Sovateltide Mediated Endothelin B Receptors Agonism and Curbing Neurological Disorders
Abstract
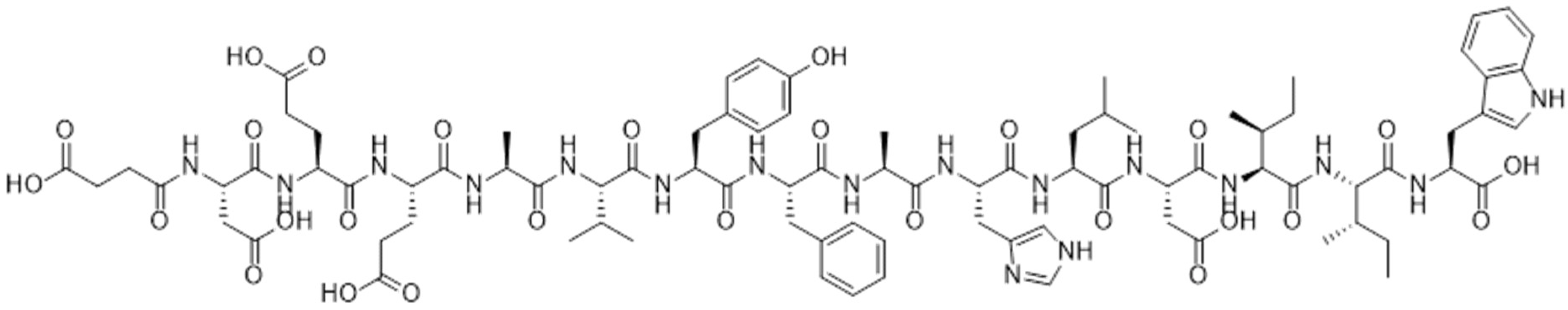
:1. Introduction
2. Toxicological Studies
3. Neurovascular Diseases and Clinical Development of Sovateltide
3.1. Stroke
3.2. Alzheimer’s Disease
3.3. Spinal Cord Injury
3.4. Neonatal Hypoxic-Ischemic Encephalopathy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, A.P.; Maguire, J.J. Endothelin. Handb. Exp. Pharmacol. 2006, 176 Pt 1, 295–329. [Google Scholar] [CrossRef]
- Sakurai, T.; Yanagisawa, M.; Takuwa, Y.; Miyazaki, H.; Kimura, S.; Goto, K.; Masaki, T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 1990, 348, 732–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, H.; Hori, S.; Aramori, I.; Ohkubo, H.; Nakanishi, S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature 1990, 348, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Oliveira, A., Jr.; Nogueira, A.I.; Pereira, R.M.; Boas, W.W.; Dos Santos, R.A.; Simoes e Silva, A.C. The renin-angiotensin system and diabetes: An update. Vasc. Health Risk Manag. 2008, 4, 787–803. [Google Scholar] [PubMed]
- Schneider, M.P.; Boesen, E.I.; Pollock, D.M. Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 731–759. [Google Scholar] [CrossRef] [Green Version]
- Birnbaumer, L. G proteins in signal transduction. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 675–705. [Google Scholar] [CrossRef] [PubMed]
- Casey, P.J.; Gilman, A.G. G protein involvement in receptor-effector coupling. J. Biol. Chem. 1988, 263, 2577–2580. [Google Scholar] [CrossRef]
- Attwood, T.K.; Findlay, J.B. Design of a discriminating fingerprint for G-protein-coupled receptors. Protein Eng. 1993, 6, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Attwood, T.K.; Findlay, J.B. Fingerprinting G-protein-coupled receptors. Protein Eng. 1994, 7, 195–203. [Google Scholar] [CrossRef]
- Kolakowski, L.F., Jr. GCRDb: A G-protein-coupled receptor database. Recept. Channels 1994, 2, 1–7. [Google Scholar] [PubMed]
- Foord, S.M.; Bonner, T.I.; Neubig, R.R.; Rosser, E.M.; Pin, J.P.; Davenport, A.P.; Spedding, M.; Harmar, A.J. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol. Rev. 2005, 57, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjarnadottir, T.K.; Gloriam, D.E.; Hellstrand, S.H.; Kristiansson, H.; Fredriksson, R.; Schioth, H.B. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 2006, 88, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Shrader, S.H.; Song, Z.H. Discovery of endogenous inverse agonists for G protein-coupled receptor 6. Biochem. Biophys. Res. Commun. 2020, 522, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Civelli, O.; Reinscheid, R.K.; Zhang, Y.; Wang, Z.; Fredriksson, R.; Schioth, H.B. G protein-coupled receptor deorphanizations. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 127–146. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, H.; Eguchi, S.; Ueno, H.; Marumo, F.; Hirata, Y. Endothelin-mediated vascular growth requires p42/p44 mitogen-activated protein kinase and p70 S6 kinase cascades via transactivation of epidermal growth factor receptor. Endocrinology 1999, 140, 4659–4668. [Google Scholar] [CrossRef]
- Robin, P.; Boulven, I.; Desmyter, C.; Harbon, S.; Leiber, D. ET-1 stimulates ERK signaling pathway through sequential activation of PKC and Src in rat myometrial cells. Am. J. Physiol. Cell Physiol. 2002, 283, C251–C260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzuca, M.Q.; Khalil, R.A. Vascular endothelin receptor type B: Structure, function and dysregulation in vascular disease. Biochem. Pharmacol. 2012, 84, 147–162. [Google Scholar] [CrossRef] [Green Version]
- MacCumber, M.W.; Ross, C.A.; Snyder, S.H. Endothelin in brain: Receptors, mitogenesis, and biosynthesis in glial cells. Proc. Natl. Acad. Sci. USA 1990, 87, 2359–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenreich, H.; Nau, T.R.; Dembowski, C.; Hasselblatt, M.; Barth, M.; Hahn, A.; Schilling, L.; Siren, A.L.; Bruck, W. Endothelin b receptor deficiency is associated with an increased rate of neuronal apoptosis in the dentate gyrus. Neuroscience 2000, 95, 993–1001. [Google Scholar] [CrossRef]
- Vidovic, M.; Chen, M.M.; Lu, Q.Y.; Kalloniatis, K.F.; Martin, B.M.; Tan, A.H.; Lynch, C.; Croaker, G.D.; Cass, D.T.; Song, Z.M. Deficiency in endothelin receptor B reduces proliferation of neuronal progenitors and increases apoptosis in postnatal rat cerebellum. Cell. Mol. Neurobiol. 2008, 28, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Srimal, R.C. Endothelin antagonizes the hypotension and potentiates the hypertension induced by clonidine. Eur. J. Pharmacol. 1993, 230, 293–300. [Google Scholar] [CrossRef]
- Gulati, A.; Rebello, S.; Chari, G.; Bhat, R. Ontogeny of endothelin and its receptors in rat brain. Life Sci. 1992, 51, 1715–1724. [Google Scholar] [CrossRef]
- Gulati, A. Endothelin Receptors, Mitochondria and Neurogenesis in Cerebral Ischemia. Curr. Neuropharmacol. 2016, 14, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, A.; Hornick, M.G.; Briyal, S.; Lavhale, M.S. A novel neuroregenerative approach using ET(B) receptor agonist, IRL-1620, to treat CNS disorders. Physiol. Res. 2018, 67, S95–S113. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Kuc, R.E.; Maguire, J.J.; Harland, S.P. ETA receptors predominate in the human vasculature and mediate constriction. J. Cardiovasc. Pharmacol. 1995, 26 (Suppl. 3), S265–S267. [Google Scholar] [CrossRef] [PubMed]
- Regard, J.B.; Sato, I.T.; Coughlin, S.R. Anatomical profiling of G protein-coupled receptor expression. Cell 2008, 135, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harland, S.P.; Kuc, R.E.; Pickard, J.D.; Davenport, A.P. Characterization of endothelin receptors in human brain cortex, gliomas, and meningiomas. J. Cardiovasc. Pharmacol. 1995, 26 (Suppl. 3), S408–S411. [Google Scholar] [CrossRef]
- Barton, M.; Yanagisawa, M. Endothelin: 30 Years from Discovery to Therapy. Hypertension 2019, 74, 1232–1265. [Google Scholar] [CrossRef]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, A.P.; Kuc, R.E.; Southan, C.; Maguire, J.J. New drugs and emerging therapeutic targets in the endothelin signaling pathway and prospects for personalized precision medicine. Physiol. Res. 2018, 67, S37–S54. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.G.; Prazad, P.; Puppala, B.; Gulati, A. Selective Endothelin-B Receptor Stimulation Increases Vascular Endothelial Growth Factor in the Rat Brain during Postnatal Development. Drug Res. 2015, 65, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Riechers, C.C.; Knabe, W.; Siren, A.L.; Gariepy, C.E.; Yanagisawa, M.; Ehrenreich, H. Endothelin B receptor deficient transgenic rescue rats: A rescue phenomenon in the brain. Neuroscience 2004, 124, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Briyal, S.; Nguyen, C.; Leonard, M.; Gulati, A. Stimulation of endothelin B receptors by IRL-1620 decreases the progression of Alzheimer’s disease. Neuroscience 2015, 301, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A. Understanding neurogenesis in the adult human brain. Indian J. Pharmacol. 2015, 47, 583–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, M.G.; Briyal, S.; Gulati, A. Endothelin B receptor agonist, IRL-1620, reduces neurological damage following permanent middle cerebral artery occlusion in rats. Brain Res. 2011, 1420, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.G.; Briyal, S.; Gulati, A. Endothelin B receptor agonist, IRL-1620, provides long-term neuroprotection in cerebral ischemia in rats. Brain Res. 2012, 1464, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S. Stroke in the 21(st) Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef] [Green Version]
- Bamford, J.; Sandercock, P.; Dennis, M.; Burn, J.; Warlow, C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991, 337, 1521–1526. [Google Scholar] [CrossRef]
- Seet, R.C.S.; Rabinstein, A.A. Symptomatic Intracranial Hemorrhage following Intravenous Thrombolysis for Acute Ischemic Stroke: A Critical Review of Case Definitions. Cerebrovasc. Dis. 2012, 34, 106–114. [Google Scholar] [CrossRef]
- Minnerup, J.; Wersching, H.; Schilling, M.; Schabitz, W.R. Analysis of early phase and subsequent phase III stroke studies of neuroprotectants: Outcomes and predictors for success. Exp. Transl. Stroke Med. 2014, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Lampl, Y.; Fleminger, G.; Gilad, R.; Galron, R.; Sarova-Pinhas, I.; Sokolovsky, M. Endothelin in cerebrospinal fluid and plasma of patients in the early stage of ischemic stroke. Stroke J. Cereb. Circ. 1997, 28, 1951–1955. [Google Scholar] [CrossRef]
- Ziv, I.; Fleminger, G.; Djaldetti, R.; Achiron, A.; Melamed, E.; Sokolovsky, M. Increased plasma endothelin-1 in acute ischemic stroke. Stroke J. Cereb. Circ. 1992, 23, 1014–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, F.C.; Ohlstein, E.H.; Hunter, A.J.; Campbell, C.A.; Hadingham, S.H.; Parsons, A.A.; Yang, Y.; Shohami, E. Selective antagonism of endothelin-A-receptors improves outcome in both head trauma and focal stroke in rat. J. Cardiovasc. Pharmacol. 2000, 36, S357–S361. [Google Scholar] [CrossRef] [PubMed]
- Briyal, S.; Gulati, A. Endothelin-A receptor antagonist BQ123 potentiates acetaminophen induced hypothermia and reduces infarction following focal cerebral ischemia in rats. Eur. J. Pharmacol. 2010, 644, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Legos, J.J.; Lenhard, S.C.; Haimbach, R.E.; Schaeffer, T.R.; Bentley, R.G.; McVey, M.J.; Chandra, S.; Irving, E.A.; Andrew, A.P.; Barone, F.C. SB 234551 selective ET(A) receptor antagonism: Perfusion/diffusion MRI used to define treatable stroke model, time to treatment and mechanism of protection. Exp. Neurol. 2008, 212, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Tatlisumak, T.; Carano, R.A.; Takano, K.; Opgenorth, T.J.; Sotak, C.H.; Fisher, M. A novel endothelin antagonist, A-127722, attenuates ischemic lesion size in rats with temporary middle cerebral artery occlusion: A diffusion and perfusion MRI study. Stroke J. Cereb. Circ. 1998, 29, 850–857; discussion 857–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.L.; Zhang, C.; Zhang, L.; Roberts, C.; Lu, M.; Kapke, A.; Cui, Y.; Ninomiya, M.; Nagafuji, T.; Albala, B.; et al. Synergistic effect of an endothelin type A receptor antagonist, S-0139, with rtPA on the neuroprotection after embolic stroke. Stroke J. Cereb. Circ. 2008, 39, 2830–2836. [Google Scholar] [CrossRef] [Green Version]
- Briyal, S.; Gulati, A.; Gupta, Y.K. Effect of combination of endothelin receptor antagonist (TAK-044) and aspirin in middle cerebral artery occlusion model of acute ischemic stroke in rats. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Briyal, S.; Pant, A.B.; Gupta, Y.K. Protective effect of endothelin antagonist (TAK-044) on neuronal cell viability in in vitro oxygen-glucose deprivation model of stroke. Indian J. Physiol. Pharmacol. 2006, 50, 157–162. [Google Scholar]
- Chuquet, J.; Benchenane, K.; Toutain, J.; MacKenzie, E.T.; Roussel, S.; Touzani, O. Selective blockade of endothelin-B receptors exacerbates ischemic brain damage in the rat. Stroke J. Cereb. Circ. 2002, 33, 3019–3025. [Google Scholar] [CrossRef] [Green Version]
- Ehrenreich, H.; Oldenburg, J.; Hasselblatt, M.; Herms, J.; Dembowski, C.; Loffler, B.M.; Bruck, W.; Kamrowski-Kruck, H.; Gall, S.; Siren, A.L.; et al. Endothelin B receptor-deficient rats as a subtraction model to study the cerebral endothelin system. Neuroscience 1999, 91, 1067–1075. [Google Scholar] [CrossRef]
- Castaneda, M.M.; Cubilla, M.A.; Lopez-Vicchi, M.M.; Suburo, A.M. Endothelinergic cells in the subependymal region of mice. Brain Res. 2010, 1321, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Kumar, A.; Morrison, S.; Shahani, B.T. Effect of centrally administered endothelin agonists on systemic and regional blood circulation in the rat: Role of sympathetic nervous system. Neuropeptides 1997, 31, 301–309. [Google Scholar] [CrossRef]
- Koyama, Y.; Takemura, M.; Fujiki, K.; Ishikawa, N.; Shigenaga, Y.; Baba, A. BQ788, an endothelin ET(B) receptor antagonist, attenuates stab wound injury-induced reactive astrocytes in rat brain. Glia 1999, 26, 268–271. [Google Scholar] [CrossRef]
- Sakurai-Yamashita, Y.; Niwa, M.; Yamashita, K.; Kataoka, Y.; Himeno, A.; Shigematsu, K.; Tsutsumi, K.; Taniyama, K. Endothelin receptors in kainic acid-induced neural lesions of rat brain. Neuroscience 1997, 81, 565–577. [Google Scholar] [CrossRef]
- Yamashita, K.; Niwa, M.; Kataoka, Y.; Shigematsu, K.; Himeno, A.; Tsutsumi, K.; Nakano-Nakashima, M.; Sakurai-Yamashita, Y.; Shibata, S.; Taniyama, K. Microglia with an endothelin ETB receptor aggregate in rat hippocampus CA1 subfields following transient forebrain ischemia. J. Neurochem. 1994, 63, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Siren, A.L.; Lewczuk, P.; Hasselblatt, M.; Dembowski, C.; Schilling, L.; Ehrenreich, H. Endothelin B receptor deficiency augments neuronal damage upon exposure to hypoxia-ischemia in vivo. Brain Res. 2002, 945, 144–149. [Google Scholar] [CrossRef]
- Puppala, B.; Awan, I.; Briyal, S.; Mbachu, O.; Leonard, M.; Gulati, A. Ontogeny of endothelin receptors in the brain, heart, and kidneys of neonatal rats. Brain Dev. 2015, 37, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.G.; Gulati, A. Endothelin B receptor agonist, IRL-1620, enhances angiogenesis and neurogenesis following cerebral ischemia in rats. Brain Res. 2013, 1528, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Briyal, S.; Ranjan, A.K.; Hornick, M.G.; Puppala, A.K.; Luu, T.; Gulati, A. Anti-apoptotic activity of ETB receptor agonist, IRL-1620, protects neural cells in rats with cerebral ischemia. Sci. Rep. 2019, 9, 10439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cifuentes, E.G.; Hornick, M.G.; Havalad, S.; Donovan, R.L.; Gulati, A. Neuroprotective Effect of IRL-1620, an Endothelin B Receptor Agonist, on a Pediatric Rat Model of Middle Cerebral Artery Occlusion. Front. Pediatr. 2018, 6, 310. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, A.K.; Briyal, S.; Gulati, A. Sovateltide (IRL-1620) activates neuronal differentiation and prevents mitochondrial dysfunction in adult mammalian brains following stroke. Sci. Rep. 2020, 10, 12737. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.K.; Briyal, S.; Khandekar, D.; Gulati, A. Sovateltide (IRL-1620) affects neuronal progenitors and prevents cerebral tissue damage after ischemic stroke. Can. J. Physiol. Pharmacol. 2020, 98, 659–666. [Google Scholar] [CrossRef]
- Gulati, A.; Agrawal, N.; Vibha, D.; Misra, U.K.; Paul, B.; Jain, D.; Pandian, J.; Borgohain, R. Safety and Efficacy of Sovateltide (IRL-1620) in a Multicenter Randomized Controlled Clinical Trial in Patients with Acute Cerebral Ischemic Stroke. CNS Drugs 2021, 35, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Devlin, N.J.; Brooks, R. EQ-5D and the EuroQol Group: Past, Present and Future. Appl. Health Econ. Health Policy 2017, 15, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrag, A.; Selai, C.; Jahanshahi, M.; Quinn, N.P. The EQ-5D--a generic quality of life measure-is a useful instrument to measure quality of life in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2000, 69, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-Cruz, C.; Martinez-Nunez, J.M.; Perez, M.E.; Kravzov-Jinich, J.; Rios-Castaneda, C.; Altagracia-Martinez, M. Evaluation of the Stroke-Specific Quality-of-Life (SSQOL) Scale in Mexico: A Preliminary Approach. Value Health Reg. Issues 2013, 2, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, A. Press Releases. Pharmazz Inc. 2022. Available online: https://www.pharmazz.com/press-releases.php (accessed on 3 March 2022).
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020. [CrossRef]
- Crous-Bou, M.; Minguillon, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimer’s Res. Ther. 2017, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Res 2018, 7, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Nisbet, R.M.; Polanco, J.C.; Ittner, L.M.; Gotz, J. Tau aggregation and its interplay with amyloid-beta. Acta Neuropathol. 2015, 129, 207–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, J.L.; Ringman, J.; Vinters, H.V. Neuropathologic correlates of trial-related instruments for Alzheimer’s disease. Am. J. Neurodegener. Dis. 2014, 3, 45–49. [Google Scholar]
- Emanuel, E.J. A Middle Ground for Accelerated Drug Approval-Lessons from Aducanumab. JAMA 2021, 326, 1367–1368. [Google Scholar] [CrossRef]
- Dunn, B.; Stein, P.; Temple, R.; Cavazzoni, P. An Appropriate Use of Accelerated Approval—Aducanumab for Alzheimer’s Disease. N. Engl. J. Med. 2021, 385, 856–857. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.C.; Knopman, D.S.; Emerson, S.S.; Ovbiagele, B.; Kryscio, R.J.; Perlmutter, J.S.; Kesselheim, A.S. Revisiting FDA Approval of Aducanumab. N. Engl. J. Med. 2021, 385, 769–771. [Google Scholar] [CrossRef]
- Palmer, J.C.; Baig, S.; Kehoe, P.G.; Love, S. Endothelin-converting enzyme-2 is increased in Alzheimer’s disease and up-regulated by Abeta. Am. J. Pathol. 2009, 175, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Koyama, Y. Endothelin systems in the brain: Involvement in pathophysiological responses of damaged nerve tissues. Biomol. Concepts 2013, 4, 335–347. [Google Scholar] [CrossRef]
- Turner, A.J.; Murphy, L.J. Molecular pharmacology of endothelin converting enzymes. Biochem. Pharmacol. 1996, 51, 91–102. [Google Scholar] [CrossRef]
- Rodriguiz, R.M.; Gadnidze, K.; Ragnauth, A.; Dorr, N.; Yanagisawa, M.; Wetsel, W.C.; Devi, L.A. Animals lacking endothelin-converting enzyme-2 are deficient in learning and memory. Genes Brain Behav. 2008, 7, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Eckman, E.A.; Watson, M.; Marlow, L.; Sambamurti, K.; Eckman, C.B. Alzheimer’s disease beta-amyloid peptide is increased in mice deficient in endothelin-converting enzyme. J. Biol. Chem. 2003, 278, 2081–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, K.I.; Taniwaki, Y.; Utsunomiya, H.; Taniwaki, T. Cerebral blood flow reduction associated with orientation for time in amnesic mild cognitive impairment and Alzheimer disease patients. J. Neuroimaging 2014, 24, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.; Kumar, A.; Shahani, B.T. Cardiovascular effects of centrally administered endothelin-1 and its relationship to changes in cerebral blood flow. Life Sci. 1996, 58, 437–445. [Google Scholar] [CrossRef]
- Gulati, A.; Rebello, S.; Roy, S.; Saxena, P.R. Cardiovascular effects of centrally administered endothelin-1 in rats. J. Cardiovasc. Pharmacol. 1995, 26 (Suppl. 3), S244–S246. [Google Scholar] [CrossRef] [PubMed]
- Paris, D.; Humphrey, J.; Quadros, A.; Patel, N.; Crescentini, R.; Crawford, F.; Mullan, M. Vasoactive effects of A beta in isolated human cerebrovessels and in a transgenic mouse model of Alzheimer’s disease: Role of inflammation. Neurol. Res. 2003, 25, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Briyal, S.; Philip, T.; Gulati, A. Endothelin-A receptor antagonists prevent amyloid-beta-induced increase in ETA receptor expression, oxidative stress, and cognitive impairment. J. Alzheimer’s Dis. 2011, 23, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Druckenbrod, N.R.; Powers, P.A.; Bartley, C.R.; Walker, J.W.; Epstein, M.L. Targeting of endothelin receptor-B to the neural crest. Genesis 2008, 46, 396–400. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, K.; Ayukawa, K.; Hara, Y.; Wada, K.; Aoki, S. Endothelin/endothelin-B receptor signals regulate ventricle-directed interkinetic nuclear migration of cerebral cortical neural progenitors. Neurochem. Int. 2011, 58, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Yagami, T.; Ueda, K.; Asakura, K.; Kuroda, T.; Hata, S.; Sakaeda, T.; Kambayashi, Y.; Fujimoto, M. Effects of endothelin B receptor agonists on amyloid beta protein (25–35)-induced neuronal cell death. Brain Res. 2002, 948, 72–81. [Google Scholar] [CrossRef]
- Leonard, M.G.; Gulati, A. Repeated administration of ET(B) receptor agonist, IRL-1620, produces tachyphylaxis only to its hypotensive effect. Pharmacol. Res. 2009, 60, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.D.; Oesterling, B.M.; Wu, C.; Gwizdz, N.; Pais, G.; Briyal, S.; Gulati, A. Evaluation of liposomal nanocarriers loaded with ETB receptor agonist, IRL-1620, using cell-based assays. Neuroscience 2016, 312, 141–152. [Google Scholar] [CrossRef]
- Briyal, S.; Shepard, C.; Gulati, A. Endothelin receptor type B agonist, IRL-1620, prevents beta amyloid (Abeta) induced oxidative stress and cognitive impairment in normal and diabetic rats. Pharmacol. Biochem. Behav. 2014, 120, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nori, S.; Ahuja, C.S.; Fehlings, M.G. Translational Advances in the Management of Acute Spinal Cord Injury: What is New? What is Hot? Neurosurgery 2017, 64, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.; Kirshblum, S. Clinical Trials in Traumatic Spinal Cord Injury. Neurotherapeutics 2018, 15, 654–668. [Google Scholar] [CrossRef] [Green Version]
- Peters, C.M.; Rogers, S.D.; Pomonis, J.D.; Egnaczyk, G.F.; Keyser, C.P.; Schmidt, J.A.; Ghilardi, J.R.; Maggio, J.E.; Mantyh, P.W. Endothelin receptor expression in the normal and injured spinal cord: Potential involvement in injury-induced ischemia and gliosis. Exp. Neurol. 2003, 180, 1–13. [Google Scholar] [CrossRef]
- Kallakuri, S.; Kreipke, C.W.; Schafer, P.C.; Schafer, S.M.; Rafols, J.A. Brain cellular localization of endothelin receptors A and B in a rodent model of diffuse traumatic brain injury. Neuroscience 2010, 168, 820–830. [Google Scholar] [CrossRef]
- McKenzie, A.L.; Hall, J.J.; Aihara, N.; Fukuda, K.; Noble, L.J. Immunolocalization of endothelin in the traumatized spinal cord: Relationship to blood-spinal cord barrier breakdown. J. Neurotrauma 1995, 12, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, Y.; He, Z.; Zhang, B.; Li, Y.; Hu, J.; Han, M.; Xu, Y.; Li, Y.; Gu, J.; et al. Targeting endothelin receptors A and B attenuates the inflammatory response and improves locomotor function following spinal cord injury in mice. Int. J. Mol. Med. 2014, 34, 74–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forner, S.; Martini, A.C.; Andrade, E.L.; Rae, G.A. Neuropathic pain induced by spinal cord injury: Role of endothelin ETA and ETB receptors. Neurosci. Lett. 2016, 617, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, M.; Kasuya, Y.; Hayashi, K.; Goto, K. SB209670, a potent endothelin receptor antagonist, prevents or delays axonal degeneration after spinal cord injury. Brain Res. 1998, 786, 235–239. [Google Scholar] [CrossRef]
- Laziz, I.; Larbi, A.; Grebert, D.; Sautel, M.; Congar, P.; Lacroix, M.C.; Salesse, R.; Meunier, N. Endothelin as a neuroprotective factor in the olfactory epithelium. Neuroscience 2011, 172, 20–29. [Google Scholar] [CrossRef]
- Yagami, T.; Ueda, K.; Sakaeda, T.; Okamura, N.; Nakazato, H.; Kuroda, T.; Hata, S.; Sakaguchi, G.; Itoh, N.; Hashimoto, Y.; et al. Effects of an endothelin B receptor agonist on secretory phospholipase A2-IIA-induced apoptosis in cortical neurons. Neuropharmacology 2005, 48, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef]
- Lawn, J.E.; Blencowe, H.; Oza, S.; You, D.; Lee, A.C.; Waiswa, P.; Lalli, M.; Bhutta, Z.; Barros, A.J.; Christian, P.; et al. Every Newborn: Progress, priorities, and potential beyond survival. Lancet 2014, 384, 189–205. [Google Scholar] [CrossRef]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [Green Version]
- Harteman, J.C.; Nikkels, P.G.; Benders, M.J.; Kwee, A.; Groenendaal, F.; de Vries, L.S. Placental pathology in full-term infants with hypoxic-ischemic neonatal encephalopathy and association with magnetic resonance imaging pattern of brain injury. J. Pediatr. 2013, 163, 968–995.e2. [Google Scholar] [CrossRef]
- Azzopardi, D.; Wyatt, J.S.; Cady, E.B.; Delpy, D.T.; Baudin, J.; Stewart, A.L.; Hope, P.L.; Hamilton, P.A.; Reynolds, E.O. Prognosis of newborn infants with hypoxic-ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr. Res. 1989, 25, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Azzopardi, D.V.; Strohm, B.; Edwards, A.D.; Dyet, L.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; Levene, M.; Marlow, N.; Porter, E.; et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 2009, 361, 1349–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bale, G.; Mitra, S.; de Roever, I.; Sokolska, M.; Price, D.; Bainbridge, A.; Gunny, R.; Uria-Avellanal, C.; Kendall, G.S.; Meek, J.; et al. Oxygen dependency of mitochondrial metabolism indicates outcome of newborn brain injury. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2019, 39, 2035–2047. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, E.P.; Ekici, B.; Tatli, B. Neonatal hypoxic ischemic encephalopathy: An update on disease pathogenesis and treatment. Expert Rev. Neurother. 2017, 17, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Morley, C.J.; Inder, T.E.; Stewart, M.J.; Smith, K.R.; McNamara, P.J.; Wright, I.M.; Kirpalani, H.M.; Darlow, B.A.; Doyle, L.W.; et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: A randomized controlled trial. Arch. Pediatr. Adolesc. Med. 2011, 165, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Gluckman, P.D.; Wyatt, J.S.; Azzopardi, D.; Ballard, R.; Edwards, A.D.; Ferriero, D.M.; Polin, R.A.; Robertson, C.M.; Thoresen, M.; Whitelaw, A.; et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet 2005, 365, 663–670. [Google Scholar] [CrossRef]
- Shankaran, S.; Laptook, A.R.; Ehrenkranz, R.A.; Tyson, J.E.; McDonald, S.A.; Donovan, E.F.; Fanaroff, A.A.; Poole, W.K.; Wright, L.L.; Higgins, R.D.; et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 2005, 353, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.D.; Brocklehurst, P.; Gunn, A.J.; Halliday, H.; Juszczak, E.; Levene, M.; Strohm, B.; Thoresen, M.; Whitelaw, A.; Azzopardi, D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ 2010, 340, c363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankaran, S.; Pappas, A.; McDonald, S.A.; Vohr, B.R.; Hintz, S.R.; Yolton, K.; Gustafson, K.E.; Leach, T.M.; Green, C.; Bara, R.; et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 2012, 366, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Gluckman, P.D.; Gunn, T.R. Selective head cooling in newborn infants after perinatal asphyxia: A safety study. Pediatrics 1998, 102, 885–892. [Google Scholar] [CrossRef]
- Higgins, R.D.; Raju, T.; Edwards, A.D.; Azzopardi, D.V.; Bose, C.L.; Clark, R.H.; Ferriero, D.M.; Guillet, R.; Gunn, A.J.; Hagberg, H.; et al. Hypothermia and other treatment options for neonatal encephalopathy: An executive summary of the Eunice Kennedy Shriver NICHD workshop. J. Pediatr. 2011, 159, 851–858.e1. [Google Scholar] [CrossRef] [Green Version]
- Thoresen, M.; Whitelaw, A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischemic encephalopathy. Pediatrics 2000, 106, 92–99. [Google Scholar] [CrossRef] [PubMed]

| Clinical Trials Involving Sovateltide | Disease | Design | Findings |
|---|---|---|---|
| 1. Phase I—CTRI/2016/11/007509 | None | Total cohorts—3. Healthy subjects in each cohort—3. Dose—3 doses of either 0.3, 0.6, or 0.9 μg/kg at an interval of 4 h as an intravenous bolus over 1 min in each subject. | Minimum Intolerable Dose (MID) of 0.9 μg/kg and Maximum Tolerated Dose (MTD) of 0.6 μg/kg of body wt. were established. No serious adverse effects in any cohort. No significant effect on vital signs. Sovateltide was found well-tolerated and safe at these doses. |
| 2. Phase II—CTRI/2017/11/010654 and NCT04046484 | Ischemic Stroke | Prospective, multicenter, randomized, placebo-controlled, double-blinded, and exploratory clinical study. Total cerebral ischemic stroke patients—40 (36 patients completed 90-day follow-up). Dose—0.3 μg/kg body wt. per dose, 3 doses per day at an interval of 3 ± 1 h as an intravenous bolus over 1 min in each subject (total dose/day: 0.9 ug/kg body wt.). Treatments—Treatments on days 1, 3, and 6 with sovateltide or an equal volume of saline. Sovateltide (n = 18; 15 males and 3 females) or saline (n = 18; 11 males and 7 females) + standard treatment and care. Clinical outcome parameters—National Institute of Health Stroke Scale (NIHSS), Modified Rankin Scale (mRS), and Barthel Index (BI). Primary objective—Testing safety and tolerability of sovateltide. Secondary objective—efficacy testing on neurological improvements using the NIHSS, mRS, and BI scales, and quality-of-life assessments using EQ-5D and SSQoL. | Improvements in mRS and BI scales in the sovateltide group on day 6 compared to day 1 indicated a quicker recovery in the sovateltide cohort. At day 90, improvement of ≥ 6 points in NIHSS in 56% of sovateltide and 43% of the saline group. A significant improvement (p = 0.0112) of ≥40 points in BI in 64% of sovateltide and 36% of saline patients. An improvement of ≥2 points in the mRS in 60% of sovateltide and 40% saline group. Higher number of patients had complete recovery with NIHSS score of 0 (p < 0.05), a BI score of 100 (p < 0.05) and an mRS score of 0 (p = 0.1193) in sovateltide cohort compared to saline. No adverse effects due to treatment were observed. These results showed the safety and efficacy of sovateltide with a better trend toward the complete recovery of ischemic stroke patients compared to saline. |
| 3. Phase III—NCT04047563 | Ischemic Stroke | Prospective, multicenter, randomized, placebo-controlled, double-blinded, and exploratory clinical study. Total cerebral ischemic stroke patients—158. Age range—18–76 years. Dose—0.3 μg/kg body wt. per dose, 3 doses per day at an interval of 3 ± 1 h as an intravenous bolus over 1 min in each subject (total dose/day: 0.9 ug/kg body wt.). Treatments—Treatments on days 1, 3, and 6 with sovateltide or an equal volume of saline + standard treatment and care. Clinical outcome parameters—National Institute of Health Stroke Scale (NIHSS), Modified Rankin Scale (mRS), and Barthel Index (BI). Primary objective—Efficacy testing on neurological improvements using the NIHSS, mRS, and BI scales. Secondary objective—Quality-of-life assessments using EQ-5D and SSQoL. Recurrence of ischemic stroke, incidence of intracerebral hemorrhage and mortality, alteration in cognition, and sovateltide related adverse events. | Enrollment of patients is complete. Results are awaited. |
| 4. Phase II—NCT04052737 | Alzheimer’s Disease (AD) | Prospective, multicentric, randomized, double-blinded, placebo-controlled study in mild to moderate Alzheimer’s disease to assess safety and efficacy of sovateltide therapy. Total participants—80. Dose—0.3 μg/kg body wt. per dose. Three doses of sovateltide, at every 3 ± 1 h (total dose/day: 0.9 µg/kg body wt.). Repeat doses every month for 6 months. In both treatment groups, subjects are provided the best available standard of care for AD. | An interim analysis of 62 patients (control n = 31 and sovateltide n = 31) after 180 days of treatment and follow-up. AD Assessment Scale cognitive subscale (ADAS-Cog) changed 1.382 ± 0.920 (from a baseline value 22.59) in control and 0.247 ± 0.955 (from a baseline value 27.85) in the sovateltide treated patients from their respective baseline. Mini-mental state examination (MMSE) score changed by 1.226 ± 0.498 (from a baseline value of 19.45) in the control group and 0.9355 ± 0.5004 (from a baseline value of 17.42) in the sovateltide group from their respective baseline value. Neuropsychiatric Inventory (NPI) score was changed by −0.548 ± 1.123 (from a baseline of 9.516) in the control group, whereas by −1.968 ± 1.077 (from a baseline of 9.871) in the sovateltide group. These data indicate a potential beneficial effect of sovateltide in AD patients with late early to moderate stage of the disease. |
| 5. Phase II—NCT04054414 | Spinal Cord Injury (SCI) | Prospective, multicentric, randomized, double-blind, parallel, saline controlled to compare the safety and efficacy of sovateltide therapy along with standard supportive care in patients of acute spinal cord injury. Total spinal cord injury patients—40. Dose—0.3 μg/kg body wt. per dose. Three doses per day (total dose/day: 0.9 µg/kg body wt.) as an intravenous bolus over 1 min at every 3 ± 1 h on day 1, 3, and day 6. Primary objective—Testing safety and tolerability of sovateltide in spinal cord injury patients. Secondary objective—Assessment of International Standards for Neurological Classification of spinal cord injury (ISNCSCI), Walking Index for Spinal Cord Injury (WISCI) Score, Spinal Cord Independence Measure (SCIM) Score, Changes in Magnetic Resonance Imaging (MRI), Computerized Tomography (CT), and electromyography (EMG). | Expected to be completed in July 2022. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranjan, A.K.; Gulati, A. Sovateltide Mediated Endothelin B Receptors Agonism and Curbing Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 3146. https://doi.org/10.3390/ijms23063146
Ranjan AK, Gulati A. Sovateltide Mediated Endothelin B Receptors Agonism and Curbing Neurological Disorders. International Journal of Molecular Sciences. 2022; 23(6):3146. https://doi.org/10.3390/ijms23063146
Chicago/Turabian StyleRanjan, Amaresh K., and Anil Gulati. 2022. "Sovateltide Mediated Endothelin B Receptors Agonism and Curbing Neurological Disorders" International Journal of Molecular Sciences 23, no. 6: 3146. https://doi.org/10.3390/ijms23063146






