Toxicological Impact of Rare Earth Elements (REEs) on the Reproduction and Development of Aquatic Organisms Using Sea Urchins as Biological Models
Abstract
:1. Introduction
2. Rare Earth Elements
3. Gadolinium
4. The Sea Urchin Embryo
5. Rare Earth Elements and the Sea Urchin Embryo
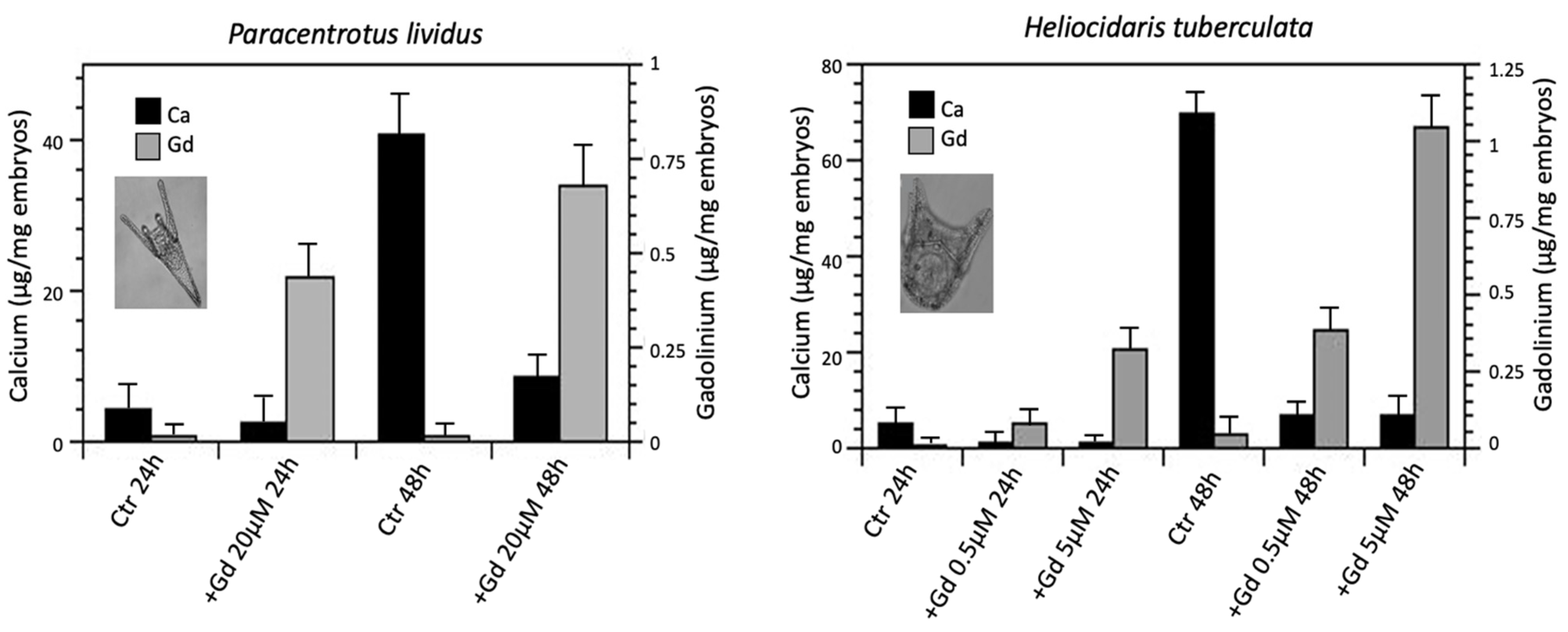
6. Gadolinium and the Sea Urchin Embryos
7. Synergistic Effects of Gadolinium Exposure and Increased Temperature on Sea Urchin Embryos
8. Gadolinium and Embryonic Gene Expression in Sea Urchins
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci. Total Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef]
- Rogowska, J.; Olkowska, E.; Ratajczyk, W.; Wolsk, L. Gadolinium as a new emerging contaminant of aquatic environments. Environ. Toxicol. Chem. 2018, 37, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Vukojević, V.; Đurđić, S.; Stefanović, V.; Trifković, J.; Čakmak, D.; Perović, V.; Mutić, J. Scandium, yttrium, and lanthanide contents in soil from Serbia and their accumulation in the mushroom Macrolepiota procera (Scop.) Singer. Environ. Sci. Pollut. Res. Int. 2019, 26, 5422–5434. [Google Scholar] [CrossRef] [PubMed]
- Brünjes, R.; Hofmann, T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020, 182, 115966. [Google Scholar] [CrossRef] [PubMed]
- Blinova, I.; Muna, M.; Heinlaan, M.; Lukjanova, A.; Kahru, A. Potential Hazard of Lanthanides and Lanthanide-Based Nanoparticles to Aquatic Ecosystems: Data Gaps, Challenges and Future Research Needs Derived from Bibliometric Analysis. Nanomaterials 2020, 10, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapasso, G.; Chiesa, S.; Freitas, R.; Pereira, E. What do we know about the ecotoxicological implications of the rare earth element gadolinium in aquatic ecosystems? Sci. Total Environ. 2021, 781, 146273. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Nolde, J.; Berger, S.; Heise, S. Aquatic ecotoxicity of lanthanum—A review and an attempt to derive water and sediment quality criteria. Ecotoxicol. Environ. Saf. 2016, 124, 213–238. [Google Scholar] [CrossRef] [Green Version]
- Arnot, J.A.; Gobas, F.A.P.C. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Ferreira, N.; Ferreira, A.; Viana, T.; Lopes, C.B.; Costa, M.; Pinto, J.; Soares, J.; Pinheiro-Torres, J.; Henriques, B.; Pereira, E. Assessment of marine macroalgae potential for gadolinium removal from contaminated aquatic systems. Sci. Total Environ. 2020, 20, 749–141488. [Google Scholar] [CrossRef] [PubMed]
- Boucek, M.M.; Snyderman, R. Calcium influx requirement for human neutrophil chemotaxis: Inhibition by lanthanum chloride. Science 1976, 193, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Pałasz, A.; Czekaj, P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim. Pol. 2000, 47, 1107–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsumori, L.M.; Bhargava, P.; Essig, M.; Maki, J.H. Magnetic resonance imaging using gadolinium-based contrast agents. J. Magn. Reson. Imaging 2014, 23, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Trapasso, G.; Coppola, F.; Queirós, V.; Henriques, B.; Soares, A.M.V.M.; Pereira, E.; Chiesa, S.; Freitas, R. How Ulva lactuca can influence the impacts induced by the rare earth element Gadolinium in Mytilus galloprovincialis? The role of macroalgae in water safety towards marine wildlife. Ecotoxicol. Environ. Saf. 2021, 215, 112101. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Ai, T.; Goerner, F.; Hu, X.; Runge, V.M.; Tweedle, M. MRI contrast agents: Basic chemistry and safety. J. Magn. Reson. Imaging 2012, 36, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Cowper, S.E.; Robin, H.S.; Steinberg, S.M.; Su, L.D.; Gupta, S.; LeBoit, P.E. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000, 356, 1000–1001. [Google Scholar] [CrossRef]
- Grobner, T. Gadolinium—A specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006, 21, 1104–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bau, M.; Dulski, P. Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet. Sci. Lett. 1996, 143, 245–255. [Google Scholar] [CrossRef]
- Parant, M.; Perrat, E.; Wagner, P.; Rosin, C.; Py, J.S.; Cossu-Leguille, C. Variations of anthropogenic gadolinium in rivers close to waste water treatment plant discharges. Environ. Sci. Pollut. Res. Int. 2018, 25, 36207–36222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hoshino, M.; Yamada, H.; Itoh, A.; Haraguchi, H. Gadolinium anomaly in the distributions of rare earth elements observed for coastal seawater and river waters around Nagoya. City Bull. Chem. Soc. Jpn. 2004, 77, 1835–1842. [Google Scholar] [CrossRef]
- Hatje, V.; Bruland, K.W.; Flegal, A.R. Increases in Anthropogenic Gadolinium Anomalies and Rare Earth Element Concentrations in San Francisco Bay over a 20 Year Record. Environ. Sci. Technol. 2016, 50, 4159–4168. [Google Scholar] [CrossRef]
- Pedreira, R.M.A.; Pahnke, K.; Böning, P.; Hatje, V. Tracking hospital effluent-derived gadolinium in Atlantic coastal waters off Brazil. Water Res. 2018, 145, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Zabrecky, J.M.; Liu, X.; Wu, Q.; Cao, C. Evidence of Anthropogenic Gadolinium in Triangle Area Waters, North Carolina, USA. Water 2021, 13, 1895. [Google Scholar] [CrossRef]
- Lingott, J.; Lindner, U.; Telgmann, L.; Esteban-Fernández, D.; Jakubowski, N.; Panne, U. Gadolinium-uptake by aquatic and terrestrial organisms-distribution determined by laser ablation inductively coupled plasma mass spectrometry. Environ. Sci. Process Impacts 2016, 18, 200–207. [Google Scholar] [CrossRef]
- Le Goff, S.; Barrat, J.A.; Chauvaud, L.; Paulet, Y.M.; Gueguen, B.; Ben Salem, D. Compound-specific recording of gadolinium pollution in coastal waters by great scallops. Sci. Rep. 2019, 29, 8015. [Google Scholar] [CrossRef]
- McClay, D.R. Evolutionary crossroads in developmental biology: Sea urchins. Development 2011, 138, 2639–2648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Bernardo, M.; Di Carlo, M. The Sea Urchin Embryo: A Model for Studying Molecular Mechanisms Involved in Human Diseases and for Testing Bioactive Compounds. In Sea Urchin—From Environment to Aquaculture and Biomedicine; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Ettensohn, C.A. Sea Urchins as a Model System for Studying Embryonic Development. Ref. Modul. Biomed. Sci. 2017. [Google Scholar] [CrossRef]
- Adonin, L.; Drozdov, A.; Barlev, N.A. Sea Urchin as a Universal Model for Studies of Gene Networks. Front. Genet. 2021, 11, 627259. [Google Scholar] [CrossRef] [PubMed]
- Zito, F.; Costa, C.; Sciarrino, S.; Cavalcante, C.; Poma, V.; Matranga, V. Cell adhesion and communication: A lesson from echinoderm embryos for the exploitation of new therapeutic tools. Prog. Mol. Subcell. Biol. 2005, 39, 7–44. [Google Scholar] [CrossRef] [PubMed]
- Livingston, B.T.; Wilt, F.H. Determination of cell fate in sea urchin embryos. Bioessays 1990, 12, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, A.G. Marine invertebrates as models for aging research. Exp. Gerontol. 2009, 44, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, R.; Agnello, M.; Bosco, L.; Roccheri, M.C. Sea urchin embryos exposed to cadmium as an experimental model for studying the relationship between autophagy and apoptosis. Mar. Environ. Res. 2014, 93, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Le Bouffant, R.; Cormier, P.; Cueff, A.; Bellé, R.; Mulner-Lorillon, O. Sea urchin embryo as a model for analysis of the signaling pathways linking DNA damage checkpoint, DNA repair and apoptosis. Cell. Mol. Life Sci. 2007, 64, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Pinsino, A.; Matranga, V.; Trinchella, F.; Roccheri, M.C. Sea urchin embryos as an in vivo model for the assessment of manganese toxicity: Developmental and stress response effects. Ecotoxicology 2010, 19, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, M.; Bennici, C.; Biondo, G.; Masullo, T.; Monastero, C.; Tagliavia, M.; Torri, M.; Costa, S.; Ragusa, M.A.; Cuttitta, A.; et al. Aberrant gene expression profiles in Mediterranean sea urchin reproductive tissues after metal exposures. Chemosphere 2019, 216, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Nikitina, L.A.; Buznikov, G.A.; Lauder, J.M.; Seidler, F.J.; Slotkin, T.A. The sea urchin embryo as a model for mammalian developmental neurotoxicity: Ontogenesis of the high-affinity choline transporter and its role in cholinergic trophic activity. Environ. Health Perspect. 2003, 111, 1730–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond-Barbosa, D.; Tennessen, J.M. Reclaiming Warburg: Using developmental biology to gain insight into human metabolic diseases. Development 2020, 14, 189340. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Okamura, H. Effects of new antifouling compounds on the development of sea urchin. Mar. Pollut. Bull. 2002, 44, 748–751. [Google Scholar] [CrossRef]
- Schröder, H.C.; Di Bella, G.; Janipour, N.; Bonaventura, R.; Russo, R.; Müller, W.E.; Matranga, V. DNA damage and developmental defects after exposure to UV and heavy metals in sea urchin cells and embryos compared to other invertebrates. Prog. Mol. Subcell. Biol. 2005, 39, 111–137. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, H.M.; Koerting, L.; Devito, S.; van den Berg, J.H.; Dubbeldam, M.; Kwadijk, C.; Murk, A.J. Early life developmental effects of marine persistent organic pollutants on the sea urchin Psammechinus miliaris. Ecotoxicol. Environ. Saf. 2011, 74, 2182–2192. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, R.; Martino, C.; Roccheri, M.C. Cadmium stress effects indicating marine pollution in different species of sea urchin employed as environmental bioindicators. Cell Stress Chaperones 2019, 24, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, R.; Martino, C.; Roccheri, M.C.; Cancemi, P. Toxic effects induced by vanadium on sea urchin embryos. Chemosphere 2021, 274, 129843. [Google Scholar] [CrossRef] [PubMed]
- Trifuoggi, M.; Pagano, G.; Oral, R.; Pavičić-Hamer, D.; Burić, P.; Kovačić, I.; Siciliano, A.; Toscanesi, M.; Thomas, P.J.; Paduano, L.; et al. Microplastic-induced damage in early embryonal development of sea urchin Sphaerechinus granularis. Environ. Res. 2019, 179, 108815. [Google Scholar] [CrossRef]
- Rendell-Bhatti, F.; Paganos, P.; Pouch, A.; Mitchell, C.; D’Aniello, S.; Godley, B.J.; Pazdro, K.; Arnone, M.I.; Jimenez-Guri, E. Developmental toxicity of plastic leachates on the sea urchin Paracentrotus lividus. Environ. Pollut. 2021, 15, 115744. [Google Scholar] [CrossRef] [PubMed]
- Oral, R.; Bustamante, P.; Warnau, M.; D’Ambra, A.; Guida, M.; Pagano, G. Cytogenetic and developmental toxicity of cerium and lanthanum to sea urchin embryos. Chemosphere 2010, 81, 194–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oral, R.; Pagano, G.; Siciliano, A.; Gravina, M.; Palumbo, A.; Castellano, I.; Migliaccio, O.; Thomas, P.J.; Guida, M.; Tommasi, F.; et al. Heavy rare earth elements affect early life stages in Paracentrotus lividus and Arbacia lixula sea urchins. Environ. Res. 2017, 154, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Kuroda, R.; Muranaka, Y.; Uto, N.; Murai, J.; Kuroda, H. Asymmetric inhibition of spicule formation in sea urchin embryos with low concentrations of gadolinium ion. Dev. Growth Differ. 2010, 52, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Guida, M.; Siciliano, A.; Oral, R.; Koçbaş, F.; Palumbo, A.; Castellano, I.; Migliaccio, O.; Thomas, P.J.; Trifuoggi, M. Comparative toxicities of selected rare earth elements: Sea urchin embryogenesis and fertilization damage with redox and cytogenetic effects. Environ. Res. 2016, 147, 453–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, C.; Bonaventura, R.; Byrne, M.; Roccheri, M.; Matranga, V. Effects of exposure to gadolinium on the development of geographically and phylogenetically distant sea urchins species. Mar. Environ. Res. 2017, 128, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, C.; Chiarelli, R.; Bosco, L.; Roccheri, M.C. Induction of skeletal abnormalities and autophagy in Paracentrotus lividus sea urchin embryos exposed to gadolinium. Mar. Environ. Res. 2017, 130, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Costa, C.; Roccheri, M.C.; Koop, D.; Scudiero, R.; Byrne, M. Gadolinium perturbs expression of skeletogenic genes, calcium uptake and larval development in phylogenetically distant sea urchin species. Aquat. Toxicol. 2018, 194, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Martino, C.; Byrne, M.; Roccheri, M.C.; Chiarelli, R. Interactive effects of increased temperature and gadolinium pollution in Paracentrotus lividus sea urchin embryos: A climate change perspective. Aquat. Toxicol. 2021, 21, 105750. [Google Scholar] [CrossRef] [PubMed]
- Trifuoggi, M.; Pagano, G.; Guida, M.; Palumbo, A.; Siciliano, A.; Gravina, M.; Lyons, D.M.; Burić, P.; Levak, M.; Thomas, P.J.; et al. Comparative toxicity of seven rare earth elements in sea urchin early life stages. Environ. Sci. Pollut. Res. Int. 2017, 24, 20803–20810. [Google Scholar] [CrossRef] [Green Version]
- Gravina, M.; Pagano, G.; Oral, R.; Guida, M.; Toscanesi, M.; Siciliano, A.; Di Nunzio, A.; Burić, P.; Lyons, D.M.; Thomas, P.J.; et al. Heavy Rare Earth Elements Affect Sphaerechinus granularis Sea Urchin Early Life Stages by Multiple Toxicity Endpoints. Bull. Environ. Contam. Toxicol. 2018, 100, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, D.T.; Smith, A.B. A combined morphological and molecular phylogeny for sea urchins (Echinoidea: Echinodermata). Phil. Trans. R. Soc. Lond. B 1995, 347, 213–234. [Google Scholar] [CrossRef]
- Foo, S.A.; Dworjanyn, S.A.; Poore, A.G.; Byrne, M. Adaptive capacity of the habitat modifying sea urchin Centrostephanus rodgersii to ocean warming and ocean acidification: Performance of early embryos. PLoS ONE 2012, 7, 42497. [Google Scholar] [CrossRef]
- Yang, X.C.; Sachs, F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science 1989, 243, 1068–1071. [Google Scholar] [CrossRef]
- Lansman, J.B. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. J. Gen. Physiol. 1990, 95, 679–696. [Google Scholar] [CrossRef] [Green Version]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on gadolinium chemistry. J. Magn. Reson. Imag. 2009, 30, 1240–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burić, P.; Jaksić, Z.; Stajner, L.; Dutour Sikirić, M.; Jurasin, D.; Cascio, C.; Calzolai, L.; Lyons, D.M. Effect of silver nanoparticles on Mediterranean sea urchin embryonal development is species specific and depends on moment of first exposure. Mar. Environ. Res. 2015, 111, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carballeira, C.; De Orte, M.R.; Viana, I.G.; Delvalls, T.A.; Carballeira, A. Assessing the toxicity of chemical compounds associated with land-based marine fish farms: The sea urchin embryo bioassay with Paracentrotus lividus and Arbacia lixula. Arch. Environ. Contam. Toxicol. 2012, 63, 249–261. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC: Geneva, Switzerland, 2019; in press. [Google Scholar]
- Mos, B.; Kaposi, K.L.; Rose, A.L.; Kelaher, B.; Dworjanyn, S.A. Moderate ocean warming mitigates, but more extreme warming exacerbates the impacts of zinc from engineered nanoparticles on a marine larva. Environ. Pollut. 2017, 228, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Bertucci, J.I.; Bellas, J. Combined effect of microplastics and global warming factors on early growth and development of the sea urchin (Paracentrotus lividus). Sci. Total Environ. 2021, 782, 146888. [Google Scholar] [CrossRef] [PubMed]
- Soars, N.A.; Prowse, T.A.A.; Byrne, M. Overview of phenotypic plasticity in echinoid larvae, Echinopluteus transversus’ type versus typical echinoplutei. Mar. Ecol. Prog. Ser. 2009, 383, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Ettensohn, C.A.; Illies, M.R.; Oliveri, P.; De Jong, D.L. Alx1, a member of the Cart1/Alx3/Alx4 subfamily of Paired-class homeodomain proteins, is an essential component of the gene network controlling skeletogenic fate specification in the sea urchin embryo. Development 2003, 130, 2917–2928. [Google Scholar] [CrossRef] [Green Version]
- Czarkwiani, A.; Dylus, D.V.; Oliveri, P. Expression of skeletogenic genes during arm regeneration in the brittle star Amphiura filiformis. Gene Expr. Patterns 2013, 13, 464–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, H.; Fujitani, H.; Morino, Y.; Miyamoto, N.; Tsuchimoto, J.; Shibata, T.F.; Nozawa, M.; Shigenobu, S.; Ogura, A.; Tachibana, K.; et al. Experimental Approach Reveals the Role of alx1 in the Evolution of the Echinoderm Larval Skeleton. PLoS ONE 2016, 11, 149067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.J.; Su, Y.H. Opposing nodal and BMP signals regulate left-right asymmetry in the sea urchin larva. PLoS Biol. 2012, 10, 1001402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koop, D.; Cisternas, P.; Morris, V.B.; Strbenac, D.; Yang, J.Y.H.; Wray, G.A.; Byrne, M. Nodal and BMP expression during the transition to pentamery in the sea urchin Heliocidaris erytrogramma: Insight into patterning the enigmatic echinoderm body plan. BMC Dev. 2017, 17, 4. [Google Scholar] [CrossRef] [Green Version]
- Duboc, V.; Röttinger, E.; Lapraz, F.; Besnardeau, L.; Lepage, T. Left-right asymmetry in the sea urchin embryo is regulated by nodal signaling on the right side. Dev. Cell. 2005, 9, 147–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessodes, N.; Haillot, E.; Duboc, V.; Röttinger, E.; Lahaye, F.; Lepage, T. Reciprocal signaling between the ectoderm and a mesendodermal left-right organizer directs left-right determination in the sea urchin embryo. PLoS Genet. 2012, 8, 1003121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adomako-Ankomah, A.; Ettensohn, C.A. Growth factors and early mesoderm morphogenesis: Insights from the sea urchin embryo. Genesis 2014, 52, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Veis, A. Organic matrix-related mineralization of sea urchin spicules, spines, test and teeth. Front. Biosci. Landmark 2011, 16, 2540–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiq, K.; Shashikant, T.; McManus, C.J.; Ettensohn, C.A. Genome-wide analysis of the skeletogenic gene regulatory network of sea urchins. Development 2014, 141, 950–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zito, F.; Koop, D.; Byrne, M.; Matranga, V. Carbonic anhydrase inhibition blocks skeletogenesis and echinochrome production in Paracentrotus lividus and Heliocidaris tuberculata embryos and larvae. Dev. Growth Differ. 2015, 57, 507–514. [Google Scholar] [CrossRef]
- Whitehead, A. Evolutionary genomics of environmental pollution. Adv. Exp. Med. Biol. 2014, 781, 321–337. [Google Scholar] [CrossRef]
- Rodríguez-Verdugo, A.; Buckley, J.; Stapley, J. The genomic basis of eco-evolutionary dynamics. Mol. Ecol. 2017, 26, 1456–1464. [Google Scholar] [CrossRef] [Green Version]
- Chiarelli, R.; Martino, C.; Agnello, M.; Bosco, L.; Roccheri, M.C. Autophagy as a defense strategy against stress: Focus on Paracentrotus lividus sea urchin embryos exposed to cadmium. Cell Stress Chaperones 2016, 21, 19–27. [Google Scholar] [CrossRef] [Green Version]

| Sea Urchin Species | Observed Alteration | REE(s) Concentration | Reference |
|---|---|---|---|
| S. granularis | Sperm toxicity Developmental defects | 10 µM Gd; 100µM La, Nd, Eu 1–100 µM Yb, La, Ce, Nd, Sm, Eu | [54] [55] |
| P. lividus | Sperm toxicity Impaired larval skeletogenesis Developmental defects | 100 µM La, Ce, Nd, Sm, Eu, Dy, Gd, Yb 20 µM Gd 1–100 µM Ho, Gd, Yb, La, Nd, Eu, Ce, Sm | [49] [47] [50,51,52] |
| A. lixula | Sperm toxicity Developmental defects | 100 µM La, Sm, Eu 1–100 µM Yb, La, Ce, Nd, Sm, Eu | [47] [54] [50] |
| H. pulcherrimus | Impaired larval skeletogenesis | 3 µM Gd | [48] |
| A. crassispina | Impaired larval skeletogenesis | 1 µM Gd | [48] |
| P. depressus | Impaired larval skeletogenesis | 1 µM Gd | [48] |
| H. tuberculata | Impaired larval skeletogenesis | 0.5 µM Gd | [50,52] |
| C. rodgersii | Impaired larval skeletogenesis | 150 µM Gd | [50] |
| Gene Name | Gd-Interference in Expression of Embryonic Skeletogenic Gene Regulatory Network | |||||
|---|---|---|---|---|---|---|
| Paracentrotus lividus | Heliocidaris tuberculata | |||||
| 20 µM Gd | 0.5 µM Gd | 5 µM Gd | ||||
| 24 hpf | 48 hpf | 24 hpf | 48 hpf | 24 hpf | 48 hpf | |
| alx-1 | −43 ± 0.07% | −57 ± 0.06% | n.d | n.d. | n.d. | n.d. |
| nodal | −59 ± 0.07% | −20 ± 0.08% | −33 ± 0.01% | = | −33 ± 0.04% | −20 ± 0.01% |
| lefty | n.d. | n.d. | −29 ± 0.04% | = | −43 ± 0.11% | = |
| bmp | n.d. | n.d. | −19 ± 0.05% | = | −29 ± 0.09% | = |
| univin | = | −48 ± 0.05% | n.d. | n.d. | n.d. | n.d. |
| vegf | = | = | +100 ± 8% | = | +179 ± 15% | = |
| vegf-r | = | = | n.d. | n.d. | n.d. | n.d. |
| fgf | = | −58 ± 0.04% | −24 ± 0.03% | −30 ± 0.09% | −19 ± 0.08% | −30 ± 0.1% |
| msp130 | = | −52 ± 0.12% | −34 ± 0.07% | = | −30 ± 0.01% | = |
| sm30 | n.d | n.d | −37 ± 0.06% | = | −35 ± 0.06% | −29 ± 0.03% |
| p16 | −28 ± 0.05% | −49 ± 0.06% | n.d. | n.d. | n.d. | n.d. |
| p19 | +35 ± 0.08% | −23 ± 0.05% | n.d. | n.d. | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martino, C.; Chianese, T.; Chiarelli, R.; Roccheri, M.C.; Scudiero, R. Toxicological Impact of Rare Earth Elements (REEs) on the Reproduction and Development of Aquatic Organisms Using Sea Urchins as Biological Models. Int. J. Mol. Sci. 2022, 23, 2876. https://doi.org/10.3390/ijms23052876
Martino C, Chianese T, Chiarelli R, Roccheri MC, Scudiero R. Toxicological Impact of Rare Earth Elements (REEs) on the Reproduction and Development of Aquatic Organisms Using Sea Urchins as Biological Models. International Journal of Molecular Sciences. 2022; 23(5):2876. https://doi.org/10.3390/ijms23052876
Chicago/Turabian StyleMartino, Chiara, Teresa Chianese, Roberto Chiarelli, Maria Carmela Roccheri, and Rosaria Scudiero. 2022. "Toxicological Impact of Rare Earth Elements (REEs) on the Reproduction and Development of Aquatic Organisms Using Sea Urchins as Biological Models" International Journal of Molecular Sciences 23, no. 5: 2876. https://doi.org/10.3390/ijms23052876
APA StyleMartino, C., Chianese, T., Chiarelli, R., Roccheri, M. C., & Scudiero, R. (2022). Toxicological Impact of Rare Earth Elements (REEs) on the Reproduction and Development of Aquatic Organisms Using Sea Urchins as Biological Models. International Journal of Molecular Sciences, 23(5), 2876. https://doi.org/10.3390/ijms23052876








