Bone Mass and Osteoblast Activity Are Sex-Dependent in Mice Lacking the Estrogen Receptor α in Chondrocytes and Osteoblast Progenitor Cells
Abstract
:1. Introduction
2. Results
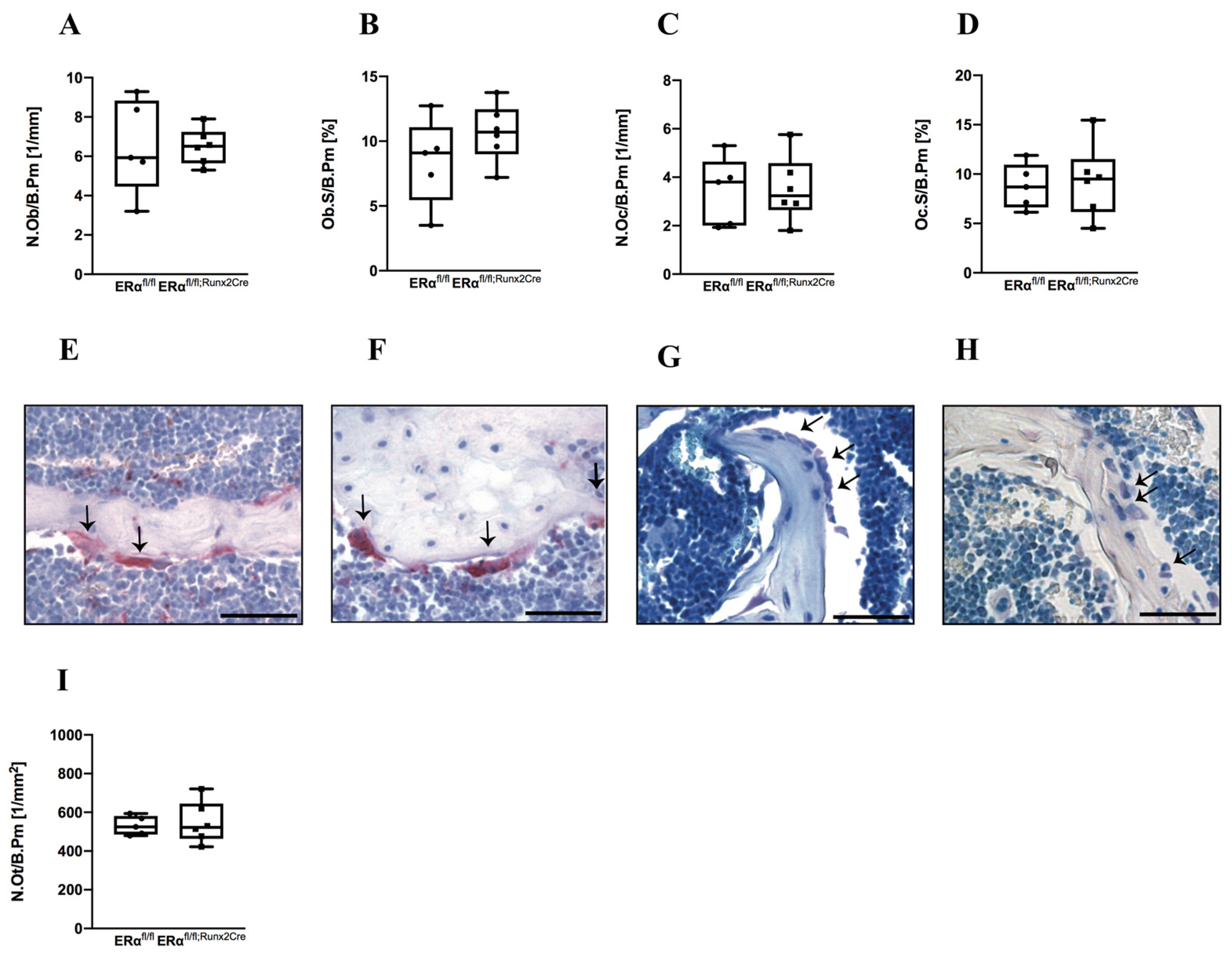
2.1. Influence of ERα Deletion in Osteoblast Progenitors on Bone in Male Mice
2.2. Influence of ER α Deletion in Osteoblast Progenitors on Bone in Female Mice
3. Discussion
4. Materials and Methods
4.1. Animal Care and Animal Models
4.2. Biomechanical Testing
4.3. μCT Analysis
4.4. Histomorphometry
4.5. Cultivation of Primary Mouse Osteoblasts
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riggs, B.L.; Khosla, S.; Melton, L.J. Sex steroids and the construction and conservation of the adult skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, F.; Sinnesael, M.; Gielen, E.; Boonen, S.; Vanderschueren, D. Skeletal sexual dimorphism: Relative contribution of sex steroids, GH-IGF1, and mechanical loading. J. Endocrinol. 2010, 207, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Laurent, M.R.; Claessens, F.; Gielen, E.; Lagerquist, M.; Vandenput, L.; Börjesson, A.E.; Ohlsson, C. Sex Steroid Actions in Male Bone. Endocr. Rev. 2014, 35, 906–960. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S. Update on Estrogens and the Skeleton. J. Clin. Endocrinol. Metab. 2010, 95, 3569–3577. [Google Scholar] [CrossRef]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699–712. [Google Scholar] [CrossRef]
- Callewaert, F.; Boonen, S.; Vanderschueren, D. Sex steroids and the male skeleton: A tale of two hormones. Trends Endocrinol. Metab. 2010, 21, 89–95. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Vandenput, L.; Boonen, S.; Lindberg, M.K.; Bouillon, R.; Ohlsson, C. Androgens and Bone. Endocr. Rev. 2004, 25, 389–425. [Google Scholar] [CrossRef]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Bellido, T.; Jilka, R.L. Sex Steroids, Cytokines and the Bone Marrow: New Concepts on the Pathogenesis of Osteoporosis. Physiol. Rev. 2007, 191, 187–202. [Google Scholar] [CrossRef]
- Bord, S.; Horner, A.; Beavan, S.; Compston, J. Estrogen Receptors? and? Are Differentially Expressed in Developing Human Bone 1. J. Clin. Endocrinol. Metab. 2001, 86, 2309–2314. [Google Scholar] [CrossRef]
- Khalid, A.B.; Krum, S.A. Estrogen receptors alpha and beta in bone. Bone 2016, 87, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-M.; Albanese, C.; Anderson, C.M.; Hilty, K.; Webb, P.; Uht, R.M.; Price, R.H.; Pestell, R.G.; Kushner, P.J. Opposing action of estrogen receptors α and β on cyclin D1 gene expression. J. Biol. Chem. 2002, 277, 24353–24360. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, M.K.; Movérare, S.; Skrtic, S.; Gao, H.; Dahlman-Wright, K.; Gustafsson, J.-A.; Ohlsson, C. Estrogen Receptor (ER)-β Reduces ERα-Regulated Gene Transcription, Supporting a “Ying Yang” Relationship between ERα and ERβ in Mice. Mol. Endocrinol. 2003, 17, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.P.; Boyd, J.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K. Estrogen Resistance Caused by a Mutation in the Estrogen-Receptor Gene in a Man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef]
- Vanderschueren, D.; Van Herck, E.; Nijs, J.; Ederveen, A.G.H.; De Coster, R.; Bouillon, R. Aromatase Inhibition Impairs Skeletal Modeling and Decreases Bone Mineral Density in Growing Male Rats*. Endocrinology 1997, 138, 2301–2307. [Google Scholar] [CrossRef]
- Vidal, O.; Lindberg, M.K.; Hollberg, K.; Baylink, D.J.; Andersson, G.; Lubahn, D.B.; Mohan, S.; Gustafsson, J.Å.; Ohlsson, C. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc. Natl. Acad. Sci. USA 2000, 97, 5474–5479. [Google Scholar] [CrossRef]
- Bouillon, R.; Bex, M.; Vanderschueren, D.; Boonen, S. Estrogens Are Essential for Male Pubertal Periosteal Bone Expansion. J. Clin. Endocrinol. Metab. 2004, 89, 6025–6029. [Google Scholar] [CrossRef]
- Rochira, V.; Zirilli, L.; Madeo, B.; Aranda, C.; Caffagni, G.; Fabre, B.; Montangero, V.E.; Roldan, E.; Maffei, L.; Carani, C. Skeletal effects of long-term estrogen and testosterone replacement treatment in a man with congenital aromatase deficiency: Evidences of a priming effect of estrogen for sex steroids action on bone. Bone 2007, 40, 1662–1668. [Google Scholar] [CrossRef]
- Gennari, L.; Merlotti, D.; Becherini, L.; Martini, G.; De Paola, V.; Calabrò, A.; Nuti, R. Estrogen Receptor Gene Polymorphisms and the Genetics of Osteoporosis: A HuGE Review. Am. J. Epidemiol. 2005, 161, 307–320. [Google Scholar] [CrossRef]
- Sims, N.; Clément-Lacroix, P.; Minet, D.; Fraslon-Vanhulle, C.; Gaillard-Kelly, M.; Resche-Rigon, M.; Baron, R. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor–deficient mice. J. Clin. Investig. 2003, 111, 1319–1327. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Fischer, V.; Ignatius, A. Differences in Fracture Healing Between Female and Male C57BL/6J Mice. Front. Physiol. 2021, 12, 2494. [Google Scholar] [CrossRef] [PubMed]
- Somjen, D.; Katzburg, S.; Lieberherr, M.; Hendel, D.; Yoles, I. DT56a stimulates gender-specific human cultured bone cells in vitro. J. Steroid Biochem. Mol. Biol. 2006, 98, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Somjen, D.; Katzburg, S.; Sharon, O.; Knoll, E.; Hendel, D.; Stern, N. Sex specific response of cultured human bone cells to ERα and ERβ specific agonists by modulation of cell proliferation and creatine kinase specific activity. J. Steroid Biochem. Mol. Biol. 2011, 125, 226–230. [Google Scholar] [CrossRef]
- Couse, J.F.; Korach, K.S. Estrogen Receptor Null Mice: What Have We Learned and Where Will They Lead Us? Endocr. Rev. 1999, 20, 358–417. [Google Scholar] [CrossRef] [PubMed]
- Vidal, O.; Lindberg, M.; Sävendahl, L.; Lubahn, D.; Ritzen, E.; Gustafsson, J.; Ohlsson, C. Disproportional Body Growth in Female Estrogen Receptor-α-Inactivated Mice. Biochem. Biophys. Res. Commun. 1999, 265, 569–571. [Google Scholar] [CrossRef]
- Lindberg, M.K.; Alatalo, S.L.; Halleen, J.M.; Mohan, S.; Gustafsson, J.; Ohlsson, C. Estrogen receptor specificity in the regulation of the skeleton in female mice. J. Endocrinol. 2001, 171, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.; Dupont, S.; Krust, A.; Clement-Lacroix, P.; Minet, D.; Resche-Rigon, M.; Gaillard-Kelly, M.; Baron, R. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-β in bone remodeling in females but not in males. Bone 2002, 30, 18–25. [Google Scholar] [CrossRef]
- Couse, J.F.; Curtis, S.W.; Washburn, T.F.; Golding, T.S.; Lubahn, D.B.; Korach, K.S.; Lindzey, J.; Smithies, O. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 1995, 9, 1441–1454. [Google Scholar] [CrossRef]
- Almeida, M.; Iyer, S.; Martin-Millan, M.; Bartell, S.M.; Han, L.; Ambrogini, E.; Onal, M.; Xiong, J.; Weinstein, R.S.; Jilka, R.L.; et al. Estrogen receptor-α signaling in osteoblast progenitors stimulates cortical bone accrual. J. Clin. Investig. 2012, 123, 394–404. [Google Scholar] [CrossRef]
- Seitz, S.; Keller, J.; Schilling, A.F.; Jeschke, A.; Marshall, R.P.; Stride, B.D.; Wintermantel, T.; Beil, F.T.; Amling, M.; Schutz, G.; et al. Pharmacological Estrogen Administration Causes a FSH-Independent Osteo-Anabolic Effect Requiring ER Alpha in Osteoblasts. PLoS ONE 2012, 7, e50301. [Google Scholar] [CrossRef]
- Rooney, A.M.; Ayobami, O.O.; Kelly, N.H.; Schimenti, J.C.; Ross, F.P.; van der Meulen, M.C. Bone mass and adaptation to mechanical loading are sexually dimorphic in adult osteoblast-specific ERα knockout mice. Bone 2022, 158, 116349. [Google Scholar] [CrossRef] [PubMed]
- Melville, K.M.; Kelly, N.H.; Khan, S.; Schimenti, J.C.; Ross, F.P.; Main, R.P.; van der Meulen, M.C.H. Female Mice Lacking Estrogen Receptor-Alpha in Osteoblasts Have Compromised Bone Mass and Strength. J. Bone Miner. Res. 2013, 29, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Melville, K.M.; Kelly, N.H.; Surita, G.; Buchalter, D.B.; Schimenti, J.C.; Main, R.P.; Ross, F.P.; Van Der Meulen, M.C.H. Effects of Deletion of ERα in Osteoblast-Lineage Cells on Bone Mass and Adaptation to Mechanical Loading Differ in Female and Male Mice. J. Bone Miner. Res. 2015, 30, 1468–1480. [Google Scholar] [CrossRef]
- Määttä, J.A.; Büki, K.G.; Gu, G.; Alanne, M.H.; Vääräniemi, J.; Liljenbäck, H.; Poutanen, M.; Härkönen, P.; Väänänen, K. Inactivation of estrogen receptor α in bone-forming cells induces bone loss in female mice. FASEB J. 2012, 27, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Windahl, S.H.; Borjesson, A.E.; Farman, H.H.; Engdahl, C.; Moverare-Skrtic, S.; Sjögren, K.; Lagerquist, M.; Kindblom, J.; Koskela, A.; Tuukkanen, J.; et al. Estrogen receptor- in osteocytes is important for trabecular bone formation in male mice. Proc. Natl. Acad. Sci. USA 2013, 110, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zhang, Y.F. Osteoinduction: A review of old concepts with new standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef]
- Park, J.; Gebhardt, M.; Golovchenko, S.; Perez-Branguli, F.; Hattori, T.; Hartmann, C.; Zhou, X.; Decrombrugghe, B.; Stock, M.; Schneider, H.; et al. Dual pathways to endochondral osteoblasts: A novel chondrocyte-derived osteoprogenitor cell identified in hypertrophic cartilage. Biol. Open 2015, 4, 608–621. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Zhou, X.; Von Der Mark, K.; Henry, S.; Norton, W.; Adams, H.; De Crombrugghe, B. Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice. PLoS Genet. 2014, 10, e1004820. [Google Scholar] [CrossRef]
- Hu, D.P.; Ferro, F.; Yang, F.; Taylor, A.J.; Chang, W.; Miclau, T.; Marcucio, R.S.; Bahney, C.S. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development 2017, 144, 221–234. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A Transcriptional Activator of Osteoblast Differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a Candidate Gene for Cleidocranial Dysplasia Syndrome, Is Essential for Osteoblast Differentiation and Bone Development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.; Gao, Y.-H.; Inada, M.; et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation owing to Maturational Arrest of Osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Sabsovich, I.; Clark, J.D.; Liao, G.; Peltz, G.; Lindsey, D.P.; Jacobs, C.R.; Yao, W.; Guo, T.-Z.; Kingery, W.S. Bone microstructure and its associated genetic variability in 12 inbred mouse strains: μCT study and in silico genome scan. Bone 2008, 42, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.T.; Colvard, D.S.; Spelsberg, T.C. Estrogen Inhibition of Periosteal Bone Formation in Rat Long Bones: Down-Regulation of Gene Expression for Bone Matrix Proteins*. Endocrinology 1990, 127, 1346–1351. [Google Scholar] [CrossRef]
- Emmanuelle, N.E.; Marie-Cécile, V.; Florence, T.; Jean-François, A.; Françoise, L.; Coralie, F.; Alexia, V. Critical role of estrogens on bone homeostasis in both male and female: From physiology to medical implications. Int. J. Mol. Sci. 2021, 22, 1–18. [Google Scholar]
- Börjesson, A.; Lagerquist, M.K.; Liu, C.; Shao, R.; Windahl, S.H.; Karlsson, C.; Sjögren, K.; Movérare-Skrtic, S.; Antal, M.C.; Krust, A.; et al. The role of estrogen receptor α in growth plate cartilage for longitudinal bone growth. J. Bone Miner. Res. 2010, 25, 2690–2700. [Google Scholar] [CrossRef]
- Steppe, L.; Krüger, B.T.; Tschaffon, M.E.A.; Fischer, V.; Tuckermann, J.; Ignatius, A.; Haffner-Luntzer, M. Estrogen Receptor α Signaling in Osteoblasts is Required for Mechanotransduction in Bone Fracture Healing. Front. Bioeng. Biotechnol. 2021, 9, 2355. [Google Scholar] [CrossRef]
- Röntgen, V.; Blakytny, R.; Matthys, R.; Landauer, M.; Wehner, T.; Göckelmann, M.; Jermendy, P.; Amling, M.; Schinke, T.; Claes, L.; et al. Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. J. Orthop. Res. 2010, 28, 1456–1462. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone mi-crostructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Kemmler, J.; Heidler, V.; Prystaz, K.; Schinke, T.; Amling, M.; Kovtun, A.; Rapp, A.E.; Ignatius, A.; Liedert, A. Inhibition of Midkine Augments Osteoporotic Fracture Healing. PLoS ONE 2016, 11, e0159278. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Mason, Z.D.; Chien, K.B.; Pfeiffer, A.J.; Barnes, G.L.; Einhorn, T.; Gerstenfeld, L.C. Micro-computed tomography assessment of fracture healing: Relationships among callus structure, composition, and mechanical function. Bone 2009, 44, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.E.; Bindl, R.; Recknagel, S.; Erbacher, A.; Müller, I.; Schrezenmeier, H.; Ehrnthaller, C.; Gebhard, F.; Ignatius, A. Fracture Healing Is Delayed in Immunodeficient NOD/scid-IL2Rγcnull Mice. PLoS ONE 2016, 11, e0147465. [Google Scholar] [CrossRef]




| Mouse Model | Females | Males | ||
|---|---|---|---|---|
| Cortical | Trabecular | Cortical | Trabecular | |
| Osteoblast lineage | ||||
| ERα fl/fl Prx1-Cre [29] | ↓ Ct. Th. | ↔ | ↓ Ct. Th. | ↔ |
| ERα fl/fl Runx2-Cre [30] | N.a. | ↓ | N.a. | N.a. |
| ERα fl/fl Runx2-Cre | ↔ | ↓ | ↑ | ↔ |
| ERα fl/fl Col1a1-Cre [29] | ↔ | ↔ | ↔ | ↔ |
| ERα fl/fl Osx1-Cre [29] | ↓ | ↔ | N.a. | N.a. |
| ERα fl/fl OC-Cre [31] | ↓ | ↓ | ↔ | ↔ |
| ERα fl/fl OC-Cre [32] | ↓ | ↓ | N.a. | N.a. |
| ERα fl/fl OC-Cre [33] C57Bl/6 background | ↓ | ↓ | ↑ Ct. Ar. ↑ Femoral length | ↑ Tb. Th, BV/TV |
| ERα fl/fl OC-Cre [34] | ↓ | ↓ Tb. BV | ↔ | ↔ |
| ERα fl/fl Dmp1-Cre [35] | ↔ | ↔ | ↔ | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steppe, L.; Bülow, J.; Tuckermann, J.; Ignatius, A.; Haffner-Luntzer, M. Bone Mass and Osteoblast Activity Are Sex-Dependent in Mice Lacking the Estrogen Receptor α in Chondrocytes and Osteoblast Progenitor Cells. Int. J. Mol. Sci. 2022, 23, 2902. https://doi.org/10.3390/ijms23052902
Steppe L, Bülow J, Tuckermann J, Ignatius A, Haffner-Luntzer M. Bone Mass and Osteoblast Activity Are Sex-Dependent in Mice Lacking the Estrogen Receptor α in Chondrocytes and Osteoblast Progenitor Cells. International Journal of Molecular Sciences. 2022; 23(5):2902. https://doi.org/10.3390/ijms23052902
Chicago/Turabian StyleSteppe, Lena, Jasmin Bülow, Jan Tuckermann, Anita Ignatius, and Melanie Haffner-Luntzer. 2022. "Bone Mass and Osteoblast Activity Are Sex-Dependent in Mice Lacking the Estrogen Receptor α in Chondrocytes and Osteoblast Progenitor Cells" International Journal of Molecular Sciences 23, no. 5: 2902. https://doi.org/10.3390/ijms23052902
APA StyleSteppe, L., Bülow, J., Tuckermann, J., Ignatius, A., & Haffner-Luntzer, M. (2022). Bone Mass and Osteoblast Activity Are Sex-Dependent in Mice Lacking the Estrogen Receptor α in Chondrocytes and Osteoblast Progenitor Cells. International Journal of Molecular Sciences, 23(5), 2902. https://doi.org/10.3390/ijms23052902






