The UV-B-Induced Transcription Factor HY5 Regulated Anthocyanin Biosynthesis in Zanthoxylum bungeanum
Abstract
:1. Introduction
2. Results
2.1. Analyses of Anthocyanin Composition and Content during the Ripening of Z. bungeanum Fruit
2.2. Analysis of Expression Levels of Anthocyanin Biosynthesis-Related Genes during the Ripening of Z. bungeanum Fruit
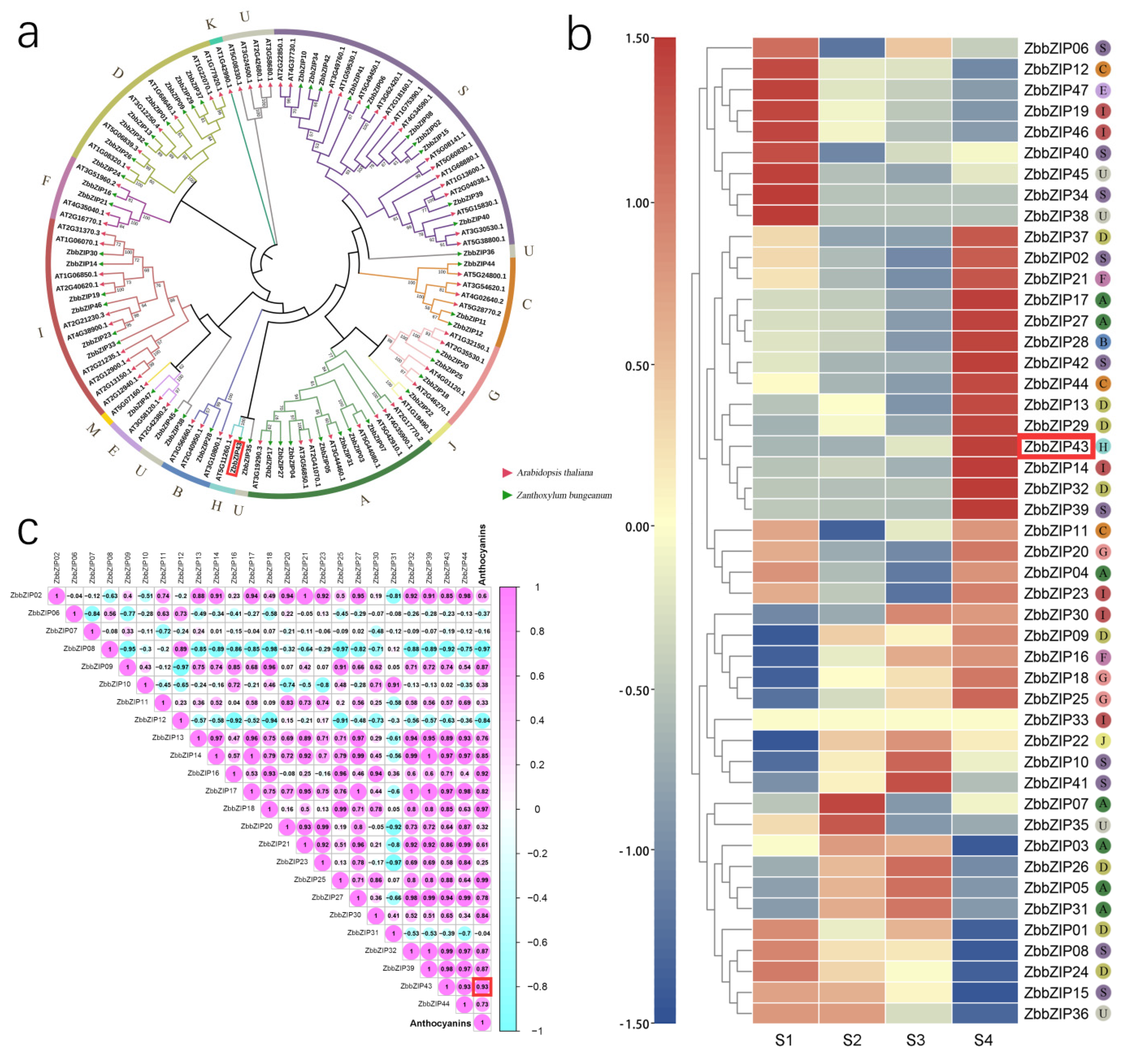
2.3. Phylogenetic Tree Analysis of HY5 and Other bZIP Family Transcription Factors in Z. bungeanum
2.4. Clone and Identification of HY5 in Z. bungeanum
2.5. Bagging Inhibited Anthocyanin Synthesis in Z. bungeanum Fruit
2.6. UV-B Irradiation Promoted Anthocyanin Synthesis in Z. bungeanum Leaves
2.7. Transient Overexpression of ZbHY5 Promoted Anthocyanin Synthesis of Z. bungeanum Leaves
2.8. ZbHY5 Can Interact with ZbMYB113 In Vivo
3. Discussion
4. Materials and Methods
4.1. De Novo Assembly and Unigene Annotation
4.2. Metabonomic Analysis and Transcriptome Analysis
4.3. The Treatment of Bagging Fruit
4.4. The Treatment of UV-B Irradiation for Leaves
4.5. Bioinformatics Analysis of ZbbZIP Transcription Factor Family
4.5.1. Excavation and Identification of ZbbZIP Transcription Factor Family Members
4.5.2. Construction of Phylogenetic Tree
4.5.3. Differential Expression Pattern Analysis of ZbbZIP Transcription Factor Family Genes
4.5.4. Correlation Analysis of Anthocyanin Content and ZbbZIP Transcription Factor Family Gene Expression
4.6. The Extraction and Measurement of Total Anthocyanins
4.7. Extraction of Total RNA and Real-Time Fluorescence Quantitative PCR (qRT-PCR) Analysis
4.8. Construction of Transient Gene Overexpression Vector
4.9. Transient Gene Overexpression Assay in Z. bungeanum Leaves
4.10. Bimolecular Fluorescence Complementation (BiFC) Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Traka, M.H.; Mithen, R.F. Plant science and human nutrition: Challenges in assessing health-promoting properties of phytochemicals. Plant Cell 2011, 23, 2483–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanin, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Song, X.H.; Yang, Q.S.; Liu, Y.; Li, J.J.; Chang, X.C.; Xian, L.H. Genome-wide identification of Pistacia R2R3-MYB gene family and function characterization of PcMYB113 during autumn leaf coloration in Pistacia chinensis. Int. J. Biol. Macromol. 2021, 192, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Zhang, A.D.; Wu, X.X.; Zhu, Z.W.; Yang, Z.F.; Zhu, Y.L. Transcriptome analysis revealed expression of genes related to anthocyanin biosynthesis in eggplant (Solanum melongena L.) under high-temperature stress. BMC Plant Biol. 2019, 19, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, A.; Brockman, A.; Aguirre, L.; Campbell, A.; Bean, A.; Cantero, A. Advances in the MYB-bHLH-WD Repeat (MBW) Pigment Regulatory Model: Addition of a WRKY Factor and Co-option of an Anthocyanin MYB for Betalain Regulation. Plant Cell Physiol. 2017, 58, 1431–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Plant Dev. 2010, 91, 29–66. [Google Scholar]
- Takos, A.M.; Jaffe, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.B.; Zawora, C.; Li, Y.; Wu, J.; Liu, L.C.; Liu, Z.C.; Cai, R.; Lian, H.L. Transcriptome sequencing reveals role of light in promoting anthocyanin accumulation of strawberry fruit. Plant Growth Regul. 2018, 86, 121–132. [Google Scholar] [CrossRef]
- Cominelli, E.; Gusmaroli, G.; Allegra, D.; Galbiati, M.; Wade, H.K.; Jenkins, G.I.; Tonelli, C. Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, P.B.; Chen, G.Q.; Wu, J.; Liu, Z.C.; Lian, H.L. FvbHLH9, functions as a positive regulator of anthocyanin biosynthesis, by forming HY5-bHLH9 transcription complex in strawberry fruit. Plant Cell Physiol. 2020, 61, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Botto, J.F. The multifaceted roles of HY5 in plant growth and development. Mol. Plant 2016, 9, 1353–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stracke, R.; Favory, J.J.; Gruber, H.; Bartelniewoehner, L.; Bartels, S.; Binkert, M. The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ. 2010, 33, 88–103. [Google Scholar]
- Favory, J.J.; Stec, A.; Gruber, H.; Rizzini, L.; Oravecz, A.; Funk, M.; Albert, A.; Cloix, C.; Jenkins, G.I.; Oakeley, E.J. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 2009, 28, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; He, H.; Wang, X.C.; Wang, X.F.; Yang, X.Z.; Li, L.; Deng, X.W. Genome-wide mapping of the HY5-mediated genenetworks in Arabidopsis that involve both transcriptional and post-transcriptional regulation. Plant J. 2011, 65, 346–358. [Google Scholar] [CrossRef]
- Shin, J.; Park, E.; Choi, G. PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 2007, 49, 981–994. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, X.D.; Zhao, Y.R.; Yang, J.; He, Y.Y.; Li, G.C. Transcription factor PyHY5 binds to the promoters of PyWD40 and PyMYB10 and regulates its expression in red pear ‘Yunhongli No. 1’. Plant Physiol. Biochem. 2020, 154, 665–674. [Google Scholar] [CrossRef]
- Wolfgang, D.; Basten, L.S.; Berend, S.; Christoph, W. The Arabidopsis bZIP transcription factor family—An update. Curr. Opin. Plant Biol. 2018, 45, 36–49. [Google Scholar]
- Zhang, Y.Q.; Zheng, S.; Liu, Z.J.; Wang, L.G.; Bi, Y.R. Both HY5 and HYH are necessary regulators for low temperature-induced anthocyanin accumulation in Arabidopsis seedlings. J. Plant Physiol. 2011, 168, 367–374. [Google Scholar] [CrossRef]
- Qiu, Z.K.; Wang, H.J.; Li, D.J.; Yu, B.W.; Hui, Q.L.; Yan, S.S.; Huang, Z.J.; Cui, X.; Cao, B.H. Identification of Candidate HY5-dependent and -independent Regulators of Anthocyanin Biosynthesis in Tomato. Plant Cell Physiol. 2019, 60, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Loyola, R.; Herrera, D.; Mas, A.; Wong, D.C.J.; Holl, J.; Cavallini, E.; Amato, A.; Azuma, A.; Ziegler, T.; Aquea, F.; et al. The photomorphogenic factors UV-B RECEPTOR 1, ELONGATED HYPOCOTYL 5, and HY5 HOMOLOGUE are part of the UV-B signalling pathway in grapevine and mediate flavonol accumulation in response to the environment. J. Exp. Bot. 2016, 67, 5429–5445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Yuan, Y.; Tang, Z.Z.; Huang, Y.; Kang, C.Y.; Deng, X.X.; Xu, Q. Retrotransposon promoter of Ruby1 controls both light-and cold-induced accumulation of anthocyanin in blood orange. Plant Cell Environ. 2019, 42, 3092–3104. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Beuning, L.; Davies, K.; Mitra, D.; Morris, B.; Kootstra, A. Expression of pigmentation genes and photo-regulation of anthocyanin biosynthesis in developing Royal Gala apple flowers. Aust J. Plant Physiol. 1998, 25, 245–252. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Ang, L.H.; Puente, P.; Deng, X.W.; Wei, N. Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 1998, 10, 673–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.S.; Li, Y.H.; Chen, L.T.; Chen, W.C.; Hsieh, W.P.; Shin, J.; Jane, W.N.; Chou, S.J.; Choi, G.; Hu, J.M.; et al. LZF1, a HY5-regulated transcriptional factor, functions in Arabidopsis de-etiolation. Plant J. 2008, 54, 205–219. [Google Scholar] [CrossRef]
- An, J.P.; Qu, F.J.; Yao, J.F.; Wang, X.N.; You, C.X.; Wang, X.F.; Hao, Y.J. The bZIP transcription factor MdHY5 regulates anthocyanin accumulation and nitrate assimilation in apple. Hortic. Res. 2017, 4, 17023. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Chi, C.; Jin, L.J.; Zhu, J.; Yu, J.Q.; Zhou, Y.H. The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant Cell Environ. 2018, 41, 1762–1775. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, Y.Q.; Song, Z.Q.; Zhang, H.Y. Repression of MYBL2 by both microRNA858a and HY5 leads to the activation of anthocyanin biosynthetic pathway in Arabidopsis. Mol. Plant 2016, 9, 1395–1405. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.H.; Chen, C.; Wang, J.; Xie, W.Y.; Wang, M.; Li, X.S.; Zhang, X.Y. Purple potato (Solanum tuberosum L.) anthocyanin attenuate alcohol-induced hepatic injury by enhancing antioxidant defense. J. Nat. Med. 2016, 70, 45–53. [Google Scholar] [CrossRef]
- Mcnellis, T.W.; Vonamim, A.G.; Araki, T.; Komeda, Y.; Miser, S.; Deng, X.W. Genetic and molecular analysis of an allelic series of cop1 mutants suggest functional roles for the multiple protein domains. Plant Cell 1994, 6, 487–500. [Google Scholar] [PubMed] [Green Version]
- Shin, D.H.; Choi, M.; Kim, K.; Bang, G.; Cho, M.; Choi, S.B.; Choi, G.; Park, Y.I. HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis. FEBS Lett. 2013, 587, 1543–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A. Gapped BLAST and PSI BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. Ital. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, R.D.; Bateman, A.; Clements, J. Pfam: The protein families database. Nucleic Acids Res. Ital. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [Green Version]
- Eddy, S.R. Profile hidden Markov models. Bioinform. Ital. 1998, 14, 755–763. [Google Scholar] [CrossRef]
- Pucker, B. Automatic identification and annotation of MYB gene family members in plants. Biorxiv 2021. [Google Scholar] [CrossRef]
- Pucker, B.; Reiher, F.; Schilbert, H.M. Automatic Identification of Players in the Flavonoid Biosynthesis with Application on the Biomedicinal Plant Croton tiglium. Plants 2020, 9, 1103. [Google Scholar] [CrossRef]
- Sun, L.W.; Yu, D.M.; Wu, Z.C.; Wang, C.; Yu, L.; Wei, A.Z. Comparative Transcriptome Analysis and Expression of Genes Reveal the Biosynthesis and Accumulation Patterns of Key Flavonoids in Different Varieties of Zanthoxylum bungeanum Leaves. J. Agric. Food Chem. 2019, 67, 13258–13268. [Google Scholar] [CrossRef]
- Zhao, B.B.; Wang, L.; Pang, S.Y.; Jia, Z.C.; Wang, L.; Li, W.X. UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Ind. Crops Prod. 2020, 151, 112483. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yan, L.Y.; Wan, L.Y.; Huai, D.X.; Kang, Y.P.; Shi, L. Genome-wide systematic characterization of bZIP transcription factors and their expression profiles during seed development and in response to salt stress in peanut. BMC Genom. 2019, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Chen, N.N.; Chen, F.; Cai, B. Genome-wide analysis and expression profile of the bZIP transcription factor gene family in grapevine (Vitis vinifera). BMC Genom. 2014, 15, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unel, N.M.; Cetin, F.; Karaca, Y.; Altunoglu, Y.C.; Baloglu, M.C. Comparative identification, characterization, and expression analysis of bZIP gene family members in watermelon and melon genomes. Plant Growth Regul. 2019, 87, 227–243. [Google Scholar] [CrossRef]
- Wang, Z.H.; Cheng, K.; Wan, L.Y.; Yan, L.Y.; Jiang, H.F.; Liu, S.Y. Genome-wide identification and analysis of the basic leucine zipper (bZIP) transcription factor gene family in Ustilaginoidea virens. Genome 2017, 60, 1051–1059. [Google Scholar]
- Liu, P.Y.; Wang, Y.L.; Meng, J.X.; Zhang, X.; Zhou, J.; Han, M.L. Transcriptome Sequencing and Expression Analysis of Genes Related to Anthocyanin Biosynthesis in Leaves of Malus ‘Profusion’ Infected by Japanese Apple Rust. Forests 2019, 10, 665. [Google Scholar] [CrossRef] [Green Version]
- Fei, X.T.; Shi, Q.Q.; Yang, T.X.; Fei, Z.X.; Wei, A.Z. Expression Stabilities of Ten Candidate Reference Genes for RT-qPCR in Zanthoxylum bungeanum Maxim. Molecules 2018, 23, 802. [Google Scholar] [CrossRef] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C-T method. Nat. Protoc. 2007, 3, 1101–1108. [Google Scholar] [CrossRef]
- Zhai, R.; Wang, Z.; Yang, C.; Wang, K.L.; Richard, E.; Liu, J.; Li, X.; Wu, Z.; Li, P.; Guan, Q.; et al. PbGA2ox8 induces vascular-related anthocyanin accumulation and contributes to red stripe formation on pear fruit. Hortic. Res. 2019, 6, 137. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Gong, J.X.; Chen, K.L.; Yao, W.K.; Zhang, B.X.; Wang, J.Y. A novel R3 MYB transcriptional repressor, MaMYBx, finely regulates anthocyanin biosynthesis in grape hyacinth. Plant Sci. 2020, 298, 110588. [Google Scholar] [CrossRef]





| Anthocyanins | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| Cyanidin-3-O-galactoside | (1.57 ± 0.02) × 107 d | (1.06 ± 0.02) × 108 c | (9.07 ± 0.29) × 107 b | (1.25 ± 0.02) × 108 a |
| Cyanidin-3-O-glucoside (Kuromanin) | (1.71 ± 0.04) × 106 d | (9.87 ± 0.4) × 106 c | (2.85 ± 0.01) × 107 b | (6.18 ± 0.22) × 107 a |
| Cyanidin-3-O-rutinoside (Keracyanin) | (6.27 ± 0.13) × 105 a | (3.96 ± 0.04) × 105 b | (3.12 ± 0.02) × 105 c | (3.33 ± 0.06) × 105 c |
| Peonidin-3-O-glucoside | (1.92 ± 0.19) × 103 a | (3.42 ± 0.54) × 103 a | (3.24 ± 1.70) × 103 a | (1.81 ± 0.34) × 103 a |
| Delphinidin-3-O-(6’’-O-acetyl) glucoside | (1.12 ± 0.03) × 106 a | (7.90 ± 0.20) × 105 b | (4.58 ± 0.19) × 105 c | (3.50 ± 0.65) × 105 c |
| Pelargonidin-3-O-rutinoside | (2.19 ± 0.04) × 105 a | (1.41 ± 0.02) × 105 b | (1.00 ± 0.03) × 105 c | (8.11 ± 0.38) × 104 d |
| Pelargonidin-3,5-O-diglucoside | (8.01 ± 0.45) × 104 a | (3.33 ± 0.02) × 104 b | (2.37 ± 0.51) × 104 c | (3.76 ± 0.43) × 104 b |
| Cyanidin-3,5-O-diglucoside (Cyanin) | (1.71 ± 0.02) × 107 d | (1.15 ± 0.01) × 108 b | (9.46 ± 0.17) × 107 c | (1.36 ± 0.03) × 108 a |
| Total anthocyanins | (3.65 ± 0.03) × 107 d | (2.32 ± 0.03) × 108 c | (2.15 ± 0.05) × 108 b | (3.23 ± 0.03) × 108 a |
| Gene Name | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| ZbHY5 | 66.33 ± 1.23 b | 153.74 ± 6.13 a | 172.40 ± 14.95 a | 189.11 ± 2.17 a |
| ZbMYB113 | 1.83 ± 0.65 c | 4.45 ± 1.21 c | 19.71 ± 0.85 b | 57.59 ± 2.73 a |
| ZbbHLH35 | 20.65 ± 1.02 b | 41.08 ± 3.93 a | 25.51 ± 1.24 b | 29.41 ± 2.65 b |
| ZbCHS | 160.66 ± 2.56 b | 147.64 ± 3.25 b | 435.95 ± 2.47 b | 746.46 ± 34.78 a |
| ZbCHI | 69.74 ± 1.84 c | 45.62 ± 2.92 d | 104.60 ± 3.88 b | 115.01 ± 2.12 a |
| ZbF3H | 465.99 ± 6.53 bc | 439.16 ± 5.81 c | 483.21 ± 12.77 b | 314.87 ± 13.38 a |
| ZbDFR | 46.28 ± 0.50 b | 10.30 ± 0.66 d | 20.39 ± 0.16 c | 52.18 ± 1.77 a |
| ZbANS | 52.82 ± 4.49 c | 41.63 ± 1.43 c | 75.15 ± 3.46 b | 151.92 ± 9.12 a |
| ZbUFGT | 36.74 ± 0.59 d | 84.75 ± 3.92 b | 103.24 ± 4.06 a | 84.03 ± 1.41 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Meng, J.; Zhang, S.; Chi, R.; Wang, C.; Wang, D.; Li, H. The UV-B-Induced Transcription Factor HY5 Regulated Anthocyanin Biosynthesis in Zanthoxylum bungeanum. Int. J. Mol. Sci. 2022, 23, 2651. https://doi.org/10.3390/ijms23052651
Zhou J, Meng J, Zhang S, Chi R, Wang C, Wang D, Li H. The UV-B-Induced Transcription Factor HY5 Regulated Anthocyanin Biosynthesis in Zanthoxylum bungeanum. International Journal of Molecular Sciences. 2022; 23(5):2651. https://doi.org/10.3390/ijms23052651
Chicago/Turabian StyleZhou, Jing, Jiaxin Meng, Shuangyu Zhang, Rufei Chi, Cheng Wang, Dongmei Wang, and Houhua Li. 2022. "The UV-B-Induced Transcription Factor HY5 Regulated Anthocyanin Biosynthesis in Zanthoxylum bungeanum" International Journal of Molecular Sciences 23, no. 5: 2651. https://doi.org/10.3390/ijms23052651
APA StyleZhou, J., Meng, J., Zhang, S., Chi, R., Wang, C., Wang, D., & Li, H. (2022). The UV-B-Induced Transcription Factor HY5 Regulated Anthocyanin Biosynthesis in Zanthoxylum bungeanum. International Journal of Molecular Sciences, 23(5), 2651. https://doi.org/10.3390/ijms23052651





