From Cancer Therapy to Winemaking: The Molecular Structure and Applications of β-Glucans and β-1, 3-Glucanases
Abstract
:1. Introduction
2. Structure of β-Glucans
2.1. Variations in β-Glucan Primary Structure
2.2. 3D Structure of β-Glucans
3. β-1,3-Glucanases
3.1. Classification of β-1,3-Glucanases
3.2. Primary Structure of β-1,3-Glucanases
3.3. 3D Structure of β-1,3-Glucanases
4. Other β-Glucan Binding Proteins
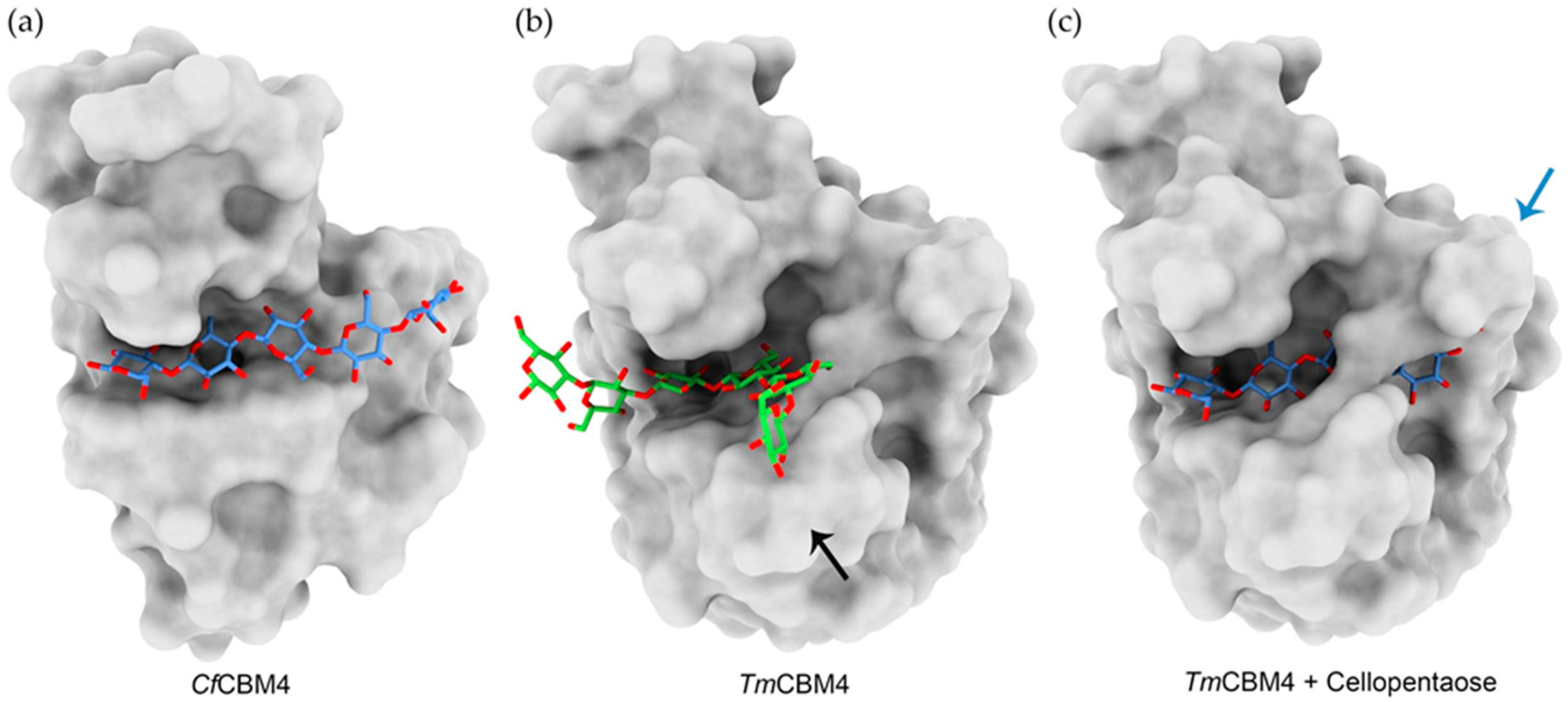
4.1. Carbohydrate Binding Modules
4.2. Dectin-1
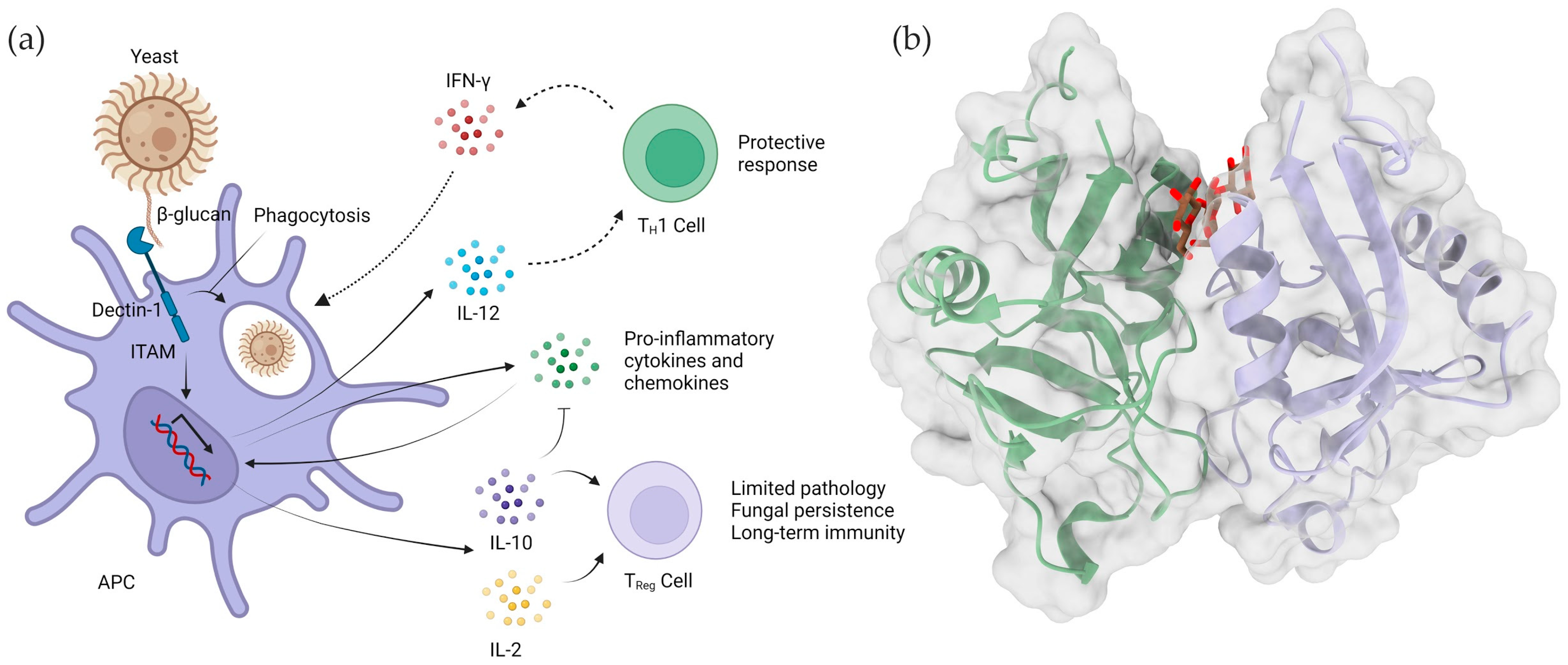
4.3. Complement Receptor 3
4.4. β-glucan Binding Proteins in Invertebrates
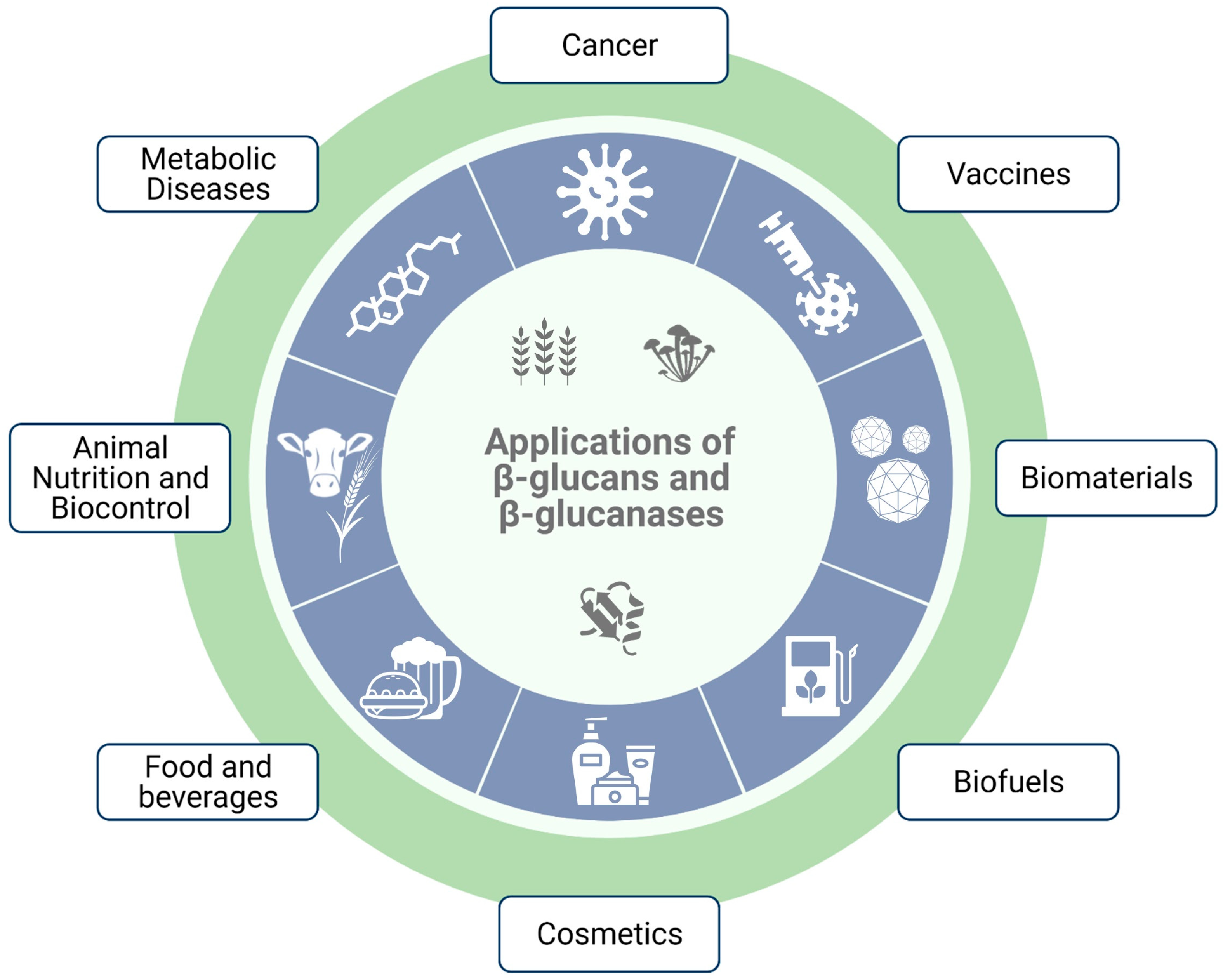
5. Applications of β-Glucans
5.1. Immunomodulating Activity
5.1.1. Anticancer Activity
| Molecule | Cancer Type | Status | Identifier |
|---|---|---|---|
| Yeast derived β-glucan + glutamine + immunoglobulin | Metastatic cancers | Phase II/III | NCT04710290 |
| Yeast derived Soluble Beta-Glucan | Advanced solid tumors | Phase I | NCT01910597 |
| Soluble β-1,3-1,6-glucan + standard antibody and chemotherapy | Breast cancer | Phase I/II | NCT00533364 |
| Soluble β-glucan + rituximab + COP/CHOP | Non-Hodgkin’s lymphoma | Phase I | NCT00533728 |
| Particulate β-glucan | Oral squamous cell carcinoma | Not Applicable | NCT04387682 |
| β-glucan MM-10-001 | Locally advanced or metastatic non-small cell lung cancer | Phase I | NCT00857025 |
| β-glucan (Imucell WGP) | Non-small cell lung cancer | Not Applicable | NCT00682032 |
| β-glucan + pembrolizumab | Melanoma stage III/IV | Not Applicable | NCT04513028 |
| β-glucan + monoclonal antibody 3F8 | Neuroblastoma | Phase I | NCT00037011 |
| β -glucan + monoclonal antibody 3F8 | Neuroblastoma | Phase I | NCT00492167 |
| β-glucan + isotretinoin + sargramostim + monoclonal antibody 3F8 | Neuroblastoma | Phase II | NCT00089258 |
| β-glucan + granulocyte-macrophage colony stimulating factor + bivalent vaccine + adjuvant OPT-821 | Neuroblastoma | Phase II | NCT04936529 |
| β-glucan + bivalent vaccine + adjuvant OPT-821 | Neuroblastoma | Phase I/II | NCT00911560 |
| Imprime PGG β-glucan + rituximab + alemtuzumab | Chronic lymphocytic leukemia | Phase I/II | NCT01269385 |
| Imprime PGG β-glucan + pembrolizumab | Malignant Neoplasm of Breast | Phase II | NCT05159778 |
| Imprime PGG β-glucan + pembrolizumab | Advanced melanoma Triple-negative breast cancer | Phase II | NCT02981303 |
| Imprime PGG C + rituximab | Relapsed/refractory indolent B cell non-Hodgkin lymphomas | Phase II | NCT02086175 |
5.1.2. Anti-Infective, Anti-Inflammatory and Wound Healing Properties
5.2. Metabolic Activity
5.3. Industrial Applications of β-Glucans
5.4. Biotechnological and Biomedical Applications of β-Glucans
5.5. Applications of β-1,3-Glucanases
6. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saito, H.; Misaki, A.; Harada, T. A Comparison of the Structure of Curdlan and Pachyman. Agric. Biol. Chem. 1968, 32, 1261–1269. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usoltseva, R.V.; Belik, A.A.; Kusaykin, M.I.; Malyarenko, O.S.; Zvyagintseva, T.N.; Ermakova, S.P. Laminarans and 1,3-β-D-Glucanases. Int. J. Biol. Macromol. 2020, 163, 1010–1025. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.; Laffey, J.G. β-Glucans. Encyclopedia 2021, 1, 831–847. [Google Scholar] [CrossRef]
- Wang, Q.; Sheng, X.; Shi, A.; Hu, H.; Yang, Y.; Liu, L.; Fei, L.; Liu, H. β-Glucans: Relationships between Modification, Conformation and Functional Activities. Molecules 2017, 22, 257. [Google Scholar] [CrossRef] [Green Version]
- da Silva Milhorini, S.; Simas, F.F.; Smiderle, F.R.; Inara de Jesus, L.; Rosado, F.R.; Longoria, E.L.; Iacomini, M. β-Glucans from the Giant Mushroom Macrocybe Titans: Chemical Characterization and Rheological Properties. Food Hydrocoll. 2022, 125, 107392. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. JoF 2020, 6, 356. [Google Scholar] [CrossRef]
- Ahnen, R.T.; Jonnalagadda, S.S.; Slavin, J.L. Role of Plant Protein in Nutrition, Wellness, and Health. Nutr. Rev. 2019, 77, 735–747. [Google Scholar] [CrossRef]
- Frank, J.; Fukagawa, N.K.; Bilia, A.R.; Johnson, E.J.; Kwon, O.; Prakash, V.; Miyazawa, T.; Clifford, M.N.; Kay, C.D.; Crozier, A.; et al. Terms and Nomenclature Used for Plant-Derived Components in Nutrition and Related Research: Efforts toward Harmonization. Nutr. Rev. 2020, 78, 451–458. [Google Scholar] [CrossRef]
- Chang, S.-C.; Saldivar, R.K.; Liang, P.-H.; Hsieh, Y.S.Y. Structures, Biosynthesis, and Physiological Functions of (1,3;1,4)-β-d-Glucans. Cells 2021, 10, 510. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kofuji, K.; Aoki, A.; Tsubaki, K.; Konishi, M.; Isobe, T.; Murata, Y. Antioxidant Activity of β-Glucan. ISRN Pharm. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, J.; Hamuro, J.; Hatanaka, H.; Hamabata, K.; Kinoshita, S. Alleviation of Seasonal Allergic Symptoms with Superfine β-1,3-Glucan: A Randomized Study. J. Allergy Clin. Immunol. 2007, 119, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef] [Green Version]
- Sivieri, K.; de Oliveira, S.M.; de Souza Marquez, A.; Pérez-Jiménez, J.; Diniz, S.N. Insights on β-Glucan as a Prebiotic Coadjuvant in the Treatment of Diabetes Mellitus: A Review. Food Hydrocoll. Health 2022, 2, 100056. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P. The Effects of β-Glucan on Fish Immunity. N. Am. J. Med. Sci. 2013, 5, 580. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, B.; Vetvicka, V. Review: β-Glucans as Effective Antibiotic Alternatives in Poultry. Molecules 2021, 26, 3560. [Google Scholar] [CrossRef]
- Byrne, K.A.; Loving, C.L.; McGill, J.L. Innate Immunomodulation in Food Animals: Evidence for Trained Immunity? Front. Immunol. 2020, 11, 1099. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. Beta Glucan: A Valuable Functional Ingredient in Foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef]
- Du, B.; Bian, Z.; Xu, B. Skin Health Promotion Effects of Natural Beta-Glucan Derived from Cereals and Microorganisms: A Review. Phytother. Res. 2014, 28, 159–166. [Google Scholar] [CrossRef]
- Déjean, G.; Tamura, K.; Cabrera, A.; Jain, N.; Pudlo, N.A.; Pereira, G.; Viborg, A.H.; Van Petegem, F.; Martens, E.C.; Brumer, H. Synergy between Cell Surface Glycosidases and Glycan-Binding Proteins Dictates the Utilization of Specific Beta(1,3)-Glucans by Human Gut Bacteroides. mBio 2020, 11, e00095-20. [Google Scholar] [CrossRef] [Green Version]
- Labourel, A.; Jam, M.; Legentil, L.; Sylla, B.; Hehemann, J.-H.; Ferrières, V.; Czjzek, M.; Michel, G. Structural and Biochemical Characterization of the Laminarinase Zg LamC GH16 from Zobellia Galactanivorans Suggests Preferred Recognition of Branched Laminarin. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 173–184. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Biochemistry of Digestion. In Comprehensive Molecular Insect Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 171–224. ISBN 978-0-444-51924-5. [Google Scholar]
- Mackay, R.M.; Baird, S.; Dove, M.J.; Erratt, J.A.; Gines, M.; Moranelli, F.; Nasim, A.; Willick, G.E.; Yaguchi, M.; Seligy, V.L. Glucanase Gene Diversity in Prokaryotic and Eukaryotic Organisms. Biosystems 1985, 18, 279–292. [Google Scholar] [CrossRef]
- Sova, V.V.; Pesentseva, M.S.; Zakharenko, A.M.; Kovalchuk, S.N.; Zvyagintseva, T.N. Glycosidases of Marine Organisms. Biochemistry 2013, 78, 746–759. [Google Scholar] [CrossRef]
- Dong, J.-Z.; Dunstan, D.I. Endochitinase and ?-1,3-Glucanase Genes Are Developmentally Regulated during Somatic Embryogenesis InPicea Glauca. Planta 1997, 201, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sova, V.V.; Shirokova, N.I.; Kusaykin, M.I.; Scobun, A.S.; Elyakova, L.A.; Zvyagintseva, T.N. β-1,3-Glucanase from Unfertilized Eggs of the Sea Urchin Strongylocentrotus Intermedius. Comparison with β-1,3-Glucanases of Marine and Terrestrial Mollusks. Biochemistry 2003, 68, 529–533. [Google Scholar] [CrossRef]
- Carvalho, V.S.D.; Gómez-Delgado, L.; Curto, M.Á.; Moreno, M.B.; Pérez, P.; Ribas, J.C.; Cortés, J.C.G. Analysis and Application of a Suite of Recombinant Endo-β(1,3)-d-Glucanases for Studying Fungal Cell Walls. Microb. Cell Fact 2021, 20, 126. [Google Scholar] [CrossRef]
- Wu, Q.; Dou, X.; Wang, Q.; Guan, Z.; Cai, Y.; Liao, X. Isolation of β-1,3-Glucanase-Producing Microorganisms from Poria Cocos Cultivation Soil via Molecular Biology. Molecules 2018, 23, 1555. [Google Scholar] [CrossRef] [Green Version]
- Brazil, C.; de Oliveira, D.F.; Duarte, R.A.; Galo, J.M.; Lucchetta, L.; da Costa dos Santos, E.; Hashimoto, E.H. β-Glucanase Addition in Brewing Malt Produced by Reduced Time of Germination. Braz. Arch. Biol. Technol. 2019, 62, e19180315. [Google Scholar] [CrossRef]
- Karunaratne, N.D.; Classen, H.L.; Ames, N.P.; Bedford, M.R.; Newkirk, R.W. Effects of Diet Hulless Barley and Beta-Glucanase Levels on Ileal Digesta Soluble Beta-Glucan Molecular Weight and Carbohydrate Fermentation in Laying Hens. Poult. Sci. 2022, 101, 101735. [Google Scholar] [CrossRef]
- Claus, H.; Mojsov, K. Enzymes for Wine Fermentation: Current and Perspective Applications. Fermentation 2018, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- de Marco, J.L.; Felix, C.R. Purification and Characterization of a Beta-Glucanase Produced by Trichoderma Harzianum Showing Biocontrol Potential. Braz. Arch. Biol. Technol. 2007, 50, 21–29. [Google Scholar] [CrossRef]
- Bai, L.; Kim, J.; Son, K.-H.; Shin, D.-H.; Ku, B.-H.; Kim, D.Y.; Park, H.-Y. Novel Anti-Fungal d-Laminaripentaose-Releasing Endo-β-1,3-Glucanase with a RICIN-like Domain from Cellulosimicrobium Funkei HY-13. Biomolecules 2021, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Yang, D.; You, X.; Liu, Y.; Hu, S.; Yan, Q.; Yang, S.; Jiang, Z. The Recognition Mechanism of Triple-Helical β-1,3-Glucan by a β-1,3-Glucanase. Chem. Commun. 2017, 53, 9368–9371. [Google Scholar] [CrossRef]
- Pluvinage, B.; Fillo, A.; Massel, P.; Boraston, A.B. Structural Analysis of a Family 81 Glycoside Hydrolase Implicates Its Recognition of β-1,3-Glucan Quaternary Structure. Structure 2017, 25, 1348–1359. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, I.; Pettolino, F.A.; Wilson, S.M.; Doblin, M.S.; Johansen, B.; Bacic, A.; Willats, W.G.T. Mixed-Linkage (1→3),(1→4)-β-d-Glucan Is Not Unique to the Poales and Is an Abundant Component of Equisetum Arvense Cell Walls. Plant J. 2008, 54, 510–521. [Google Scholar] [CrossRef]
- Pérez-Mendoza, D.; Rodríguez-Carvajal, M.Á.; Romero-Jiménez, L.; de Araujo Farias, G.; Lloret, J.; Gallegos, M.T.; Sanjuán, J. Novel Mixed-Linkage β-Glucan Activated by c-Di-GMP in Sinorhizobium meliloti. Proc. Natl. Acad. Sci. USA 2015, 112, E757–E765. [Google Scholar] [CrossRef] [Green Version]
- Olafsdottir, E.S.; Ingólfsdottir, K. Polysaccharides from Lichens: Structural Characteristics and Biological Activity. Planta Med. 2001, 67, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Lechat, H.; Amat, M.; Mazoyer, J.; Buleon, A.; Lahaye, M. Structure and distribution of glucomannan and sulfated glucan in the cell walls of the red alga Kappaphycus alvarezii (Gigartinales, Rhodophyta). J. Phycol. 2000, 36, 891–902. [Google Scholar] [CrossRef]
- Ao, J.; Free, S.J. Genetic and Biochemical Characterization of the GH72 Family of Cell Wall Transglycosylases in Neurospora Crassa. Fungal Genet. Biol. 2017, 101, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Burton, R.A.; Fincher, G.B. (1,3;1,4)-β-D-Glucans in Cell Walls of the Poaceae, Lower Plants, and Fungi: A Tale of Two Linkages. Mol. Plant 2009, 2, 873–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, S.C.; Nesselrode, B.H.W.A.; Miller, J.G.; Mewburn, B.R. Mixed-linkage (1→3,1→4)-β-d-glucan Is a Major Hemicellulose of Equisetum (Horsetail) Cell Walls. New Phytol. 2008, 179, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Tenhaken, R.; Driouich, A.; Lütz-Meindl, U. Occurrence and characterization of arabinogalactan-like proteins and hemicelluloses in Micrasterias (Streptophyta) 1. J. Phycol. 2008, 44, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Salmeán, A.A.; Duffieux, D.; Harholt, J.; Qin, F.; Michel, G.; Czjzek, M.; Willats, W.G.T.; Hervé, C. Insoluble (1 → 3), (1 → 4)-β-D-Glucan Is a Component of Cell Walls in Brown Algae (Phaeophyceae) and Is Masked by Alginates in Tissues. Sci. Rep. 2017, 7, 2880. [Google Scholar] [CrossRef]
- Honegger, R.; Haisch, A. Immunocytochemical Location of the (1→3) (1→4)-β-Glucan Lichenin in the Lichen-Forming Ascomycete Cetraria islandica (Icelandic Moss) 1. New Phytol. 2001, 150, 739–746. [Google Scholar] [CrossRef]
- Fontaine, T.; Simenel, C.; Dubreucq, G.; Adam, O.; Delepierre, M.; Lemoine, J.; Vorgias, C.E.; Diaquin, M.; Latgé, J.-P. Molecular Organization of the Alkali-Insoluble Fraction OfAspergillus Fumigatus Cell Wall. J. Biol. Chem. 2000, 275, 27594–27607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal B-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manabe, N.; Yamaguchi, Y. 3D Structural Insights into β-Glucans and Their Binding Proteins. Int. J. Mol. Sci. 2021, 22, 1578. [Google Scholar] [CrossRef]
- Sasaki, T.; Takasuka, N. Further Study of the Structure of Lentinan, an Anti-Tumor Polysaccharide from Lentinus Edodes. Carbohydr. Res. 1976, 47, 99–104. [Google Scholar] [CrossRef]
- Nanba, H.; Kubo, K. Antitumor Substance Extracted from Grifola. U.S. Patent 5,854,404, 29 December 1998. [Google Scholar]
- Tabata, K.; Ito, W.; Kojima, T.; Kawabata, S.; Misaki, A. Ultrasonic Degradation of Schizophyllan, an Antitumor Polysaccharide Produced by Schizophyllum Commune Fries. Carbohydr. Res. 1981, 89, 121–135. [Google Scholar] [CrossRef]
- Ogawa, K.; Tsurugi, J.; Watanabe, T. The Dependence of the Conformation of a (1→3)-β-D-Glucan on Chain-Length in Alkaline Solution. Carbohydr. Res. 1973, 29, 397–403. [Google Scholar] [CrossRef]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef] [PubMed]
- Gajdošová, A.; Petruláková, Z.; Havrlentová, M.; Červená, V.; Hozová, B.; Šturdík, E.; Kogan, G. The Content of Water-Soluble and Water-Insoluble β-d-Glucans in Selected Oats and Barley Varieties. Carbohydr. Polym. 2007, 70, 46–52. [Google Scholar] [CrossRef]
- Sletmoen, M.; Stokke, B.T. Higher Order Structure of (1,3)-β-D-Glucans and Its Influence on Their Biological Activities and Complexation Abilities. Biopolymers 2008, 89, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Chuah, C.T.; Sarko, A.; Deslandes, Y.; Marchessault, R.H. Packing Analysis of Carbohydrates and Polysaccharides. Part 14. Triple-Helical Crystalline Structure of Curdlan and Paramylon Hydrates. Macromolecules 1983, 16, 1375–1382. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Uehara, N.; Saito, H. Conformation-Dependent Change in Antitumor Activity of Linear and Branched (1.RAR.3)-.BETA.-D-Glucans on the Basis of Conformational Elucidation by Carbon-13 Nuclear Magnetic Resonance Spectroscopy. Chem. Pharm. Bull. 1992, 40, 1221–1226. [Google Scholar] [CrossRef] [Green Version]
- Kulicke, W.-M.; Lettau, A.I.; Thielking, H. Correlation between Immunological Activity, Molar Mass, and Molecular Structure of Different (1→3)-β-d-Glucans. Carbohydr. Res. 1997, 297, 135–143. [Google Scholar] [CrossRef]
- Young, S.-H.; Dong, W.-J.; Jacobs, R.R. Observation of a Partially Opened Triple-Helix Conformation in 1→3-β-Glucan by Fluorescence Resonance Energy Transfer Spectroscopy. J. Biol. Chem. 2000, 275, 11874–11879. [Google Scholar] [CrossRef] [Green Version]
- Okobira, T.; Miyoshi, K.; Uezu, K.; Sakurai, K.; Shinkai, S. Molecular Dynamics Studies of Side Chain Effect on the β-1,3- d -Glucan Triple Helix in Aqueous Solution. Biomacromolecules 2008, 9, 783–788. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Norisuye, T.; Fujita, H. Triple Helix of Schizophyllum Commune Polysaccharide in Dilute Solution. 4. Light Scattering and Viscosity in Dilute Aqueous Sodium Hydroxide. Macromolecules 1981, 14, 1220–1225. [Google Scholar] [CrossRef]
- Bluhm, T.L.; Sarko, A. The Triple Helical Structure of Lentinan, a Linear β-(1 → 3)-D-Glucan. Can. J. Chem. 1977, 55, 293–299. [Google Scholar] [CrossRef]
- Oda, M.; Tanabe, Y.; Noda, M.; Inaba, S.; Krayukhina, E.; Fukada, H.; Uchiyama, S. Structural and Binding Properties of Laminarin Revealed by Analytical Ultracentrifugation and Calorimetric Analyses. Carbohydr. Res. 2016, 431, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Norisuye, T.; Yanaki, T.; Fujita, H. Triple Helix of Aschizophyllum Commune Polysaccharide in Aqueous Solution. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 547–558. [Google Scholar] [CrossRef]
- Yanaki, T.; Tabata, K.; Kojima, T. Melting Behaviour of a Triple Helical Polysaccharide Schizophyllan in Aqueous Solution. Carbohydr. Polym. 1985, 5, 275–283. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Xu, X. Morphologies and Conformation Transition Oflentinan in Aqueous NaOH Solution. Biopolymers 2004, 75, 187–195. [Google Scholar] [CrossRef]
- Bluhm, T.L.; Deslandes, Y.; Marchessault, R.H.; Pérez, S.; Rinaudo, M. Solid-State and Solution Conformation of Scleroglucan. Carbohydr. Res. 1982, 100, 117–130. [Google Scholar] [CrossRef]
- Guo, X.; Kang, J.; Xu, Z.; Guo, Q.; Zhang, L.; Ning, H.; Cui, S.W. Triple-Helix Polysaccharides: Formation Mechanisms and Analytical Methods. Carbohydr. Polym. 2021, 262, 117962. [Google Scholar] [CrossRef]
- Martin, K.; McDougall, B.M.; McIlroy, S.; Jayus; Chen, J.; Seviour, R.J. Biochemistry and Molecular Biology of Exocellular Fungal β-(1,3)- and β-(1,6)-Glucanases. FEMS Microbiol. Rev. 2007, 31, 168–192. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Lyu, Q. Biochemical Characterization of a Novel Endo-1,3-β-Glucanase from the Scallop Chlamys Farreri. Mar. Drugs 2020, 18, 466. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-Glucanases: Their Biological Functions and Transgenic Expression against Phytopathogenic Fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef]
- Sun, L.; Gurnon, J.R.; Adams, B.J.; Graves, M.V.; Van Etten, J.L. Characterization of a β-1,3-Glucanase Encoded by Chlorella Virus PBCV-1. Virology 2000, 276, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueguen, Y.; Voorhorst, W.G.B.; van der Oost, J.; de Vos, W.M. Molecular and Biochemical Characterization of an Endo-β-1,3-Glucanase of the Hyperthermophilic Archaeon Pyrococcus furiosus. J. Biol. Chem. 1997, 272, 31258–31264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hien, N.H.; Fleet, G.H. Separation and Characterization of Six (1 Leads to 3)-Beta-Glucanases from Saccharomyces Cerevisiae. J. Bacteriol. 1983, 156, 1204–1213. [Google Scholar] [CrossRef] [Green Version]
- Rotchanapreeda, T.; Kumsang, Y.; Sae-Chew, P.; Rujirawat, T.; Lohnoo, T.; Yingyong, W.; Payattikul, P.; Reamtong, O.; Krajaejun, T. Expression, Purification, and Characterization of the Recombinant Exo-1,3-β-Glucanase (Exo1) of the Pathogenic Oomycete Pythium insidiosum. Heliyon 2020, 6, e04237. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mendoza, J.; Santiago-Hernández, A.; Alvarez-Zúñiga, M.T.; Gutiérrez-Antón, M.; Aguilar-Osorio, G.; Hidalgo-Lara, M.E. Purification and Biochemical Characterization of a Novel Thermophilic Exo-β-1,3-Glucanase from the Thermophile Biomass-Degrading Fungus Thielavia Terrestris Co3Bag1. Electron. J. Biotechnol. 2019, 41, 60–71. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Nakade, K.; Konno, N. Endo-β-1,3-Glucanase GLU1, from the Fruiting Body of Lentinula Edodes, Belongs to a New Glycoside Hydrolase Family. Appl. Environ. Microbiol. 2011, 77, 8350–8354. [Google Scholar] [CrossRef] [Green Version]
- Bragatto, I.; Genta, F.A.; Ribeiro, A.F.; Terra, W.R.; Ferreira, C. Characterization of a β-1,3-Glucanase Active in the Alkaline Midgut of Spodoptera Frugiperda Larvae and Its Relation to β-Glucan-Binding Proteins. Insect Biochem. Mol. Biol. 2010, 40, 861–872. [Google Scholar] [CrossRef]
- El-Shora, H.M.; El-Sharkawy, R.M.; Khateb, A.M.; Darwish, D.B. Production and Immobilization of β-Glucanase from Aspergillus Niger with Its Applications in Bioethanol Production and Biocontrol of Phytopathogenic Fungi. Sci. Rep. 2021, 11, 21000. [Google Scholar] [CrossRef]
- Hartl, L.; Gastebois, A.; Aimanianda, V.; Latgé, J.-P. Characterization of the GPI-Anchored Endo β-1,3-Glucanase Eng2 of Aspergillus Fumigatus. Fungal Genet. Biol. 2011, 48, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Co, R.; Hug, L.A. A Need for Improved Cellulase Identification from Metagenomic Sequence Data. Appl. Environ. Microbiol. 2020, 87, e01928-20. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The Carbohydrate-Active Enzymes Database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hettle, A.; Fillo, A.; Abe, K.; Massel, P.; Pluvinage, B.; Langelaan, D.N.; Smith, S.P.; Boraston, A.B. Properties of a Family 56 Carbohydrate-Binding Module and Its Role in the Recognition and Hydrolysis of β-1,3-Glucan. J. Biol. Chem. 2017, 292, 16955–16968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyani, D.C.; Reichenbach, T.; Aspeborg, H.; Divne, C. A Homodimeric Bacterial Exo-β-1,3-Glucanase Derived from Moose Rumen Microbiome Shows a Structural Framework Similar to Yeast Exo-β-1,3-Glucanases. Enzym. Microb. Technol. 2021, 143, 109723. [Google Scholar] [CrossRef] [PubMed]
- Takashima, T.; Taku, T.; Yamanaka, T.; Fukamizo, T.; Numata, T.; Ohnuma, T. Crystal Structure and Biochemical Characterization of CJP38, a β-1,3-Glucanase and Allergen of Cryptomeria Japonica Pollen. Mol. Immunol. 2019, 116, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Millet, N.; Moya-Nilges, M.; Sachse, M.; Krijnse Locker, J.; Latgé, J.; Mouyna, I. Aspergillus Fumigatus Exoβ(1-3)Glucanases Family GH55 Are Essential for Conidial Cell Wall Morphogenesis. Cell. Microbiol. 2019, 21, e13102. [Google Scholar] [CrossRef] [PubMed]
- Michel, G.; Chantalat, L.; Duee, E.; Barbeyron, T.; Henrissat, B.; Kloareg, B.; Dideberg, O. The κ-Carrageenase of P. Carrageenovora Features a Tunnel-Shaped Active Site. Structure 2001, 9, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Ilari, A.; Fiorillo, A.; Angelaccio, S.; Florio, R.; Chiaraluce, R.; van der Oost, J.; Consalvi, V. Crystal Structure of a Family 16 Endoglucanase from the Hyperthermophile Pyrococcus Furiosus- Structural Basis of Substrate Recognition: Substrate Recognition in Family 16 Endoglucanase. FEBS J. 2009, 276, 1048–1058. [Google Scholar] [CrossRef]
- Feng, J.; Xu, S.; Feng, R.; Kovalevsky, A.; Zhang, X.; Liu, D.; Wan, Q. Identification and Structural Analysis of a Thermophilic β-1,3-Glucanase from Compost. Bioresour. Bioprocess. 2021, 8, 102. [Google Scholar] [CrossRef]
- Wojtkowiak, A.; Witek, K.; Hennig, J.; Jaskolski, M. Two High-Resolution Structures of Potato Endo-1,3-β-Glucanase Reveal Subdomain Flexibility with Implications for Substrate Binding. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 713–723. [Google Scholar] [CrossRef]
- Fibriansah, G.; Masuda, S.; Koizumi, N.; Nakamura, S.; Kumasaka, T. The 1.3 Å Crystal Structure of a Novel Endo-β-1,3-Glucanase of Glycoside Hydrolase Family 16 from Alkaliphilic Nocardiopsis Sp. Strain F96. Proteins 2007, 69, 683–690. [Google Scholar] [CrossRef]
- Gilbert, H.J.; Knox, J.P.; Boraston, A.B. Advances in Understanding the Molecular Basis of Plant Cell Wall Polysaccharide Recognition by Carbohydrate-Binding Modules. Curr. Opin. Struct. Biol. 2013, 23, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Herve, C.; Rogowski, A.; Blake, A.W.; Marcus, S.E.; Gilbert, H.J.; Knox, J.P. Carbohydrate-Binding Modules Promote the Enzymatic Deconstruction of Intact Plant Cell Walls by Targeting and Proximity Effects. Proc. Natl. Acad. Sci. USA 2010, 107, 15293–15298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontes, C.M.G.A.; Gilbert, H.J. Cellulosomes: Highly Efficient Nanomachines Designed to Deconstruct Plant Cell Wall Complex Carbohydrates. Annu. Rev. Biochem. 2010, 79, 655–681. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Nurizzo, D.; Notenboom, V.; Ducros, V.; Rose, D.R.; Kilburn, D.G.; Davies, G.J. Differential Oligosaccharide Recognition by Evolutionarily-Related β-1,4 and β-1,3 Glucan-Binding Modules. J. Mol. Biol. 2002, 319, 1143–1156. [Google Scholar] [CrossRef]
- Van Bueren, A.L.; Morland, C.; Gilbert, H.J.; Boraston, A.B. Family 6 Carbohydrate Binding Modules Recognize the Non-Reducing End of β-1,3-Linked Glucans by Presenting a Unique Ligand Binding Surface. J. Biol. Chem. 2005, 280, 530–537. [Google Scholar] [CrossRef] [Green Version]
- Tamashiro, T.; Tanabe, Y.; Ikura, T.; Ito, N.; Oda, M. Critical Roles of Asp270 and Trp273 in the α-Repeat of the Carbohydrate-Binding Module of Endo-1,3-β-Glucanase for Laminarin-Binding Avidity. Glycoconj. J. 2012, 29, 77–85. [Google Scholar] [CrossRef]
- Martín-Cuadrado, A.B.; del Dedo, J.E.; de Medina-Redondo, M.; Fontaine, T.; del Rey, F.; Latgé, J.P.; de Aldana, C.R.V. The Schizosaccharomyces pombe Endo-1,3-β-Glucanase Eng1 Contains a Novel Carbohydrate Binding Module Required for Septum Localization. Mol. Microbiol. 2008, 69, 188–200. [Google Scholar] [CrossRef]
- Dvortsov, I.A.; Lunina, N.A.; Chekanovskaya, L.A.; Schwarz, W.H.; Zverlov, V.V.; Velikodvorskaya, G.A. Carbohydrate-Binding Properties of a Separately Folding Protein Module from β-1,3-Glucanase Lic16A of Clostridium Thermocellum. Microbiology 2009, 155, 2442–2449. [Google Scholar] [CrossRef] [Green Version]
- Kanagawa, M.; Satoh, T.; Ikeda, A.; Adachi, Y.; Ohno, N.; Yamaguchi, Y. Structural Insights into Recognition of Triple-Helical β-Glucans by an Insect Fungal Receptor. J. Biol. Chem. 2011, 286, 29158–29165. [Google Scholar] [CrossRef] [Green Version]
- Duan, C.-J.; Feng, Y.-L.; Cao, Q.-L.; Huang, M.-Y.; Feng, J.-X. Identification of a Novel Family of Carbohydrate-Binding Modules with Broad Ligand Specificity. Sci. Rep. 2016, 6, 19392. [Google Scholar] [CrossRef] [Green Version]
- Venditto, I.; Luis, A.S.; Rydahl, M.; Schückel, J.; Fernandes, V.O.; Vidal-Melgosa, S.; Bule, P.; Goyal, A.; Pires, V.M.R.; Dourado, C.G.; et al. Complexity of the Ruminococcus Flavefaciens Cellulosome Reflects an Expansion in Glycan Recognition. Proc. Natl. Acad. Sci. USA 2016, 113, 7136–7141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y.C. The β-Glucan Receptor, Dectin-1, Is Predominantly Expressed on the Surface of Cells of the Monocyte/Macrophage and Neutrophil Lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariizumi, K.; Shen, G.-L.; Shikano, S.; Xu, S.; Ritter, R.; Kumamoto, T.; Edelbaum, D.; Morita, A.; Bergstresser, P.R.; Takashima, A. Identification of a Novel, Dendritic Cell-Associated Molecule, Dectin-1, by Subtractive CDNA Cloning. J. Biol. Chem. 2000, 275, 20157–20167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schorey, J.; Lawrence, C. The Pattern Recognition Receptor Dectin-1: From Fungi to Mycobacteria. CDT 2008, 9, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.S.; Feizi, T.; Zhang, Y.; Stoll, M.S.; Lawson, A.M.; Díaz-Rodríguez, E.; Campanero-Rhodes, M.A.; Costa, J.; Gordon, S.; Brown, G.D.; et al. Ligands for the β-Glucan Receptor, Dectin-1, Assigned Using “Designer” Microarrays of Oligosaccharide Probes (Neoglycolipids) Generated from Glucan Polysaccharides. J. Biol. Chem. 2006, 281, 5771–5779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanashima, S.; Ikeda, A.; Tanaka, H.; Adachi, Y.; Ohno, N.; Takahashi, T.; Yamaguchi, Y. NMR Study of Short β(1-3)-Glucans Provides Insights into the Structure and Interaction with Dectin-1. Glycoconj. J. 2014, 31, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; O’Callaghan, C.A.; Marshall, A.S.J.; Gilbert, R.J.C.; Siebold, C.; Gordon, S.; Brown, G.D.; Jones, E.Y. Structure of the Fungal β-Glucan-Binding Immune Receptor Dectin-1: Implications for Function. Protein Sci. 2007, 16, 1042–1052. [Google Scholar] [CrossRef]
- Manabe, Y.; Marchetti, R.; Takakura, Y.; Nagasaki, M.; Nihei, W.; Takebe, T.; Tanaka, K.; Kabayama, K.; Chiodo, F.; Hanashima, S.; et al. The Core Fucose on an IgG Antibody Is an Endogenous Ligand of Dectin-1. Angew. Chem. Int. Ed. 2019, 58, 18697–18702. [Google Scholar] [CrossRef]
- Steele, C.; Marrero, L.; Swain, S.; Harmsen, A.G.; Zheng, M.; Brown, G.D.; Gordon, S.; Shellito, J.E.; Kolls, J.K. Alveolar Macrophage–Mediated Killing of Pneumocystis Carinii f. Sp. Muris Involves Molecular Recognition by the Dectin-1 β-Glucan Receptor. J. Exp. Med. 2003, 198, 1677–1688. [Google Scholar] [CrossRef] [Green Version]
- Gantner, B.N.; Simmons, R.M.; Underhill, D.M. Dectin-1 Mediates Macrophage Recognition of Candida Albicans Yeast but Not Filaments. EMBO J. 2005, 24, 1277–1286. [Google Scholar] [CrossRef] [Green Version]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative Induction of Inflammatory Responses by Dectin-1 and Toll-like Receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.J.; Gordon, S. Dectin-1 Mediates the Biological Effects of β-Glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, N.C.; Slack, E.C.; Edwards, A.D.; Nolte, M.A.; Schulz, O.; Schweighoffer, E.; Williams, D.L.; Gordon, S.; Tybulewicz, V.L.; Brown, G.D.; et al. Syk-Dependent Cytokine Induction by Dectin-1 Reveals a Novel Pattern Recognition Pathway for C Type Lectins. Immunity 2005, 22, 507–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romani, L. Immunity to Fungal Infections. Nat. Rev. Immunol. 2004, 4, 11–24. [Google Scholar] [CrossRef]
- Brown, G.D. Dectin-1: A Signalling Non-TLR Pattern-Recognition Receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef]
- Ross, G.D.; Větvička, V. CR3 (CD11b, CD18): A Phagocyte and NK Cell Membrane Receptor with Multiple Ligand Specificities and Functions. Clin. Exp. Immunol. 2008, 92, 181–184. [Google Scholar] [CrossRef]
- Le Cabec, V.; Cols, C.; Maridonneau-Parini, I. Nonopsonic Phagocytosis of Zymosan and Mycobacterium kansasii by CR3 (CD11b/CD18) Involves Distinct Molecular Determinants and Is or Is Not Coupled with NADPH Oxidase Activation. Infect. Immun. 2000, 68, 4736–4745. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Borland, G.; Huang, J.; Mizukami, I.F.; Petty, H.R.; Todd, R.F.; Ross, G.D. Function of the Lectin Domain of Mac-1/Complement Receptor Type 3 (CD11b/CD18) in Regulating Neutrophil Adhesion. J. Immunol. 2002, 169, 6417–6426. [Google Scholar] [CrossRef] [Green Version]
- Tsikitis, V.L.; Morin, N.A.; Harrington, E.O.; Albina, J.E.; Reichner, J.S. The Lectin-Like Domain of Complement Receptor 3 Protects Endothelial Barrier Function from Activated Neutrophils. J. Immunol. 2004, 173, 1284–1291. [Google Scholar] [CrossRef]
- van Bruggen, R.; Drewniak, A.; Jansen, M.; van Houdt, M.; Roos, D.; Chapel, H.; Verhoeven, A.J.; Kuijpers, T.W. Complement Receptor 3, Not Dectin-1, Is the Major Receptor on Human Neutrophils for β-Glucan-Bearing Particles. Mol. Immunol. 2009, 47, 575–581. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune Recognition of Fungal β-Glucans: Immune Recognition of Fungal β-Glucans. Cell. Microbiol. 2005, 7, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Hiromasa, Y.; Takahashi, D.; VanderVelde, D.; Fabrick, J.A.; Kanost, M.R.; Krishnamoorthi, R. An Initial Event in the Insect Innate Immune Response: Structural and Biological Studies of Interactions between β-1,3-Glucan and the N-Terminal Domain of β-1,3-Glucan Recognition Protein. Biochemistry 2013, 52, 161–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahasi, K.; Ochiai, M.; Horiuchi, M.; Kumeta, H.; Ogura, K.; Ashida, M.; Inagaki, F. Solution Structure of the Silkworm ΒGRP/GNBP3 N-Terminal Domain Reveals the Mechanism for β-1,3-Glucan-Specific Recognition. Proc. Natl. Acad. Sci. USA 2009, 106, 11679–11684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishima, Y.; Quintin, J.; Aimanianda, V.; Kellenberger, C.; Coste, F.; Clavaud, C.; Hetru, C.; Hoffmann, J.A.; Latgé, J.-P.; Ferrandon, D.; et al. The N-Terminal Domain of Drosophila Gram-Negative Binding Protein 3 (GNBP3) Defines a Novel Family of Fungal Pattern Recognition Receptors. J. Biol. Chem. 2009, 284, 28687–28697. [Google Scholar] [CrossRef] [Green Version]
- Adachi, Y.; Ishii, M.; Kanno, T.; Tetsui, J.; Ishibashi, K.I.; Yamanaka, D.; Miura, N.; Ohno, N. N-Terminal (1→3)-β-d-Glucan Recognition Proteins from Insects Recognize the Difference in Ultra-Structures of (1→3)-β-d-Glucan. Int. J. Mol. Sci. 2019, 20, 3498. [Google Scholar] [CrossRef] [Green Version]
- Seki, N.; Muta, T.; Oda, T.; Iwaki, D.; Kuma, K.; Miyata, T.; Iwanaga, S. Horseshoe Crab (1,3)-Beta-D-Glucan-Sensitive Coagulation Factor, G. A Serine Protease Zymogen Heterodimer with Similarities to Beta-Glucan-Binding Proteins. J. Biol. Chem. 1994, 269, 1370–1374. [Google Scholar] [CrossRef]
- Takaki, Y.; Seki, N.; Kawabata, S.; Iwanaga, S.; Muta, T. Duplicated Binding Sites for (1→3)-β-d-Glucan in the Horseshoe Crab Coagulation Factor G. J. Biol. Chem. 2002, 277, 14281–14287. [Google Scholar] [CrossRef] [Green Version]
- Theel, E.S.; Doern, C.D. Point-Counterpoint: β-d-Glucan Testing Is Important for Diagnosis of Invasive Fungal Infections. J. Clin. Microbiol. 2013, 51, 3478–3483. [Google Scholar] [CrossRef] [Green Version]
- Saitô, H.; Yoshioka, Y.; Uehara, N.; Aketagawa, J.; Tanaka, S.; Shibata, Y. Relationship between Conformation and Biological Response for (1→3)-β-d-Glucans in the Activation of Coagulation Factor G from Limulus Amebocyte Lysate and Host-Mediated Antitumor Activity. Demonstration of Single-Helix Conformation as a Stimulant. Carbohydr. Res. 1991, 217, 181–190. [Google Scholar] [CrossRef]
- Duysburgh, C.; Van den Abbeele, P.; Kamil, A.; Fleige, L.; De Chavez, P.J.; Chu, Y.; Barton, W.; O’Sullivan, O.; Cotter, P.D.; Quilter, K.; et al. In Vitro–in Vivo Validation of Stimulatory Effect of Oat Ingredients on Lactobacilli. Pathogens 2021, 10, 235. [Google Scholar] [CrossRef]
- Fuller, R.; Butt, H.; Noakes, P.S.; Kenyon, J.; Yam, T.S.; Calder, P.C. Influence of Yeast-Derived 1,3/1,6 Glucopolysaccharide on Circulating Cytokines and Chemokines with Respect to Upper Respiratory Tract Infections. Nutrition 2012, 28, 665–669. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Y.; Lv, X.; Shen, X.; Ni, X.; Ding, K. Structure of a β-Glucan from Grifola Frondosa and Its Antitumor Effect by Activating Dectin-1/Syk/NF-ΚB Signaling. Glycoconj. J. 2012, 29, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Hao, Y.; Zhu, M.; Zhai, Y.; Yang, L.; Liu, Y.; Cheng, G. Incorporation of Laminarin-Based Hydrogel with Graphene Foam To Enhance the Toughness of Scaffold and Regulate the Stem Cell Behavior. ACS Biomater. Sci. Eng. 2019, 5, 5295–5304. [Google Scholar] [CrossRef] [PubMed]
- De Smet, R.; Demoor, T.; Verschuere, S.; Dullaers, M.; Ostroff, G.R.; Leclercq, G.; Allais, L.; Pilette, C.; Dierendonck, M.; De Geest, B.G.; et al. β-Glucan Microparticles Are Good Candidates for Mucosal Antigen Delivery in Oral Vaccination. J. Control. Release 2013, 172, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. β-Glucan Recognition by the Innate Immune System. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Riggi, S.J.; Di Luzio, N.R. Identification of a Reticuloendothelial Stimulating Agent in Zymosan. Am. J. Physiol. Leg. Content 1961, 200, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Soltanian, S.; Stuyven, E.; Cox, E.; Sorgeloos, P.; Bossier, P. Beta-Glucans as Immunostimulant in Vertebrates and Invertebrates. Crit. Rev. Microbiol. 2009, 35, 109–138. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. A New Receptor for β-Glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Taylor, P.R.; Tsoni, S.V.; Willment, J.A.; Dennehy, K.M.; Rosas, M.; Findon, H.; Haynes, K.; Steele, C.; Botto, M.; Gordon, S.; et al. Dectin-1 Is Required for β-Glucan Recognition and Control of Fungal Infection. Nat. Immunol. 2007, 8, 31–38. [Google Scholar] [CrossRef]
- Rice, P.J.; Kelley, J.L.; Kogan, G.; Ensley, H.E.; Kalbfleisch, J.H.; Browder, I.W.; Williams, D.L. Human Monocyte Scavenger Receptors Are Pattern Recognition Receptors for (1-->3)-Beta-D-Glucans. J. Leukoc. Biol. 2002, 72, 140–146. [Google Scholar]
- Zimmerman, J.W.; Lindermuth, J.; Fish, P.A.; Palace, G.P.; Stevenson, T.T.; DeMong, D.E. A Novel Carbohydrate-Glycosphingolipid Interaction between a β-(1–3)-Glucan Immunomodulator, PGG-Glucan, and Lactosylceramide of Human Leukocytes. J. Biol. Chem. 1998, 273, 22014–22020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.D.; Taylor, P.R.; Reid, D.M.; Willment, J.A.; Williams, D.L.; Martinez-Pomares, L.; Wong, S.Y.C.; Gordon, S. Dectin-1 Is A Major β-Glucan Receptor On Macrophages. J. Exp. Med. 2002, 196, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetvicka, V.; Thornton, B.P.; Ross, G.D. Soluble Beta-Glucan Polysaccharide Binding to the Lectin Site of Neutrophil or Natural Killer Cell Complement Receptor Type 3 (CD11b/CD18) Generates a Primed State of the Receptor Capable of Mediating Cytotoxicity of IC3b-Opsonized Target Cells. J. Clin. Investig. 1996, 98, 50–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Iwabuchi, K.; Nagaoka, I.; Adachi, Y.; Ohno, N.; Tamura, H.; Seyama, K.; Fukuchi, Y.; Nakayama, H.; Yoshizaki, F.; et al. Induction of Human Neutrophil Chemotaxis by Candida Albicans-Derived -1,6-Long Glycoside Side-Chain-Branched -Glucan. J. Leukoc. Biol. 2006, 80, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Bohn, J.A.; BeMiller, J.N. (1→3)-β-d-Glucans as Biological Response Modifiers: A Review of Structure-Functional Activity Relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Cobb, B.A.; Wang, Q.; Tzianabos, A.O.; Kasper, D.L. Polysaccharide Processing and Presentation by the MHCII Pathway. Cell 2004, 117, 677–687. [Google Scholar] [CrossRef]
- LeibundGut-Landmann, S.; Osorio, F.; Brown, G.D.; Reis e Sousa, C. Stimulation of Dendritic Cells via the Dectin-1/Syk Pathway Allows Priming of Cytotoxic T-Cell Responses. Blood 2008, 112, 4971–4980. [Google Scholar] [CrossRef] [Green Version]
- LeibundGut-Landmann, S.; Groß, O.; Robinson, M.J.; Osorio, F.; Slack, E.C.; Tsoni, S.V.; Schweighoffer, E.; Tybulewicz, V.; Brown, G.D.; Ruland, J.; et al. Syk- and CARD9-Dependent Coupling of Innate Immunity to the Induction of T Helper Cells That Produce Interleukin 17. Nat. Immunol. 2007, 8, 630–638. [Google Scholar] [CrossRef]
- Osorio, F.; LeibundGut-Landmann, S.; Lochner, M.; Lahl, K.; Sparwasser, T.; Eberl, G.; Reis e Sousa, C. DC Activated via Dectin-1 Convert Treg into IL-17 Producers. Eur. J. Immunol. 2008, 38, 3274–3281. [Google Scholar] [CrossRef]
- Dillon, S. Yeast Zymosan, a Stimulus for TLR2 and Dectin-1, Induces Regulatory Antigen-Presenting Cells and Immunological Tolerance. J. Clin. Investig. 2006, 116, 916–928. [Google Scholar] [CrossRef]
- Karumuthil-Melethil, S.; Perez, N.; Li, R.; Vasu, C. Induction of Innate Immune Response through TLR2 and Dectin 1 Prevents Type 1 Diabetes. J. Immunol. 2008, 181, 8323–8334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netea, M.G.; Quintin, J.; van der Meer, J.W.M. Trained Immunity: A Memory for Innate Host Defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintin, J. Fungal Mediated Innate Immune Memory, What Have We Learned? Semin. Cell Dev. Biol. 2019, 89, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.-J.; Wijmenga, C.; et al. Candida Albicans Infection Affords Protection against Reinfection via Functional Reprogramming of Monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Nakao, I.; Uchino, H.; Orita, K.; Kaido, I.; Kimura, T.; Goto, Y.; Kondo, T.; Takino, T.; Taguchi, T.; Nakajima, T.; et al. Clinical evaluation of schizophyllan (SPG) in advanced gastric cancer-a randomized comparative study by an envelope method. Gan Kagaku Ryoho 1983, 10, 1146–1159. [Google Scholar]
- Kodama, N.; Komuta, K.; Nanba, H. Effect of Maitake (Grifola frondosa) D-Fraction on the Activation of NK Cells in Cancer Patients. J. Med. Food 2003, 6, 371–377. [Google Scholar] [CrossRef]
- Standish, L.J.; Wenner, C.A.; Sweet, E.S.; Bridge, C.; Nelson, A.; Martzen, M.; Novack, J.; Torkelson, C. Trametes Versicolor Mushroom Immune Therapy in Breast Cancer. J. Soc. Integr. Oncol. 2008, 6, 122–128. [Google Scholar]
- Kashimoto, N.; Ishii, S.; Myojin, Y.; Ushijima, M.; Hayama, M.; Watanabe, H. A Water-Soluble Extract from Cultured Medium of Ganoderma Lucidum (Reishi) Mycelia Attenuates the Small Intestinal Injury Induced by Anti-Cancer Drugs. Oncol. Lett. 2010, 1, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Yoshida, R.; Kanada, Y.; Fukuda, Y.; Yagyu, T.; Inagaki, K.; Kondo, T.; Kurita, N.; Suzuki, M.; Kanayama, N.; et al. Suppressing Effects of Daily Oral Supplementation of Beta-Glucan Extracted from Agaricus Blazei Murill on Spontaneous and Peritoneal Disseminated Metastasis in Mouse Model. J. Cancer Res. Clin. Oncol. 2005, 131, 527–538. [Google Scholar] [CrossRef]
- Mao, G.-H.; Zhang, Z.-H.; Fei, F.; Ding, Y.-Y.; Zhang, W.-J.; Chen, H.; Ali, S.S.; Zhao, T.; Feng, W.-W.; Wu, X.-Y.; et al. Effect of Grifola Frondosa Polysaccharide on Anti-Tumor Activity in Combination with 5-Fu in Heps-Bearing Mice. Int. J. Biol. Macromol. 2019, 121, 930–935. [Google Scholar] [CrossRef]
- Shimizu, Y.; Hasumi, K.; Masubuchi, K. Augmenting Effect of Sizofiran on the Immunofunction of Regional Lymph Nodes in Cervical Cancer. Cancer 1992, 69, 1184–1194. [Google Scholar] [PubMed]
- Meng, X.; Liang, H.; Luo, L. Antitumor Polysaccharides from Mushrooms: A Review on the Structural Characteristics, Antitumor Mechanisms and Immunomodulating Activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Teplyakova, T.V.; Shintyapina, A.B.; Korolenko, T.A. Effects of Medicinal Fungi-Derived β-Glucan on Tumor Progression. JoF 2021, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Tangen, J.-M.; Mahmood, F.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Nentwich, I.; Therkelsen, S.P.; Tjønnfjord, G.E.; Johnson, E. Antitumor, Anti-Inflammatory and Antiallergic Effects of Agaricus Blazei Mushroom Extract and the Related Medicinal Basidiomycetes Mushrooms, Hericium Erinaceus and Grifola Frondosa: A Review of Preclinical and Clinical Studies. Nutrients 2020, 12, 1339. [Google Scholar] [CrossRef] [PubMed]
- Ina, K.; Kataoka, T.; Ando, T. The Use of Lentinan for Treating Gastric Cancer. ACAMC 2013, 13, 681–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonelli, M.; Donelli, D.; Firenzuoli, F. Lentinan for Integrative Cancer Treatment: An Umbrella Review. Proceedings 2020, 83, 8733. [Google Scholar] [CrossRef]
- Taguchi, T. Effects of lentinan in advanced or recurrent cases of gastric, colorectal, and breast cancer. Gan Kagaku Ryoho 1983, 10, 387–393. [Google Scholar]
- Inoue, M.; Tanaka, Y.; Sugita, N.; Yamasaki, M.; Yamanaka, T.; Minagawa, J.; Nakamuro, K.; Tani, T.; Okudaira, Y.; Karita, T.; et al. Improvement of Long-Term Prognosis in Patients with Ovarian Cancers by Adjuvant Sizofiran Immunotherapy: A Prospective Randomized Controlled Study. Biotherapy 1993, 6, 13–18. [Google Scholar] [CrossRef]
- Weitberg, A.B. A Phase I/II Trial of Beta-(1,3)/(1,6) D-Glucan in the Treatment of Patients with Advanced Malignancies Receiving Chemotherapy. J. Exp. Clin. Cancer Res. 2008, 27, 40. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, H.; Shimura, T.; Mizoshita, T.; Kubota, E.; Mori, Y.; Mizushima, T.; Wada, T.; Ogasawara, N.; Tanida, S.; Sasaki, M.; et al. Lentinan with S-1 and Paclitaxel for Gastric Cancer Chemotherapy Improve Patient Quality of Life. Hepatogastroenterology 2009, 56, 547–550. [Google Scholar]
- Gu, X.-M. Combination Therapy with Lentinan Improves Outcomes in Patients with Esophageal Carcinoma. Mol. Med. Rep. 2011, 5, 745–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, Y.; Miyahara, E.; Hihara, J. Efficacy and Safety of Orally Administered Lentinula Edodes Mycelia Extract for Patients Undergoing Cancer Chemotherapy: A Pilot Study. Am. J. Chin. Med. 2011, 39, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, Y.; Maeda, N.; Yamamoto, S.; Yoshino, S.; Oka, M. Evaluation of Host Quality of Life and Immune Function in Breast Cancer Patients Treated with Combination of Adjuvant Chemotherapy and Oral Administration of Lentinula Edodes Mycelia Extract. OTT 2013, 6, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, W.-S.; Kim, D.-J.; Chae, G.-T.; Lee, J.-M.; Bae, S.-M.; Sin, J.-I.; Kim, Y.-W.; Namkoong, S.-E.; Lee, I.P. Natural Killer Cell Activity and Quality of Life Were Improved by Consumption of a Mushroom Extract, Agaricus Blazei Murill Kyowa, in Gynecological Cancer Patients Undergoing Chemotherapy. Int. J. Gynecol. Cancer 2004, 14, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Krizan, J.; Sima, P.; Stakheev, D.; Caja, F.; Rajsiglova, L.; Horak, V.; Saieh, M. Immunostimulatory Properties and Antitumor Activities of Glucans. Int. J. Oncol. 2013, 43, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Ezekowitz, R.A.; Sim, R.B.; Hill, M.; Gordon, S. Local Opsonization by Secreted Macrophage Complement Components. Role of Receptors for Complement in Uptake of Zymosan. J. Exp. Med. 1984, 159, 244–260. [Google Scholar] [CrossRef]
- Tani, M.; Tanimura, H.; Yamaue, H.; Tsunoda, T.; Iwahashi, M.; Noguchi, K.; Tamai, M.; Hotta, T.; Mizobata, S. Augmentation of Lymphokine-Activated Killer Cell Activity by Lentinan. Anticancer Res. 1993, 13, 1773–1776. [Google Scholar]
- Qi, C.; Cai, Y.; Gunn, L.; Ding, C.; Li, B.; Kloecker, G.; Qian, K.; Vasilakos, J.; Saijo, S.; Iwakura, Y.; et al. Differential Pathways Regulating Innate and Adaptive Antitumor Immune Responses by Particulate and Soluble Yeast-Derived β-Glucans. Blood 2011, 117, 6825–6836. [Google Scholar] [CrossRef]
- Gelderman, K.A.; Tomlinson, S.; Ross, G.D.; Gorter, A. Complement Function in MAb-Mediated Cancer Immunotherapy. Trends Immunol. 2004, 25, 158–164. [Google Scholar] [CrossRef]
- Hong, F.; Yan, J.; Baran, J.T.; Allendorf, D.J.; Hansen, R.D.; Ostroff, G.R.; Xing, P.X.; Cheung, N.-K.V.; Ross, G.D. Mechanism by Which Orally Administered β-1,3-Glucans Enhance the Tumoricidal Activity of Antitumor Monoclonal Antibodies in Murine Tumor Models. J. Immunol. 2004, 173, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Chihara, G.; Maeda, Y.; Hamuro, J.; Sasaki, T.; Fukuoka, F. Inhibition of Mouse Sarcoma 180 by Polysaccharides from Lentinus Edodes (Berk.) Sing. Nature 1969, 222, 687–688. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Allendorf, D.J.; Hansen, R.; Marroquin, J.; Cramer, D.E.; Harris, C.L.; Yan, J. Combined Yeast β-Glucan and Antitumor Monoclonal Antibody Therapy Requires C5a-Mediated Neutrophil Chemotaxis via Regulation of Decay-Accelerating Factor CD55. Cancer Res. 2007, 67, 7421–7430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modak, S.; Koehne, G.; Vickers, A.; O’Reilly, R.J.; Cheung, N.-K.V. Rituximab Therapy of Lymphoma Is Enhanced by Orally Administered (1→3),(1→4)-d-β-Glucan. Leuk. Res. 2005, 29, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Salvador, C.; Li, B.; Hansen, R.; Cramer, D.E.; Kong, M.; Yan, J. Yeast-Derived β-Glucan Augments the Therapeutic Efficacy Mediated by Anti–Vascular Endothelial Growth Factor Monoclonal Antibody in Human Carcinoma Xenograft Models. Clin. Cancer Res. 2008, 14, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Kim, J.A.; Huang, A.Y.-C. Optimizing Tumor Microenvironment for Cancer Immunotherapy: β-Glucan-Based Nanoparticles. Front. Immunol. 2018, 9, 341. [Google Scholar] [CrossRef] [Green Version]
- Zent, C.S.; Call, T.G.; Bowen, D.A.; Conte, M.J.; LaPlant, B.R.; Witzig, T.E.; Ansell, S.M.; Weiner, G.J. Early Treatment of High Risk Chronic Lymphocytic Leukemia with Alemtuzumab, Rituximab and Poly-(1-6)-Beta-Glucotriosyl-(1-3)- Beta-Glucopyranose Beta-Glucan Is Well Tolerated and Achieves High Complete Remission Rates. Leuk. Lymphoma 2015, 56, 2373–2378. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Sadjadian, P.; Kollmeier, J.; Lowe, J.; Mattson, P.; Trout, J.R.; Gargano, M.; Patchen, M.L.; Walsh, R.; Beliveau, M.; et al. A Randomized, Open-Label, Multicenter, Phase II Study Evaluating the Efficacy and Safety of BTH1677 (1,3–1,6 Beta Glucan; Imprime PGG) in Combination with Cetuximab and Chemotherapy in Patients with Advanced Non-Small Cell Lung Cancer. Investig. New Drugs 2017, 35, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Chan, A.S.H.; Jonas, A.B.; Kangas, T.; Ottoson, N.R.; Graff, J.R.; Bose, N. Imprime PGG, a Yeast β-Glucan PAMP Elicits a Coordinated Immune Response in Combination with Anti-PD1 Antibody. J. Immunol. 2016, 196, 214–216. [Google Scholar]
- Uhlik, M.T.; Bose, N.; Cox, J.; Mattson, P.; Gargano, M.; O’Day, S.; Borges, V.; Chmielowski, B.; Rao, R.; Abu-Khalaf, M.; et al. Abstract PD1-02: Response and Clinical Benefit Assessment of the Combination of the Dectin-1 Agonist Imprime PGG and Anti-PD-1 Pembrolizumab in Chemotherapy-Resistant Metastatic Triple Negative Breast Cancer (TNBC). Cancer Res. 2020, 80, PD1-02. [Google Scholar] [CrossRef]
- Cognigni, V.; Ranallo, N.; Tronconi, F.; Morgese, F.; Berardi, R. Potential Benefit of β-Glucans as Adjuvant Therapy in Immuno-Oncology: A Review. Explor. Target. Anti Tumor Ther. 2021, 2, 122–138. [Google Scholar] [CrossRef]
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Kramer, K.; Ragupathi, G.; Cheung, N.-K.V. Phase I Trial of a Bivalent Gangliosides Vaccine in Combination with β-Glucan for High-Risk Neuroblastoma in Second or Later Remission. Clin. Cancer Res. 2014, 20, 1375–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasrollahi, Z.; Mohammadi, S.R.; Mollarazi, E.; Yadegari, M.H.; Hassan, Z.M.; Talaei, F.; Dinarvand, R.; Akbari, H.; Atyabi, F. Functionalized Nanoscale β-1,3-Glucan to Improve Her2+ Breast Cancer Therapy: In Vitro and in Vivo Study. J. Control. Release 2015, 202, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Home—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 5 August 2021).
- Richter, J.; Svozil, V.; Král, V.; Rajnohová Dobiášová, L.; Vetvicka, V. β-Glucan Affects Mucosal Immunity in Children with Chronic Respiratory Problems under Physical Stress: Clinical Trials. Ann. Transl. Med. 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Svozil, V.; Král, V.; Rajnohová Dobiášová, L.; Stiborová, I.; Vetvicka, V. Clinical Trials of Yeast-Derived β-(1,3) Glucan in Children: Effects on Innate Immunity. Ann. Transl. Med. 2014, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. Β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2021, 65, 1901071. [Google Scholar] [CrossRef] [PubMed]
- Graubaum, H.-J.; Busch, R.; Stier, H.; Gruenwald, J. A Double-Blind, Randomized, Placebo-Controlled Nutritional Study Using an Insoluble Yeast Beta-Glucan to Improve the Immune Defense System. FNS 2012, 03, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Fuller, R.; Moore, M.V.; Lewith, G.; Stuart, B.L.; Ormiston, R.V.; Fisk, H.L.; Noakes, P.S.; Calder, P.C. Yeast-Derived β-1,3/1,6 Glucan, Upper Respiratory Tract Infection and Innate Immunity in Older Adults. Nutrition 2017, 39–40, 30–35. [Google Scholar] [CrossRef]
- Talbott, S.M.; Talbott, J.A.; Talbott, T.L.; Dingler, E. β-Glucan Supplementation, Allergy Symptoms, and Quality of Life in Self-described Ragweed Allergy Sufferers. Food Sci. Nutr. 2013, 1, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Therkelsen, S.P.; Hetland, G.; Lyberg, T.; Lygren, I.; Johnson, E. Cytokine Levels After Consumption of a Medicinal Agaricus Blazei Murill-Based Mushroom Extract, AndoSanTM, in Patients with Crohn’s Disease and Ulcerative Colitis in a Randomized Single-Blinded Placebo-Controlled Study. Scand. J. Immunol. 2016, 84, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Therkelsen, S.P.; Hetland, G.; Lyberg, T.; Lygren, I.; Johnson, E. Effect of a Medicinal Agaricus Blazei Murill-Based Mushroom Extract, AndoSanTM, on Symptoms, Fatigue and Quality of Life in Patients with Ulcerative Colitis in a Randomized Single-Blinded Placebo Controlled Study. PLoS ONE 2016, 11, e0150191. [Google Scholar] [CrossRef]
- Chaudhari, V.; Buttar, H.S.; Bagwe-Parab, S.; Tuli, H.S.; Vora, A.; Kaur, G. Therapeutic and Industrial Applications of Curdlan With Overview on Its Recent Patents. Front. Nutr. 2021, 8, 646988. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, S.D.V.; Cordeiro, S.L.; Cavalcanti, J.E.C.; Melchuna, K.M.; da Silva Lima, A.M.; Filho, I.A.; Medeiros, A.C.; Rocha, K.B.F.; Oliveira, E.M.; Faria, E.D.B.; et al. Effects of Purified Saccharomyces Cerevisiae (1→3)-β-Glucan on Venous Ulcer Healing. Int. J. Mol. Sci. 2012, 13, 8142–8158. [Google Scholar] [CrossRef] [PubMed]
- Zykova, S.N.; Balandina, K.A.; Vorokhobina, N.V.; Kuznetsova, A.V.; Engstad, R.; Zykova, T.A. Macrophage Stimulating Agent Soluble Yeast Β-1,3/1,6-glucan as a Topical Treatment of Diabetic Foot and Leg Ulcers: A Randomized, Double Blind, Placebo-controlled Phase II Study. J. Diabetes Investig. 2014, 5, 392–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration (FDA). Health Claims: Soluble Fibre from Certain Foods and Coronary Heart Disease (CHD); FDA: Silver Spring, MA, USA, 2005; pp. 17162–76150.
- Hsu, M.-J.; Lee, S.-S.; Lin, W.-W. Polysaccharide Purified from Ganoderma Lucidum Inhibits Spontaneous and Fas-Mediated Apoptosis in Human Neutrophils through Activation of the Phosphatidylinositol 3 Kinase/Akt Signaling Pathway. J. Leukoc. Biol. 2002, 72, 207–216. [Google Scholar]
- Zurbau, A.; Noronha, J.C.; Khan, T.A.; Sievenpiper, J.L.; Wolever, T.M.S. The Effect of Oat β-Glucan on Postprandial Blood Glucose and Insulin Responses: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2021, 75, 1540–1554. [Google Scholar] [CrossRef]
- McRorie, J.W.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Joyce, S.A.; Kamil, A.; Fleige, L.; Gahan, C.G.M. The Cholesterol-Lowering Effect of Oats and Oat Beta Glucan: Modes of Action and Potential Role of Bile Acids and the Microbiome. Front. Nutr. 2019, 6, 171. [Google Scholar] [CrossRef]
- Tiwari, U.; Cummins, E. Meta-Analysis of the Effect of β-Glucan Intake on Blood Cholesterol and Glucose Levels. Nutrition 2011, 27, 1008–1016. [Google Scholar] [CrossRef]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M. Cholesterol-Lowering Effects of Oat β-Glucan: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The Effect of Oat β -Glucan on LDL-Cholesterol, Non-HDL-Cholesterol and ApoB for CVD Risk Reduction: A Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef] [Green Version]
- Ripsin, C.M.; Keenan, J.M.; Jacobs, D.R.; Elmer, P.J.; Welch, R.R.; Van Horn, L.; Liu, K.; Turnbull, W.H.; Thye, F.W.; Kestin, M. Oat Products and Lipid Lowering. A Meta-Analysis. JAMA 1992, 267, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheung, P.C.K. Application of Natural β -Glucans as Biocompatible Functional Nanomaterials. Food Sci. Hum. Wellness 2019, 8, 315–319. [Google Scholar] [CrossRef]
- Zhou, A.L.; Hergert, N.; Rompato, G.; Lefevre, M. Whole Grain Oats Improve Insulin Sensitivity and Plasma Cholesterol Profile and Modify Gut Microbiota Composition in C57BL/6J Mice. J. Nutr. 2015, 145, 222–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsou, E.K.; Panopoulou, N.; Turunen, K.; Spiliotis, V.; Kyriacou, A. Prebiotic Potential of Barley Derived β-Glucan at Low Intake Levels: A Randomised, Double-Blinded, Placebo-Controlled Clinical Study. Food Res. Int. 2010, 43, 1086–1092. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Yuan, R. Applications of Natural Polymer-Based Hydrogels in the Food Industry. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–410. ISBN 978-0-12-816421-1. [Google Scholar]
- Aljewicz, M.; Florczuk, A.; Dąbrowska, A. Influence of β-Glucan Structures and Contents on the Functional Properties of Low-Fat Ice Cream During Storage. Pol. J. Food Nutr. Sci. 2020, 70, 233–240. [Google Scholar] [CrossRef]
- Xin, Y.; Lee, J.Y.; Kim, J.; Kim, Y. Effect of Curdlan on Textural and Cooking Qualities of Noodles Made with Tofu. J. Food Process. Preserv. 2018, 42, e13661. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, C.; Xia, X.; Liu, Q.; Kong, B. Enhancement of the Textural and Gel Properties of Frankfurters by Adding Thermo-reversible or Thermo-irreversible Curdlan Gels. J. Food Sci. 2019, 84, 1068–1077. [Google Scholar] [CrossRef]
- Szpicer, A.; Onopiuk, A.; Półtorak, A.; Wierzbicka, A. The Influence of Oat β-Glucan Content on the Physicochemical and Sensory Properties of Low-Fat Beef Burgers. CyTA J. Food 2020, 18, 315–327. [Google Scholar] [CrossRef]
- Qian, Y.; Bian, L.; Wang, K.; Chia, W.Y.; Khoo, K.S.; Zhang, C.; Chew, K.W. Preparation and Characterization of Curdlan/Nanocellulose Blended Film and Its Application to Chilled Meat Preservation. Chemosphere 2021, 266, 128948. [Google Scholar] [CrossRef]
- Laroche, C.; Michaud, P. New Developments and Prospective Applications for β (1,3) Glucans. BIOT 2007, 1, 59–73. [Google Scholar] [CrossRef]
- Lee, C.H.; Chin, K.B. Changes in Physicochemical Properties of Pork Myofibrillar Protein Combined with Corn Starch and Application to Low-fat Pork Patties. Int. J. Food Sci. Technol. 2020, 55, 157–164. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.M.; Bae, I.Y.; Park, H.-G.; Gyu Lee, H.; Lee, S. (1-3)(1-6)-β-Glucan-Enriched Materials from Lentinus Edodes Mushroom as a High-Fibre and Low-Calorie Flour Substitute for Baked Foods: β-Glucan-Enriched Materials from Lentinus Edodes Mushroom. J. Sci. Food Agric. 2011, 91, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgos, V.; Biliaderis, C.; Kiosseoglou, V.; Doxastakis, G. Stability and Rheology of Egg-Yolk-Stabilized Concentrated Emulsions Containing Cereal β-Glucans of Varying Molecular Size. Food Hydrocoll. 2004, 18, 987–998. [Google Scholar] [CrossRef]
- Sharafbafi, N.; Tosh, S.M.; Alexander, M.; Corredig, M. Phase Behaviour, Rheological Properties, and Microstructure of Oat β-Glucan-Milk Mixtures. Food Hydrocoll. 2014, 41, 274–280. [Google Scholar] [CrossRef]
- Rinaldi, L.; Rioux, L.-E.; Britten, M.; Turgeon, S.L. In Vitro Bioaccessibility of Peptides and Amino Acids from Yogurt Made with Starch, Pectin, or β-Glucan. Int. Dairy J. 2015, 46, 39–45. [Google Scholar] [CrossRef]
- Tudorica, C.M.; Jones, T.E.R.; Kuri, V.; Brennan, C.S. The Effects of Refined Barleyβ-Glucan on the Physico-Structural Properties of Low-Fat Dairy Products: Curd Yield, Microstructure, Texture and Rheology. J. Sci. Food Agric. 2004, 84, 1159–1169. [Google Scholar] [CrossRef]
- Ozanne, H.; Toumi, H.; Roubinet, B.; Landemarre, L.; Lespessailles, E.; Daniellou, R.; Cesaro, A. Laminarin Effects, a β-(1,3)-Glucan, on Skin Cell Inflammation and Oxidation. Cosmetics 2020, 7, 66. [Google Scholar] [CrossRef]
- Zulli, F.; Suter, F.; Biltz, H.; Nissen, H.P. Improving Skin Function with CM-Glucan, a Biological Response Modifier from Yeast. Int. J. Cosmet. Sci. 1998, 20, 79–86. [Google Scholar] [CrossRef]
- Anwar, M.I.; Muhammad, F.; Awais, M.M.; Akhtar, M. A Review of β-Glucans as a Growth Promoter and Antibiotic Alternative against Enteric Pathogens in Poultry. Worlds Poult. Sci. J. 2017, 73, 651–661. [Google Scholar] [CrossRef]
- Meena, D.K.; Das, P.; Kumar, S.; Mandal, S.C.; Prusty, A.K.; Singh, S.K.; Akhtar, M.S.; Behera, B.K.; Kumar, K.; Pal, A.K.; et al. Beta-Glucan: An Ideal Immunostimulant in Aquaculture (a Review). Fish Physiol. Biochem. 2013, 39, 431–457. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P. The Effects of β-Glucan on Pig Growth and Immunity. TOBIOCJ 2014, 1, 89–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, V.K.; Farnell, M.B.; Ferro, P.J.; Swaggerty, C.L.; Bahl, A.; Kogut, M.H. Purified β-Glucan as an Abiotic Feed Additive up-Regulates the Innate Immune Response in Immature Chickens against Salmonella Enterica Serovar Enteritidis. Int. J. Food Microbiol. 2005, 98, 309–318. [Google Scholar] [CrossRef]
- Huff, G.R.; Huff, W.E.; Farnell, M.B.; Rath, N.C.; Solis de los Santos, F.; Donoghue, A.M. Bacterial Clearance, Heterophil Function, and Hematological Parameters of Transport-Stressed Turkey Poults Supplemented with Dietary Yeast Extract. Poult. Sci. 2010, 89, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Guo, Y.; Wang, Z. β-1,3/1,6-Glucan Alleviated Intestinal Mucosal Barrier Impairment of Broiler Chickens Challenged with Salmonella Enterica Serovar Typhimurium. Poult. Sci. 2013, 92, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.M.; Sumners, L.H.; Kim, S.; McElroy, A.P.; Bedford, M.R.; Dalloul, R.A. Immune Responses to Dietary β-Glucan in Broiler Chicks during an Eimeria Challenge. Poult. Sci. 2010, 89, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Eicher, S.D.; McKee, C.A.; Carroll, J.A.; Pajor, E.A. Supplemental Vitamin C and Yeast Cell Wall β-Glucan as Growth Enhancers in Newborn Pigs and as Immunomodulators after an Endotoxin Challenge after Weaning1. J. Anim. Sci. 2006, 84, 2352–2360. [Google Scholar] [CrossRef]
- Martins, C.R.; Custódio, C.A.; Mano, J.F. Multifunctional Laminarin Microparticles for Cell Adhesion and Expansion. Carbohydr. Polym. 2018, 202, 91–98. [Google Scholar] [CrossRef]
- Salgado, M.; Rodríguez-Rojo, S.; Reis, R.L.; Cocero, M.J.; Duarte, A.R.C. Preparation of Barley and Yeast β-Glucan Scaffolds by Hydrogel Foaming: Evaluation of Dexamethasone Release. J. Supercrit. Fluids 2017, 127, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xu, Z.; Wu, Y.; Li, H.; Liu, W. A High Strength Semi-Degradable Polysaccharide-Based Hybrid Hydrogel for Promoting Cell Adhesion and Proliferation. J. Mater. Sci. 2018, 53, 6302–6312. [Google Scholar] [CrossRef]
- Zargarzadeh, M.; Silva, A.S.; Nunes, C.; Coimbra, M.A.; Custódio, C.A.; Mano, J.F. Self-Glucose Feeding Hydrogels by Enzyme Empowered Degradation for 3D Cell Culture. Mater. Horiz. 2022, 9, 694–707. [Google Scholar] [CrossRef]
- Choi, Y.; Jang, J.; Koo, H.-J.; Tanaka, M.; Lee, K.-H.; Choi, J. Alginate-Chitosan Hydrogel Patch with Beta-Glucan Nanoemulsion for Antibacterial Applications. Biotechnol. Bioproc. E 2021, 26, 71–77. [Google Scholar] [CrossRef]
- Fujiwara, N.; Izumi, H.; Morimoto, Y.; Sakurai, K.; Mochizuki, S. Complex Consisting of Antisense DNA and β-Glucan Promotes Internalization into Cell through Dectin-1 and Hybridizes with Target MRNA in Cytosol. Cancer Gene Ther. 2019, 26, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Morishita, H.; Sakurai, K. Complex Consisting of β-Glucan and Antigenic Peptides with Cleavage Site for Glutathione and Aminopeptidases Induces Potent Cytotoxic T Lymphocytes. Bioconjugate Chem. 2017, 28, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Srivastava, A.K.; Dev, A.; Kaundal, B.; Choudhury, S.R.; Karmakar, S. 1, 3β-Glucan Anchored, Paclitaxel Loaded Chitosan Nanocarrier Endows Enhanced Hemocompatibility with Efficient Anti-Glioblastoma Stem Cells Therapy. Carbohydr. Polym. 2018, 180, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zou, S.; Wang, K.; Liang, R.; Fan, X.; Wang, B.; Liu, M.; Fang, L.; Liu, W.; Wu, Z.; et al. Synthesis, Characterization and In Vitro Evaluation of Dual PH/Redox Sensitive Marine Laminarin-Based Nanomedicine Carrier Biomaterial for Cancer Therapy. J. Biomed. Nanotechnol. 2018, 14, 1568–1577. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P. Β-glucan as a New Tool in Vaccine Development. Scand. J. Immunol. 2020, 91, e12833. [Google Scholar] [CrossRef]
- Soares, E.; Cordeiro, R.; Faneca, H.; Borges, O. Polymeric Nanoengineered HBsAg DNA Vaccine Designed in Combination with Β-glucan. Int. J. Biol. Macromol. 2019, 122, 930–939. [Google Scholar] [CrossRef]
- Mirza, Z.; Soto, E.R.; Dikengil, F.; Levitz, S.M.; Ostroff, G.R. Beta-Glucan Particles as Vaccine Adjuvant Carriers. In Vaccines for Invasive Fungal Infections; Kalkum, M., Semis, M., Eds.; Springer: New York, NY, USA, 2017; Volume 1625, pp. 143–157. ISBN 978-1-4939-7103-9. [Google Scholar]
- Labourel, A.; Jam, M.; Jeudy, A.; Hehemann, J.-H.; Czjzek, M.; Michel, G. The β-Glucanase ZgLamA from Zobellia Galactanivorans Evolved a Bent Active Site Adapted for Efficient Degradation of Algal Laminarin. J. Biol. Chem. 2014, 289, 2027–2042. [Google Scholar] [CrossRef] [Green Version]
- Ebhodaghe, S.O.; Imanah, O.E.; Ndibe, H. Biofuels from Microalgae Biomass: A Review of Conversion Processes and Procedures. Arab. J. Chem. 2022, 15, 103591. [Google Scholar] [CrossRef]
- Gilani, S.; Gracia, M.I.; Barnard, L.; Dersjant-Li, Y.; Millán, C.; Gibbs, K. Effects of a Xylanase and Beta-Glucanase Enzyme Combination on Growth Performance of Broilers Fed Maize-Soybean Meal-Based Diets. J. Appl. Anim. Nutr. 2021, 9, 77–83. [Google Scholar] [CrossRef]
- Józefiak, D.; Rutkowski, A.; Jensen, B.B.; Engberg, R.M. The Effect of β-Glucanase Supplementation of Barley- and Oat-Based Diets on Growth Performance and Fermentation in Broiler Chicken Gastrointestinal Tract. Br. Poult. Sci. 2006, 47, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang, C.C.; Johansen, C.; Christgau, S.; Adler-Nissen, J. Antimicrobial Enzymes: Applications and Future Potential in the Food Industry. Trends Food Sci. Technol. 1995, 6, 390–396. [Google Scholar] [CrossRef]
- Ramos, O.S.; Malcata, F.X. Food-Grade Enzymes. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 555–569. ISBN 978-0-08-088504-9. [Google Scholar]
- Dimopoulou, M.; Lonvaud-Funel, A.; Dols-Lafargue, M. Polysaccharide Production by Grapes Must and Wine Microorganisms. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 293–314. ISBN 978-3-319-60020-8. [Google Scholar]
- Hüfner, E.; Haßelbeck, G. Application of Microbial Enzymes During Winemaking. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 635–658. ISBN 978-3-319-60020-8. [Google Scholar]
- Blättel, V.; Larisika, M.; Pfeiffer, P.; Nowak, C.; Eich, A.; Eckelt, J.; König, H. β-1,3-Glucanase from Delftia Tsuruhatensis Strain MV01 and Its Potential Application in Vinification. Appl. Environ. Microbiol. 2011, 77, 983–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces Cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 1435. [Google Scholar] [CrossRef] [PubMed]
- Gacto, M.; Vicente-Soler, J.; Cansado, J.; Villa, T.G. Characterization of an Extracellular Enzyme System Produced by Micromonospora Chalcea with Lytic Activity on Yeast Cells. J. Appl. Microbiol. 2000, 88, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Miyanishi, N.; Inaba, Y.; Okuma, H.; Imada, C.; Watanabe, E. Amperometric Determination of Laminarin Using Immobilized β-1,3-Glucanase. Biosens. Bioelectron. 2004, 19, 557–562. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]




| β-1,3-glucanase Class | Domain | GH Families | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 5 | 8 | 9 | 16 | 17 | 51 | 55 | 64 | 81 | 128 | 152 | 157 | 158 | ||
| EC 3.2.1.58 | Bacteria | 2 | 2 | ||||||||||||
| Fungi | 15 | 13 | |||||||||||||
| Plants | 2 | ||||||||||||||
| EC 3.2.1.39 | Archaea | 1 | |||||||||||||
| Bacteria | 1 | 27 | 3 | 1 | 5 | 3 | 3 | 1 | 3 | ||||||
| Fungi | 2 | 1 | 2 | 10 | 1 | 1 | |||||||||
| Plants | 28 | 1 | |||||||||||||
| Animals | 14 | ||||||||||||||
| EC 3.2.1.6 | Bacteria | 3 | 2 | 2 | 11 | 1 | |||||||||
| Fungi | 6 | ||||||||||||||
| Unclassified | 3 | ||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caseiro, C.; Dias, J.N.R.; de Andrade Fontes, C.M.G.; Bule, P. From Cancer Therapy to Winemaking: The Molecular Structure and Applications of β-Glucans and β-1, 3-Glucanases. Int. J. Mol. Sci. 2022, 23, 3156. https://doi.org/10.3390/ijms23063156
Caseiro C, Dias JNR, de Andrade Fontes CMG, Bule P. From Cancer Therapy to Winemaking: The Molecular Structure and Applications of β-Glucans and β-1, 3-Glucanases. International Journal of Molecular Sciences. 2022; 23(6):3156. https://doi.org/10.3390/ijms23063156
Chicago/Turabian StyleCaseiro, Catarina, Joana Nunes Ribeiro Dias, Carlos Mendes Godinho de Andrade Fontes, and Pedro Bule. 2022. "From Cancer Therapy to Winemaking: The Molecular Structure and Applications of β-Glucans and β-1, 3-Glucanases" International Journal of Molecular Sciences 23, no. 6: 3156. https://doi.org/10.3390/ijms23063156
APA StyleCaseiro, C., Dias, J. N. R., de Andrade Fontes, C. M. G., & Bule, P. (2022). From Cancer Therapy to Winemaking: The Molecular Structure and Applications of β-Glucans and β-1, 3-Glucanases. International Journal of Molecular Sciences, 23(6), 3156. https://doi.org/10.3390/ijms23063156







