SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection
Abstract
:1. Introduction
2. Overview of NAFLD Pathogenesis
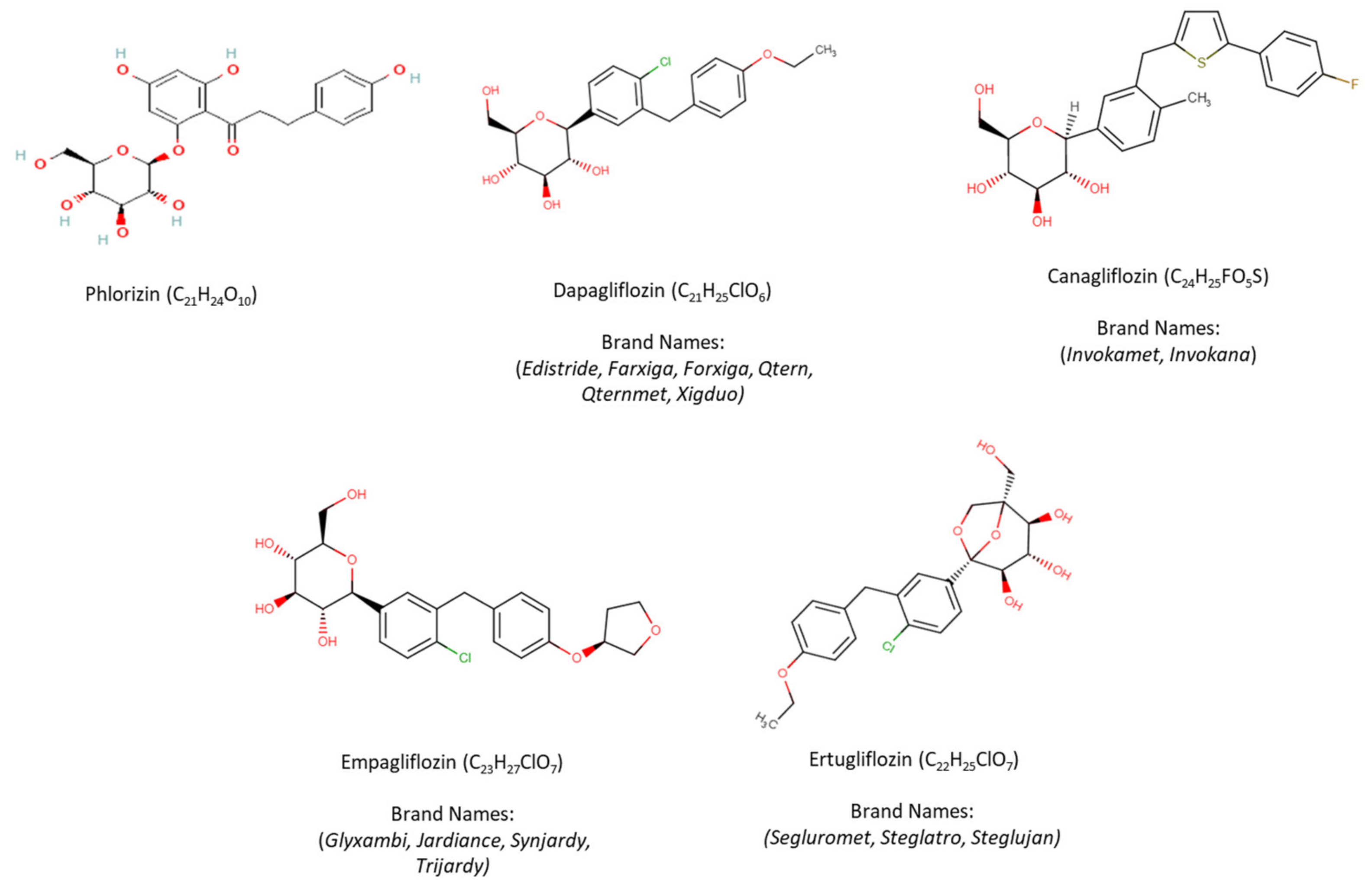
3. SGLT-2i Overview
4. SGLT-2 Inhibitors in NAFLD
4.1. Laboratory Experiments
4.1.1. In Vitro Data
4.1.2. Animal Studies
| Study/Reference | Animal Model, Dose, & Duration | Effect on Body Weight and Liver Weight | Effect on Laboratory Values | Mechanism of Action | Effect on Insulin Sensitivity & Glucose Homeostasis | NAFLD Activity Score (NAS) & Fibrosis/Steatosis |
|---|---|---|---|---|---|---|
| Perakakis, N., et al., 2021 [99] | Male C57BL/6JRj on AMLN diet (HFD, fructose + cholesterol) 10 mg/kg/day 12 (w) | No effect | - | ⬇ Hepatic lactosylceramides | ⬇ Blood glucose levels No effect on Insulin sensitivity | ⬇ Lobular inflammation ⬇ NAS No effect on the hepatic steatosis and fibrosis |
| Meng, Z., et al., 2021 [117] | Male C57BL/6J on HFD + streptozotocin injection (T2DM with NAFLD) 10 mg/kg/day 8 (w) | ⬇ Body weight ⬇ Liver/Bw | ⬇ ALT ⬇ TG & TC ⬆ HDL | ⬇ Lipogenesis markers and lipid uptake genes (SREBP1, ChREBP, FASN, ACCα, SCDα, CD36) ⬆ Autophagy activation (AMPK/mTOR & BECLIN1, LC3BII) ⬆ IL-17/IL-23 axis inhibition (IL-23p19, IL-23, IL-1β, IL-17A, RORγt, p-STAT3/t-STAT3, IL-17) ⬇ M1 macrophage marker (CD11C, CD86, NOS2) ⬇ Th17-related chemokines and chemokine receptors (CCL20, CCR6, CCR4, CXCL1/CXCL2, CXCL1, CXCR2) | ⬇ Blood glucose levels | ⬇ Hepatic steatosis ⬇ NAS |
| Nasiri-Ansari, N., et al., 2021 [30] | Male ApoE knockout mice on HFD 10 mg/kg/day 10 (w) | No effects | ⬇ ALT, TG, TC levels ⬇ Serum TG/HDL levels | ⬇ Lipogenesis markers (SREBP1, Pck1, FASN) ⬇ Inflammatory markers (MCP-1, F4/80) ⬇ ER stress markers (GRP78, IRE1α, XBP1, ELF2α, ATF4, CHOP, GRP94) Autophagy markers (⬇ mTOR and P62 & ⬆ pAMPK/AMPK, BECLIN) ⬇ Apoptosis markers (Bax/Bcl-2 Ratio, cleaved Caspase-3) | ⬇ Blood glucose levels | ⬇ Lobular inflammation ⬇ Hepatic steatosis ⬇ NAS No effect on hepatic fibrosis |
| Petito-da-Silva, et al., 2019 [107] | Male C57Bl/6 mice on HFD 10 mg/kg/day 5 (w) | ⬇ BW ⬇ Liver/Bw | No effect on ALT | ⬇ Lipogenic genes (Fas, SREBP1c, PPARγ) ⬇ ΕR- stress markers (CHOP, ATF4, GADD45) Fatty acid β-oxidation (⬆ PPAR-α, ⬇ Acox1) ⬇ lipid droplet-associated protein (Fsp27/cidec) ⬇ Inflammatory markers (Nfκb, TNF-α) | Improved Glucose intelorence Improved Insulin sensitivity | ⬇ Hepatic TC ⬇ Hepatic steatosis |
| Jojima, T., et al., 2016 [136] | Male C57BL/6J on HFD + early STZ injection 10 mg/kg/day 3 (w) | ⬇ Liver/BW | ⬇ GA ⬇ ALT | ⬇ Inflammatory markers (IL6, TNF-α, MCP-1, SOCS3) ⬇ Plasma DPP-4 activity (CD26/DPP-4) | ⬇ Plasma glucose Levels | ⬇ Hepatic TG ⬇ NAS ⬇ Hepatic fibrosis |
| Hüttl, M., et al., 2021 [115] | HHTg & Wistar rats 10 mg/kg/day 8 (w) | No effect on BW | ⬇ TAG No effect on serum ALT | ⬇ Lipogenicgenes (Fas, Scd-1, SREBP1c, PPARγ) Improvement of hepatic lipid metabolism (⬆ Nrf, Cyp2e1, ⬇ FGF21, Cyp4a1, Cyp1a1, Cyp2b1) ⬇ Inflammatory markers (MCP-1) ⬆ Oxidative stress markers (⬇ Hepatic GSH/GSSG, SOD) ⬇ Hepatokines (Fetuin-A) | Improved Glucose intolerance Improved Insulin sensitivity | ⬇ Hepatic TG ⬇ lipotoxic diacylglycerols ⬇ Fibrosis |
| Study/Reference | Animal Model Dose & Duration | Effect on Body & Liver Weight | Effect on Laboratory Values | Mechanism of Action | Effect on Insulin Sensitivity & Glucose Homeostasis | NAFLD Activity Score (NAS) & Fibrosis/Steatosis |
|---|---|---|---|---|---|---|
| Yoshino, K., et al., 2021 [111] | obese diabetic KK-Ay mice putative dose of ~17 mg/kg/day 3 (w) | No effect on body weight No effect epididymal fat weigh ⬇ Liver/Bw | ⬇ TG No effect on serum ALT | ⬆ Prostaglandin E2 (PGE2) and resolvin E3 | ⬇ Plasma glucose levels | ⬇ Hepatic TG |
| Tanaka K., et al., 2020 [108] | Male C57BL/6J mice on HFD 30 mg/kg/day 4 (w) | ⬇ BW | ⬇ ALT ⬆ TG, ketone bodies | ⬆ lipid-dependent energy expenditure ⬇ Respiratory Qquotients ⬇ Lipogenesis markers (PPAR, FAS, Scd1) ⬆ Fatty acid β-oxidation markers (CPT1a, PGC1a, PGC1b) ⬇ Inflammatory markers (IL-1b) | Improved glucose intolerance Improved insulin sensitivity ⬇ lasma glucose & insulin levels | ⬇ Hepatic TG ⬇ NAS |
| Jojima, T., et al., 2019 [87] | STAM mice 30 mg/kg/day 4 & 11 (w) | ⬇ Liver/Bw (11 w) | ⬇ TG (11 w) ⬇ ALT (11 w) | ⬇ Inflammatory marker and fibrosis marker [SOCS-3, collagen 3 (4 w)] ⬇ Lipogenesis markers [FAS (11 w)] ⬇ Inhibits progression of NASH to Hepatocarcinogenesis GS & AFP | ⬇ Plasma glucose levels | ⬇ NAS (11 w) ⬇ Hepatic fibrosis (4 w) ⬇ Tumor number (11 w) |
| Shiba, K., et al., 2018 [101] | MC4R-KO mice on HFD 20–30 mg/kg/day 8, 20 & 52 (w) | ⬇ Liver weight (8 w) ⬆ Body weight (8 and 52 w) | ⬆ TG (8 and 20 w) ⬇ ALT (8 and 20 w) | ⬇ Lipogenic markers genes [Acc1 and Scd1 (8 and 20 w), Fasn (8 w)] ⬇ Gluconeogenic markers (G6pc, Pck1) ⬇ Inflammatory markers [F4/80 gene (20 w), TNFa (20 w), Cd11c (8 and 20 w)] ⬇ Fibrosis markers [Col1a1, TIMP-1 (8 and 20w), Acta2, Tgf1b (20 w)] | Improved insulin sensitivity and hyperglycemia (8 and 20 w) | ⬇ Hepatic steatosis (8 w) ⬇ Hepatic fibrosis (20 w) ⬇ NAS (20 w) ⬇ Tumor number (52 w) ⬇ Hepatic TG content (8 and 20 w) |
| Ozutsumi, T., et al., 2020 [110] | F344 rats on CDAA diet 10 mg/kg/day 16 (w) | No effect on body weight No effect on Liver/BW | ⬇ ALT | ⬇ Fibrosis markers (αSMA, TGF-β1, α1(I)-procollagen) ⬇ Inflammatory markers (CCL2, TNF-α, IL-6) ⬇ Hepatocarcinogenesis markers (GST-P, VEGF, CD31) ⬇ Oxidative stress markers (MDA, 8-OHdG) | No effect on insulin sensitivity No effect on plasma glucose levels | ⬇ Hepatic fibrosis & Steatosis ⬇ Hepatic Cirrhosis ⬇ Hepatic inflammation ⬇ Hepatic ballooning ⬇ NAS |
| Kabil, Sl, et al., 2018 [129] | Male Wister rats injected with STZ on HFD 10 and 20 mg/kg/day 8 (w) | ⬇ Liver weight ⬇ BW (20 mg) | ⬇ ALT ⬆ TC, TG & NEFA | ⬆ Hepatic lipolytic factor ZAG ⬇ Inflammatory markers (serum TNF-α, hepatic IL-6) Serum apoptotic markers (⬇ Caspase3, ⬆ Bcl-2) Hepatic oxidative stress (⬇ MDA, ⬆ SOD and GPx activity) Serum antioxidant enzyme activity (⬇ TOS and ⬆ TAS) | No effect on fasting insulin levels Fasting blood glucose | ⬇ Hepatic inflammation ⬇ Hepatic TC, TG, NEFA ⬇ Hepatic inflammation Hepatic Steatosis ⬇ NAS |
| Study/Reference | Animal Model Dose & Duration | Effect on Body and Liver Weight | Effect on Laboratory Values | Mechanism of Action | Effect on Insulin Sensitivity & Glucose Homeostasis | NAFLD Activity Score (NAS) & Fibrosis/Steatosis |
|---|---|---|---|---|---|---|
| Han, T., et al., 2021 [105] | Male C57BL/6 J and ob/ob mice on HFD 1 mg/kg/day 4 (w) | No effect on BW | ⬇ TC | ⬆ β-oxidation (PPAR-α, CPT1, PGC1α) ⬇ Inflammatory markers (MCP1) | ⬇ Fasting blood glucose | ⬇ Hepatic oxidative stress ⬇ Hepatic lipid accumulation ⬇ Hepatic steatosis |
| Luo, J., et al., 2021 [84] | Male NIH mice on HFD 25 mg/kg/day 4 (w) | No effect on BW ⬆ Food intake | ⬇ ALT | ⬇ Lipogenic markers (SREBP1, ACC, FASN) ⬆ β-oxidation markers (PPARα, CPT1a) Regulation of lipid metabolism ⬆ pAMPK and ⬇ pmTOR | - | ⬇ Hepatic steatosis ⬇ Hepatic ballooning ⬇ HepaticTC, TG |
| Tang, L., et al., 2017 [132] | db/db mice 1.0 mg/kg/day via diet gel 4 (w) | No effect on BW | ⬇ ALT ⬇ TG | ⬇ Inflammatory markers (MPO, F4/80) ⬇ Oxidative stress markers (ROS) ⬇ Fibrosis markers (FN, Col I, Col III, LM) | ⬇ Plasma glucose levels | ⬇ Hepatic injury ⬇ Hepatic fibrosis ⬇ Hepatic inflammation |
| Yabiku, K., et al., 2020 [109] | Male C57BL/6J mice on HFD or HFD + MCDD 0.1 or 1.0 mg/kg/day 2 (w) | ⬇ BW (0.1 and 1 mg) in both diets | ⬇ ALT (0.1 and 1 mg) Mice on HFD | - | Improved glucose tolerance and insulin sensitivity | - |
| Omori, K., et al., 2019 [125] | db/db mice on ND 1.0 mg/kg/day 8 (w) | No effect on BW ⬇ Liver weight | ⬇ TG ⬇ Plasma C-peptide | No significant differences in the expression of fatty acid oxidation markers No significant differences in the expression of inflammatory markers ⬇ Fatty acid uptake and storage markers (PPARγ targeted genes as compared to Gla group) | Improved glucose tolerance | No significant changes in hepatic TG, Palmitate, Oleate, and Stearate content |
| Li, L., et al., 2021 [85] | ZDF rats 1 mg/kg/day 9 (w) | ⬇ BW ⬇ Liver weight ⬇ Liver weight/BW | ⬇ TG, TC, LDL, HDL | ⬇ Lipogenic markers (SREBP1, ACC1, p-ACC) ⬆ Fatty acid oxidation markers (ACOX1, CPT1, pACOX) Autophagy-related markers (⬆ LC3B, Beclin1, activation of AMPK/mTOR pathway and ⬇ P62) | ⬇ Plasma glucose and insulin levels | ⬇ Hepatic lipid accumulation ⬇ Hepatic steatosis |
| ElMahdy, M.K., et al., 2020 [130] | Male Wistar rats on HCHF diet 1 mg/kg/day 5 (w) | No significant effects on liver weight | ⬇ ALT, AST ⬇ TC, TG, LDL ⬆ HDL | ⬇ Inflammatory markers (TNF-α, IL-1β, IL-18) | - | ⬇ Hepatic steatosis |
| Study/Reference | Animal Model Dose & Duration | Effect on Body Weight &Body Composition | Effect on Laboratory Values | Mechanism of Action | Effect on Insulin Sensitivity & Glucose Homeostasis | NAFLD Activity Score (NAS) & Fibrosis/Steatosis |
|---|---|---|---|---|---|---|
| Ipragliflozin | ||||||
| Tahara, A. & Takasu, T., 2020 [100] | KK-Ay mice on HFD 0.1, 0.3, 1 and 3 mg/kg/day Alone or with Metformin 4 (w) | ⬇ BW weight (1 & 3 mg) ⬇ Liver weight (0.3, 1 & 3 mg) | ⬇ TG (0.3, 1 & 3 mg) ⬇ TC (1 & 3 mg) ⬇ AST (1 & 3 mg) ⬇ ALT (0.3, 1 & 3 mg) | ⬇ Inflammatory Markers [serum TNF-α, IL-6, MCP-1 and CRP (1 and 3mg); Liver TNF-α (3 mg) and IL-6, MCP-1 and CRP (1 & 3 mg)] ⬇ Serum and hepatic oxidative stress markers [TBARS and protein carbonyl (1 & 3 mg)] | Improve glucose intolerance Improved Insulin resistance Improved hyperlipidemia | ⬇ Hepatic TG, TC (1 & 3 mg) ⬇ Hepatic Hyperthrophy (1 & 3 mg) ⬇ Hepatic Inflammation (1 & 3 mg) ⬇ Hepatic fibrosis & steatosis (3 mg) |
| Tahara, A., et al., 2019 [113] | KK-Ay mice on HFD 0.1, 0.3, 1 and 3 mg/kg/day Alone or with Pioglitazone 4 (w) | ⬇ BW weight (1 & 3 mg) ⬇ Liver weight (0.3, 1 & 3 mg) | ⬇ TC (0.3, 1 & 3 mg) ⬇ TG (1 & 3 mg) ⬇ AST (1 & 3 mg) ⬇ ALT (1 & 3 mg) | ⬇ Genes involved in regulation of insulin sensitivity (Plasma adipocytokines, Leptin & FGF-21) ⬇ Inflammatory Markers [serum TNF-α, IL-6, MCP-1 and CRP (1 and 3 mg); Liver TNF-α (3 mg) and IL-6, MCP-1 and CRP (1 and 3 mg)] ⬇ Serum and hepatic oxidative stress markers [TBARS and protein carbonyl (1 & 3 mg)] | ⬇ Plasma glucose and insulin levels (0.3, 1 and 3 mg) | ⬇ Hepatic TG (0.3, 1 & 3 mg) ⬇ Hepatic TC (1 & 3 mg) ⬇ Hepatic Hyperthrophy (1 & 3 mg) ⬇ Hepatic Inflammation (3 mg) ⬇ Hepatic fibrosis (3 mg) |
| Komiya, Ch, et al., 2016 [112] | ob/ob and WT mice on HFD 11 mg/kg/day 4 (w) | ⬇ Hyperphagia ⬇ BW weight ⬇ Liver weight | ⬇ ALT ⬇ TG ⬇ Plasma glucagon | ⬇ Lipogenic markers genes (SREBP1, Fasn, Acc1, Scd1) ⬇ Gluconeogenic markers (Pck1) ⬇ Inflammatory markers (F4/80, Cd11c) | Improved Insulin resistance Improved fasting glucose levels | ⬇ Hepatic TG ⬇ Hepatic lipid ⬇ Hepatic steatosis |
| Honda, Y., et al., 2016 [114] | C57BL/6J male mice on AMLN diet 40 mg/kg/day 8 (w) | No effect on BW ⬇ Liver weight | ⬇ ALT, AST ⬇ FFA | ⬆ β-oxidation (PPAR-α, CPT1, MTTP) ⬇ Hepatocytes apoptosis (TUNEL) ⬇ Lipogenic markers genes (SREBP1 & Acc1) | Improved Insulin resistance | ⬇ Hepatic TG & FFA ⬇ Hepatic fibrosis ⬇ Hepatocyte ballooning ⬇ Lobular inflammation ⬇ NAS |
| Hayashizaki-Someya, Y., et al., 2015 [144] | Male Wistar rats on CDAA diet 0.3 and 3 mg/kg/day 5 (w) | ⬇ BW weight (3 mg) | No effect on ALT, AST | - | No effect on fasting blood glucose levels | ⬇ Hepatic TG (3 mg) ⬇ Hepatic lipid droplet size ⬇ Hepatic fibrosis (0.3 & 3 mg) ⬇ Hepatic HP |
| Yoshioka, N., et al., 2021 [126] | Mc4r KO mice on HFD and injected with single dose of diethylnitrosamine 5 mg/kg/day 12 (w) | ⬇ BW weight ⬇ Liver weight | ⬇ ALT, AST ⬇ LDH | ⬇ Lipogenic markers genes (Fasn in non-tumor) ⬇ Fibrosis markers (Emr1, Itgax in non-tumor) ⬇ Cell senescence markers (Cxcl1 in tumor lesion; p21, Cxcl1, MMp12, mmp13 in non-tumor) ⬆ β-oxidation (PPAR-α in tumor lesion; PPAR-α, CPT1, PGC1 in non-tumor) ⬇ Cell apoptosis (Bax and Pcna) | ⬇ Plasma glucose & insulin levels | ⬇ Hepatic TG ⬇ Lobular inflammation ⬇ Hepatocyte ballooning ⬇ NAS ⬇ Hepatic steatosis & fibrosis ⬇ Hepatic tumor number & size |
| (Remogliflozin) | ||||||
| Nakano, S., et al., 2015 [134] | C57BL/6J mice on HFD32 13.2 ± 2.2 and 33.9 ± 2.0 mg/kg/day 4 (w) | ⬇ Liver weight ⬇ Liver/BW | ⬇ ALT & AST | ⬇ Inflammatory markers [Hepatic TNF-α (13.2 mg), hepatic MCP-1 (13.2 and 33.9 mg)] ⬇ Oxidative stress (serum and hepatic TBARS) | Improved non fasting glucose levels | ⬇ Hepatic TG ⬇ Hepatic fibrosis |
| Tofogliflozin | ||||||
| Obara, K., et al., 2017 [89] | db/db mice on HFD and injected with single dose of diethylnitrosamine 1 and 10 mg/kg/day 14 (w) | ⬇ Liver weight (10 mg) | ⬇ ALT (10 mg) ⬇ FFA (1 & 10 mg) | ⬇ Inflammatory markers (10 mg) (F4/80) | ⬇ Plasma glucose levels Improved insulin insensitivity | ⬇ Foci of cellular alteration (10 mg) ⬇ Hepatic pre-neoplastic lesions (10 mg) ⬇ Hepatocyte balooning (10 mg) ⬇ Hepatic steatosis (10 mg) ⬇ NAS (1 & 10 mg) |
| Luseogliflozin | ||||||
| Qiang, Sh, et al., 2015 [142] | C57BL/6 mice injected with STZ on HFDT Mixing in food at 0.1%. w/w food 8 (w) | ⬇ Liver weight | ⬇ ALT ⬆ TG, NEFA | ⬇ Hepatic fibrosis markers (collagen1a1, collagen1a2, TGF, SMA, TIMP1) ⬇ Inflammatory markers (MCP-1, IL1, IL-12, IL-6, f4/80) | ⬇ Plasma glucose levels | ⬇ Hepatic TC, TG &NEFA |
4.2. Human Trials
| Study | Study Design | No of Pts | SGLT-2i/Drug Used (No of Pts) | Control Group | Treatment Duration (Weeks) | NAFLD Diagnosis ** | Key Results |
|---|---|---|---|---|---|---|---|
| Eriksson, J., et al., 2018 [150] | Randomised, double-blind, prospective | 84 | Dapagliflozin (42) | OM-3CA or placebo | 12 | MRI | Reduction of serum transaminases, CK-18, FGF-21 in Dapagliflozin group and liver fat in Dapagliflozin + OM-3CA group |
| Kahl, S., et al., 2020, [161] | Randomised, double-blind, prospective | 84 * | Empagliflozin (42) | Placebo | 24 | MRI | LFC improvement only in empagliflozin |
| Chehrehgosha, H., et al., 2021 [165] | Randomised, double-blind, prospective | 78 | Empagliflozin (21) | Pioglitzone or placebo | 24 | TE | Better CAP, LS, no difference vs. pioglitzone for serum transaminases or FIB-4 |
| Gaborit, B., et al., 2021 [167] | Randomised, double-blind, prospective | 34 | Empagliflozin (18) | Placebo | 12 | MRI | Reduction in liver fat vs. placebo |
| Bando, Y., et al., 2017 [145] | Randomised, open label, prospective | 62 | Ipragliflozin (40) | SOC | 12 | C/T | Improvement in serum transaminases. VFA, L/S ratio compared to SOC |
| Ito, D., et al., 2017 [147] | Randomized, open label, prospective | 66 | Ipragliflozin (32) | Pioglitazone | 24 | C/T or U/S | Improvement of L/S ratio, ALT, ferritin not statistically significant between 2 groups; ipragliflozin more weight and VFA reduction |
| Kuchay, M.S., et al., 2018 [152] | Randomized, open label, prospective | 42 | Empagliflozin (22) | SOC | 20 | MRI | Reduction of liver fat and ALT |
| Shibuya, T., et al., 2018 [154] | Randomized, open label, prospective | 32 | Luseogliflozin (16) | Metformin | 26 (6 months) | C/T or U/S | Improvement in L/S ratio compared to baseline |
| Shimizu, M., et al., 2019 [155] | Randomized, open label, prospective | 57 | Dapagliflozin (33) | SOC | 24 | U/S | Improvement of CAP and LS, especially for high LS at the trial beginning |
| Han, E., et al., 2020 [160] | Randomized, open label, prospective | 44 | Ipragliflozin (+metformin +pioglitazone) (29) | Metformin + pioglitazone | 24 | U/S | Better FLI, CAP, NAFLD liver fat score |
| Kinoshita, T., et al., 2020 [162] | Randomized, open label, prospective | 98 | Dapagliflozin (32) | Pioglitazone (33) Glimepiride (33) | 28 | C/T | Improvement of L/S ratio and ALT with pioglitazone and dapagliflozin |
| Takahashi, H., et al., 2021 [168] | Randomized, open label, prospective | 55 | Ipragliflozin (27) | SOC, except pioglitazone, GLP1 | 72 | LB | Statistically significant improvement in NASH resolution and fibrosis improvement in SGLT-2i vs. SOC |
| Yoneda, M., et al., 2021 [169] | Randomized, open label, prospective | 40 | Topogliflozin (21) | Pioglitzone | 24 | MRI | Decrease of liver steatosis in both groups, body weight decrease in topogliflozin |
| Arai, T., et al., 2021 [164] | Open label, Prospective | 100 | Canagliflozin (29) Ipragliflozin (12) Tofogliflozin (6) Dapagliflozin (4) Luseogliflozin (4) Empagliflozin (1) | SOC | 48 | U/S | Decrease in LS and CAP in SGLT-2i during treatment, statistically significant decrease in SGLT-2i vs SOC in ALT, FIB-4 |
| Akuta, N., et al., 2017 [146] | Single-arm, Prospective | 5 | Canagliflozin (5) | N/A | 24 | LB | Improvement of NAS score, liver steatosis; fibrosis improvement in 2 pts |
| Itani, T., et al., 2018 [151] | Single arm, Prospective | 35 | Canagliflozin (35) | N/A | 26 (6 months) | U/S | Improvement in ALT, ferritin, FIB-4 at 3 and 6 months |
| Miyake, T., et al., 2018 [153] | Single arm, Prospective | 43 | Ipragliflozin (43) | N/A | 24 | 12 LB, 41 U/S | Reduction in serum transaminases, CAP, not statistically significant reduction in fibrosis |
| Sumida, Y., et al., 2019 [156] | Single-arm, Prospective | 40 | Luseogliflozin (40) | N/A | 24 | U/S | Reduction in transaminases, serum ferritin and liver fat in MRI |
| Akuta, N., et al., 2019 [157] | Single arm, Prospective | 9 | Canagliflozin (9) | N/A | 24 | LB | Histological improvement in all patients |
| Akuta, N., et al., 2020 [159] | Single arm, Prospective | 7 | Canagliflozin (7) | N/A | 24 | LB | Histopathological improvement at 24 weeks sustained to >1 year, transaminases and ferritin better at 24 weeks |
| Seko, Y., et al., 2017 [148] | Retrospective | 45 | Canagliflozin (18) Ipragliflozin (6) | Sitagliptin | 24 | LB | Significant decrease in serum transaminases with both drugs, not statistically significant between SGLT-2i and sitagliptin |
| Choi, D.H., et al., 2018 [149] | Retrospective | 102 (all abnormal ALT) | Dapagliflozin + Metformin (50) | DPP4 + Metformin | 44.4 ± 18.4 for dapagliflozin and 50.4 ± 21.6 for DPP4 | U/S | Statistically significant decrease in dapagliflozin vs. DPP4 |
| Yamashima, M., et al., 2019 [158] | Retrospective | 22 | Ipragliflozin (18) Dapagliflozin (2) Tofogliflozin (1) Empagliflozin (1) | N/A | 52 (22 pts) and 104 (15 pts) | 12 LB, 10 U/S | Lower serum transaminases levels at 12 and 24 months, better CAR and shear wave velocity at 12 months |
| Yano, K., et al., 2020 [163] | Retrospective | 69 | Dapagliflozin (10) Canagliflozin (7) Ipragliflozin (3) Empagliflozin (2) | SOC | 162 | LB | Improvement of serum transaminases in both groups (No head to head comparison) |
| Euh, W., et al., 2021 [166] | Retrospective | 283 | Dapagliflozin (58) Empagliflozin (34) Ipragliflozin (3) | SOC, except GLP-1 and Insulin | 39 | U/S | Statistically significant reduction in ALT and body weight in SLT2i vs. SOC |
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef]
- Hui, J.M.; Kench, J.G.; Chitturi, S.; Sud, A.; Farrell, G.C.; Byth, K.; Hall, P.; Khan, M.; George, J. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology 2003, 38, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Leone, N.; Vanni, E.; Marchesini, G.; Brunello, F.; Carucci, P.; Musso, A.; De Paolis, P.; Capussotti, L.; Salizzoni, M.; et al. Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002, 123, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Yki-Jarvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Petroni, M.L.; Brodosi, L.; Bugianesi, E.; Marchesini, G. Management of non-alcoholic fatty liver disease. BMJ 2021, 372, m4747. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Fouad, Y.; Waked, I.; Bollipo, S.; Gomaa, A.; Ajlouni, Y.; Attia, D. What’s in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020, 40, 1254–1261. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, L.; Pafundi, P.C.; Galiero, R.; Caturano, A.; Morone, M.V.; Silvestri, C.; Giordano, M.; Salvatore, T.; Sasso, F.C. Mechanisms of Non-Alcoholic Fatty Liver Disease in the Metabolic Syndrome. A Narrative Review. Antioxidants 2021, 10, 270. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Aglitti, A.; Bucci, T.; Caruso, R.; Salvatore, T.; Sasso, F.C.; Tripodi, M.F.; Persico, M. Liver biopsy in type 2 diabetes mellitus: Steatohepatitis represents the sole feature of liver damage. PLoS ONE 2017, 12, e0178473. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef]
- Fargion, S.; Porzio, M.; Fracanzani, A.L. Nonalcoholic fatty liver disease and vascular disease: State-of-the-art. World J. Gastroenterol. 2014, 20, 13306–13324. [Google Scholar] [CrossRef]
- Galiero, R.; Caturano, A.; Vetrano, E.; Cesaro, A.; Rinaldi, L.; Salvatore, T.; Marfella, R.; Sardu, C.; Moscarella, E.; Gragnano, F.; et al. Pathophysiological mechanisms and clinical evidence of relationship between Nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease. Rev. Cardiovasc. Med. 2021, 22, 755–768. [Google Scholar] [CrossRef]
- Petta, S.; Argano, C.; Colomba, D.; Camma, C.; Di Marco, V.; Cabibi, D.; Tuttolomondo, A.; Marchesini, G.; Pinto, A.; Licata, G.; et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: Association with the severity of liver disease. J. Hepatol. 2015, 62, 928–933. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). FDA Approves New Treatment for a Type of Heart Failure. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure (accessed on 10 December 2021).
- U.S. Food and Drug Administration (FDA). FDA Approves Treatment for Chronic Kidney Disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease (accessed on 15 December 2021).
- Kim, J.W.; Lee, Y.J.; You, Y.H.; Moon, M.K.; Yoon, K.H.; Ahn, Y.B.; Ko, S.H. Effect of sodium-glucose cotransporter 2 inhibitor, empagliflozin, and alpha-glucosidase inhibitor, voglibose, on hepatic steatosis in an animal model of type 2 diabetes. J. Cell Biochem. 2018, 20, 8534–8546. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- Mahady, S.E.; George, J. Exercise and diet in the management of nonalcoholic fatty liver disease. Metabolism 2016, 65, 1172–1182. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Marra, F.; Bertolani, C. Adipokines in liver diseases. Hepatology 2009, 50, 957–969. [Google Scholar] [CrossRef]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V., Jr. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, K.; Kong, A.; Zhou, Y.; Chen, D.; Gu, J.; Shi, H. Dysregulation of autophagy acts as a pathogenic mechanism of non-alcoholic fatty liver disease (NAFLD) induced by common environmental pollutants. Ecotoxicol. Environ. Saf. 2021, 217, 112256. [Google Scholar] [CrossRef]
- Nasiri-Ansari, N.; Nikolopoulou, C.; Papoutsi, K.; Kyrou, I.; Mantzoros, C.S.; Kyriakopoulos, G.; Chatzigeorgiou, A.; Kalotychou, V.; Randeva, M.S.; Chatha, K.; et al. Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE−/− Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int. J. Mol. Sci. 2021, 22, 818. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, X.; Lu, Y.; Wang, E.; Zhang, Z.; Yang, J.; Zhang, H.; Li, X. Hepatic steatosis exacerbated by endoplasmic reticulum stress-mediated downregulation of FXR in aging mice. J. Hepatol. 2014, 60, 847–854. [Google Scholar] [CrossRef]
- Zhang, X.; Han, J.; Man, K.; Li, X.; Du, J.; Chu, E.S.; Go, M.Y.; Sung, J.J.; Yu, J. CXC chemokine receptor 3 promotes steatohepatitis in mice through mediating inflammatory cytokines, macrophages and autophagy. J. Hepatol. 2016, 64, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Alkhouri, N.; Carter-Kent, C.; Feldstein, A.E. Apoptosis in nonalcoholic fatty liver disease: Diagnostic and therapeutic implications. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Zhang, X.J.; Li, H. The Role of Innate Immune Cells in Nonalcoholic Steatohepatitis. Hepatology 2019, 70, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Friedman, S.L. Inflammatory and fibrotic mechanisms in NAFLD-Implications for new treatment strategies. J. Intern. Med. 2021, 291, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Ertunc, M.E.; Hotamisligil, G.S. Lipid signaling and lipotoxicity in metaflammation: Indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, D.; Kotronen, A.; Westerbacka, J.; Puig, O.; Arkkila, P.; Kiviluoto, T.; Laitinen, S.; Kolak, M.; Fisher, R.M.; Hamsten, A.; et al. Gene expression in human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1281–G1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; de Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5; quiz e14–e15. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Noworolski, S.M.; Erkin-Cakmak, A.; Korn, N.J.; Wen, M.J.; Tai, V.W.; Jones, G.M.; Palii, S.P.; Velasco-Alin, M.; Pan, K.; et al. Effects of Dietary Fructose Restriction on Liver Fat, De Novo Lipogenesis, and Insulin Kinetics in Children with Obesity. Gastroenterology 2017, 153, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Viscarra, J.; Kim, S.J.; Sul, H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689. [Google Scholar] [CrossRef] [Green Version]
- Abdelmalek, M.F.; Lazo, M.; Horska, A.; Bonekamp, S.; Lipkin, E.W.; Balasubramanyam, A.; Bantle, J.P.; Johnson, R.J.; Diehl, A.M.; Clark, J.M. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 2012, 56, 952–960. [Google Scholar] [CrossRef] [Green Version]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.; Wang, Y.; Keshaw, H.; Xu, L.Y.; Lam, K.S.; Cooper, G.J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Invest. 2003, 112, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Myers, M.G.; Cowley, M.A.; Münzberg, H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef] [Green Version]
- Sabio, G.; Das, M.; Mora, A.; Zhang, Z.; Jun, J.Y.; Ko, H.J.; Barrett, T.; Kim, J.K.; Davis, R.J. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008, 322, 1539–1543. [Google Scholar] [CrossRef] [Green Version]
- Aubert, J.; Begriche, K.; Knockaert, L.; Robin, M.A.; Fromenty, B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: Mechanisms and pathophysiological role. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 630–637. [Google Scholar] [CrossRef]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative Stress in Non-alcoholic Fatty Liver Disease. An Updated Mini Review. Front. Med. 2021, 8, 595371. [Google Scholar] [CrossRef]
- Cases, S.; Smith, S.J.; Zheng, Y.W.; Myers, H.M.; Lear, S.R.; Sande, E.; Novak, S.; Collins, C.; Welch, C.B.; Lusis, A.J.; et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef] [Green Version]
- Cases, S.; Stone, S.J.; Zhou, P.; Yen, E.; Tow, B.; Lardizabal, K.D.; Voelker, T.; Farese, R.V., Jr. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 2001, 276, 38870–38876. [Google Scholar] [CrossRef] [Green Version]
- Luukkonen, P.K.; Zhou, Y.; Sädevirta, S.; Leivonen, M.; Arola, J.; Orešič, M.; Hyötyläinen, T.; Yki-Järvinen, H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1167–1175. [Google Scholar] [CrossRef] [Green Version]
- Han, M.S.; Park, S.Y.; Shinzawa, K.; Kim, S.; Chung, K.W.; Lee, J.H.; Kwon, C.H.; Lee, K.W.; Lee, J.H.; Park, C.K.; et al. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J. Lipid Res. 2008, 49, 84–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fassnacht, M.; Kreissl, M.C.; Weismann, D.; Allolio, B. New targets and therapeutic approaches for endocrine malignancies. Pharmacol. Ther. 2009, 123, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Yang, L.; McCall, S.; Huang, J.; Yu, X.X.; Pandey, S.K.; Bhanot, S.; Monia, B.P.; Li, Y.X.; Diehl, A.M. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007, 45, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Mirshahi, F.; Cheung, O.; Natarajan, R.; Maher, J.W.; Kellum, J.M.; Sanyal, A.J. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 2008, 134, 568–576. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, D.; Pagliassotti, M.J. Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol. Cell Biochem. 2007, 303, 105–113. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, D.; Topczewski, F.; Pagliassotti, M.J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E275–E281. [Google Scholar] [CrossRef]
- Li, C.; Xu, M.M.; Wang, K.; Adler, A.J.; Vella, A.T.; Zhou, B. Macrophage polarization and meta-inflammation. Transl. Res. 2018, 191, 29–44. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef]
- Gomes, A.L.; Teijeiro, A.; Burén, S.; Tummala, K.S.; Yilmaz, M.; Waisman, A.; Theurillat, J.P.; Perna, C.; Djouder, N. Metabolic Inflammation-Associated IL-17A Causes Non-alcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 2016, 30, 161–175. [Google Scholar] [CrossRef]
- Choi, C.I. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors from Natural Products: Discovery of Next-Generation Antihyperglycemic Agents. Molecules 2016, 21, 1136. [Google Scholar] [CrossRef] [Green Version]
- Hardman, T.C.; Rutherford, P.; Duprey, S.W.; Wierzbicki, A.S. Sodium-glucose co-transporter 2 inhibitors: From apple tree to ‘Sweet Pee’. Curr. Pharm. Des. 2010, 16, 3830–3838. [Google Scholar] [CrossRef]
- Blaschek, W. Natural Products as Lead Compounds for Sodium Glucose Cotransporter (SGLT) Inhibitors. Planta Med. 2017, 83, 985–993. [Google Scholar] [CrossRef]
- Vick, H.; Diedrich, D.F.; Baumann, K. Reevaluation of renal tubular glucose transport inhibition by phlorizin analogs. Am. J. Physiol. 1973, 224, 552–557. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The Bioavailability, Extraction, Biosynthesis and Distribution of Natural Dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Lehmann, A.; Hornby, P.J. Intestinal SGLT1 in metabolic health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G887–G898. [Google Scholar] [CrossRef] [Green Version]
- Katsuno, K.; Fujimori, Y.; Takemura, Y.; Hiratochi, M.; Itoh, F.; Komatsu, Y.; Fujikura, H.; Isaji, M. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J. Pharmacol. Exp. Ther. 2007, 320, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Oku, A.; Ueta, K.; Arakawa, K.; Ishihara, T.; Nawano, M.; Kuronuma, Y.; Matsumoto, M.; Saito, A.; Tsujihara, K.; Anai, M.; et al. T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes 1999, 48, 1794–1800. [Google Scholar] [CrossRef]
- Meng, W.; Ellsworth, B.A.; Nirschl, A.A.; McCann, P.J.; Patel, M.; Girotra, R.N.; Wu, G.; Sher, P.M.; Morrison, E.P.; Biller, S.A.; et al. Discovery of dapagliflozin: A potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2008, 51, 1145–1149. [Google Scholar] [CrossRef]
- Nomura, S.; Sakamaki, S.; Hongu, M.; Kawanishi, E.; Koga, Y.; Sakamoto, T.; Yamamoto, Y.; Ueta, K.; Kimata, H.; Nakayama, K.; et al. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J. Med. Chem. 2010, 53, 6355–6360. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef]
- Markham, A.; Elkinson, S. Luseogliflozin: First global approval. Drugs 2014, 74, 945–950. [Google Scholar] [CrossRef]
- Poole, R.M.; Dungo, R.T. Ipragliflozin: First global approval. Drugs 2014, 74, 611–617. [Google Scholar] [CrossRef]
- Poole, R.M.; Prossler, J.E. Tofogliflozin: First global approval. Drugs 2014, 74, 939–944. [Google Scholar] [CrossRef]
- Xu, J.; Hirai, T.; Koya, D.; Kitada, M. Effects of SGLT2 Inhibitors on Atherosclerosis: Lessons from Cardiovascular Clinical Outcomes in Type 2 Diabetic Patients and Basic Researches. J. Clin. Med. 2021, 11, 137. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Isaji, M. Sodium-glucose cotransporter inhibitors for diabetes. Curr. Opin. Investig. Drugs 2007, 8, 285–292. [Google Scholar]
- Isaji, M. SGLT2 inhibitors: Molecular design and potential differences in effect. Kidney Int. Suppl. 2011, 79 (Suppl. S20), S14–S19. [Google Scholar] [CrossRef] [Green Version]
- Okada, J.; Yamada, E.; Saito, T.; Yokoo, H.; Osaki, A.; Shimoda, Y.; Ozawa, A.; Nakajima, Y.; Pessin, J.E.; Okada, S.; et al. Dapagliflozin Inhibits Cell Adhesion to Collagen I and IV and Increases Ectodomain Proteolytic Cleavage of DDR1 by Increasing ADAM10 Activity. Molecules 2020, 25, 495. [Google Scholar] [CrossRef] [Green Version]
- Kaji, K.; Nishimura, N.; Seki, K.; Sato, S.; Saikawa, S.; Nakanishi, K.; Furukawa, M.; Kawaratani, H.; Kitade, M.; Moriya, K.; et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int. J. Cancer 2018, 142, 1712–1722. [Google Scholar] [CrossRef] [Green Version]
- Hawley, S.A.; Ford, R.J.; Smith, B.K.; Gowans, G.J.; Mancini, S.J.; Pitt, R.D.; Day, E.A.; Salt, I.P.; Steinberg, G.R.; Hardie, D.G. The Na+/Glucose Cotransporter Inhibitor Canagliflozin Activates AMPK by Inhibiting Mitochondrial Function and Increasing Cellular AMP Levels. Diabetes 2016, 65, 2784–2794. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Sun, P.; Wang, Y.; Chen, Y.; Niu, Y.; Ding, Y.; Xu, N.; Zhang, Y.; Xie, W. Dapagliflozin attenuates steatosis in livers of high-fat diet-induced mice and oleic acid-treated L02 cells via regulating AMPK/mTOR pathway. Eur. J. Pharmacol. 2021, 907, 174304. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Q.; Huang, W.; Han, Y.; Tan, H.; An, M.; Xiang, Q.; Zhou, R.; Yang, L.; Cheng, Y. Dapagliflozin Alleviates Hepatic Steatosis by Restoring Autophagy via the AMPK-mTOR Pathway. Front. Pharmacol. 2021, 12, 589273. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, M.; Yin, F.; Wang, Y.; Li, X.; Wu, Y.; Ye, C.; Liu, J. Trilobatin, a Novel SGLT1/2 Inhibitor, Selectively Induces the Proliferation of Human Hepatoblastoma Cells. Molecules 2019, 24, 3390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jojima, T.; Wakamatsu, S.; Kase, M.; Iijima, T.; Maejima, Y.; Shimomura, K.; Kogai, T.; Tomaru, T.; Usui, I.; Aso, Y. The SGLT2 Inhibitor Canagliflozin Prevents Carcinogenesis in a Mouse Model of Diabetes and Non-Alcoholic Steatohepatitis-Related Hepatocarcinogenesis: Association with SGLT2 Expression in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 5237. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Rafei, M.K.; Thabet, N.M.; Rashed, L.A.; Moustafa, E.M. Canagliflozin, a SGLT-2 inhibitor, relieves ER stress, modulates autophagy and induces apoptosis in irradiated HepG2 cells: Signal transduction between PI3K/AKT/GSK-3beta/mTOR and Wnt/beta-catenin pathways; in vitro. J. Cancer Res. Ther. 2021, 17, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Obara, K.; Shirakami, Y.; Maruta, A.; Ideta, T.; Miyazaki, T.; Kochi, T.; Sakai, H.; Tanaka, T.; Seishima, M.; Shimizu, M. Preventive effects of the sodium glucose cotransporter 2 inhibitor tofogliflozin on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic mice. Oncotarget 2017, 8, 58353–58363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, H.; Lee, J.C.; Huang, H.C.; Huang, H.; Liu, H.K.; Huang, C. Delayed intervention with a novel SGLT2 inhibitor NGI001 suppresses diet-induced metabolic dysfunction and non-alcoholic fatty liver disease in mice. Br. J. Pharmacol. 2020, 177, 239–253. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, Y.; Liu, Q.; Tang, Q.; Zhang, Z.; Zhang, J.; Huang, C.; Huang, H.; Zhang, G.; Zhou, J.; et al. Canagliflozin Facilitates Reverse Cholesterol Transport through Activation of AMPK/ABC Transporter Pathway. Drug Des. Devel. Ther. 2021, 15, 2117–2128. [Google Scholar] [CrossRef]
- Wang, B.; Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018, 14, 452–463. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, J.; Yu, S.J.; Ma, H.L.; Chen, J.; Ding, X.F.; Chen, G.; Liang, Y.; Zhang, Q. Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed. Pharmacother. 2020, 132, 110821. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, S.; Yang, C.; Du, R.; Zhao, J.; Li, J.; Xu, Y.; Qin, Y.; Gao, Y.; Huang, W. Ginsenoside Rg1 Ameliorates Palmitic Acid-Induced Hepatic Steatosis and Inflammation in HepG2 Cells via the AMPK/NF-kappaB Pathway. Int. J. Endocrinol. 2019, 2019, 7514802. [Google Scholar] [CrossRef] [Green Version]
- Soret, P.A.; Magusto, J.; Housset, C.; Gautheron, J. In Vitro and In Vivo Models of Non-Alcoholic Fatty Liver Disease: A Critical Appraisal. J. Clin. Med. 2020, 10, 36. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Goldin, R.D. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Pathol. 2006, 87, 1–16. [Google Scholar] [CrossRef]
- Ibrahim, S.H.; Hirsova, P.; Malhi, H.; Gores, G.J. Animal Models of Nonalcoholic Steatohepatitis: Eat, Delete, and Inflame. Dig. Dis. Sci. 2016, 61, 1325–1336. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, A.; Terauchi, Y. Lessons from mouse models of high-fat diet-induced NAFLD. Int. J. Mol. Sci. 2013, 14, 21240–21257. [Google Scholar] [CrossRef] [Green Version]
- Perakakis, N.; Chrysafi, P.; Feigh, M.; Veidal, S.S.; Mantzoros, C.S. Empagliflozin Improves Metabolic and Hepatic Outcomes in a Non-Diabetic Obese Biopsy-Proven Mouse Model of Advanced NASH. Int. J. Mol. Sci. 2021, 22, 6332. [Google Scholar] [CrossRef]
- Tahara, A.; Takasu, T. Therapeutic Effects of SGLT2 Inhibitor Ipragliflozin and Metformin on NASH in Type 2 Diabetic Mice. Endocr. Res. 2020, 45, 147–161. [Google Scholar] [CrossRef]
- Shiba, K.; Tsuchiya, K.; Komiya, C.; Miyachi, Y.; Mori, K.; Shimazu, N.; Yamaguchi, S.; Ogasawara, N.; Katoh, M.; Itoh, M.; et al. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci. Rep. 2018, 8, 2362. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.; Wilding, J.P.H.; Barber, T.M.; Alam, U.; Cuthbertson, D.J. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: Mechanistic possibilities. Obes. Rev. 2019, 20, 816–828. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Yang, W.; Gao, X.; Chen, Y.; Zhou, L.; Zhang, S.; Han, X.; Ji, L. The Association between the Dosage of SGLT2 Inhibitor and Weight Reduction in Type 2 Diabetes Patients: A Meta-Analysis. Obesity 2018, 26, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherney, D.Z.I.; Dekkers, C.C.J.; Barbour, S.J.; Cattran, D.; Abdul Gafor, A.H.; Greasley, P.J.; Laverman, G.D.; Lim, S.K.; Di Tanna, G.L.; Reich, H.N.; et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): A randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020, 8, 582–593. [Google Scholar] [CrossRef]
- Han, T.; Fan, Y.J.; Gao, J.; Fatima, M.R.; Zhang, Y.L.; Ding, Y.M.; Bai, L.A.; Wang, C.X. Sodium glucose cotransporter 2 inhibitor dapagliflozin depressed adiposity and ameliorated hepatic steatosis in high-fat diet induced obese mice. Adipocyte 2021, 10, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Kubota, N.; Kubota, T.; Iwamoto, M.; Sato, H.; Sakurai, Y.; Takamoto, I.; Katsuyama, H.; Suzuki, Y.; Fukazawa, M.; et al. Tofogliflozin Improves Insulin Resistance in Skeletal Muscle and Accelerates Lipolysis in Adipose Tissue in Male Mice. Endocrinology 2016, 157, 1029–1042. [Google Scholar] [CrossRef]
- Petito-da-Silva, T.I.; Souza-Mello, V.; Barbosa-da-Silva, S. Empaglifozin mitigates NAFLD in high-fat-fed mice by alleviating insulin resistance, lipogenesis and ER stress. Mol. Cell Endocrinol. 2019, 498, 110539. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahashi, H.; Katagiri, S.; Sasaki, K.; Ohsugi, Y.; Watanabe, K.; Rasadul, I.M.D.; Mine, K.; Nagafuchi, S.; Iwata, T.; et al. Combined effect of canagliflozin and exercise training on high-fat diet-fed mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E492–E503. [Google Scholar] [CrossRef]
- Yabiku, K.; Nakamoto, K.; Tsubakimoto, M. Effects of Sodium-Glucose Cotransporter 2 Inhibition on Glucose Metabolism, Liver Function, Ascites, and Hemodynamics in a Mouse Model of Nonalcoholic Steatohepatitis and Type 2 Diabetes. J. Diabetes Res. 2020, 2020, 1682904. [Google Scholar] [CrossRef]
- Ozutsumi, T.; Namisaki, T.; Shimozato, N.; Kaji, K.; Tsuji, Y.; Kaya, D.; Fujinaga, Y.; Furukawa, M.; Nakanishi, K.; Sato, S.; et al. Combined Treatment with Sodium-Glucose Cotransporter-2 Inhibitor (Canagliflozin) and Dipeptidyl Peptidase-4 Inhibitor (Teneligliptin) Alleviates NASH Progression in A Non-Diabetic Rat Model of Steatohepatitis. Int. J. Mol. Sci. 2020, 21, 2164. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, K.; Hosooka, T.; Shinohara, M.; Aoki, C.; Hosokawa, Y.; Imamori, M.; Ogawa, W. Canagliflozin ameliorates hepatic fat deposition in obese diabetic mice: Role of prostaglandin E2. Biochem. Biophys. Res. Commun. 2021, 557, 62–68. [Google Scholar] [CrossRef]
- Komiya, C.; Tsuchiya, K.; Shiba, K.; Miyachi, Y.; Furuke, S.; Shimazu, N.; Yamaguchi, S.; Kanno, K.; Ogawa, Y. Ipragliflozin Improves Hepatic Steatosis in Obese Mice and Liver Dysfunction in Type 2 Diabetic Patients Irrespective of Body Weight Reduction. PLoS ONE 2016, 11, e0151511. [Google Scholar] [CrossRef] [Green Version]
- Tahara, A.; Takasu, T. SGLT2 inhibitor ipragliflozin alone and combined with pioglitazone prevents progression of nonalcoholic steatohepatitis in a type 2 diabetes rodent model. Physiol. Rep. 2019, 7, e14286. [Google Scholar] [CrossRef] [Green Version]
- Honda, Y.; Imajo, K.; Kato, T.; Kessoku, T.; Ogawa, Y.; Tomeno, W.; Kato, S.; Mawatari, H.; Fujita, K.; Yoneda, M.; et al. The Selective SGLT2 Inhibitor Ipragliflozin Has a Therapeutic Effect on Nonalcoholic Steatohepatitis in Mice. PLoS ONE 2016, 11, e0146337. [Google Scholar] [CrossRef] [Green Version]
- Huttl, M.; Markova, I.; Miklankova, D.; Zapletalova, I.; Poruba, M.; Haluzik, M.; Vaneckova, I.; Malinska, H. In a Prediabetic Model, Empagliflozin Improves Hepatic Lipid Metabolism Independently of Obesity and before Onset of Hyperglycemia. Int. J. Mol. Sci. 2021, 22, 11513. [Google Scholar] [CrossRef]
- Steven, S.; Oelze, M.; Hanf, A.; Kroller-Schon, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Godtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, X.; Li, T.; Fang, T.; Cheng, Y.; Han, L.; Sun, B.; Chen, L. The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. Int. Immunopharmacol. 2021, 94, 107492. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol. Sci. 2017, 38, 649–665. [Google Scholar] [CrossRef]
- Chambel, S.S.; Santos-Goncalves, A.; Duarte, T.L. The Dual Role of Nrf2 in Nonalcoholic Fatty Liver Disease: Regulation of Antioxidant Defenses and Hepatic Lipid Metabolism. Biomed. Res. Int. 2015, 2015, 597134. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Kong, Y.; Peng, D. Fibroblast growth factor 21 in lipid metabolism and non-alcoholic fatty liver disease. Clin. Chim. Acta 2019, 498, 30–37. [Google Scholar] [CrossRef]
- Eberle, D.; Hegarty, B.; Bossard, P.; Ferre, P.; Foufelle, F. SREBP transcription factors: Master regulators of lipid homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef]
- Kern, M.; Kloting, N.; Mark, M.; Mayoux, E.; Klein, T.; Bluher, M. The SGLT2 inhibitor empagliflozin improves insulin sensitivity in db/db mice both as monotherapy and in combination with linagliptin. Metabolism 2016, 65, 114–123. [Google Scholar] [CrossRef]
- Bakan, I.; Laplante, M. Connecting mTORC1 signaling to SREBP-1 activation. Curr. Opin. Lipidol. 2012, 23, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Souza-Mello, V. Peroxisome proliferator-activated receptors as targets to treat non-alcoholic fatty liver disease. World J. Hepatol. 2015, 7, 1012–1019. [Google Scholar] [CrossRef]
- Omori, K.; Nakamura, A.; Miyoshi, H.; Takahashi, K.; Kitao, N.; Nomoto, H.; Kameda, H.; Cho, K.Y.; Takagi, R.; Hatanaka, K.C.; et al. Effects of dapagliflozin and/or insulin glargine on beta cell mass and hepatic steatosis in db/db mice. Metabolism 2019, 98, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Tanaka, M.; Ochi, K.; Watanabe, A.; Ono, K.; Sawada, M.; Ogi, T.; Itoh, M.; Ito, A.; Shiraki, Y.; et al. The sodium-glucose cotransporter-2 inhibitor Tofogliflozin prevents the progression of nonalcoholic steatohepatitis-associated liver tumors in a novel murine model. Biomed. Pharmacother. 2021, 140, 111738. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.M.; Meers, G.M.; Booth, F.W.; Fritsche, K.L.; Hardin, C.D.; Thyfault, J.P.; Ibdah, J.A. PGC-1alpha overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G979–G992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrysavgis, L.; Papatheodoridi, A.M.; Chatzigeorgiou, A.; Cholongitas, E. The impact of sodium glucose co-transporter 2 inhibitors on non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2021, 36, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Kabil, S.L.; Mahmoud, N.M. Canagliflozin protects against non-alcoholic steatohepatitis in type-2 diabetic rats through zinc alpha-2 glycoprotein up-regulation. Eur. J. Pharmacol. 2018, 828, 135–145. [Google Scholar] [CrossRef] [PubMed]
- ElMahdy, M.K.; Helal, M.G.; Ebrahim, T.M. Potential anti-inflammatory effect of dapagliflozin in HCHF diet- induced fatty liver degeneration through inhibition of TNF-alpha, IL-1beta, and IL-18 in rat liver. Int. Immunopharmacol. 2020, 86, 106730. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur. J. Pharmacol. 2013, 715, 246–255. [Google Scholar] [CrossRef]
- Tang, L.; Wu, Y.; Tian, M.; Sjostrom, C.D.; Johansson, U.; Peng, X.R.; Smith, D.M.; Huang, Y. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E563–E576. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Nakano, S.; Katsuno, K.; Isaji, M.; Nagasawa, T.; Buehrer, B.; Walker, S.; Wilkison, W.O.; Cheatham, B. Remogliflozin Etabonate Improves Fatty Liver Disease in Diet-Induced Obese Male Mice. J. Clin. Exp. Hepatol. 2015, 5, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Ookawara, M.; Matsuda, K.; Watanabe, M.; Moritoh, Y. The GPR40 Full Agonist SCO-267 Improves Liver Parameters in a Mouse Model of Nonalcoholic Fatty Liver Disease without Affecting Glucose or Body Weight. J. Pharmacol. Exp. Ther. 2020, 375, 21–27. [Google Scholar] [CrossRef]
- Jojima, T.; Tomotsune, T.; Iijima, T.; Akimoto, K.; Suzuki, K.; Aso, Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP-4 inhibitor), prevents steatohepatitis in a novel mouse model of non-alcoholic steatohepatitis and diabetes. Diabetol. Metab. Syndr. 2016, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Hupa-Breier, K.L.; Dywicki, J.; Hartleben, B.; Wellhoner, F.; Heidrich, B.; Taubert, R.; Mederacke, Y.E.; Lieber, M.; Iordanidis, K.; Manns, M.P.; et al. Dulaglutide Alone and in Combination with Empagliflozin Attenuate Inflammatory Pathways and Microbiome Dysbiosis in a Non-Diabetic Mouse Model of NASH. Biomedicines 2021, 9, 353. [Google Scholar] [CrossRef]
- Kuwako, K.I.; Okano, H. Versatile Roles of LKB1 Kinase Signaling in Neural Development and Homeostasis. Front. Mol. Neurosci. 2018, 11, 354. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Yang, L.; Liu, P.; Zhang, Y.; Wang, X. MiR-149 attenuates endoplasmic reticulum stress-induced inflammation and apoptosis in nonalcoholic fatty liver disease by negatively targeting ATF6 pathway. Immunol. Lett. 2020, 222, 40–48. [Google Scholar] [CrossRef]
- Heyens, L.J.M.; Busschots, D.; Koek, G.H.; Robaeys, G.; Francque, S. Liver Fibrosis in Non-alcoholic Fatty Liver Disease: From Liver Biopsy to Non-invasive Biomarkers in Diagnosis and Treatment. Front. Med. 2021, 8, 615978. [Google Scholar] [CrossRef]
- Zisser, A.; Ipsen, D.H.; Tveden-Nyborg, P. Hepatic Stellate Cell Activation and Inactivation in NASH-Fibrosis-Roles as Putative Treatment Targets? Biomedicines 2021, 9, 365. [Google Scholar] [CrossRef]
- Qiang, S.; Nakatsu, Y.; Seno, Y.; Fujishiro, M.; Sakoda, H.; Kushiyama, A.; Mori, K.; Matsunaga, Y.; Yamamotoya, T.; Kamata, H.; et al. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol. Metab. Syndr. 2015, 7, 104. [Google Scholar] [CrossRef] [Green Version]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastro Hepat. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Hayashizaki-Someya, Y.; Kurosaki, E.; Takasu, T.; Mitori, H.; Yamazaki, S.; Koide, K.; Takakura, S. Ipragliflozin, an SGLT2 inhibitor, exhibits a prophylactic effect on hepatic steatosis and fibrosis induced by choline-deficient l-amino acid-defined diet in rats. Eur. J. Pharmacol. 2015, 754, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Bando, Y.; Ogawa, A.; Ishikura, K.; Kanehara, H.; Hisada, A.; Notumata, K.; Okafuji, K.; Toya, D. The effects of ipragliflozin on the liver-to-spleen attenuation ratio as assessed by computed tomography and on alanine transaminase levels in Japanese patients with type 2 diabetes mellitus. Diabetol. Int. 2017, 8, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Akuta, N.; Watanabe, C.; Kawamura, Y.; Arase, Y.; Saitoh, S.; Fujiyama, S.; Sezaki, H.; Hosaka, T.; Kobayashi, M.; Kobayashi, M.; et al. Effects of a sodium-glucose cotransporter 2 inhibitor in nonalcoholic fatty liver disease complicated by diabetes mellitus: Preliminary prospective study based on serial liver biopsies. Hepatol. Commun. 2017, 1, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Shimizu, S.; Inoue, K.; Saito, D.; Yanagisawa, M.; Inukai, K.; Akiyama, Y.; Morimoto, Y.; Noda, M.; Shimada, A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care 2017, 40, 1364–1372. [Google Scholar] [CrossRef] [Green Version]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Umemura, A.; Nishikawa, T.; et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol. Res. 2017, 47, 1072–1078. [Google Scholar] [CrossRef]
- Choi, D.H.; Jung, C.H.; Mok, J.O.; Kim, C.H.; Kang, S.K.; Kim, B.Y. Effect of Dapagliflozin on Alanine Aminotransferase Improvement in Type 2 Diabetes Mellitus with Non-alcoholic Fatty Liver Disease. Endocrinol. Metab. 2018, 33, 387–394. [Google Scholar] [CrossRef]
- Eriksson, J.W.; Lundkvist, P.; Jansson, P.A.; Johansson, L.; Kvarnström, M.; Moris, L.; Miliotis, T.; Forsberg, G.B.; Risérus, U.; Lind, L.; et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: A double-blind randomised placebo-controlled study. Diabetologia 2018, 61, 1923–1934. [Google Scholar] [CrossRef] [Green Version]
- Itani, T.; Ishihara, T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: A prospective cohort study. Obes. Sci. Pract. 2018, 4, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Farooqui, K.J.; Singh, M.K.; Wasir, J.S.; Bansal, B.; Kaur, P.; Jevalikar, G.; Gill, H.K.; et al. Effect of Empagliflozin on Liver Fat in Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care 2018, 41, 1801–1808. [Google Scholar] [CrossRef] [Green Version]
- Miyake, T.; Yoshida, S.; Furukawa, S.; Sakai, T.; Tada, F.; Senba, H.; Yamamoto, S.; Koizumi, Y.; Yoshida, O.; Hirooka, M.; et al. Ipragliflozin Ameliorates Liver Damage in Non-alcoholic Fatty Liver Disease. Open Med. 2018, 13, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, T.; Fushimi, N.; Kawai, M.; Yoshida, Y.; Hachiya, H.; Ito, S.; Kawai, H.; Ohashi, N.; Mori, A. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diabetes Obes. Metab. 2018, 20, 438–442. [Google Scholar] [CrossRef]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Murotani, K.; Saito, M.; Tamasawa, A.; Osonoi, Y.; Yoneda, M.; Osonoi, T. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective, single-arm trial (LEAD trial). Hepatol. Res. 2019, 49, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akuta, N.; Kawamura, Y.; Watanabe, C.; Nishimura, A.; Okubo, M.; Mori, Y.; Fujiyama, S.; Sezaki, H.; Hosaka, T.; Kobayashi, M.; et al. Impact of sodium glucose cotransporter 2 inhibitor on histological features and glucose metabolism of non-alcoholic fatty liver disease complicated by diabetes mellitus. Hepatol. Res. 2019, 49, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Yamashima, M.; Miyaaki, H.; Miuma, S.; Shibata, H.; Sasaki, R.; Haraguchi, M.; Fukushima, M.; Nakao, K. The Long-term Efficacy of Sodium Glucose Co-transporter 2 Inhibitor in Patients with Non-alcoholic Fatty Liver Disease. Intern. Med. 2019, 58, 1987–1992. [Google Scholar] [CrossRef] [Green Version]
- Akuta, N.; Kawamura, Y.; Fujiyama, S.; Sezaki, H.; Hosaka, T.; Kobayashi, M.; Kobayashi, M.; Saitoh, S.; Suzuki, F.; Suzuki, Y.; et al. SGLT2 Inhibitor Treatment Outcome in Nonalcoholic Fatty Liver Disease Complicated with Diabetes Mellitus: The Long-term Effects on Clinical Features and Liver Histopathology. Intern. Med. 2020, 59, 1931–1937. [Google Scholar] [CrossRef]
- Han, E.; Lee, Y.H.; Lee, B.W.; Kang, E.S.; Cha, B.S. Ipragliflozin Additively Ameliorates Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Controlled with Metformin and Pioglitazone: A 24-Week Randomized Controlled Trial. J. Clin. Med. 2020, 9, 259. [Google Scholar] [CrossRef] [Green Version]
- Kahl, S.; Gancheva, S.; Straßburger, K.; Herder, C.; Machann, J.; Katsuyama, H.; Kabisch, S.; Henkel, E.; Kopf, S.; Lagerpusch, M.; et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care 2020, 43, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Shimoda, M.; Nakashima, K.; Fushimi, Y.; Hirata, Y.; Tanabe, A.; Tatsumi, F.; Hirukawa, H.; Sanada, J.; Kohara, K.; et al. Comparison of the effects of three kinds of glucose-lowering drugs on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, open-label, three-arm, active control study. J. Diabetes Investig. 2020, 11, 1612–1622. [Google Scholar] [CrossRef]
- Yano, K.; Seko, Y.; Takahashi, A.; Okishio, S.; Kataoka, S.; Takemura, M.; Okuda, K.; Mizuno, N.; Taketani, H.; Umemura, A.; et al. Effect of Sodium Glucose Cotransporter 2 Inhibitors on Renal Function in Patients with Nonalcoholic Fatty Liver Disease and Type 2 Diabetes in Japan. Diagnostics 2020, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Mikami, S.; Ono, H.; Kawano, T.; Yoshida, Y.; Tanabe, T.; Okubo, T.; Hayama, K.; et al. Effect of sodium-glucose cotransporter 2 inhibitor in patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus: A propensity score-matched analysis of real-world data. Ther. Adv. Endocrinol. Metab. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Chehrehgosha, H.; Sohrabi, M.R.; Ismail-Beigi, F.; Malek, M.; Reza Babaei, M.; Zamani, F.; Ajdarkosh, H.; Khoonsari, M.; Fallah, A.E.; Khamseh, M.E. Empagliflozin Improves Liver Steatosis and Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Diabetes Ther. 2021, 12, 843–861. [Google Scholar] [CrossRef]
- Euh, W.; Lim, S.; Kim, J.W. Sodium-Glucose Cotransporter-2 Inhibitors Ameliorate Liver Enzyme Abnormalities in Korean Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 613389. [Google Scholar] [CrossRef]
- Gaborit, B.; Ancel, P.; Abdullah, A.E.; Maurice, F.; Abdesselam, I.; Calen, A.; Soghomonian, A.; Houssays, M.; Varlet, I.; Eisinger, M.; et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: The EMPACEF study. Cardiovasc. Diabetol. 2021, 20, 57. [Google Scholar] [CrossRef]
- Takahashi, H.; Kessoku, T.; Kawanaka, M.; Nonaka, M.; Hyogo, H.; Fujii, H.; Nakajima, T.; Imajo, K.; Tanaka, K.; Kubotsu, Y.; et al. Ipragliflozin Improves the Hepatic Outcomes of Patients with Diabetes with NAFLD. Hepatol. Commun. 2021, 6, 120–132. [Google Scholar] [CrossRef]
- Yoneda, M.; Honda, Y.; Ogawa, Y.; Kessoku, T.; Kobayashi, T.; Imajo, K.; Ozaki, A.; Nogami, A.; Taguri, M.; Yamanaka, T.; et al. Comparing the effects of tofogliflozin and pioglitazone in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus (ToPiND study): A randomized prospective open-label controlled trial. BMJ Open Diabetes Res. Care 2021, 9, e001990. [Google Scholar] [CrossRef]
- Pradhan, R.; Yin, H.; Yu, O.; Azoulay, L. Glucagon-Like Peptide 1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Nonalcoholic Fatty Liver Disease Among Patients with Type 2 Diabetes. Diabetes Care 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Sinha, B.; Datta, D.; Ghosal, S. Meta-analysis of the effects of sodium glucose cotransporter 2 inhibitors in non-alcoholic fatty liver disease patients with type 2 diabetes. JGH Open 2021, 5, 219–227. [Google Scholar] [CrossRef]
- Mo, M.; Huang, Z.; Liang, Y.; Liao, Y.; Xia, N. The safety and efficacy evaluation of Sodium-Glucose co-transporter 2 inhibitors for patients with non-alcoholic fatty Liver disease: An updated meta-analysis. Dig. Liver Dis. 2021, in press. [Google Scholar] [CrossRef]
- Wong, C.; Yaow, C.Y.L.; Ng, C.H.; Chin, Y.H.; Low, Y.F.; Lim, A.Y.L.; Muthiah, M.D.; Khoo, C.M. Sodium-Glucose Co-Transporter 2 Inhibitors for Non-Alcoholic Fatty Liver Disease in Asian Patients with Type 2 Diabetes: A Meta-Analysis. Front. Endocrinol. 2020, 11, 609135. [Google Scholar] [CrossRef] [PubMed]
- Tobita, H.; Yazaki, T.; Kataoka, M.; Kotani, S.; Oka, A.; Mishiro, T.; Oshima, N.; Kawashima, K.; Ishimura, N.; Naora, K.; et al. Comparison of dapagliflozin and teneligliptin in nonalcoholic fatty liver disease patients without type 2 diabetes mellitus: A prospective randomized study. J. Clin. Biochem. Nutr. 2021, 68, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; Xiong, J.; Solis-Herrera, C.; Merovci, A.; Eldor, R.; Tripathy, D.; DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Dapagliflozin Enhances Fat Oxidation and Ketone Production in Patients with Type 2 Diabetes. Diabetes Care 2016, 39, 2036–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Luo, Y.; Wang, X.; Orlicky, D.J.; Myakala, K.; Yang, P.; Levi, M. The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Renal and Liver Disease in Western Diet Induced Obesity Mice. Int. J. Mol. Sci. 2018, 19, 137. [Google Scholar] [CrossRef] [Green Version]
- Bonner, C.; Kerr-Conte, J.; Gmyr, V.; Queniat, G.; Moerman, E.; Thévenet, J.; Beaucamps, C.; Delalleau, N.; Popescu, I.; Malaisse, W.J.; et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat. Med. 2015, 21, 512–517. [Google Scholar] [CrossRef]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Heise, T.; Bizzotto, R.; Mari, A.; Pieber, T.R.; Muscelli, E. Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects without Diabetes and Patients with Type 2 Diabetes. Diabetes 2016, 65, 1190–1195. [Google Scholar] [CrossRef] [Green Version]
- Yokono, M.; Takasu, T.; Hayashizaki, Y.; Mitsuoka, K.; Kihara, R.; Muramatsu, Y.; Miyoshi, S.; Tahara, A.; Kurosaki, E.; Li, Q.; et al. SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur. J. Pharmacol. 2014, 727, 66–74. [Google Scholar] [CrossRef]
- Terami, N.; Ogawa, D.; Tachibana, H.; Hatanaka, T.; Wada, J.; Nakatsuka, A.; Eguchi, J.; Horiguchi, C.S.; Nishii, N.; Yamada, H.; et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 2014, 9, e100777. [Google Scholar] [CrossRef]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J. Pharm. Pharmacol. 2014, 66, 975–987. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Butler, A.E.; Sahebkar, A. Sodium-glucose cotransporter inhibitors and oxidative stress: An update. J. Cell Physiol. 2019, 234, 3231–3237. [Google Scholar] [CrossRef]
- Oshima, H.; Miki, T.; Kuno, A.; Mizuno, M.; Sato, T.; Tanno, M.; Yano, T.; Nakata, K.; Kimura, Y.; Abe, K.; et al. Empagliflozin, an SGLT2 Inhibitor, Reduced the Mortality Rate after Acute Myocardial Infarction with Modification of Cardiac Metabolomes and Antioxidants in Diabetic Rats. J. Pharmacol. Exp. Ther. 2019, 368, 524–534. [Google Scholar] [CrossRef] [Green Version]
- Osorio, H.; Coronel, I.; Arellano, A.; Pacheco, U.; Bautista, R.; Franco, M.; Escalante, B. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid Med. Cell Longev. 2012, 2012, 542042. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Androutsakos, T.; Nasiri-Ansari, N.; Bakasis, A.-D.; Kyrou, I.; Efstathopoulos, E.; Randeva, H.S.; Kassi, E. SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection. Int. J. Mol. Sci. 2022, 23, 3107. https://doi.org/10.3390/ijms23063107
Androutsakos T, Nasiri-Ansari N, Bakasis A-D, Kyrou I, Efstathopoulos E, Randeva HS, Kassi E. SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection. International Journal of Molecular Sciences. 2022; 23(6):3107. https://doi.org/10.3390/ijms23063107
Chicago/Turabian StyleAndroutsakos, Theodoros, Narjes Nasiri-Ansari, Athanasios-Dimitrios Bakasis, Ioannis Kyrou, Efstathios Efstathopoulos, Harpal S. Randeva, and Eva Kassi. 2022. "SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection" International Journal of Molecular Sciences 23, no. 6: 3107. https://doi.org/10.3390/ijms23063107
APA StyleAndroutsakos, T., Nasiri-Ansari, N., Bakasis, A.-D., Kyrou, I., Efstathopoulos, E., Randeva, H. S., & Kassi, E. (2022). SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection. International Journal of Molecular Sciences, 23(6), 3107. https://doi.org/10.3390/ijms23063107









