Reactive Oxygen Species Cause Exercise-Induced Angina in a Myocardial Ischaemia-Reperfusion Injury Model
Abstract
1. Introduction
2. Results
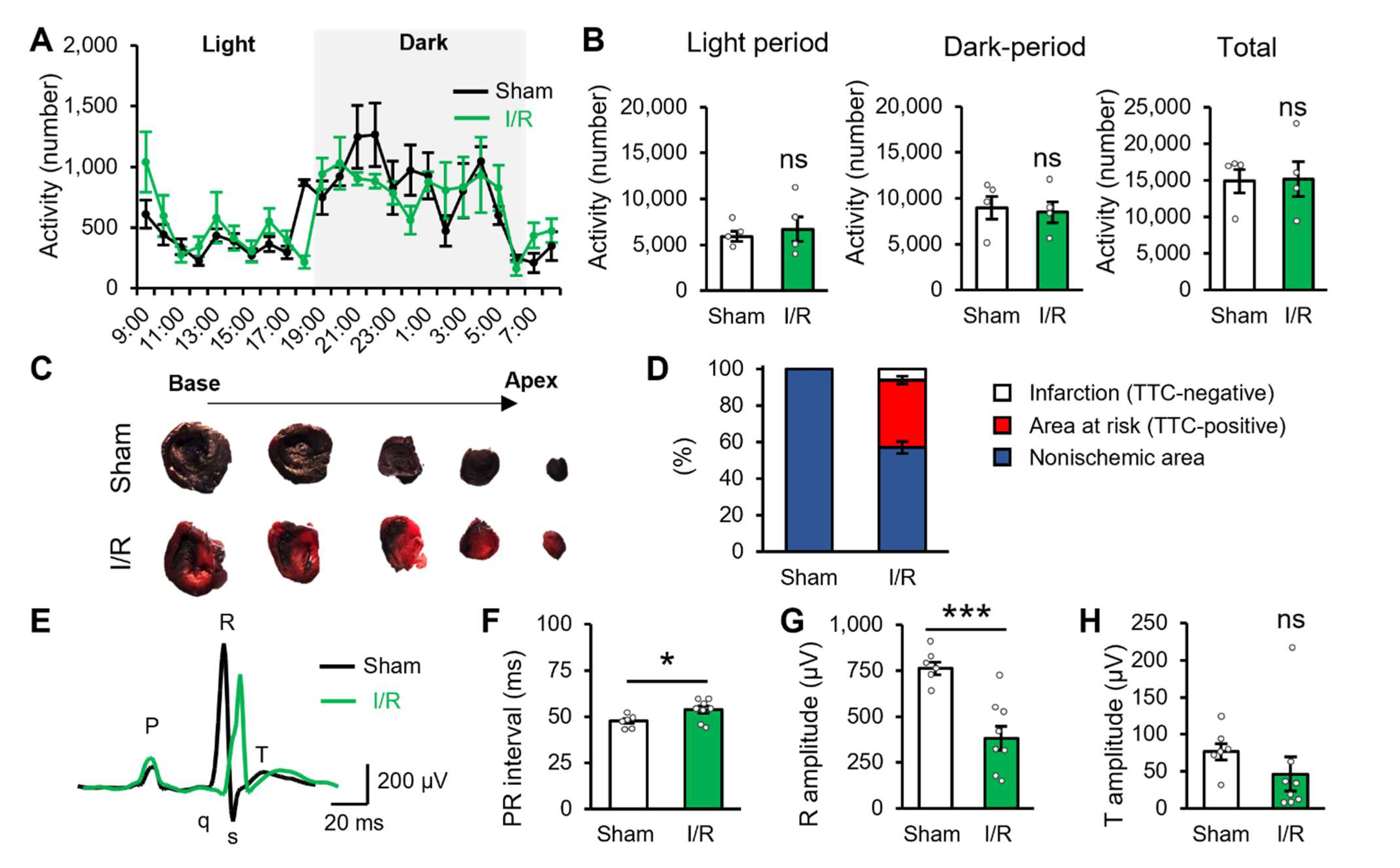
2.1. Myocardial Ischaemic/Reperfusion Produces Potential Cardiac Dysfunction
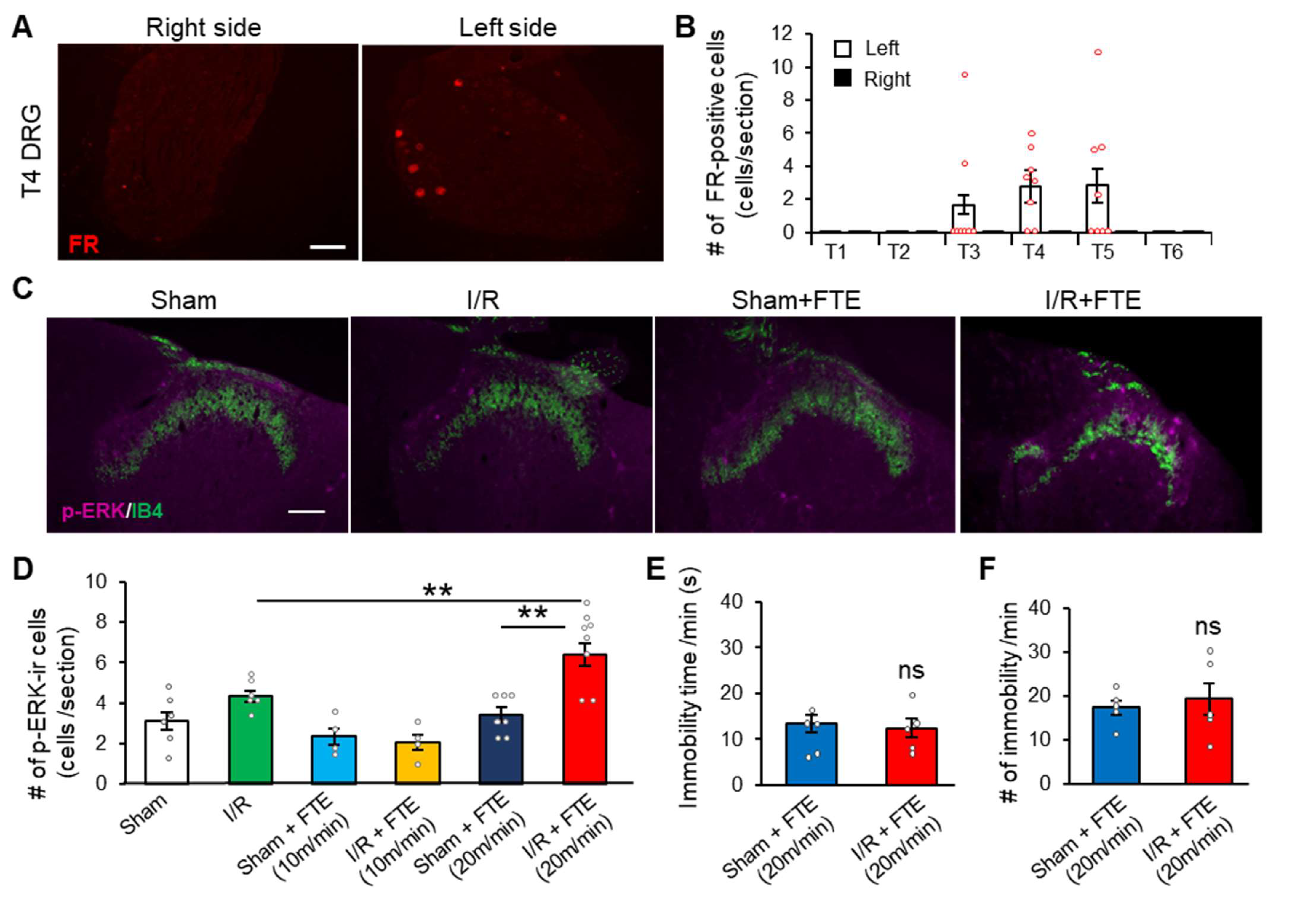
2.2. FTE Evokes Angina in I/R Model Animals
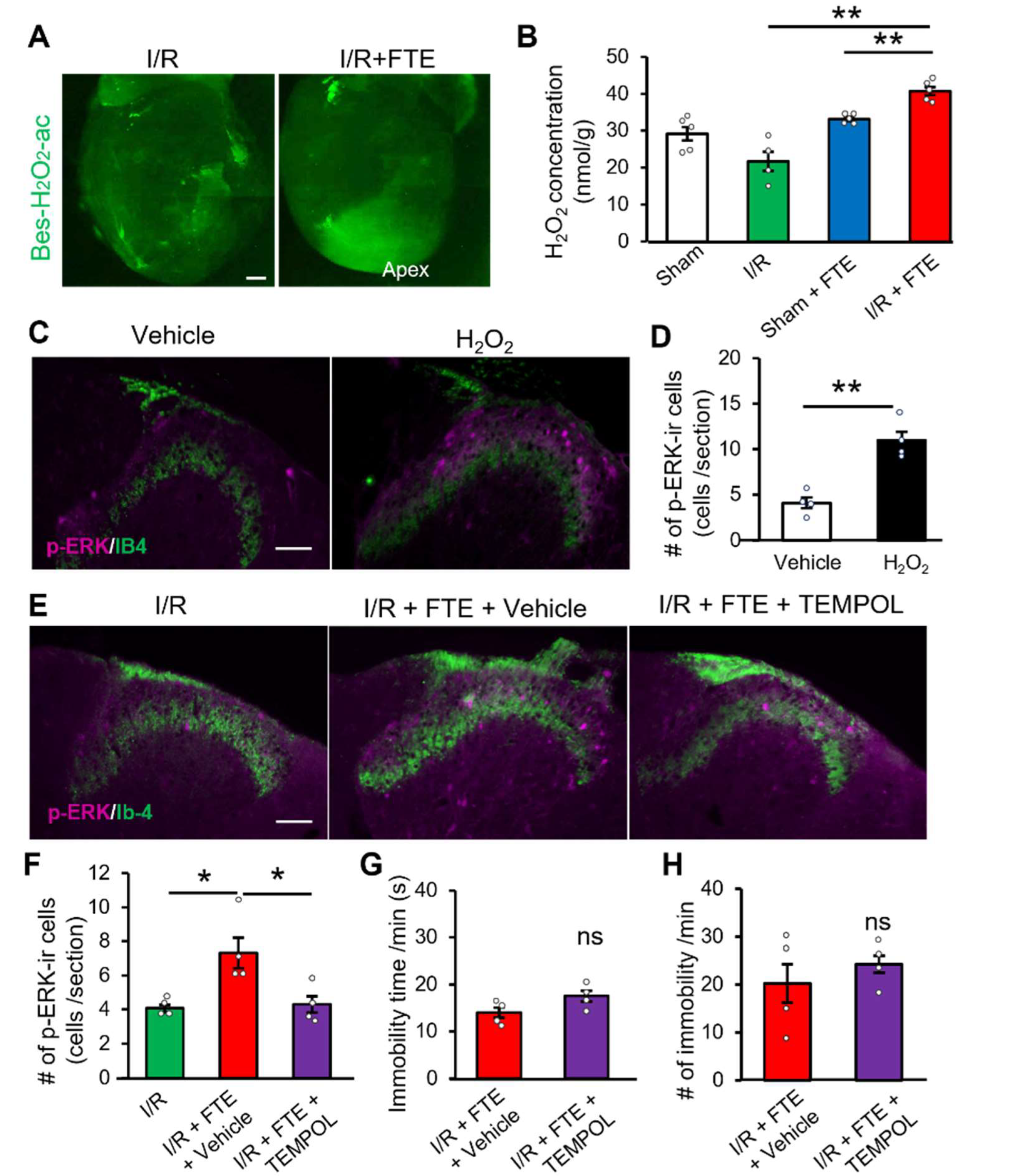
2.3. Hydrogen Peroxide Release Provokes Exercise-Induced Angina in the I/R Model
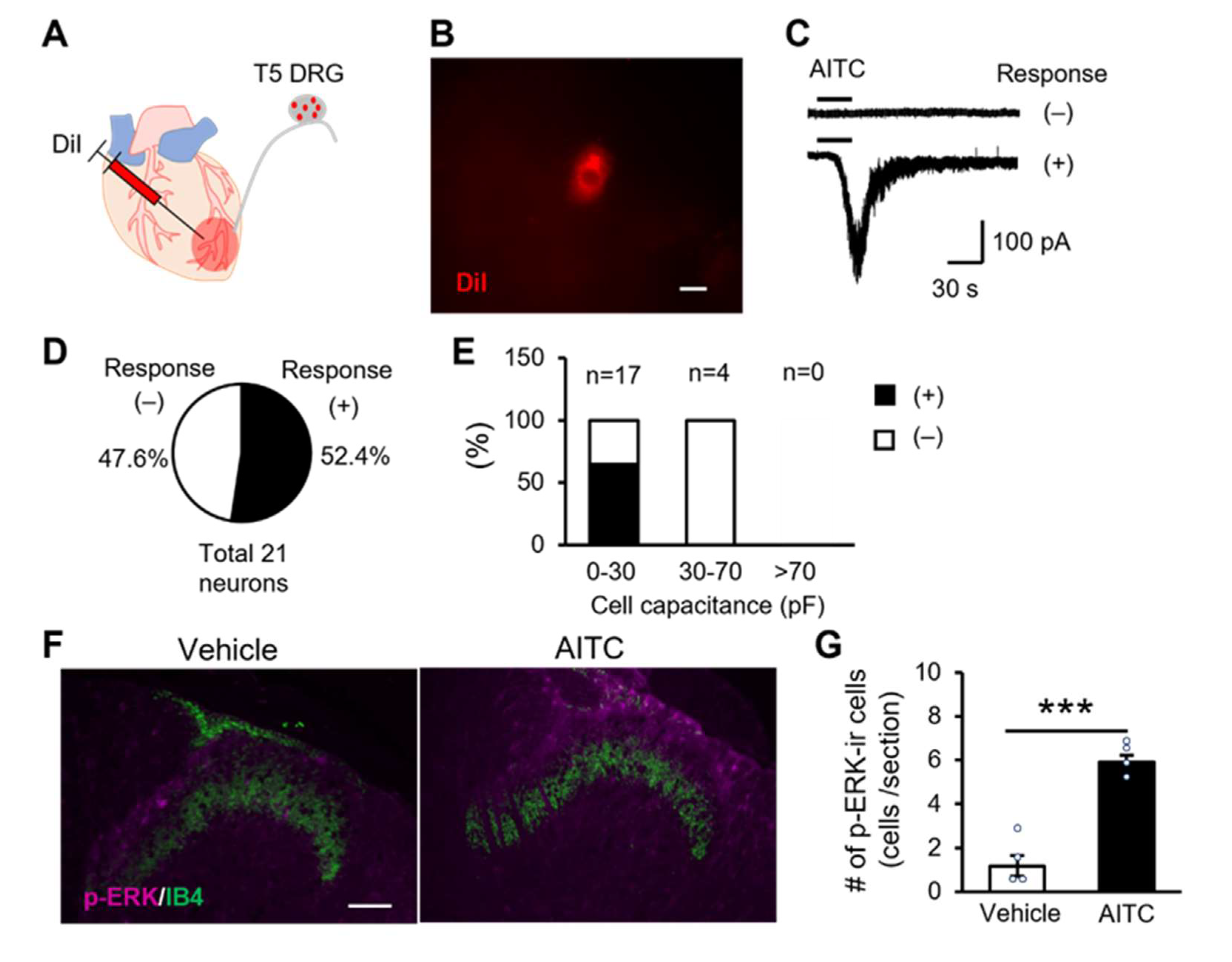
2.4. TRPA1 Channels Expressing Cardiac Sensory Neurons Mediate Cardiac Pain
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Myocardial I/R Injury Model
4.3. Intracardiac Injection
4.4. Electrocardiography
4.5. FTE
4.6. Drug Application
4.7. Perfusion Fixation through the Abdominal Aorta
4.8. Immunohistochemistry
4.9. Patch-Clamp Recording
4.10. Hydrogen Peroxide Assay
4.11. Assessment of Myocardial Infarct Size and the Risk Area
4.12. Recording of Daily Animal Activity
4.13. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCI | Percutaneous coronary intervention |
| CAD | Coronary artery disease |
| STEMI | ST-segment elevation myocardial infarction |
| DRG | Dorsal root ganglion |
| TRPA1 | Transient receptor potential ankyrin-1 |
| p-ERK | Phosphorylated extracellular signal-regulated kinase |
| FTE | Forced treadmill exercise |
| ECG | Electrocardiogram |
References
- Crea, F.; Bairey Merz, C.N.; Beltrame, J.F.; Berry, C.; Camici, P.G.; Kaski, J.C.; Ong, P.; Pepine, C.J.; Sechtem, U.; Shimokawa, H. Mechanisms and diagnostic evaluation of persistent or recurrent angina following percutaneous coronary revascularization. Eur. Heart J. 2019, 40, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Venkitachalam, L.; Kip, K.E.; Mulukutla, S.R.; Selzer, F.; Laskey, W.; Slater, J.; Cohen, H.A.; Wilensky, R.L.; Williams, D.O.; Marroquin, O.C.; et al. Temporal trends in patient-reported angina at 1 year after percutaneous coronary revascularization in the stent era: A report from the National Heart, Lung, and Blood Institute-sponsored 1997–2006 dynamic registry. Circ. Cardiovasc. Qual. Outcomes 2009, 2, 607–615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weintraub, W.S.; Spertus, J.A.; Kolm, P.; Maron, D.J.; Zhang, Z.; Claudine Jurkovitz, M.D.; Wei Zhang, M.S.; Hartigan, P.M.; Cheryl Lewis, R.N.; Veledar, E.; et al. Effect of PCI on quality of life in patients with stable coronary disease. N. Engl. J. Med. 2008, 359, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yehuda, O.; Kazi, D.S.; Bonafede, M.; Wade, S.W.; Machacz, S.F.; Stephens, L.A.; Hlatky, M.A.; Hernandez, J.B. Angina and associated healthcare costs following percutaneous coronary intervention: A real-world analysis from a multi-payer database. Catheter. Cardiovasc. Interv. 2016, 88, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Joner, M.; Kastrati, A. Stent thrombosis and restenosis: What have we learned and where are we going? The Andreas Grüntzig Lecture ESC 2014. Eur. Heart J. 2015, 36, 3320–3331. [Google Scholar] [CrossRef] [PubMed]
- Foreman, R.D.; Garrett, K.M.; Blair, R.W. Mechanisms of cardiac pain. Compr. Physiol. 2015, 5, 929–960. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.C.; Oravitz, J.J.; DeGroat, W.C. Tracing of afferent and efferent pathways in the left inferior cardiac nerve of the cat using retrograde and transganglionic transport of horseradish peroxidase. Brain Res. 1984, 321, 111–118. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signaling in the cardiovascular system. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef]
- Zahner, M.R.; Li, D.P.; Chen, S.R.; Pan, H.L. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J. Physiol. 2003, 551, 515–523. [Google Scholar] [CrossRef]
- Sutherland, S.P.; Benson, C.J.; Adelman, J.P.; McCleskey, E.W. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 711–716. [Google Scholar] [CrossRef]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian transient receptor potential TRPA1 channels: From structure to disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y. TRPs and pain. Semin. Immunopathol. 2016, 38, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Kaski, J.C.; Crea, F.; Gersh, B.J.; Camici, P.G. Reappraisal of ischemic heart disease. Circulation 2018, 138, 1463–1480. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, G.; Tritto, I. Myocardial reperfusion injury. Eur. Heart J. Suppl. 2002, 4, 1121–1135. [Google Scholar] [CrossRef]
- Gunata, M.; Parlakpinar, H. A review of myocardial ischaemia/reperfusion injury: Pathophysiology, experimental models, biomarkers, genetics and pharmacological treatment. Cell Biochem. Funct. 2021, 39, 190–217. [Google Scholar] [CrossRef]
- Vergona, R.A.; Herrott, C.; Garippa, R.; Hirkaler, G. Mechanisms of methacholine-induced coronary vasospasm in an experimental model of variant angina in the anethetized rat. Life Sci. 1984, 35, 1877–1884. [Google Scholar] [CrossRef]
- Duong, H.; Masarweh, O.M.; Campbell, G.; Win, T.T.; Joolhar, F. Isoproterenol causing coronary vasospasm and ST elevations during tilt table testing. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620966862. [Google Scholar] [CrossRef]
- Al-Zahrani, Y.A.; Al-Harthi, S.E.; Khan, L.M.; El-Bassossy, H.M.; Edris, S.M.; Sattar, M.A.A. The possible antianginal effect of allopurinol in vasopressin-induced ischemic model in rats. Saudi Pharm. J. 2015, 23, 487–498. [Google Scholar] [CrossRef]
- Ji, R.R.; Baba, H.; Brenner, G.J.; Woolf, C.J. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat. Neurosci. 1999, 2, 1114–1119. [Google Scholar] [CrossRef]
- Dai, Y.; Iwata, K.; Fukuoka, T.; Kondo, E.; Tokunaga, A.; Yamanaka, H.; Tachibana, T.; Liu, Y.; Noguchi, K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J. Neurosci. 2002, 22, 7737–7745. [Google Scholar] [CrossRef]
- Custodio-Patsey, L.; Donahue, R.R.; Fu, W.; Lambert, J.; Smith, B.N.; Taylor, B.K. Sex differences in kappa opioid receptor inhibition of latent postoperative pain sensitization in dorsal horn. Neuropharmacology 2020, 163, 107726. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dai, Y.; Fukuoka, T.; Yamanaka, H.; Obata, K.; Tokunaga, A.; Noguchi, K. Enhancement of stimulation-induced ERK activation in the spinal dorsal horn and gracile nucleus neurons in rats with peripheral nerve injury. Eur. J. Neurosci. 2004, 19, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, F.; Russo, A.; Salio, C. Fos and pERK immunoreactivity in spinal cord slices: Comparative analysis of in vitro models for testing putative antinociceptive molecules. Ann. Anat. 2014, 196, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Stowe, D.F.; Camara, A.K.S. Mitochondrial reactive oxygen species production in excitable cells: Modulators of mitochondrial and cell function. Antioxids. Redox Signal. 2009, 11, 1373–1414. [Google Scholar] [CrossRef] [PubMed]
- Vandeplassche, G.; Hermans, C.; Thoné, F.; Borgers, M. Mitochondrial hydrogen peroxide generation by NADH-oxidase activity following regional myocardial ischemia in the dog. J. Mol. Cell. Cardiol. 1989, 21, 383–392. [Google Scholar] [CrossRef]
- Trevisan, G.; Hoffmeister, C.; Rossato, M.F.; Oliveira, S.M.; Silva, M.A.; Ineu, R.P.; Guerra, G.P.; Materazzi, S.; Fusi, C.; Nassini, R.; et al. Transient receptor potential ankyrin 1 receptor stimulation by hydrogen peroxide is critical to trigger pain during monosodium urate-induced inflammation in rodents. Arthritis Rheum. 2013, 65, 2984–2995. [Google Scholar] [CrossRef]
- Verma, S.; Fedak, P.W.M.; Weisel, R.D.; Butany, J.; Rao, V.; Maitland, A.; Li, R.K.; Dhillon, B.; Yau, T.M. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation 2002, 105, 2332–2336. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current mechanistic concepts in ischemia and reperfusion injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef]
- Bhasin, D.; Gupta, P.; Gupta, A. Chest pain with ST-segment elevation: Looking behind the Masquerade. Circulation 2020, 142, 86–88. [Google Scholar] [CrossRef]
- Graichen, C.; Tanner, C. Biochemical markers in diagnosis of acute coronary syndrome. Am. Fam. Phys. 2018, 98, 186A–186B. [Google Scholar]
- Richardson, P.J.; Hill, L.S. Relationship between hypertension and angina pectoris. Br. J. Clin. Pharmacol. 1979, 7, 249S–253S. [Google Scholar] [CrossRef] [PubMed]
- Balian, V.; Galli, M.; Marcassa, C.; Cecchin, G.; Child, M.; Barlocco, F.; Petrucci, E.; Filippini, G.; Michi, R.; Onofri, M. Intracoronary ST-segment shift soon after elective percutaneous coronary intervention accurately predicts periprocedural myocardial injury. Circulation 2006, 114, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Kondo, T.; Miwa, H.; Yang, Y.; Wang, S.; Kanda, H.; Kogure, Y.; Imamura, N.; Fujimura, T.; Kono, T.; et al. Eosinophil-associated microinflammation in the gastroduodenal tract contributes to gastric hypersensitivity in a rat model of early-life adversity. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 320, G206–G216. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Park, S.K.; Zhou, J.L.; Taglialatela, G.; Chung, K.; Coggeshall, R.E.; Chung, J.M. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 2004, 111, 116–124. [Google Scholar] [CrossRef]
- Sun, M.S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.L.; Guo, Z.N. Free radical damage in ischemia reperfusion injury: An obstacle in acute ischemic stroke after revascularization therapy. Oxid. Med. Cell. Longev. 2017, 2018, 3804979. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Campagna, R.; Mateuszuk, Ł.; Wojnar-Lason, K.; Kaczara, P.; Tworzydło, A.; Kij, A.; Bujok, R.; Mlynarski, J.; Wang, Y.; Sartini, D.; et al. Nicotinamide N-methyltransferase in endothelium protects against oxidant stress-induced endothelial injury. Biochim. Biophys. Acta-Mol. Cell Res. 2021, 1868, 119082. [Google Scholar] [CrossRef]
- Szczesny-Malysiak, E.; Stojak, M.; Campagna, R.; Grosicki, M.; Jamrozik, M.; Kaczara, P.; Chlopicki, S. Bardoxolone methyl displays detrimental effects on endothelial bioenergetics, suppresses endothelial et-1 release, and increases endothelial permeability in human microvascular endothelium. Oxid. Med. Cell. Longev. 2020, 2020, 4678252. [Google Scholar] [CrossRef]
- Waters, D.D.; Szlachcic, J.; Bourassa, M.G.; Scholl, J.M.; Théroux, P. Exercise testing in patients with variant angina: Results, correlation with clinical and angiographic features and prognostic significance. Circulation 1982, 65, 265–274. [Google Scholar] [CrossRef]
- Bailly, C.; Hecquet, P.E.; Kouach, M.; Thuru, X.; Goossens, J.F. Chemical reactivity and uses of 1-phenyl-3-methyl-5-pyrazolone (PMP), also known as edaravone. Bioorg. Med. Chem. 2020, 28, 115463. [Google Scholar] [CrossRef]
- Petrov, D.; Mansfield, C.; Moussy, A.; Hermine, O. ALS clinical trials review: 20 years of failure. Are we any closer to registering a new treatment? Front. Aging Neurosci. 2017, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, Y.; Yoshimura, S.; Sakai, N.; Yamagami, H.; Egashira, Y.; Shirakawa, M.; Uchida, K.; Kageyama, H.; Tomogane, Y. Effect of edaravone on favorable outcome in patients with acute cerebral large vessel occlusion: Subanalysis of RESCUE-Japan registry. Neurol. Med. Chir. 2015, 55, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Conklin, D.J.; Guo, Y.; Nystoriak, M.A.; Jagatheesan, G.; Obal, D.; Kilfoil, P.J.; Hoetker, J.D.; Guo, L.; Bolli, R.; Bhatnagar, A. TRPA1 channel contributes to myocardial ischemia-reperfusion injury. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H889–H899. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.D.; Ustinova, E.E. Capsaicin receptors mediate free radical-induced activation of cardiac afferent endings. Cardiovasc. Res. 1998, 38, 348–355. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with Trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef]
- Sawada, Y.; Hosokawa, H.; Matsumura, K.; Kobayashi, S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur. J. Neurosci. 2008, 27, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Hoebart, C.; Rojas-Galvan, N.S.; Ciotu, C.I.; Aykac, I.; Reissig, L.F.; Weninger, W.J.; Kiss, A.; Podesser, B.K.; Fischer, M.J.M.; Heber, S. No functional TRPA1 in cardiomyocytes. Acta Physiol. 2021, 232, e13659. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to cause oxidative stress, the catalase issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Son, Y.; Kim, S.; Chung, H.T.; Pae, H.O. Reactive Oxygen Species in the Activation of MAP Kinases, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 528, ISBN 9780124058811. [Google Scholar]
- Touyz, R.M.; Schiffrin, E.L. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem. Cell Biol. 2004, 122, 339–352. [Google Scholar] [CrossRef]
- Morad, H.; Luqman, S.; Tan, C.H.; Swann, V.; McNaughton, P.A. TRPM2 ion channels steer neutrophils towards a source of hydrogen peroxide. Sci. Rep. 2021, 11, 9339. [Google Scholar] [CrossRef] [PubMed]
- DelloStritto, D.J.; Connell, P.J.; Dick, G.M.; Fancher, I.S.; Klarich, B.; Fahmy, J.N.; Kang, P.T.; Chen, Y.R.; Damron, D.S.; Thodeti, C.K.; et al. Differential regulation of TRPV1 channels by H2O2: Implications for diabetic microvascular dysfunction. Basic Res. Cardiol. 2016, 111, 21. [Google Scholar] [CrossRef] [PubMed]
- Naylor, J.; Al-Shawaf, E.; McKeown, L.; Manna, P.T.; Porter, K.E.; O’Regan, D.; Muraki, K.; Beech, D.J. TRPC5 channel sensitivities to antioxidants and hydroxylated stilbenes. J. Biol. Chem. 2011, 286, 5078–5086. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wen, H. Heat activation mechanism of TRPV1: New insights from molecular dynamics simulation. Temperature 2019, 6, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Alawi, K.M.; Russell, F.A.; Aubdool, A.A.; Srivastava, S.; Riffo-Vasquez, Y.; Baldissera, L.; Thakore, P.; Saleque, N.; Fernandes, E.S.; Walsh, D.A.; et al. Transient receptor potential canonical 5 (TRPC5) protects against pain and vascular inflammation in arthritis and joint inflammation. Ann. Rheum. Dis. 2017, 76, 252–260. [Google Scholar] [CrossRef]
- So, K.; Tei, Y.; Zhao, M.; Miyake, T.; Hiyama, H.; Shirakawa, H.; Imai, S.; Mori, Y.; Nakagawa, T.; Matsubara, K.; et al. Hypoxia-induced sensitisation of TRPA1 in painful dysesthesia evoked by transient hindlimb ischemia/reperfusion in mice. Sci. Rep. 2016, 6, 23261. [Google Scholar] [CrossRef]
- Alkhani, H.; Ase, A.R.; Grant, R.; O’Donnell, D.; Groschner, K.; Séguéla, P. Contribution of TRPC3 to store-operated calcium entry and inflammatory transductions in primary nociceptors. Mol. Pain 2014, 10, 43. [Google Scholar] [CrossRef]

| Sham | I/R | p-Value a | |
|---|---|---|---|
| Sample size, n | 6 | 8 | |
| Heart Rate (BPM) | 421.0 ± 8.4 | 407.0 ±7.0 | 0.25 |
| RR Interval (ms) | 142.8 ± 3.0 | 147.8 ± 2.7 | 0.28 |
| P Duration (ms) | 20.0 ± 1.0 | 24.0 ± 1.5 | 0.08 |
| QRS Interval (ms) | 13.0 ± 1.0 | 11 ± 0.9 | 0.22 |
| QT Interval (ms) | 63.4 ± 3.0 | 40.6 ± 8.5 | 0.06 |
| Q Amplitude (µV) | 12.0 ± 2.0 | 51.1 ± 23.2 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Kanda, H.; Tsujino, T.; Kogure, Y.; Zhu, F.; Yamamoto, S.; Sakaguchi, T.; Noguchi, K.; Dai, Y. Reactive Oxygen Species Cause Exercise-Induced Angina in a Myocardial Ischaemia-Reperfusion Injury Model. Int. J. Mol. Sci. 2022, 23, 2820. https://doi.org/10.3390/ijms23052820
Wang X, Kanda H, Tsujino T, Kogure Y, Zhu F, Yamamoto S, Sakaguchi T, Noguchi K, Dai Y. Reactive Oxygen Species Cause Exercise-Induced Angina in a Myocardial Ischaemia-Reperfusion Injury Model. International Journal of Molecular Sciences. 2022; 23(5):2820. https://doi.org/10.3390/ijms23052820
Chicago/Turabian StyleWang, Xiaohang, Hirosato Kanda, Takeshi Tsujino, Yoko Kogure, Feng Zhu, Satoshi Yamamoto, Taichi Sakaguchi, Koichi Noguchi, and Yi Dai. 2022. "Reactive Oxygen Species Cause Exercise-Induced Angina in a Myocardial Ischaemia-Reperfusion Injury Model" International Journal of Molecular Sciences 23, no. 5: 2820. https://doi.org/10.3390/ijms23052820
APA StyleWang, X., Kanda, H., Tsujino, T., Kogure, Y., Zhu, F., Yamamoto, S., Sakaguchi, T., Noguchi, K., & Dai, Y. (2022). Reactive Oxygen Species Cause Exercise-Induced Angina in a Myocardial Ischaemia-Reperfusion Injury Model. International Journal of Molecular Sciences, 23(5), 2820. https://doi.org/10.3390/ijms23052820






