. [Google Scholar] [CrossRef]
- Gerwins, P.; Claesson-Welsh, L. Anti-Vascular Endothelial Growth Factor-Based Angiostatics. In Encyclopedia of Cancer; Elsevier: Amsterdam, The Netherlands, 2002; pp. 135–141. [Google Scholar]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Hamerlik, P.; Lathia, J.D.; Rasmussen, R.; Wu, Q.; Bartkova, J.; Lee, M.; Moudry, P.; Bartek, J.; Fischer, W.; Lukas, J.; et al. Autocrine VEGF–VEGFR2–Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J. Exp. Med. 2012, 209, 507–520. [Google Scholar] [CrossRef]
- Xu, C.; Wu, X.; Zhu, J. VEGF Promotes Proliferation of Human Glioblastoma Multiforme Stem-Like Cells through VEGF Receptor 2. Sci. World J. 2013, 2013, 417413. [Google Scholar] [CrossRef]
- Jain, S.; Chalif, E.J.; Aghi, M.K. Interactions Between Anti-Angiogenic Therapy and Immunotherapy in Glioblastoma. Front. Oncol. 2022, 11, 5702. [Google Scholar] [CrossRef] [PubMed]
- Keunen, O.; Johansson, M.; Oudin, A.; Sanzey, M.; Rahim, S.A.A.; Fack, F.; Thorsen, F.; Taxt, T.; Bartos, M.; Jirik, R.; et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 3749–3754. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Sorensen, A.G.; di Tomaso, E.; Zhang, W.-T.; Duda, D.G.; Cohen, K.S.; Kozak, K.R.; Cahill, D.P.; Chen, P.-J.; Zhu, M.; et al. AZD2171, a Pan-VEGF Receptor Tyrosine Kinase Inhibitor, Normalizes Tumor Vasculature and Alleviates Edema in Glioblastoma Patients. Cancer Cell 2007, 11, 83–95. [Google Scholar] [CrossRef]
- Lieu, C.; Heymach, J.; Overman, M.; Tran, H.; Kopetz, S. Beyond VEGF: Inhibition of the Fibroblast Growth Factor Pathway and Antiangiogenesis. Clin. Cancer Res. 2011, 17, 6130–6139. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Pascual, A.; Mitchell, K.; Siebzehnrubl, F.A.; Lathia, J.D. FGF2: A novel druggable target for glioblastoma? Expert Opin. Ther. Targets 2020, 24, 311–318. [Google Scholar] [CrossRef]
- Oushy, S.; Hellwinkel, J.E.; Wang, M.; Nguyen, G.J.; Gunaydin, D.; Harland, T.A.; Anchordoquy, T.J.; Graner, M.W. Glioblastoma multiforme-derived extracellular vesicles drive normal astrocytes towards a tumour-enhancing phenotype. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160477. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.A.; Fukumoto, M.; Igarashi, K.; Oda, Y.; Kikuchi, H.; Hatanaka, M. Correlation of basic fibroblast growth factor expression levels with the degree of malignancy and vascularity in human gliomas. J. Neurosurg. 1992, 76, 792–798. [Google Scholar] [CrossRef]
- Bian, X.W.; Du, L.L.; Shi, J.Q.; Cheng, Y.S.; Liu, F.X. Correlation of bFGF, FGFR-1 and VEGF expression with vascularity and malignancy of human astrocytomas. Anal. Quant. Cytol. Histol. 2000, 22, 267–274. [Google Scholar]
- Toyoda, K.; Tanaka, K.; Nakagawa, S.; Thuy, D.H.D.; Ujifuku, K.; Kamada, K.; Hayashi, K.; Matsuo, T.; Nagata, I.; Niwa, M. Initial Contact of Glioblastoma Cells with Existing Normal Brain Endothelial Cells Strengthen the Barrier Function via Fibroblast Growth Factor 2 Secretion: A New In Vitro Blood–Brain Barrier Model. Cell. Mol. Neurobiol. 2013, 33, 489–501. [Google Scholar] [CrossRef]
- Audero, E.; Cascone, I.; Zanon, I.; Previtali, S.C.; Piva, R.; Schiffer, D.; Bussolino, F. Expression of Angiopoietin-1 in Human Glioblastomas Regulates Tumor-Induced Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 536–541. [Google Scholar] [CrossRef]
- Emdad, L.; Lee, S.-G.; Su, Z.Z.; Jeon, H.Y.; Boukerche, H.; Sarkar, D.; Fisher, P.B. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21300–21305. [Google Scholar] [CrossRef]
- Yoo, B.K.; Emdad, L.; Lee, S.-G.; Su, Z.; Santhekadur, P.; Chen, D.; Gredler, R.; Fisher, P.B.; Sarkar, D. Astrocyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacol. Ther. 2011, 130, 1–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puebla, M.; Tapia, P.J.; Espinoza, H. Key Role of Astrocytes in Postnatal Brain and Retinal Angiogenesis. Int. J. Mol. Sci. 2022, 23, 2646. https://doi.org/10.3390/ijms23052646
Puebla M, Tapia PJ, Espinoza H. Key Role of Astrocytes in Postnatal Brain and Retinal Angiogenesis. International Journal of Molecular Sciences. 2022; 23(5):2646. https://doi.org/10.3390/ijms23052646
Chicago/Turabian StylePuebla, Mariela, Pablo J. Tapia, and Hilda Espinoza. 2022. "Key Role of Astrocytes in Postnatal Brain and Retinal Angiogenesis" International Journal of Molecular Sciences 23, no. 5: 2646. https://doi.org/10.3390/ijms23052646
APA StylePuebla, M., Tapia, P. J., & Espinoza, H. (2022). Key Role of Astrocytes in Postnatal Brain and Retinal Angiogenesis. International Journal of Molecular Sciences, 23(5), 2646. https://doi.org/10.3390/ijms23052646





