The Impact of Sub-Millisecond Damage Fixation Kinetics on the In Vitro Sparing Effect at Ultra-High Dose Rate in UNIVERSE
Abstract
:1. Introduction
2. Results
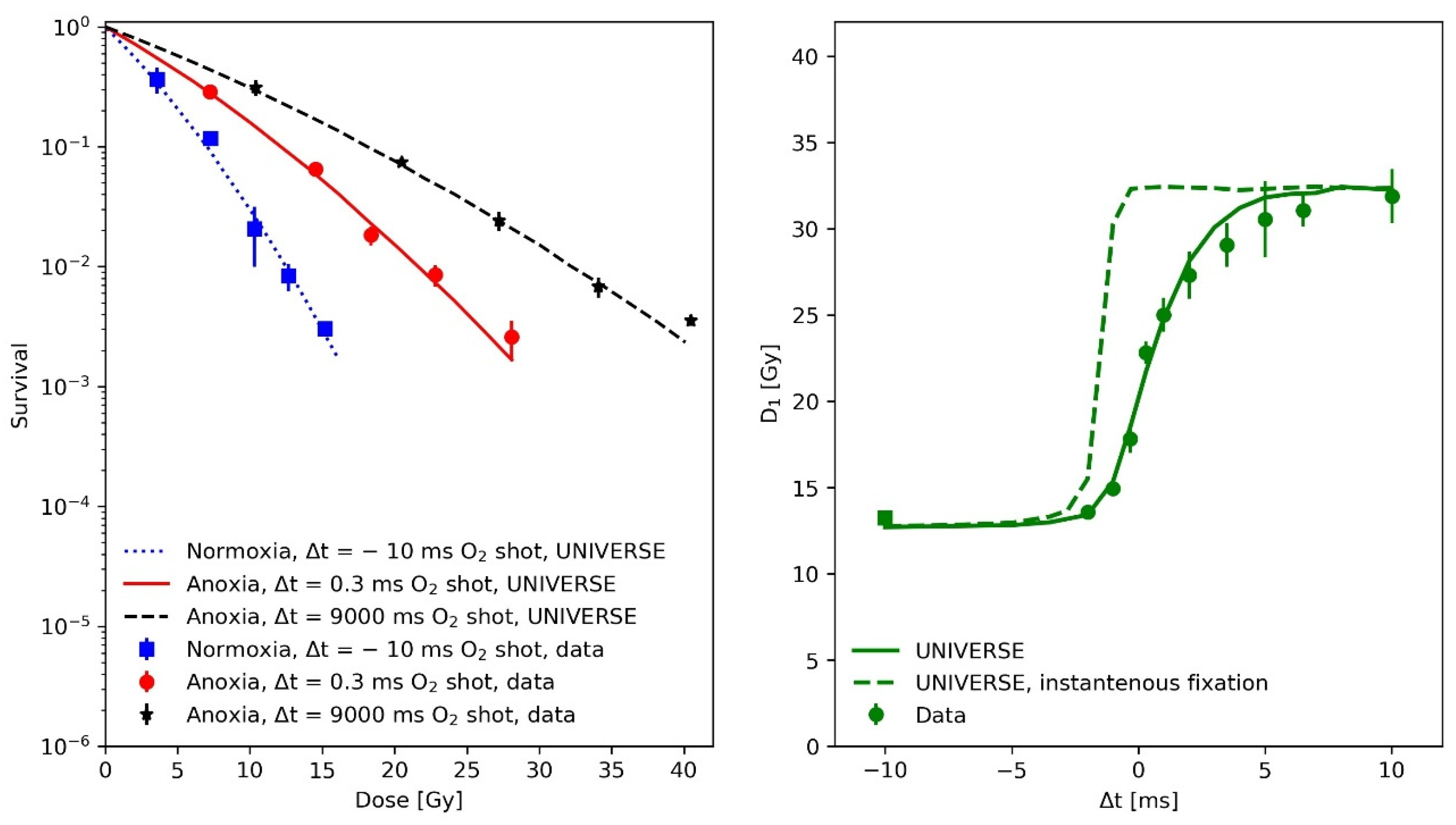
2.1. Modeling the Sub-Milliseconds Kinetics of Damage Fixation in UNIVERSE
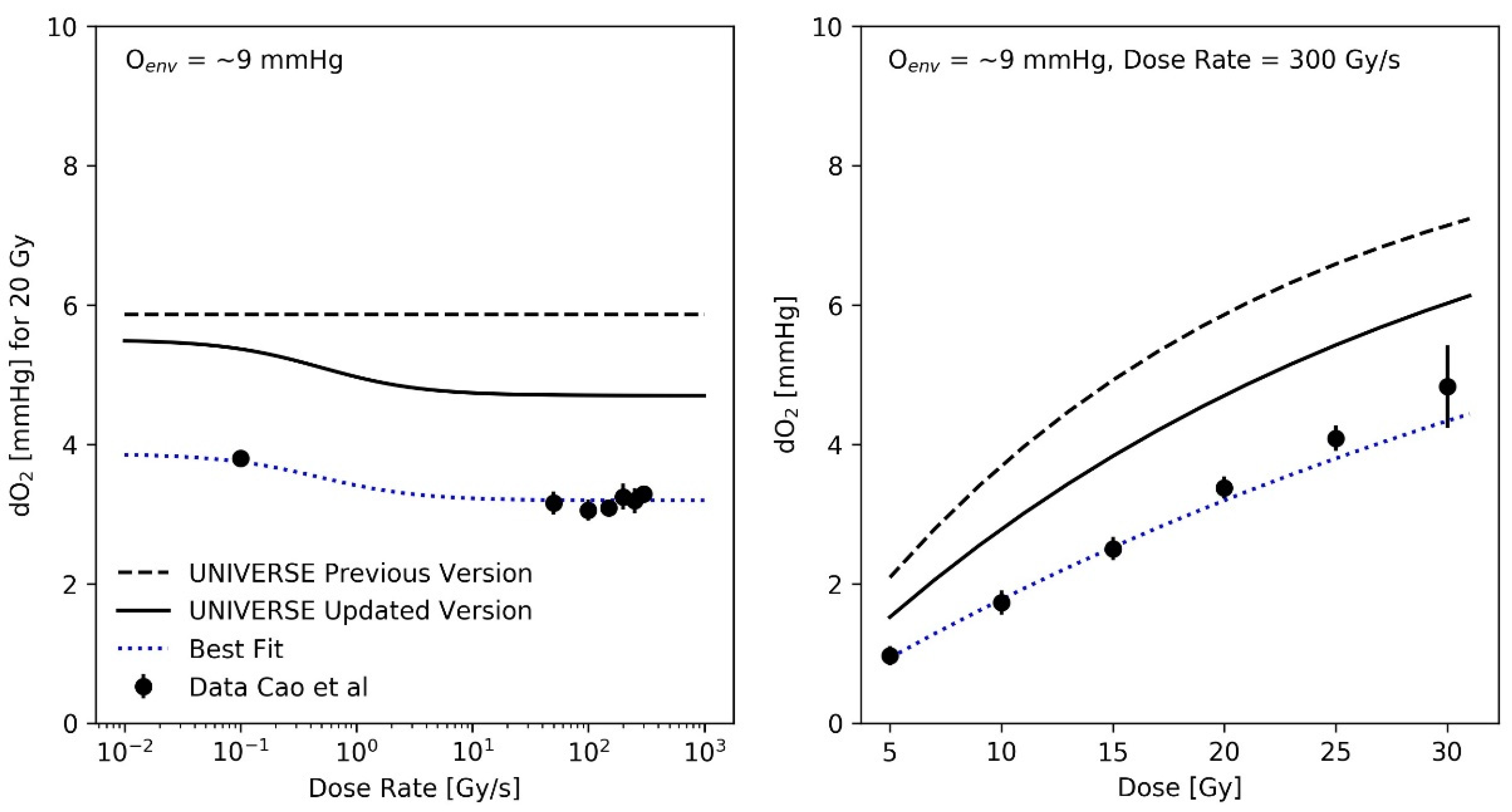
2.2. Resulting O2 Depletion in UNIVERSE vs. Experimental Data in the Literature
2.3. Recalculation of Previous Benchmarks
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Data from Literature
5.2. Modeling Approach
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LET | Linear energy transfer |
| SDR | Standard dose rate |
| uHDR | Ultra high dose rate |
| UNIVERSE | UNIfied and VERSatile bio response Engine |
| DSB | (DNA) Double strand break |
| iDSB | Isolated DSB |
| cDSB | Complex DSB |
| OER | Oxygen enhancement ratio |
| HRF | Hypoxia reduction factor |
| CHO | Chinese hamster ovary |
References
- Town, C.D. Effect of High Dose Rates on Survival of Mammalian Cells. Nature 1967, 215, 847–848. [Google Scholar] [CrossRef] [PubMed]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh Dose-Rate FLASH Irradiation Increases the Differential Response between Normal and Tumor Tissue in Mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef] [PubMed]
- Vozenin, M.-C.; Hendry, J.H.; Limoli, C.L. Biological Benefits of Ultra-High Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin. Oncol. 2019, 31, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front. Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G. Mechanisms Underlying FLASH Radiotherapy, a Novel Way to Enlarge the Differential Responses to Ionizing Radiation between Normal and Tumor Tissues. Radiat. Med. Prot. 2020, 1, 35–40. [Google Scholar] [CrossRef]
- Weber, U.A.; Scifoni, E.; Durante, M. FLASH Radiotherapy with Carbon Ion Beams. Med. Phys. 2021. [Google Scholar] [CrossRef]
- Labarbe, R.; Hotoiu, L.; Barbier, J.; Favaudon, V. A Physicochemical Model of Reaction Kinetics Supports Peroxyl Radical Recombination as the Main Determinant of the FLASH Effect. Radiother. Oncol. 2020, 153, 303–310. [Google Scholar] [CrossRef]
- Petersson, K.; Adrian, G.; Butterworth, K.; McMahon, S.J. A Quantitative Analysis of the Role of Oxygen Tension in FLASH Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 539–547. [Google Scholar] [CrossRef]
- Joiner, M.; van der Kogel, A. (Eds.) Basic Clinical Radiobiology, 5th ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018; ISBN 978-1-4441-7963-7. [Google Scholar]
- Grimes, D.R.; Partridge, M. A Mechanistic Investigation of the Oxygen Fixation Hypothesis and Oxygen Enhancement Ratio. Biomed. Phys. Eng. Express 2015, 1, 045209. [Google Scholar] [CrossRef]
- Pratx, G.; Kapp, D.S. A Computational Model of Radiolytic Oxygen Depletion during FLASH Irradiation and Its Effect on the Oxygen Enhancement Ratio. Phys. Med. Biol. 2019, 64, 185005. [Google Scholar] [CrossRef] [Green Version]
- Liew, H.; Mein, S.; Dokic, I.; Haberer, T.; Debus, J.; Abdollahi, A.; Mairani, A. Deciphering Time-Dependent DNA Damage Complexity, Repair and Oxygen Tension: A Mechanistic Model for FLASH-Dose-Rate Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.; Montay-Gruel, P.; Gonçalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical Translation of FLASH Radiotherapy: Why and How? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.E.; Maughan, R.L.; Michael, B.D. Fast Kinetics of the Oxygen Effect in Irradiated Mammalian Cells. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1978, 33, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.C.; Michaels, H.B.; Epp, E.R.; Peterson, E.C. Oxygen Diffusion into Mammalian Cells Following Ultrahigh Dose Rate Irradiation and Lifetime Estimates of Oxygen-Sensitive Species. Radiat. Res. 1978, 76, 522–532. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, R.; Esipova, T.V.; Allu, S.R.; Ashraf, R.; Rahman, M.; Gunn, J.R.; Bruza, P.; Gladstone, D.J.; Williams, B.B.; et al. Quantification of Oxygen Depletion During FLASH Irradiation In Vitro and In Vivo. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 240–248. [Google Scholar] [CrossRef]
- Michaels, H.B.; Epp, E.R.; Ling, C.C.; Peterson, E.C. Oxygen Sensitization of CHO Cells at Ultrahigh Dose Rates: Prelude to Oxygen Diffusion Studies. Radiat. Res. 1978, 76, 510–521. [Google Scholar] [CrossRef]
- Esipova, T.V.; Barrett, M.J.P.; Erlebach, E.; Masunov, A.E.; Weber, B.; Vinogradov, S.A. Oxyphor 2P: A High-Performance Probe for Deep-Tissue Longitudinal Oxygen Imaging. Cell Metab. 2019, 29, 736–744.e7. [Google Scholar] [CrossRef] [Green Version]
- Jansen, J.; Knoll, J.; Beyreuther, E.; Pawelke, J.; Skuza, R.; Hanley, R.; Brons, S.; Pagliari, F.; Seco, J. Does FLASH Deplete Oxygen? Experimental Evaluation for Photons, Protons, and Carbon Ions. Med. Phys. 2021, 48, 3982–3990. [Google Scholar] [CrossRef]
- Epp, E.R.; Weiss, H.; Djordjevic, B.; Santomasso, A. The Radiosensitivity of Cultured Mammalian Cells Exposed to Single High Intensity Pulses of Electrons in Various Concentrations of Oxygen. Radiat. Res. 1972, 52, 324–332. [Google Scholar] [CrossRef]
- Adrian, G.; Konradsson, E.; Lempart, M.; Bäck, S.; Ceberg, C.; Petersson, K. The FLASH Effect Depends on Oxygen Concentration. Br. J. Radiol. 2019, 93, 20190702. [Google Scholar] [CrossRef]
- Han, Y.; Liu, X.-M.; Liu, H.; Li, S.-C.; Wu, B.-C.; Ye, L.-L.; Wang, Q.-W.; Chen, Z.-L. Cultivation of Recombinant Chinese Hamster Ovary Cells Grown as Suspended Aggregates in Stirred Vessels. J. Biosci. Bioeng. 2006, 102, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Gerweck, L.E.; Dullea, R.; Zaidi, S.T.; Budach, W.; Hartford, A. Influence of Experimental Factors on Intrinsic Radiosensitivity Assays at Low Doses of Radiation: Cell Multiplicity. Radiat. Res. 1994, 138, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Spitz, D.R.; Buettner, G.R.; Petronek, M.S.; St-Aubin, J.J.; Flynn, R.T.; Waldron, T.J.; Limoli, C.L. An Integrated Physico-Chemical Approach for Explaining the Differential Impact of FLASH versus Conventional Dose Rate Irradiation on Cancer and Normal Tissue Responses. Radiother. Oncol. 2019, 139, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Radiotherapy Using High-Intensity Pulsed Radiation Beams (FLASH): A Radiation-Chemical Perspective. Radiat. Res. 2020, 194, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.J.; Hall, E.J.; Forster, D.W.; Storr, T.H.; Goodman, M.J. Survival of Mammalian Cells Exposed to X Rays at Ultra-High Dose-Rates. Br. J. Radiol. 1969, 42, 102–107. [Google Scholar] [CrossRef]
- Liew, H.; Klein, C.; Zenke, F.T.; Abdollahi, A.; Debus, J.; Dokic, I.; Mairani, A. Modeling the Effect of Hypoxia and DNA Repair Inhibition on Cell Survival After Photon Irradiation. Int. J. Mol. Sci. 2019, 20, 6054. [Google Scholar] [CrossRef] [Green Version]
- Liew, H.; Meister, S.; Mein, S.; Tessonnier, T.; Kopp, B.; Held, T.; Haberer, T.; Abdollahi, A.; Debus, J.; Dokic, I.; et al. Combined DNA Damage Repair Interference and Ion Beam Therapy: Development, Benchmark, and Clinical Implications of a Mechanistic Biological Model. Int. J. Radiat. Oncol. Biol. Phys. 2021, 112, 802–817. [Google Scholar] [CrossRef]
- Liew, H.; Mein, S.; Debus, J.; Dokic, I.; Mairani, A. Modeling Direct and Indirect Action on Cell Survival After Photon Irradiation under Normoxia and Hypoxia. Int. J. Mol. Sci. 2020, 21, 3471. [Google Scholar] [CrossRef]
- Liang, Y.; Fu, Q.; Wang, X.; Liu, F.; Yang, G.; Luo, C.; Ouyang, Q.; Wang, Y. Relative Biological Effectiveness for Photons: Implication of Complex DNA Double-Strand Breaks as Critical Lesions. Phys. Med. Biol. 2017, 62, 2153–2175. [Google Scholar] [CrossRef]
- Stewart, R.D.; Yu, V.K.; Georgakilas, A.G.; Koumenis, C.; Park, J.H.; Carlson, D.J. Effects of Radiation Quality and Oxygen on Clustered DNA Lesions and Cell Death. Radiat. Res. 2011, 176, 587–602. [Google Scholar] [CrossRef]
- Sachs, R.K.; van den Engh, G.; Trask, B.; Yokota, H.; Hearst, J.E. A Random-Walk/Giant-Loop Model for Interphase Chromosomes. Proc. Natl. Acad. Sci. USA 1995, 92, 2710–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, P.J.; Olive, P.L.; Bryant, P.E. Higher-Order Chromatin Structure-Dependent Repair of DNA Double-Strand Breaks: Modeling the Elution of DNA from Nucleoids. Radiat. Res. 1997, 148, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.J.; MacPhail, S.H.; Banáth, J.P.; Olive, P.L. Higher-Order Chromatin Structure-Dependent Repair of DNA Double-Strand Breaks: Factors Affecting Elution of DNA from Nucleoids. Radiat. Res. 1998, 149, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; van den Engh, G.; Hearst, J.E.; Sachs, R.K.; Trask, B.J. Evidence for the Organization of Chromatin in Megabase Pair-Sized Loops Arranged along a Random Walk Path in the Human G0/G1 Interphase Nucleus. J. Cell Biol. 1995, 130, 1239–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostashevsky, J. A Polymer Model for the Structural Organization of Chromatin Loops and Minibands in Interphase Chromosomes. Mol. Biol. Cell 1998, 9, 3031–3040. [Google Scholar] [CrossRef] [Green Version]
- Elsässer, T.; Weyrather, W.K.; Friedrich, T.; Durante, M.; Iancu, G.; Krämer, M.; Kragl, G.; Brons, S.; Winter, M.; Weber, K.-J.; et al. Quantification of the Relative Biological Effectiveness for Ion Beam Radiotherapy: Direct Experimental Comparison of Proton and Carbon Ion Beams and a Novel Approach for Treatment Planning. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1177–1183. [Google Scholar] [CrossRef]
- Friedrich, T.; Durante, M.; Scholz, M. Modeling Cell Survival after Photon Irradiation Based on Double-Strand Break Clustering in Megabase Pair Chromatin Loops. Radiat. Res. 2012, 178, 385–394. [Google Scholar] [CrossRef]
- Tommasino, F.; Friedrich, T.; Scholz, U.; Taucher-Scholz, G.; Durante, M.; Scholz, M. A DNA Double-Strand Break Kinetic Rejoining Model Based on the Local Effect Model. Radiat. Res. 2013, 180, 524–538. [Google Scholar] [CrossRef] [Green Version]
- Tommasino, F.; Friedrich, T.; Jakob, B.; Meyer, B.; Durante, M.; Scholz, M. Induction and Processing of the Radiation-Induced Gamma-H2AX Signal and Its Link to the Underlying Pattern of DSB: A Combined Experimental and Modelling Study. PLoS ONE 2015, 10, e0129416. [Google Scholar] [CrossRef] [Green Version]
- Carlson, D.J.; Stewart, R.D.; Semenenko, V.A. Effects of Oxygen on Intrinsic Radiation Sensitivity: A Test of the Relationship between Aerobic and Hypoxic Linear-Quadratic (LQ) Model Parameters. Med. Phys. 2006, 33, 3105–3115. [Google Scholar] [CrossRef]
- Alper, T.; Howard-Flanders, P. Role of Oxygen in Modifying the Radiosensitivity of E. Coli B. Nature 1956, 178, 978–979. [Google Scholar] [CrossRef] [PubMed]
- Mairani, A.; Böhlen, T.T.; Dokic, I.; Cabal, G.; Brons, S.; Haberer, T. Modelling of Cell Killing Due to Sparsely Ionizing Radiation in Normoxic and Hypoxic Conditions and an Extension to High LET Radiation. Int. J. Radiat. Biol. 2013, 89, 782–793. [Google Scholar] [CrossRef] [PubMed]



| Cell Line | Reference | ||||||
|---|---|---|---|---|---|---|---|
| V79 379A | 9.1 × 10−3 | 0.08 | 60 | 300 | 2.6 | 0.129 | Watts et al. 1978 |
| CHO | 5.9 × 10−3 * | 0.19 * | 80.22 * | 300 * | 3.1 * | 0.2 | Ling et al. 1978 |
| HeLa | 6.7 × 10−3 * | 0.21 * | 14 * | 130 * | 3.4 * | 0.41 * | Epp et al. 1972 |
| DU145 | 5.9 × 10−3 * | 0.17 * | 4 * | 100 * | 3.1 * | 0.27 * | Adrian et al. 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liew, H.; Mein, S.; Tessonnier, T.; Abdollahi, A.; Debus, J.; Dokic, I.; Mairani, A. The Impact of Sub-Millisecond Damage Fixation Kinetics on the In Vitro Sparing Effect at Ultra-High Dose Rate in UNIVERSE. Int. J. Mol. Sci. 2022, 23, 2954. https://doi.org/10.3390/ijms23062954
Liew H, Mein S, Tessonnier T, Abdollahi A, Debus J, Dokic I, Mairani A. The Impact of Sub-Millisecond Damage Fixation Kinetics on the In Vitro Sparing Effect at Ultra-High Dose Rate in UNIVERSE. International Journal of Molecular Sciences. 2022; 23(6):2954. https://doi.org/10.3390/ijms23062954
Chicago/Turabian StyleLiew, Hans, Stewart Mein, Thomas Tessonnier, Amir Abdollahi, Jürgen Debus, Ivana Dokic, and Andrea Mairani. 2022. "The Impact of Sub-Millisecond Damage Fixation Kinetics on the In Vitro Sparing Effect at Ultra-High Dose Rate in UNIVERSE" International Journal of Molecular Sciences 23, no. 6: 2954. https://doi.org/10.3390/ijms23062954





