2-Aminoimidazoles Inhibit Mycobacterium abscessus Biofilms in a Zinc-Dependent Manner
Abstract
1. Introduction
2. Results
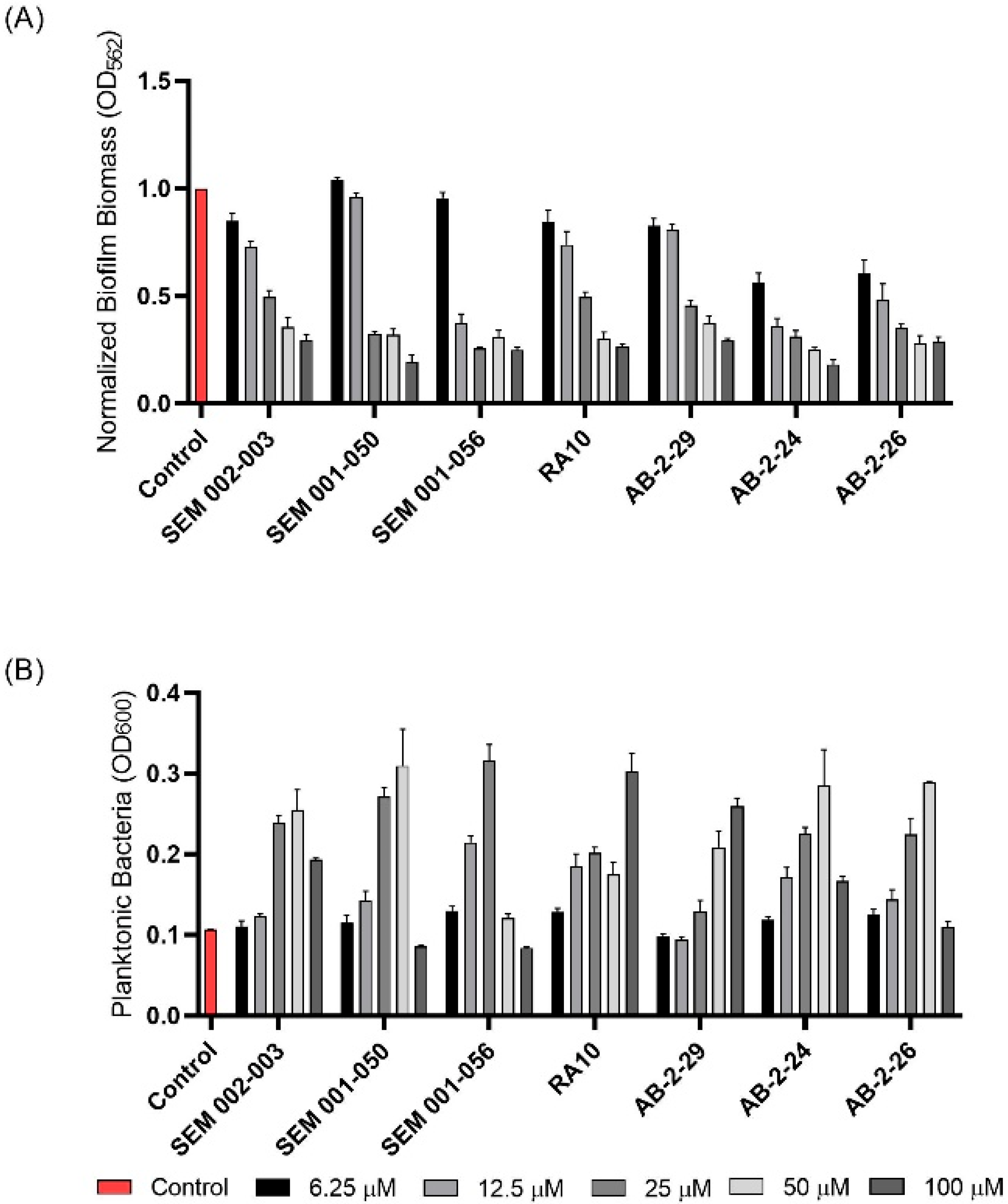
2.1. Inhibition of M. abscessus Biofilm Formation by 2-AI Compounds
2.2. Preliminary Investigations into the Mechanism of Biofilm Inhibition by AB-2-29 in M. abscessus
2.3. Alteration of M. abscessus Response to Zinc Starvation by AB-2-29
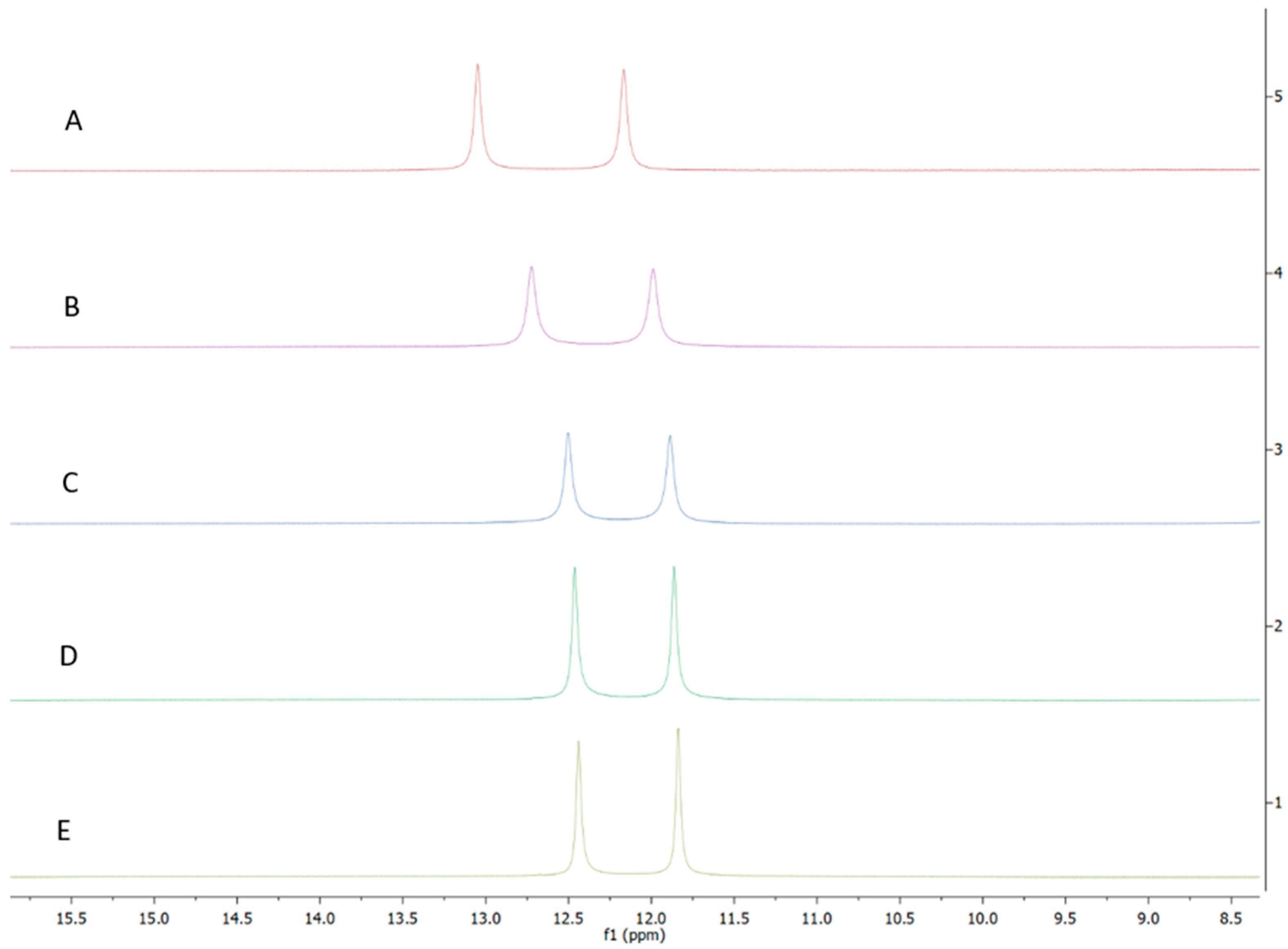
2.4. AB-2-29 Binds Zinc
2.5. Potentiation of the Biofilm Inhibitory Properties of AB-2-29 by Zinc
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Media
4.2. Biofilm Assay
4.3. Drug-Susceptibility Testing
4.4. Membrane Permeabilization Assay
4.5. Membrane Potential and Electrochemical Proton Gradient Measurements in Intact M. abscessus Bacilli
4.6. Assay for Succinate-Driven Proton Translocation into M. abscessus Inverted Membrane Vesicles (IMVs)
4.7. RNA Extraction, Reverse Transcription and RNAseq
4.8. Atomic Absorption Spectroscopy
4.9. Chemical Synthesis
4.10. Zinc and Iron Binding Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Floto, R.A.; Haworth, C.S. The growing threat of nontuberculous mycobacteria in CF. J. Cyst. Fibros. 2015, 14, 1–2. [Google Scholar] [CrossRef]
- Martiniano, S.L.; Nick, J.A.; Daley, C.L. Nontuberculous mycobacterial infections in cystic fibrosis. Thorac. Surg. Clin. 2019, 29, 95–108. [Google Scholar] [CrossRef]
- Park, I.K.; Olivier, K.N. Nontuberculous mycobacteria in cystic fibrosis and non–cystic fibrosis bronchiectasis. Semin. Respir. Crit. Care Med. 2015, 36, 217–224. [Google Scholar] [CrossRef]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, 905–913. [Google Scholar] [CrossRef]
- Haworth, C.S.; Banks, J.; Capstick, T.; Fisher, A.J.; Gorsuch, T.; Laurenson, I.F.; Leitch, A.; Loebinger, M.R.; Milburn, H.J.; Nightingale, M.; et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017, 72, ii1–ii64. [Google Scholar] [CrossRef]
- Egorova, A.; Jackson, M.; Gavrilyuk, V.; Makarov, V. Pipeline of anti-Mycobacterium abscessus small molecules: Repurposable drugs and promising novel chemical entities. Med. Res. Rev. 2021, 41, 2350–2387. [Google Scholar] [CrossRef]
- Maurer, F.P.; Bruderer, V.L.; Ritter, C.; Castelberg, C.; Bloemberg, G.V.; Böttger, E.C. Lack of antimicrobial bactericidal activity in mycobacterium abscessus. Antimicrob. Agents Chemother. 2014, 58, 3828–3836. [Google Scholar] [CrossRef]
- Wu, M.-L.; Aziz, D.B.; Dartois, V.; Dick, T. NTM drug discovery: Status, gaps and the way forward. Drug Discov. Today 2018, 23, 1502–1519. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Nash, K.A.; Wallace, R.J., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582. [Google Scholar] [CrossRef]
- Luthra, S.; Rominski, A.; Sander, P. The Role of Antibiotic-Target-Modifying and Antibiotic-Modifying Enzymes in Mycobacterium abscessus Drug Resistance. Front. Microbiol. 2018, 9, 2179. [Google Scholar] [CrossRef]
- Belardinelli, J.M.; Li, W.; Avanzi, C.; Angala, S.K.; Lian, E.; Wiersma, C.J.; Palčeková, Z.; Martin, K.H.; Angala, B.; de Moura, V.C.N.; et al. Unique Features of Mycobacterium abscessus Biofilms Formed in Synthetic Cystic Fibrosis Medium. Front. Microbiol. 2021, 12, 743126. [Google Scholar] [CrossRef]
- Fennelly, K.P.; Ojano-Dirain, C.; Yang, Q.; Liu, L.; Lu, L.; Progulske-Fox, A.; Wang, G.P.; Antonelli, P.; Schultz, G. Biofilm Formation by Mycobacterium abscessus in a Lung Cavity. Am. J. Respir. Crit. Care Med. 2016, 193, 692–693. [Google Scholar] [CrossRef]
- Høiby, N. A short history of microbial biofilms and biofilm infections. APMIS 2017, 125, 272–275. [Google Scholar] [CrossRef]
- Qvist, T.; Eickhardt, S.; Kragh, K.N.; Andersen, C.B.; Iversen, M.; Høiby, N.; Bjarnsholt, T. Chronic pulmonary disease with Mycobacterium abscessus complex is a biofilm infection. Eur. Respir. J. 2015, 46, 1823–1826. [Google Scholar] [CrossRef]
- Ackart, D.F.; Lindsey, E.A.; Podell, B.K.; Melander, R.J.; Basaraba, R.J.; Melander, C. Reversal of Mycobacterium tuberculosis phenotypic drug resistance by 2-aminoimidazole-based small molecules. Pathog. Dis. 2014, 70, 370–378. [Google Scholar] [CrossRef]
- Blackledge, M.S.; Worthington, R.J.; Melander, C. Biologically inspired strategies for combating bacterial biofilms. Curr. Opin. Pharmacol. 2013, 13, 699–706. [Google Scholar] [CrossRef]
- Brackett, S.M.; Cox, K.E.; Barlock, S.L.; Huggins, W.M.; Ackart, D.F.; Bassaraba, R.J.; Melander, R.J.; Melander, C. Meridianin D analogues possess antibiofilm activity against Mycobacterium smegmatis. RSC Med. Chem. 2020, 11, 92–97. [Google Scholar] [CrossRef]
- Cox, K.E.; Melander, C. Anti-biofilm activity of quinazoline derivatives against Mycobacterium smegmatis. MedChemComm 2019, 10, 1177–1179. [Google Scholar] [CrossRef]
- Jeon, A.B.; Obregón-Henao, A.; Ackart, D.F.; Podell, B.K.; Belardinelli, J.M.; Jackson, M.; Nguyen, T.V.; Blackledge, M.; Melander, R.J.; Melander, C.; et al. 2-aminoimidazoles potentiate ß-lactam antimicrobial activity against Mycobacterium tuberculosis by reducing ß-lactamase secretion and increasing cell envelope permeability. PLoS ONE 2017, 12, e0180925. [Google Scholar] [CrossRef]
- Martin, S.E.; Nguyen, C.M.; Basaraba, R.J.; Melander, C. Analogue synthesis reveals decoupling of antibiofilm and beta-lactam potentiation activities of a lead 2-aminoimidazole adjuvant against Mycobacterium smegmatis. Chem. Biol. Drug Des. 2018, 92, 1403–1408. [Google Scholar] [CrossRef]
- Melander, R.J.; Melander, C. Innovative Strategies for Combating Biofilm-Based Infections. Adv. Exp. Med. Biol. 2015, 831, 69–91. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Minrovic, B.M.; Melander, R.J.; Melander, C. Identification of Anti-Mycobacterial Biofilm Agents Based on the 2-Aminoimidazole Scaffold. ChemMedChem 2019, 14, 927–937. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Peszko, M.; Melander, R.J.; Melander, C.; Worthington, R. Using 2-aminobenzimidazole derivatives to inhibit Mycobacterium smegmatis biofilm formation. MedChemComm 2019, 10, 456–459. [Google Scholar] [CrossRef]
- Zeiler, M.J.; Melander, R.J.; Melander, C.C. Second-Generation Meridianin Analogues Inhibit the Formation of Mycobacterium smegmatis Biofilms and Sensitize Polymyxin-Resistant Gram-Negative Bacteria to Colistin. ChemMedChem 2020, 15, 1672–1679. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Blackledge, M.S.; Lindsey, E.A.; Minrovic, B.M.; Ackart, D.F.; Jeon, A.B.; Obregón-Henao, A.; Melander, R.J.; Basaraba, R.J.; Melander, C. The Discovery of 2-Aminobenzimidazoles That Sensitize Mycobacterium smegmatis and M. tuberculosis to beta-Lactam Antibiotics in a Pattern Distinct from beta-Lactamase Inhibitors. Angew. Chem. Int. Ed. Engl. 2017, 56, 3940–3944. [Google Scholar] [CrossRef]
- Wiersma, C.J.; Belardinelli, J.M.; Avanzi, C.; Angala, S.K.; Everall, I.; Angala, B.; Kendall, E.; De Moura, V.C.N.; Verma, D.; Benoit, J.; et al. Cell Surface Remodeling of Mycobacterium abscessus under Cystic Fibrosis Airway Growth Conditions. ACS Infect. Dis. 2020, 6, 2143–2154. [Google Scholar] [CrossRef]
- Frei, R.; Breitbach, A.S.; Blackwell, H.E. 2-Aminobenzimidazole Derivatives Strongly Inhibit and Disperse Pseudomonas aeruginosa Biofilms. Angew. Chem. Int. Ed. 2012, 51, 5226–5229. [Google Scholar] [CrossRef]
- Harris, T.L.; Worthington, R.J.; Hittle, L.E.; Zurawski, D.V.; Ernst, R.; Melander, C. Small Molecule Downregulation of PmrAB Reverses Lipid A Modification and Breaks Colistin Resistance. ACS Chem. Biol. 2014, 9, 122–127. [Google Scholar] [CrossRef]
- Harris, T.L.; Worthington, R.J.; Melander, C. Potent Small-Molecule Suppression of Oxacillin Resistance in Methicillin-Resistant Staphylococcus aureus. Angew. Chem. Int. Ed. 2012, 51, 11254–11257. [Google Scholar] [CrossRef]
- Milton, M.E.; Allen, C.L.; Feldmann, E.A.; Bobay, B.; Jung, D.K.; Stephens, M.D.; Melander, R.J.; Theisen, K.E.; Zeng, D.; Thompson, R.J.; et al. Structure of the Francisella response regulator QseB receiver domain, and characterization of QseB inhibition by antibiofilm 2-aminoimidazole-based compounds. Mol. Microbiol. 2017, 106, 223–235. [Google Scholar] [CrossRef]
- Milton, M.E.; Minrovic, B.M.; Harris, D.L.; Kang, B.; Jung, D.; Lewis, C.P.; Thompson, R.J.; Melander, R.J.; Zeng, D.; Melander, C.; et al. Re-sensitizing Multidrug Resistant Bacteria to Antibiotics by Targeting Bacterial Response Regulators: Characterization and Comparison of Interactions between 2-Aminoimidazoles and the Response Regulators BfmR from Acinetobacter baumannii and QseB from Francisella spp. Front. Mol. Biosci. 2018, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J.; Bobay, B.G.; Stowe, S.D.; Olson, A.L.; Peng, L.; Su, Z.; Actis, L.A.; Melander, C.; Cavanagh, J. Identification of BfmR, a Response Regulator Involved in Biofilm Development, as a Target for a 2-Aminoimidazole-Based Antibiofilm Agent. Biochemistry 2012, 51, 9776–9778. [Google Scholar] [CrossRef] [PubMed]
- Worthington, R.J.; Blackledge, M.S.; Melander, C. Small-molecule inhibition of bacterial two-component systems to combat antibiotic resistance and virulence. Futur. Med. Chem. 2013, 5, 1265–1284. [Google Scholar] [CrossRef] [PubMed]
- Jeon, A.B.; Ackart, D.F.; Li, W.; Jackson, M.; Melander, R.J.; Melander, C.; Abramovitch, R.B.; Chicco, A.J.; Basaraba, R.J.; Obregón-Henao, A. 2-aminoimidazoles collapse mycobacterial proton motive force and block the electron transport chain. Sci. Rep. 2019, 9, 1513. [Google Scholar] [CrossRef]
- Hurdle, J.G.; O’neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef]
- Stowe, S.D.; Thompson, R.J.; Peng, L.; Su, Z.; Blackledge, M.S.; Draughn, G.L.; Coe, W.H.; Johannes, E.; Lapham, V.K.; MacKenzie, J.; et al. Membrane-permeabilizing activity of reverse-amide 2-aminoimidazole antibiofilm agents against Acinetobacter baumannii. Curr. Drug Deliv. 2015, 12, 223–230. [Google Scholar] [CrossRef]
- Mikhaylina, A.; Ksibe, A.Z.; Scanlan, D.; Blindauer, C.A. Bacterial zinc uptake regulator proteins and their regulons. Biochem. Soc. Trans. 2018, 46, 983–1001. [Google Scholar] [CrossRef]
- Goethe, E.; Laarmann, K.; Lührs, J.; Jarek, M.; Meens, J.; Lewin, A.; Goethe, R. Critical Role of Zur and SmtB in Zinc Homeostasis of Mycobacterium smegmatis. mSystems 2020, 5, e00880-19. [Google Scholar] [CrossRef]
- Maciag, A.; Dainese, E.; Rodriguez, G.M.; Milano, A.; Provvedi, R.; Pasca, M.R.; Smith, I.; Palu, G.; Riccardi, G.; Manganelli, R. Global analysis of the Mycobacterium tuberculosis Zur (FurB) regulon. J. Bacteriol. 2007, 189, 730–740. [Google Scholar] [CrossRef]
- Mrázek, J.; Xie, S. Pattern locator: A new tool for finding local sequence patterns in genomic DNA sequences. Bioinformatics 2006, 22, 3099–3100. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. WebLogo: A Sequence Logo Generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, E.A.; Brackett, C.M.; Mullikin, T.; Alcaraz, C.; Melander, C. The discovery of N-1 substituted 2-aminobenzimidazoles as zinc-dependent S. aureus biofilm inhibitors. MedChemComm 2012, 3, 1462–1465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rogers, S.A.; Huigens, R.W., III; Melander, C. A 2-aminobenzimidazole that inhibits and disperses gram-positive biofilms through a zinc-dependent mechanism. J. Am. Chem. Soc. 2009, 131, 9868–9869. [Google Scholar] [CrossRef]
- Mathuthu, E.; van Rensburg, A.J.; Du Plessis, D.; Mason, S. EDTA as a chelating agent in quantitative 1H-NMR of biologically important ions. Biochem. Cell Biol. 2021, 99, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Hatfull, G.F. The role of iron in Mycobacterium smegmatis biofilm formation: The exochelin siderophore is essential in limiting iron conditions for biofilm formation but not for planktonic growth. Mol. Microbiol. 2007, 66, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Agertt, V.A.; Bonez, P.C.; Rossi, G.G.; Flores, V.D.C.; Siqueira, F.D.S.; Mizdal, C.R.; Marques, L.L.; De Oliveira, G.N.M.; De Campos, M.M.A. Identification of antimicrobial activity among new sulfonamide metal complexes for combating rapidly growing mycobacteria. BioMetals 2016, 29, 807–816. [Google Scholar] [CrossRef]
- Bonez, P.C.; Agertt, V.A.; Rossi, G.G.; Siqueira, F.D.S.; Siqueira, J.D.; Marques, L.L.; de Oliveira, G.N.M.; Santos, R.C.V.; de Campos, M.M.A. Sulfonamides complexed with metals as mycobacterial biofilms inhibitors. J. Clin. Tuberc. Other Mycobact. Dis. 2021, 23, 100217. [Google Scholar] [CrossRef]
- Smith, D.J.; Anderson, G.; Bell, S.; Reid, D. Elevated metal concentrations in the CF airway correlate with cellular injury and disease severity. J. Cyst. Fibros. 2014, 13, 289–295. [Google Scholar] [CrossRef]
- Li, W.; Stevens, C.M.; Pandya, A.N.; Darzynkiewicz, Z.M.; Bhattarai, P.; Tong, W.; Gonzalez-Juarrero, M.; North, E.J.; Zgurskaya, H.I.; Jackson, M.C. Direct Inhibition of MmpL3 by Novel Antitubercular Compounds. ACS Infect. Dis. 2019, 5, 1001–1012. [Google Scholar] [CrossRef]


| Compound | MIC (M) | IC50 (M) | |||||
|---|---|---|---|---|---|---|---|
| Mmas CIP 108297 | Mabs ATCC 19977 | Mabs NJH12 | Mmas 1239 | Mabs NJH9 | Mmas NJH18 | ||
EL-05-047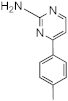 | >100 | >100 | n.d. | 50–100 | n.d. | 50–100 | 100 |
2B8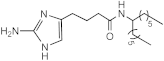 | 25 | 12.5–25 | n.d. | 25 | n.d. | 12.5–25 | 25 |
SEM-002-004 | >50 | >200 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-075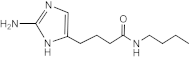 | >50 | >200 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-073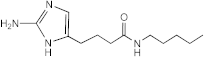 | >50 | >200 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-078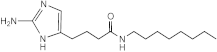 | 100–200 | 50 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-002-003 | 50–100 (Mmas CIP 108297 and Mabs ATCC 19977) 50 (Mabs NJH9, Mabs NJH12 and Mmas NJH18) | 25 | n.d. | 12.5–25 | n.d. | 12.5–25 | 50 |
RA10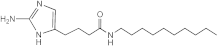 | >50 | 25 | n.d. | n.d. | n.d. | n.d. | n.d. |
RA12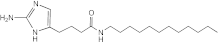 | 50 | >50 | n.d. | n.d. | n.d. | n.d. | n.d. |
RA13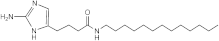 | >50 | 50 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-034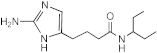 | >50 | >200 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-044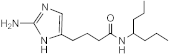 | >50 | >200 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-046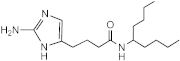 | 100–200 | 50 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-056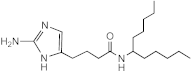 | 50 | 12.5–25 | 12.5–25 | 25 | 25 | 12.5–25 | 25 |
SEM-001-049 | 12.5–25 | 25 | n.d. | n.d. | n.d. | n.d. | n.d. |
SEM-001-050 | 50 (Mabs NJH9, Mabs NJH12, Mmas NJH18, Mmas CIP108297) 100 (Mabs ATCC 19977) | 12.5–25 | n.d. | 12.5–25 | n.d. | 12.5–25 | 12.5–25 |
SEM-001-057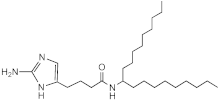 | 50–100 | 50 | n.d. | n.d. | n.d. | n.d. | n.d. |
VN03-049 | 50–100 | 25 | n.d. | n.d. | n.d. | n.d. | n.d. |
VN03-063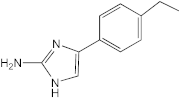 | 25–50 | 25 | n.d. | n.d. | n.d. | n.d. | n.d. |
VN03-074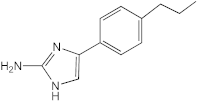 | 50–100 | 25–50 | n.d. | n.d. | n.d. | n.d. | n.d. |
4C3 | 25–50 | 12.5 | n.d. | n.d. | n.d. | n.d. | n.d. |
4B10 | 12.5–25 | 12.5 | n.d. | n.d. | n.d. | n.d. | n.d. |
4C2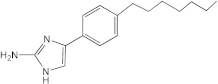 | 50 | 12.5–25 | n.d. | n.d. | n.d. | n.d. | n.d. |
VN03-064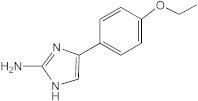 | 25 | 25 | n.d. | n.d. | n.d. | n.d. | n.d. |
AB-2-29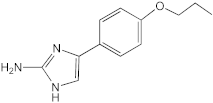 | >100 | 25 | 25 | 12.5–25 | 50 | 12.5 | 25 |
AB-2-24 | 64 | 12.5 | 25–50 | 12.5 | 25 | 12.5–25 | 12.5–25 |
AB-2-26 | 64 | 12.5–25 | 25–50 | 12.5–25 | 25 | 6.25–12.5 | 12.5–25 |
7.079 (meridianin)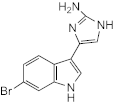 | >100 | 100 | 100 | 50 | n.d. | 50–100 | 100 |
7.025 (meridianin)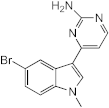 | >100 | >100 | 100 | 50–100 | n.d. | >100 | 50–100 |
8.001 (meridianin) | >100 | >100 | >100 | >100 | n.d. | >100 | >100 |
| Gene | Description | Putative Function | Log2 Fold-Change | |
|---|---|---|---|---|
| AB-2-29 vs. Ctrl 3 h | AB-2-29 vs. Ctrl 24 h | |||
| MAB_0331c | 30S ribosomal protein S18 RpsR2 | Zn-independent ribosomal proteins | −8.04 | −9.31 |
| MAB_0332c | 30S ribosomal protein S14 RpsN2 | −7.76 | −8.93 | |
| MAB_0333c | 50S ribosomal protein L33 RpmG1 | −9.72 | −6.26 | |
| MAB_0334c * | 50S ribosomal protein L28 RpmB2 | −9.30 | −7.83 | |
| MAB_0335 * | Probable cobalamin synthesis protein | Cobalamin biosynthesis | −7.95 | −10.46 |
| MAB_0336 | 50S ribosomal protein L31 type B | Zn-independent ribosomal protein | −8.52 | −9.38 |
| MAB_0575c | Putative ABC-transporter transmembrane protein | ZnuABC transporter (Zn import) | −2.91 | −2.39 |
| MAB_0576c | Putative ABC-transporter ATP-binding protein | −4.05 | −2.89 | |
| MAB_0577c * | Putative ABC-transporter solute binding protein | −5.54 | −5.40 | |
| MAB_0809c * | Conserved hypothetical PPE family protein | Unknown | −5.77 | −4.66 |
| MAB_1680 * | Hypothetical protein | Zn-siderophore biosynthesis and transport | −9.76 | −8.14 |
| MAB_1681 | Hypothetical protein | −9.84 | −7.80 | |
| MAB_1682 | Probable NAD-dependent epimerase/dehydratase | −9.04 | −8.69 | |
| MAB_1683 | Putative fatty acid desaturase | −10.30 | −7.96 | |
| MAB_1684 | Diaminobutyrate-−2-oxoglutarate aminotransferase | −9.48 | −8.31 | |
| MAB_1685 | Putative decarboxylase | −7.35 | −7.01 | |
| MAB_1686 | Hypothetical protein | −8.48 | −7.31 | |
| MAB_1687 | Hypothetical protein | −8.09 | −8.20 | |
| MAB_1688 | Hypothetical protein | −7.85 | −7.90 | |
| MAB_1689 | Probable ABC-transporter ATP-binding subunit DrrA | −7.99 | −7.48 | |
| MAB_1690 | Putative ABC-transporter transmembrane protein | −8.82 | −6.46 | |
| MAB_1691 | Hypothetical protein | −9.86 | −8.31 | |
| MAB_1692 | Putative polyketide synthase Pks16/acyl-CoA synthetase | −7.95 | −7.05 | |
| MAB_1693 | Conserved hypothetical protein (YrbE family?) | −8.46 | −7.52 | |
| MAB_1694 | Putative YrbE family protein | −8.65 | −6.58 | |
| MAB_1695 | Putative Mce family protein | −8.60 | −5.87 | |
| MAB_1696 | Putative Mce family protein | −7.56 | −6.09 | |
| MAB_1697 | Putative Mce family protein | −7.06 | −6.29 | |
| MAB_1698 | Putative Mce family protein | −7.13 | −5.57 | |
| MAB_1699 | Putative Mce family protein | −7.33 | −4.90 | |
| MAB_1700 | Putative Mce family protein | −6.82 | −5.02 | |
| MAB_1701 | Hypothetical protein | −5.82 | −3.61 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belardinelli, J.M.; Li, W.; Martin, K.H.; Zeiler, M.J.; Lian, E.; Avanzi, C.; Wiersma, C.J.; Nguyen, T.V.; Angala, B.; de Moura, V.C.N.; et al. 2-Aminoimidazoles Inhibit Mycobacterium abscessus Biofilms in a Zinc-Dependent Manner. Int. J. Mol. Sci. 2022, 23, 2950. https://doi.org/10.3390/ijms23062950
Belardinelli JM, Li W, Martin KH, Zeiler MJ, Lian E, Avanzi C, Wiersma CJ, Nguyen TV, Angala B, de Moura VCN, et al. 2-Aminoimidazoles Inhibit Mycobacterium abscessus Biofilms in a Zinc-Dependent Manner. International Journal of Molecular Sciences. 2022; 23(6):2950. https://doi.org/10.3390/ijms23062950
Chicago/Turabian StyleBelardinelli, Juan M., Wei Li, Kevin H. Martin, Michael J. Zeiler, Elena Lian, Charlotte Avanzi, Crystal J. Wiersma, Tuan Vu Nguyen, Bhanupriya Angala, Vinicius C. N. de Moura, and et al. 2022. "2-Aminoimidazoles Inhibit Mycobacterium abscessus Biofilms in a Zinc-Dependent Manner" International Journal of Molecular Sciences 23, no. 6: 2950. https://doi.org/10.3390/ijms23062950
APA StyleBelardinelli, J. M., Li, W., Martin, K. H., Zeiler, M. J., Lian, E., Avanzi, C., Wiersma, C. J., Nguyen, T. V., Angala, B., de Moura, V. C. N., Jones, V., Borlee, B. R., Melander, C., & Jackson, M. (2022). 2-Aminoimidazoles Inhibit Mycobacterium abscessus Biofilms in a Zinc-Dependent Manner. International Journal of Molecular Sciences, 23(6), 2950. https://doi.org/10.3390/ijms23062950







