Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis and Characterization of SB1–SB4
3.2. Hydrolysis Study in Solution
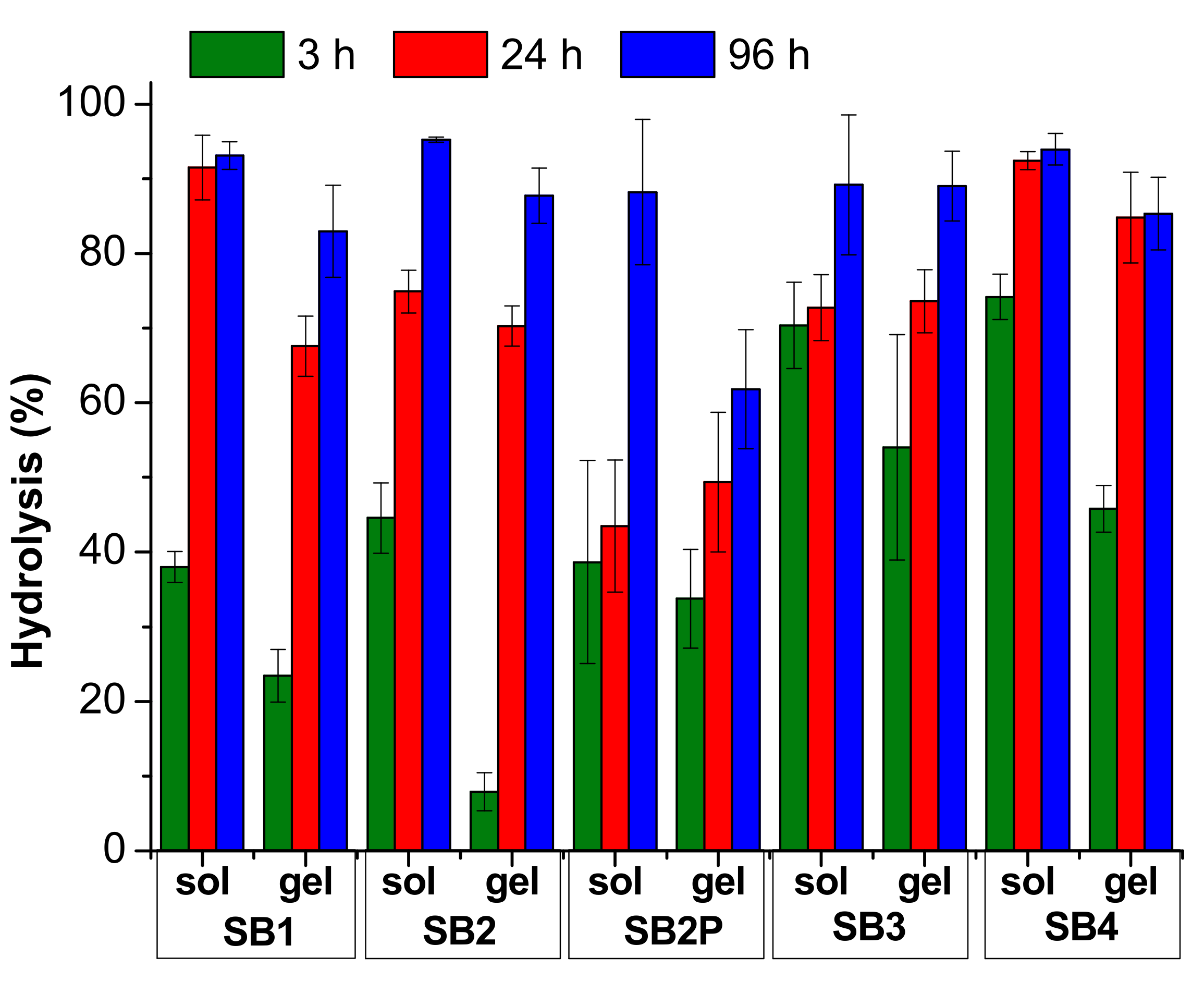
3.3. Hydrolysis Study in Acidic Solution
3.4. Gel Preparation
3.5. Optical Microscope Images
3.6. Single-Crystal X-ray Diffraction
3.7. Rheology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 6th ed.; Wiley-VCH: Weinheim, Germany, 2016; ISBN 9783527693153. [Google Scholar]
- Ohloff, G.; Pickenhagen, W.; Kraft, P. Scent and Chemistry; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Berger, R.G. (Ed.) Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9783540493389. [Google Scholar]
- Ciriminna, R.; Pagliaro, M. Sol–gel microencapsulation of odorants and flavors: Opening the route to sustainable fragrances and aromas. Chem. Soc. Rev. 2013, 42, 9243–9250. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, I.; Landfester, K.; Taden, A. pH-Sensitive Nanocapsules with Barrier Properties: Fragrance Encapsulation and Controlled Release. Macromolecules 2014, 47, 5768–5773. [Google Scholar] [CrossRef]
- Günay, K.A.; Benczédi, D.; Herrmann, A.; Klok, H.A. Peptide-Enhanced Selective Surface Deposition of Polymer-Based Fragrance Delivery Systems. Adv. Funct. Mater. 2017, 27, 1603843. [Google Scholar] [CrossRef]
- Günay, K.A.; Berthier, D.L.; Jerri, H.A.; Benczédi, D.; Klok, H.A.; Herrmann, A. Selective Peptide-Mediated Enhanced Deposition of Polymer Fragrance Delivery Systems on Human Hair. ACS Appl. Mater. Interfaces 2017, 9, 24238–24249. [Google Scholar] [CrossRef]
- Herrmann, A. Controlled Release of Volatiles under Mild Reaction Conditions: From Nature to Everyday Products. Angew. Chem. Int. Ed. 2007, 46, 5836–5863. [Google Scholar] [CrossRef]
- Herrmann, A. Profragrance Chemistry as an Interdisciplinary Research Area and Key Technology for Fragrance Delivery. CHIMIA 2017, 71, 414–419. [Google Scholar] [CrossRef]
- Herrmann, A. Controlled release of volatile compounds using the Norrish type II reaction. In Photochemistry; The Royal Society of Chemistry: Cambridge, UK, 2019; Volume 46, pp. 242–264. ISBN 978-1-78801-336-9. [Google Scholar]
- Tree-Udom, T.; Wanichwecharungruang, S.P.; Seemork, J.; Arayachukeat, S. Fragrant chitosan nanospheres: Controlled release systems with physical and chemical barriers. Carbohydr. Polym. 2011, 86, 1602–1609. [Google Scholar] [CrossRef]
- Liu, M.; Yan, C.; Han, J.; Guo, Z.; Zhu, W.H.; Xiao, Z.; Wu, Y.; Huang, J. pH-activated polymeric profragrances for dual-controllable perfume release. AIChE J. 2021, 67, 8–16. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.; Alajarin, M.; Pastor, A.; Berna, J. Mechanically Interlocked Profragrances for the Controlled Release of Scents. J. Org. Chem. 2021, 86, 15045–15054. [Google Scholar] [CrossRef]
- Schiff, H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Irawan, C.; Nur, L.; Mellisani, B.; Arinzani, H. Synthesis and characterization of citral-methylanthranilate schiff base, relationship between synthesis time and some physical properties. Rasayan J. Chem. 2019, 12, 951–958. [Google Scholar] [CrossRef]
- Irawan, C.; Islamiyati, D.; Utami, A.; Putri, I.D.; Perdana Putri, R.; Wibowo, S. Aurantiol Schiff base as A Raw Material in Fragrance Industry Synthesized by Simple Condensation Method and Its Characterization Using GC-MS. Orient. J. Chem. 2020, 36, 577–580. [Google Scholar] [CrossRef]
- Tchakalova, V.; Lutz, E.; Lamboley, S.; Moulin, E.; Benczédi, D.; Giuseppone, N.; Herrmann, A. Design of Stimuli-Responsive Dynamic Covalent Delivery Systems for Volatile Compounds (Part 2): Fragrance-Releasing Cleavable Surfactants in Functional Perfumery Applications. Chem. A Eur. J. 2021, 27, 13468–13476. [Google Scholar] [CrossRef] [PubMed]
- Sell, C.S. (Ed.) The Chemistry of Fragrances: From Perfumer to Consumer, 2nd ed.; RSC Publishing: Dorchester, UK, 2006. [Google Scholar]
- Ryan, D.M.; Anderson, S.B.; Senguen, F.T.; Youngman, R.E.; Nilsson, B.L. Self-assembly and hydrogelation promoted by F5-phenylalanine. Soft Matter 2010, 6, 475–479. [Google Scholar] [CrossRef]
- Chen, L.; Revel, S.; Morris, K.; Serpell, L.C.; Adams, D.J. Effect of molecular structure on the properties of naphthalene-dipeptide hydrogelators. Langmuir 2010, 26, 13466–13471. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Häring, M.; Haldar, D.; Díaz Díaz, D. Phenylalanine and derivatives as versatile low-molecular-weight gelators: Design, structure and tailored function. Biomater. Sci. 2018, 6, 38–59. [Google Scholar] [CrossRef] [PubMed]
- Podder, D.; Chowdhury, S.R.; Nandi, S.K.; Haldar, D. Tripeptide based super-organogelators: Structure and function. New J. Chem. 2019, 43, 3743–3749. [Google Scholar] [CrossRef]
- Fleming, S.; Ulijn, R.V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. [Google Scholar] [CrossRef]
- Zanna, N.; Focaroli, S.; Merlettini, A.; Gentilucci, L.; Teti, G.; Falconi, M.; Tomasini, C. Thixotropic Peptide-Based Physical Hydrogels Applied to Three-Dimensional Cell Culture. ACS Omega 2017, 2, 2374–2381. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Das, P.K. Antibacterial hydrogels of amino acid-based cationic amphiphiles. Biotechnol. Bioeng. 2008, 100, 756–764. [Google Scholar] [CrossRef]
- Falcone, N.; Kraatz, H.B. Supramolecular Assembly of Peptide and Metallopeptide Gelators and Their Stimuli-Responsive Properties in Biomedical Applications. Chem. Eur. J. 2018, 24, 14316–14328. [Google Scholar] [CrossRef]
- Ravarino, P.; Domenico, N.D.; Barbalinardo, M.; Faccio, D.; Falini, G.; Giuri, D.; Tomasini, C. Fluorine Effect in the Gelation Ability of Low Molecular. Gels 2022, 8, 98. [Google Scholar] [CrossRef]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Rigid, self-assembled hydrogel composed of a modified aromatic dipeptide. Adv. Mater. 2006, 18, 1365–1370. [Google Scholar] [CrossRef]
- Adams, D.J.; Butler, M.F.; Frith, W.J.; Kirkland, M.; Mullen, L.; Sanderson, P. A new method for maintaining homogeneity during liquid–hydrogel transitions using low molecular weight hydrogelators. Soft Matter 2009, 5, 1856–1862. [Google Scholar] [CrossRef]
- Chen, L.; Pont, G.; Morris, K.; Lotze, G.; Squires, A.; Serpell, L.C.; Adams, D.J. Salt-induced hydrogelation of functionalised-dipeptides at high pH. Chem. Commun. 2011, 47, 12071–12073. [Google Scholar] [CrossRef]
- Giuri, D.; Barbalinardo, M.; Zanna, N.; Paci, P.; Montalti, M.; Cavallini, M.; Valle, F.; Calvaresi, M.; Tomasini, C. Tuning mechanical properties of pseudopeptide supramolecular hydrogels by graphene doping. Molecules 2019, 24, 4345. [Google Scholar] [CrossRef] [Green Version]
- Guidetti, G.; Giuri, D.; Zanna, N.; Calvaresi, M.; Montalti, M.; Tomasini, C. Biocompatible and Light-Penetrating Hydrogels for Water Decontamination. ACS Omega 2018, 3, 8122–8128. [Google Scholar] [CrossRef]
- Adhikari, B.; Banerjee, A. Short-peptide-based hydrogel: A template for the in situ synthesis of fluorescent silver nanoclusters by using sunlight. Chem. Eur. J. 2010, 16, 13698–13705. [Google Scholar] [CrossRef]
- Yan, X.; Cui, Y.; He, Q.; Wang, K.; Li, J. Organogels based on self-assembly of diphenylalanine peptide and their application to immobilize quantum dots. Chem. Mater. 2008, 20, 1522–1526. [Google Scholar] [CrossRef]
- Giuri, D.; Jurković, L.; Fermani, S.; Kralj, D.; Falini, G.; Tomasini, C. Supramolecular Hydrogels with Properties Tunable by Calcium Ions: A Bio-Inspired Chemical System. ACS Appl. Bio Mater. 2019, 2, 5819–5828. [Google Scholar] [CrossRef]
- Tomasini, C.; Castellucci, N.; Caputo, V.C.; Milli, L.; Battistelli, G.; Fermani, S.; Falini, G. Shaping calcite crystals by customized self-assembling pseudopeptide foldamers. CrystEngComm 2015, 17, 116–123. [Google Scholar] [CrossRef]
- Naskar, J.; Palui, G.; Banerjee, A. Tetrapeptide-based hydrogels: For encapsulation and slow release of an anticancer drug at physiological ph. J. Phys. Chem. B 2009, 113, 11787–11792. [Google Scholar] [CrossRef]
- Okesola, B.O.; Wu, Y.; Derkus, B.; Gani, S.; Wu, D.; Knani, D.; Smith, D.K.; Adams, D.J.; Mata, A. Supramolecular Self-Assembly to Control Structural and Biological Properties of Multicomponent Hydrogels. Chem. Mater. 2019, 31, 7883–7897. [Google Scholar] [CrossRef] [Green Version]
- Majumder, J.; Deb, J.; Das, M.R.; Jana, S.S.; Dastidar, P. Designing a simple organic salt-based supramolecular topical gel capable of displaying in vivo self-delivery application. Chem. Commun. 2014, 50, 1671–1674. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, H.; Li, R.; Hao, J.; Wang, J.; Li, S.; Zhang, Y. A Kind of Aromatic Aldehyde Schiff Base and Its Preparation Method and Application. CN107033027A, 11 August 2016. [Google Scholar]
- Cordes, E.H.; Jencks, W.P. On the mechanism of Schiff base formation and hydrolysis. J. Am. Chem. Soc. 1962, 84, 832–837. [Google Scholar] [CrossRef]
- Frankebach, G.M. Particles for Malodor Reduction. U.S. Patent No. 9,714,401, 25 July 2017. [Google Scholar]
- Lant, N.J.; Hollinghead, J.A.; Frankebach, G.M. Cleaning Compositions Including Nuclease Enzyme and Malodor Reduction Materials. U.S. Patent Application No. 15/613,377, 14 December 2017. [Google Scholar]
- Irawan, C.; Islamiyati, D.; Utami, A.; Putri, R.P.; Wibowo, S. Synthesis Study of Precursor Mixture of Verdantiol, Aurantiol and Lyrame Schiff Base and Its Characterization Using GC-MS. Orient. J. Chem. 2019, 35, 1244–1247. [Google Scholar] [CrossRef]
- Jabło, M. Intramolecular Hydrogen Bonding 2021. Molecules 2021, 26, 6319. [Google Scholar]
- Braga, D.; d’Agostino, S.; Grepioni, F. Shape Takes the Lead: Templating Organic 3D-Frameworks around Organometallic Sandwich Compounds. Organometallics 2012, 31, 1688–1695. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. B 1990, 46 Pt 2, 256–262. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Thorn, A.; Dittrich, B.; Sheldrick, G.M. Enhanced rigid-bond restraints. Acta Crystallogr. Sect. A Found. Crystallogr. 2012, 68, 448–451. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
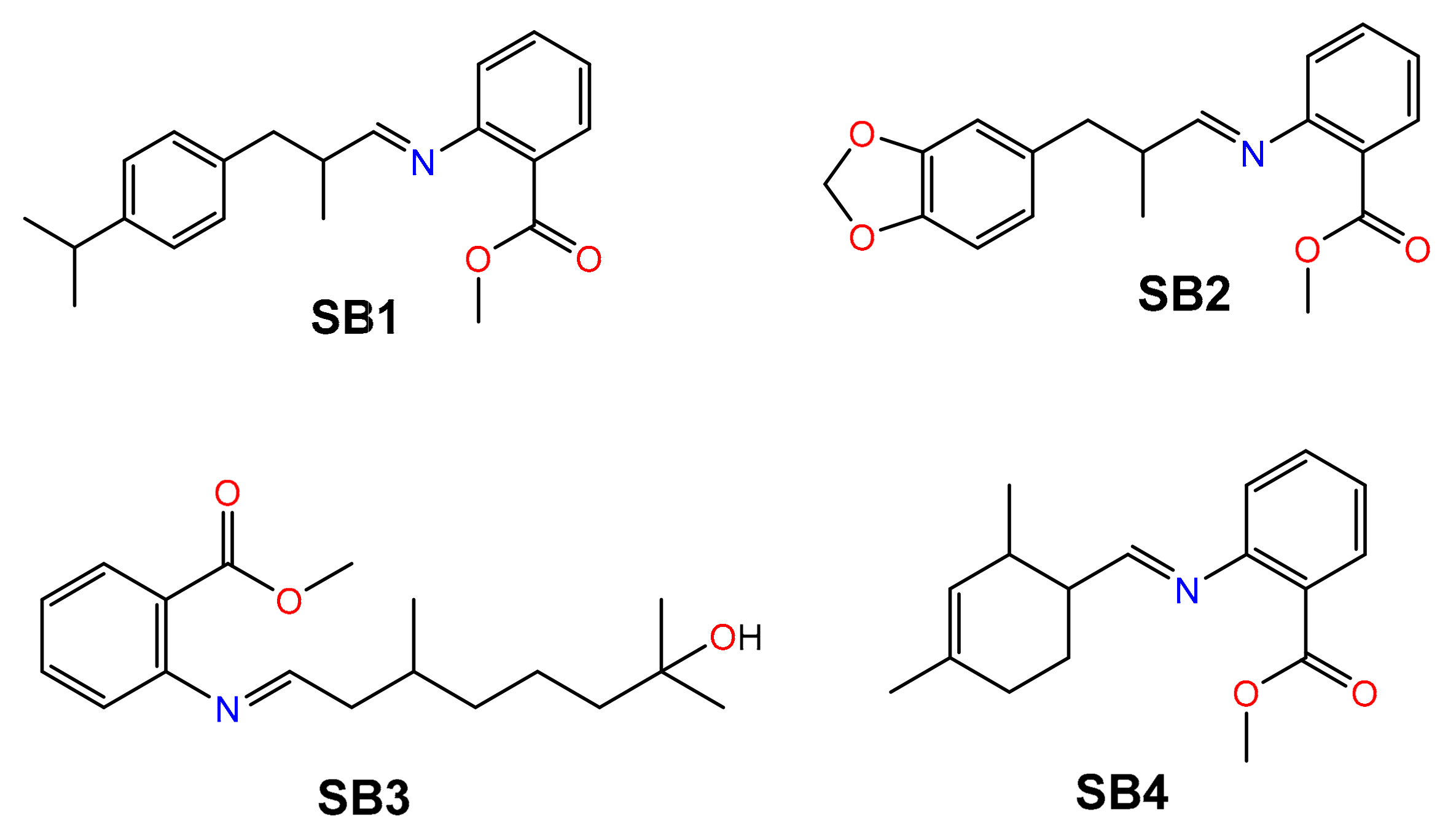
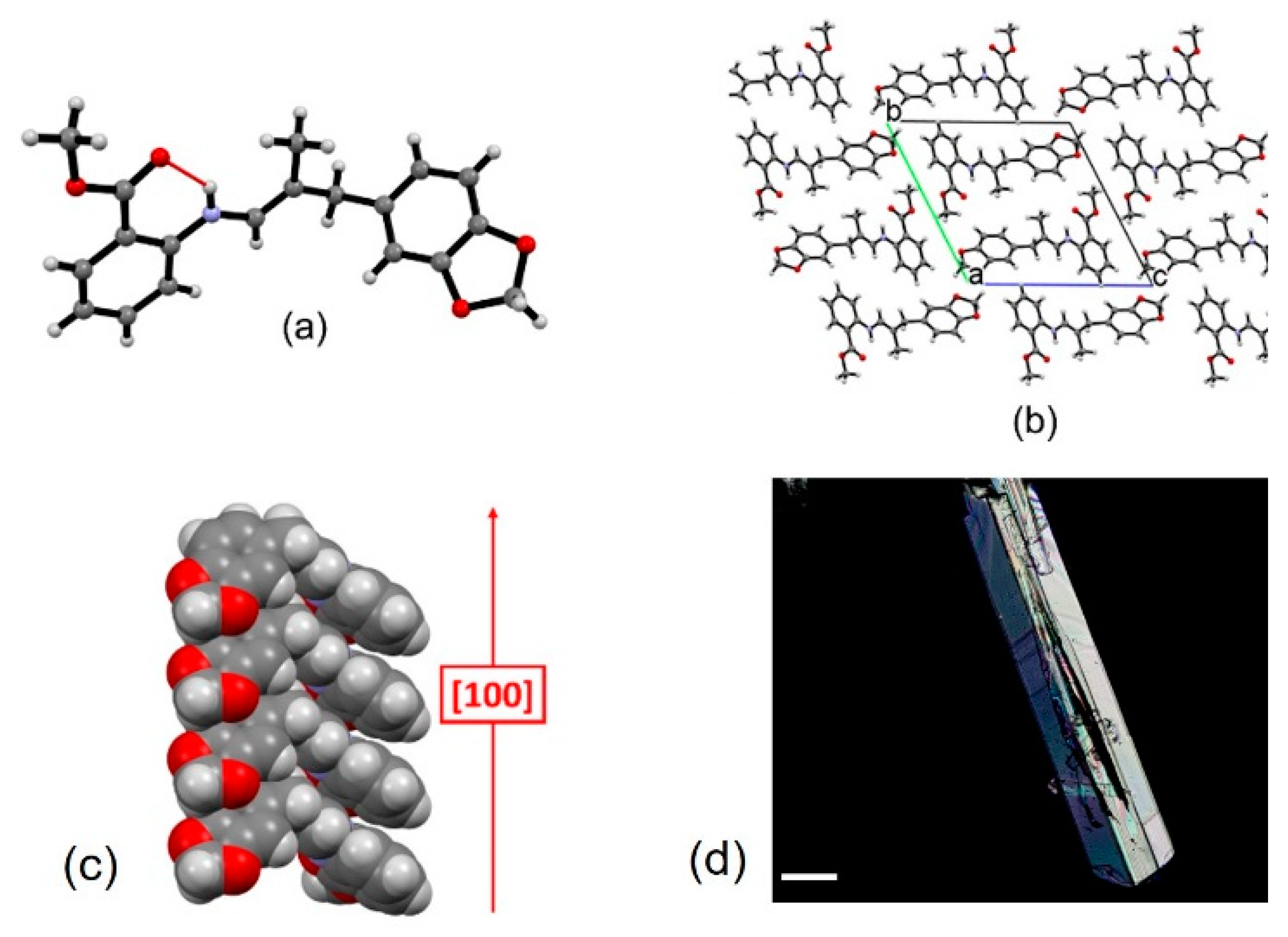
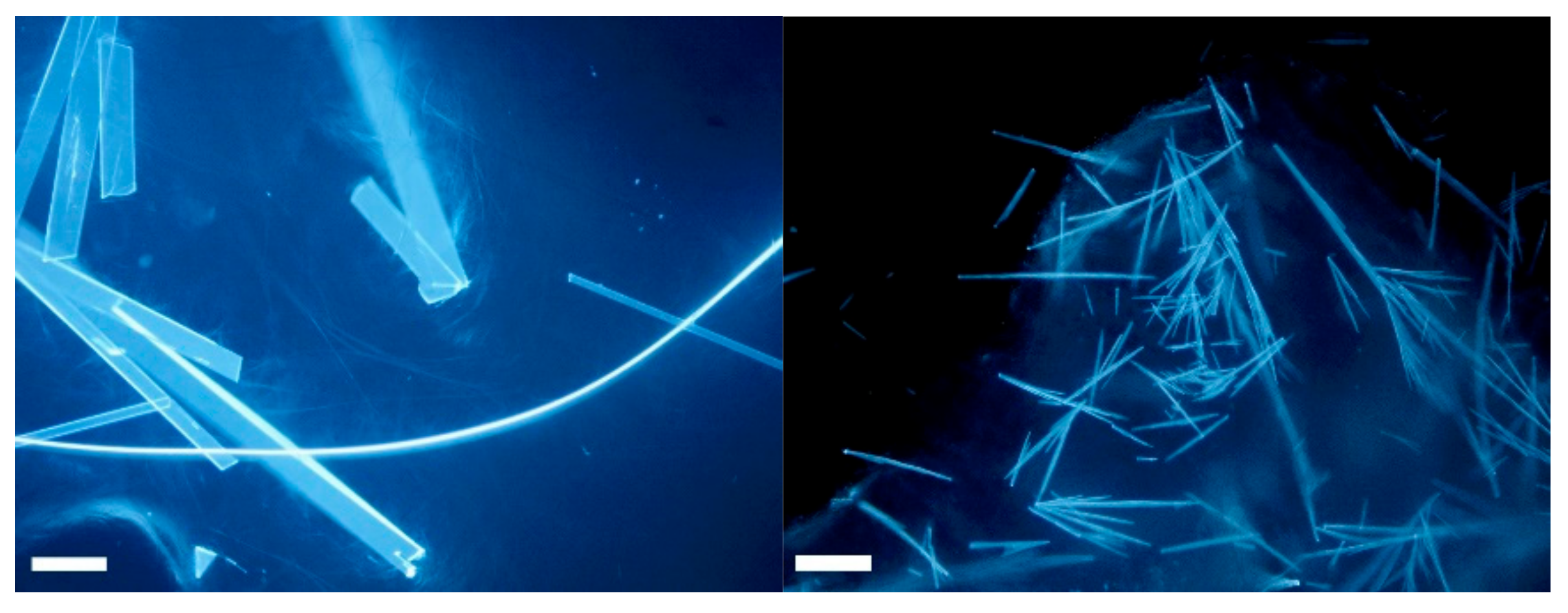
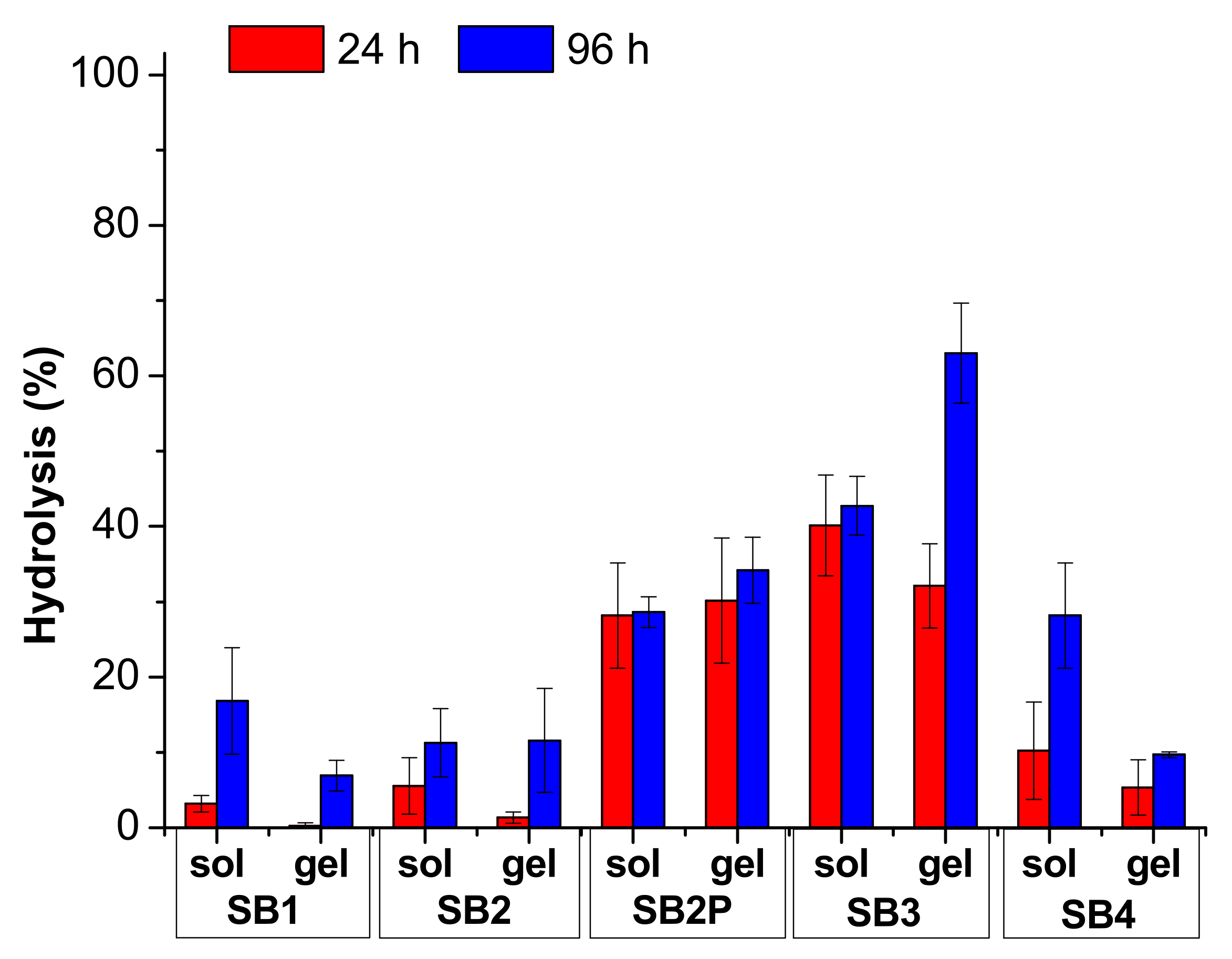
| Schiff Base | Aldehyde | Ratio A:MA | Time (min) | Conversion (%) |
|---|---|---|---|---|
| SB1 | A1 | 2:1 | 30 | 93.3 |
| SB2 | A2 | 2:1 | 30 | 97.8 |
| SB3 | A3 | 1:2 | 120 | 82.0 |
| SB4 | A4 | 2:1 | 60 | 81.8 |
| Schiff Base | EtOH/H2O Ratio | Acetic Acid (mmol/mL) | Starting pH | Hydrolysis at 24 h (%) |
|---|---|---|---|---|
| SB1 | 85:15 | - | 5.92 | 3.23 ± 1.87 |
| SB1 | 70:30 | - | 5.03 | 3.20 ± 1.10 |
| SB1 | 70:30 | 0.02 | 3.55 | 91.49 ± 4.34 |
| SB2 | 85:15 | - | 5.71 | 2.58 ± 0.51 |
| SB2 | 70:30 | - | 5.14 | 5.55 ± 3.76 |
| SB2 | 70:30 | 0.02 | 3.62 | 74.91 ± 2.86 |
| SB2P | 85:15 | - | 4.81 | 23.08 ± 7.56 |
| SB2P | 70:30 | - | 4.83 | 28.18 ± 7.00 |
| SB2P | 70:30 | 0.02 | 3.60 | 43.49 ± 8.83 |
| SB3 | 85:15 | - | 5.66 | 43.06 ± 0.60 |
| SB3 | 70:30 | - | 5.27 | 40.14 ± 6.72 |
| SB3 | 70:30 | 0.02 | 3.57 | 72.73 ± 4.41 |
| SB4 | 85:15 | - | 5.93 | 11.93 ± 1.27 |
| SB4 | 70:30 | - | 5.69 | 10.23 ± 6.47 |
| SB4 | 70:30 | 0.02 | 3.74 | 92.41 ± 1.19 |
| Gelator | Ratio EtOH/H2O | G’ (kPa) | G” (kPa) | Final pH |
|---|---|---|---|---|
| A (ester moiety) | 85:15 | 125.41 ± 23.02 | 41.41 ± 3.89 | 6.42 ± 0.16 |
| A (ester moiety) | 70:30 | 87.72 ± 22.46 | 16.49 ± 3.21 | 5.98 ± 0.19 |
| B (acidic moiety) | 70:30 | 43.01 ± 11.02 | 12.04 ± 3.10 | 3.80 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicastro, G.; Black, L.M.; Ravarino, P.; d’Agostino, S.; Faccio, D.; Tomasini, C.; Giuri, D. Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels. Int. J. Mol. Sci. 2022, 23, 3105. https://doi.org/10.3390/ijms23063105
Nicastro G, Black LM, Ravarino P, d’Agostino S, Faccio D, Tomasini C, Giuri D. Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels. International Journal of Molecular Sciences. 2022; 23(6):3105. https://doi.org/10.3390/ijms23063105
Chicago/Turabian StyleNicastro, Gloria, Louise Mary Black, Paolo Ravarino, Simone d’Agostino, Davide Faccio, Claudia Tomasini, and Demetra Giuri. 2022. "Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels" International Journal of Molecular Sciences 23, no. 6: 3105. https://doi.org/10.3390/ijms23063105
APA StyleNicastro, G., Black, L. M., Ravarino, P., d’Agostino, S., Faccio, D., Tomasini, C., & Giuri, D. (2022). Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels. International Journal of Molecular Sciences, 23(6), 3105. https://doi.org/10.3390/ijms23063105








