Nanoplastics and Arsenic Co-Exposures Exacerbate Oncogenic Biomarkers under an In Vitro Long-Term Exposure Scenario
Abstract
:1. Introduction
2. Methods
2.1. PSNPLs and y-PSNPLs Characterization
2.2. Cell Culture Conditions
2.3. PSNPLs Uptake by PTP Cells
2.4. PSNPLs/AsIII Interaction Detection
2.5. AsIII Internalization by PTP Cells
2.6. In Vitro Chronic PSNPLs and Arsenic (Co)Exposure
2.7. Comet Assay
2.8. Cell Morphology and Spindle-like-Cell Proportion Calculation
2.9. Anchorage-Independent Growth Induction
2.10. Invasion and Migration Induction
2.11. Tumorsphere Formation Induction
3. Results
3.1. PS Materials Characterization
3.2. Determination and Quantification of y-PSNPLs Uptake by PTP Cells
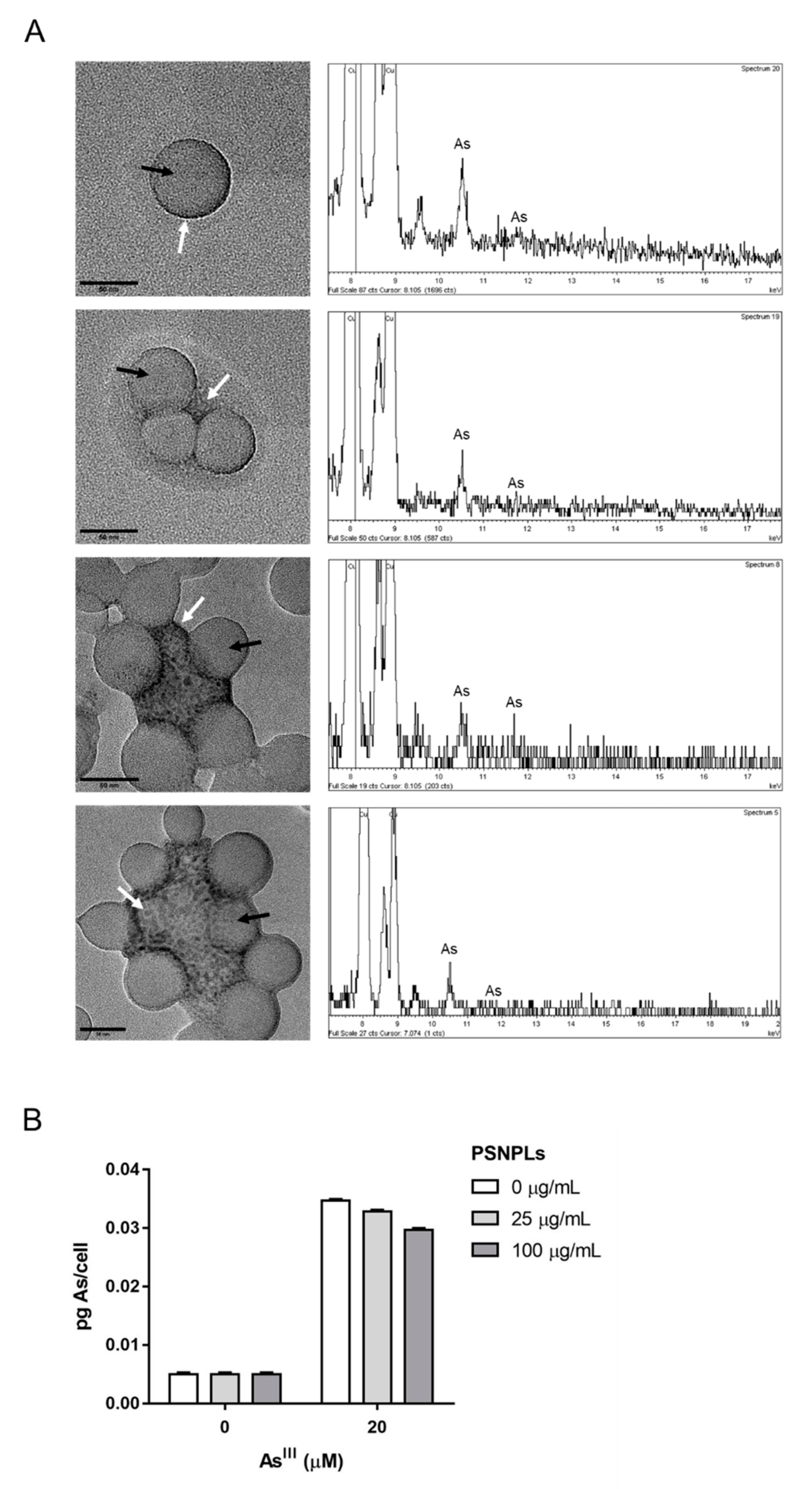
3.3. Visualization of the PSNPLs/AsIII Interaction in Dispersion, and Quantification of AsIII Uptake by PTP Cells
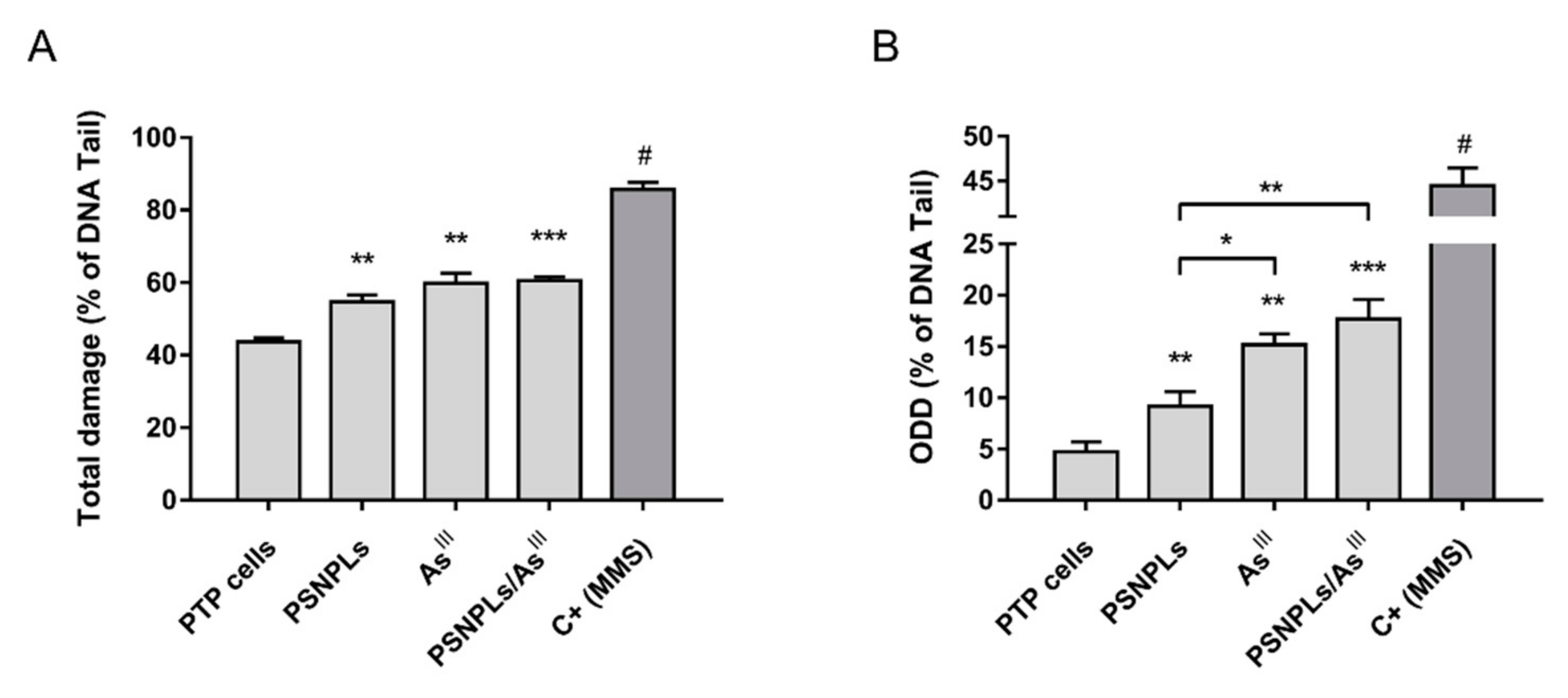
3.4. Both the Co-Exposure and the Single Pollutants Exposure Induce DNA Damage
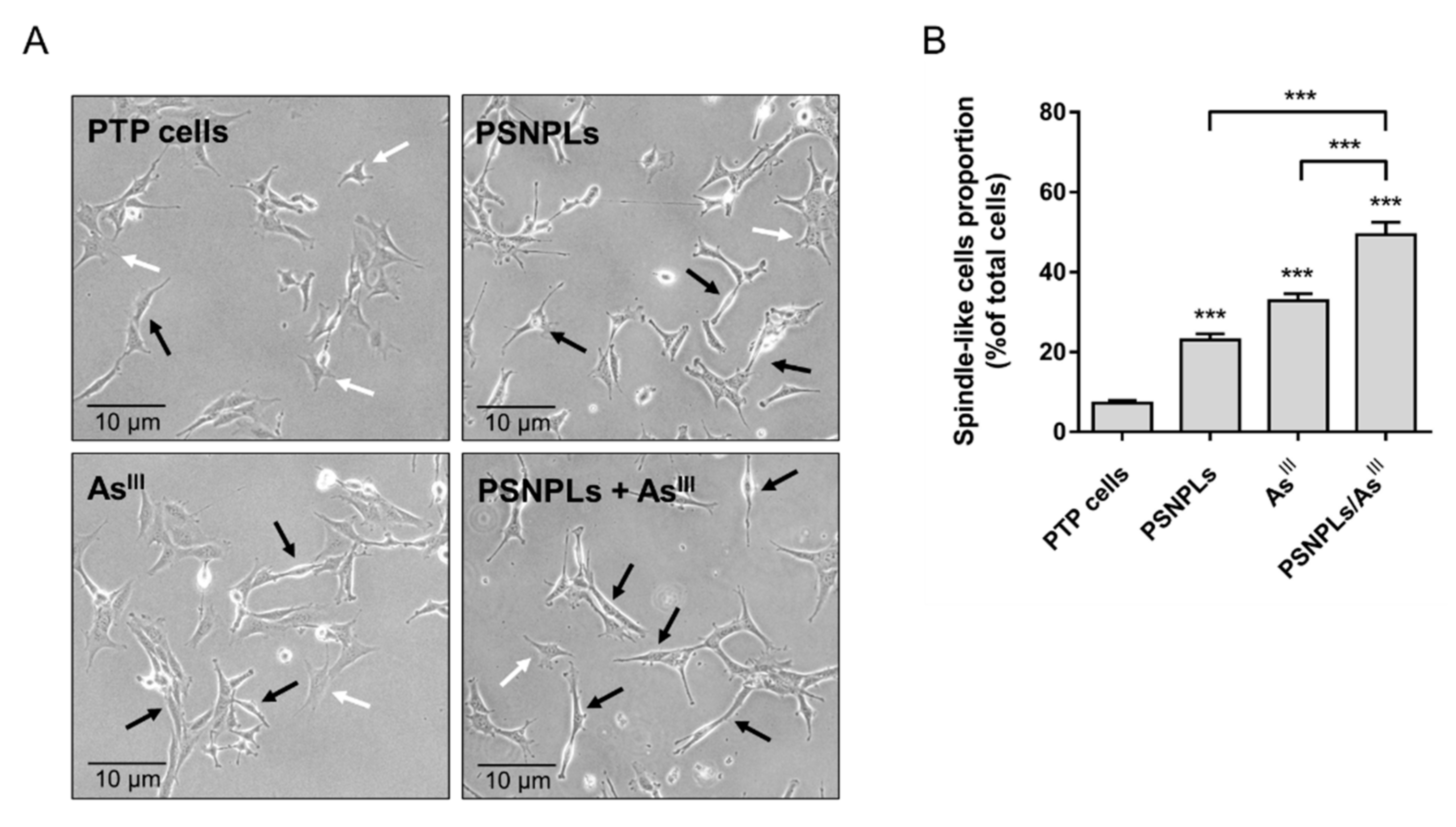
3.5. The PTP Oncogenic Phenotype Is Exacerbated by the Long-Term Co-Exposure to AsIII and PSNPLs
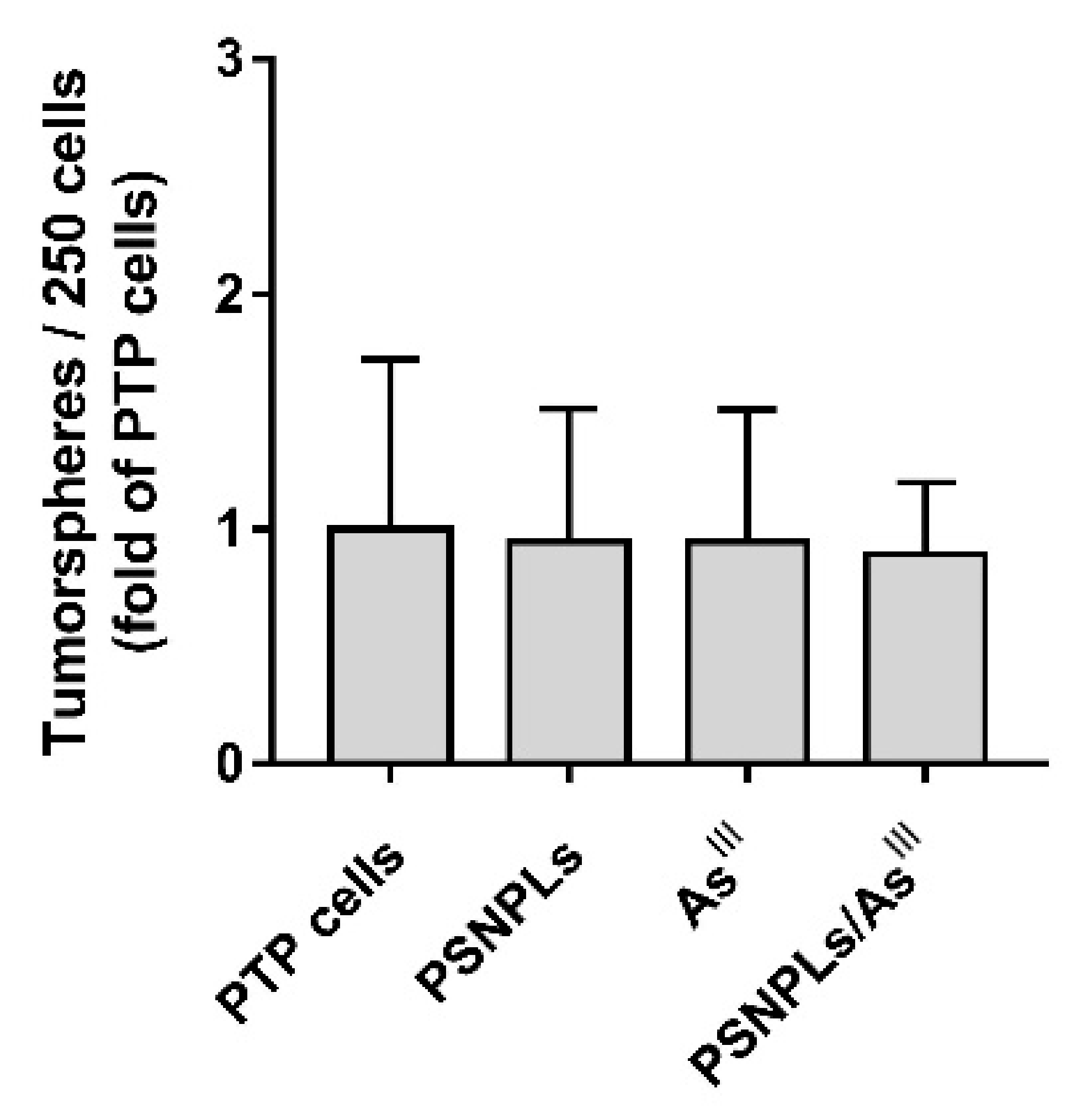
3.6. The Increased Aggressiveness of PTC’s Oncogenic Phenotype Is Not Related to an Increasing Stem-like Cells Population
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouwmeester, H.; Hollman, P.C.H.; Peters, R.J.B. Potential health impact of environmentally released micro- and nanoplastics in the human food production chain: Experiences from Nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef] [Green Version]
- Brandon, J.A.; Freibott, A.; Sala, L.M. Patterns of suspended and salp-ingested microplastic debris in the North Pacific investigated with epifluorescence microscopy. Limn. Ocean. Lett. 2020, 5, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Wanner, P. Plastic in agricultural soils—A global risk for groundwater systems and drinking water supplies?—A review. Chemosphere 2021, 264, 128453. [Google Scholar] [CrossRef]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Büchel, C.; et al. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicol. In Vitro 2019, 61, 104610. [Google Scholar] [CrossRef]
- Domenech, J.; Hernández, A.; Rubio, L.; Marcos, R.; Cortés, C. Interactions of polystyrene nanoplastics with in vitro models of the human intestinal barrier. Arch. Toxicol. 2020, 94, 2997–3012. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Jiao, Y.; Chen, Q.; Wu, D.; Yu, P.; Li, Y.; Cai, M.; Zhao, Y. Polystyrene nanoplastic induces ROS production and affects the MAPK-HIF-1/NFkB-mediated antioxidant system in Daphnia pulex. Aquat. Toxicol. 2020, 220, 105420. [Google Scholar] [CrossRef]
- Rubio, L.; Barguilla, I.; Domenech, J.; Marcos, R.; Hernández, A. Biological effects, including oxidative stress and genotoxic damage, of polystyrene nanoparticles in different human hematopoietic cell lines. J. Hazard. Mater. 2020, 398, 122900. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, S.; Domenech, J.; Barguilla, I.; Cortés, C.; Marcos, R.; Hernández, A. Genotoxic and immunomodulatory effects in human white blood cells after ex vivo exposure to polystyrene nanoplastics. Environ. Sci. Nano 2020, 7, 3431–3446. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255 Pt 3, 113363. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; Cortés, C.; Vela, L.; Marcos, R.; Hernández, A. Polystyrene nanoplastics as carriers of metals. Interactions of polystyrene nanoparticles with silver nanoparticles and silver nitrate, and its effects on human intestinal Caco-2 cells. Biomolecules 2021, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Hofmann, T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar] [CrossRef]
- Lin, W.; Jiang, R.; Wu, J.; Wei, S.; Yin, L.; Xiao, X.; Hu, S.; Shen, Y.; Ouyang, G. Sorption properties of hydrophobic organic chemicals to micro-sized polystyrene particles. Sci. Total Environ. 2019, 690, 565–572. [Google Scholar] [CrossRef]
- Massos, A.; Turner, A. Cadmium, lead, and bromine in beached microplastics. Environ. Pollut. 2017, 227, 139–145. [Google Scholar] [CrossRef]
- Prunier, J.; Maurice, L.; Perez, E.; Gigault, J.; Pierson Wickmann, A.C.; Davranche, M.; Ter Halle, A. Trace metals in polyethylene debris from the North Atlantic subtropical gyre. Environ. Pollut. 2019, 245, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic pollution. In Arsenic Pollution: A Global Synthesis; Wiley-Blackwell, A John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- IARC. Arsenic, metals fibres and dusts. Monographs on the evaluation of carcinogenic risk to humans. Int. Agency Res. Cancer 2012, 100, 407–443. [Google Scholar]
- Stueckle, T.A.; Lu, Y.; Davis, M.E.; Wang, L.; Jiang, B.-H.; Holaskova, I.; Schafer, R.; Barnett, J.B.; Rojanasakul, Y. Chronic occupational exposure to arsenic induces carcinogenic gene signaling networks and neoplastic transformation in human lung epithelial cells. Toxicol. Appl. Pharmacol. 2012, 61, 204–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treas, J.; Tyagi, T.; Singh, K.P. Chronic exposure to arsenic, estrogen, and their combination causes increased growth and transformation in human prostate epithelial cells potentially by hypermethylation-mediated silencing of MLH1. Prostate 2013, 73, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tokar, E.J.; Waalkes, M.P. Arsenic-induced cancer cell phenotype in human breast epithelia is estrogen receptor-independent but involves aromatase activation. Arch. Toxicol. 2014, 88, 263–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, J.; Diwan, B.A.; Sun, Y.; Liu, J.; Qu, W.; He, Y.; Styblo, M.; Waalkes, M.P. Arsenic-induced malignant transformation of human keratinocytes: Involvement of Nrf2. Free Radic. Biol. Med. 2008, 45, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ling, M.; Xu, Y.; Wang, S.; Li, Z.; Zhou, J.; Wang, X.; Liu, Q. The repressive effect of NF-κB on p53 by mot-2 is involved in human keratinocyte transformation induced by low levels of arsenite. Toxicol. Sci. 2010, 116, 174–182. [Google Scholar] [CrossRef]
- Shearer, M.J.J.; Wold, E.A.; Umbaugh, C.S.; Lichti, C.F.; Nilsson, C.L.; Figueiredo, M.L. Inorganic arsenic-related changes in the stromal tumor microenvironment in a prostate cancer cell-conditioned media. Environ. Health Perspect. 2016, 124, 1009–1015. [Google Scholar] [CrossRef]
- Panno, S.V.; Kelly, W.R.; Scott, J.; Zheng, W.; McNeish, R.E.; Holm, N.; Hoellein, T.J.; Baranski, E.L. Microplastic contamination in karst groundwater systems. Ground Water 2019, 57, 189–196. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. Adsorption mechanism of As(III) on polytetrafluoroethylene particles of different size. Environ. Pollut. 2019, 254, 112950. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere 2020, 239, 124792. [Google Scholar] [CrossRef]
- Bach, J.; Peremartí, J.; Annangi, B.; Marcos, R.; Hernández, A. Oxidative DNA damage enhances the carcinogenic potential of in vitro chronic arsenic exposures. Arch. Toxicol. 2016, 90, 1893–1905. [Google Scholar] [CrossRef]
- Annangi, B.; Bach, J.; Vales, G.; Rubio, L.; Marcos, R.; Hernández, A. Long-term exposures to low doses of cobalt nanoparticles induce cell transformation enhanced by oxidative damage. Nanotoxicology 2015, 9, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Annangi, B.; Rubio, L.; Alaraby, M.; Bach, J.; Marcos, R.; Hernández, A. Acute and long-term in vitro effects of zinc oxide nanoparticles. Arch. Toxicol. 2016, 90, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.; Sampayo-Reyes, A.; Marcos, R.; Hernández, A. Ogg1 genetic background determines the genotoxic potential of environmentally relevant arsenic exposures. Arch. Toxicol. 2014, 88, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Lunde, A.; Glover, J.C. A versatile toolbox for semi-automatic cell-by-cell object-based colocalization analysis. Sci. Rep. 2020, 10, 19027. [Google Scholar] [CrossRef]
- Ballesteros, S.; Domenech, J.; Velázquez, A.; Marcos, R.; Hernández, A. Ex vivo exposure to different types of graphene-based nanomaterials consistently alters human blood secretome. J. Hazard. Mater. 2021, 414, 125471. [Google Scholar] [CrossRef]
- Correia-Prata, J.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Stock, V.; Böhmert, L.; Lisicki, E.; Block, R.; Cara-Carmona, J.; Pack, L.K.; Selb, R.; Lichtenstein, D.; Voss, L.; Henderson, C.J.; et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 2019, 93, 1817–1833. [Google Scholar] [CrossRef]
- Cortés, C.; Domenech, J.; Salazar, M.; Pastor, S.; Marcos, R.; Hernández, A. Nanoplastics as a potential environmental health factor: Effects of polystyrene nanoparticles on human intestinal epithelial Caco-2 cells. Environ. Sci. Nano 2020, 7, 272–285. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveetill, S.; Tang, B.L. Toxicity of microplastics and nanoplastics in Mammalian systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stapleton, P.A. Toxicological considerations of nano-sized plastics. AIMS Environ. Sci. 2019, 6, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Reg. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Wang, J.; Nie, Y.; Chen, S.; Hei, T.K.; Deng, Z.; Wu, L.; Zhao, G.; Xu, A. Amplification of arsenic genotoxicity by TiO2 nanoparticles in mammalian cells: New insights from physicochemical interactions and mitochondria. Nanotoxicology 2017, 11, 978–995. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A. Co-exposure to SiO2 nanoparticles and arsenic induced augmentation of oxidative stress and mitochondria-dependent apoptosis in human cells. Int. J. Environ. Res. Public Health 2019, 16, 3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef]
- Zhou, Q.; Xi, S. A review on arsenic carcinogenesis: Epidemiology, metabolism, genotoxicity and epigenetic changes. Reg. Toxicol. Pharmacol. 2018, 99, 78–88. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Tokar, E.J.; Diwan, B.A.; Waalkes, M.P. Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ. Health Perspect. 2010, 118, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Person, R.J.; Olive Ngalame, N.N.; Makia, N.L.; Bell, M.W.; Waalkes, M.P.; Tokar, E.J. Chronic inorganic arsenic exposure in vitro induces a cancer cell phenotype in human peripheral lung epithelial cells. Toxicol. Appl. Pharmacol. 2015, 286, 36–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganapathy, S.; Liu, J.; Xiong, R.; Yu, T.; Makriyannis, A.; Chen, C. Chronic low dose arsenic exposure preferentially perturbs mitotic phase of the cell cycle. Genes Cancer 2019, 10, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afify, S.M.; Seno, M. Conversion of stem cells to cancer stem cells: Undercurrent of cancer initiation. Cancers 2019, 11, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngalame, N.N.O.; Luz, A.L.; Makia, N.; Tokar, E.J. Arsenic alters exosome quantity and cargo to mediate stem cell recruitment into a cancer stem cell-like phenotype. Toxicol. Sci. 2018, 165, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Ooki, A.; Begum, A.; Marchionni, L.; Vandenbussche, C.J.; Mao, S.; Kates, M.; Hoque, M.O. Arsenic promotes the COX2/PGE2-SOX2 axis to increase the malignant stemness properties of urothelial cells. Int. J. Cancer 2018, 143, 113–126. [Google Scholar] [CrossRef]
- Xiao, T.; Xue, J.; Shi, M.; Chen, C.; Luo, F.; Xu, H.; Chen, X.; Sun, B.; Sun, Q.; Yang, Q.; et al. Circ008913, via miR-889 regulation of DAB2IP/ZEB1, is involved in the arsenite-induced acquisition of CSC-like properties by human keratinocytes in carcinogenesis. Metallomics 2018, 10, 1328–1338. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, P.; Xie, J.; Lin, H.P.; Kumagai, K.; Harkema, J.; Yang, C. Arsenic and benzo[a]pyrene co-exposure acts synergistically in inducing cancer stem cell-like property and tumorigenesis by epigenetically down-regulating SOCS3 expression. Environ. Int. 2020, 137, 105560. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barguilla, I.; Domenech, J.; Rubio, L.; Marcos, R.; Hernández, A. Nanoplastics and Arsenic Co-Exposures Exacerbate Oncogenic Biomarkers under an In Vitro Long-Term Exposure Scenario. Int. J. Mol. Sci. 2022, 23, 2958. https://doi.org/10.3390/ijms23062958
Barguilla I, Domenech J, Rubio L, Marcos R, Hernández A. Nanoplastics and Arsenic Co-Exposures Exacerbate Oncogenic Biomarkers under an In Vitro Long-Term Exposure Scenario. International Journal of Molecular Sciences. 2022; 23(6):2958. https://doi.org/10.3390/ijms23062958
Chicago/Turabian StyleBarguilla, Irene, Josefa Domenech, Laura Rubio, Ricard Marcos, and Alba Hernández. 2022. "Nanoplastics and Arsenic Co-Exposures Exacerbate Oncogenic Biomarkers under an In Vitro Long-Term Exposure Scenario" International Journal of Molecular Sciences 23, no. 6: 2958. https://doi.org/10.3390/ijms23062958
APA StyleBarguilla, I., Domenech, J., Rubio, L., Marcos, R., & Hernández, A. (2022). Nanoplastics and Arsenic Co-Exposures Exacerbate Oncogenic Biomarkers under an In Vitro Long-Term Exposure Scenario. International Journal of Molecular Sciences, 23(6), 2958. https://doi.org/10.3390/ijms23062958





