Synthesis, In Silico, and Biological Evaluation of a Borinic Tryptophan-Derivative That Induces Melatonin-like Amelioration of Cognitive Deficit in Male Rat
Abstract
:1. Introduction
2. Results and Discussion
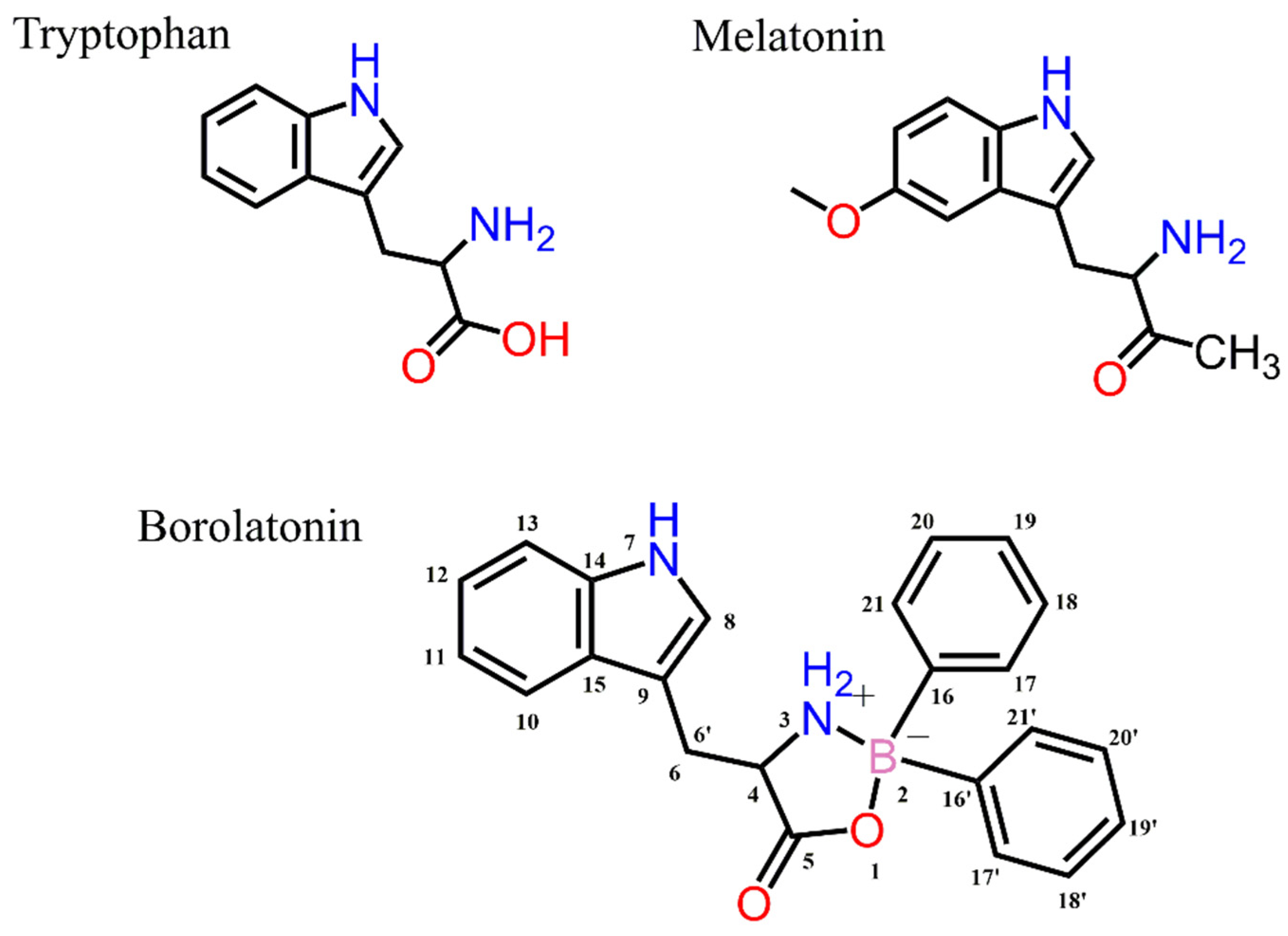
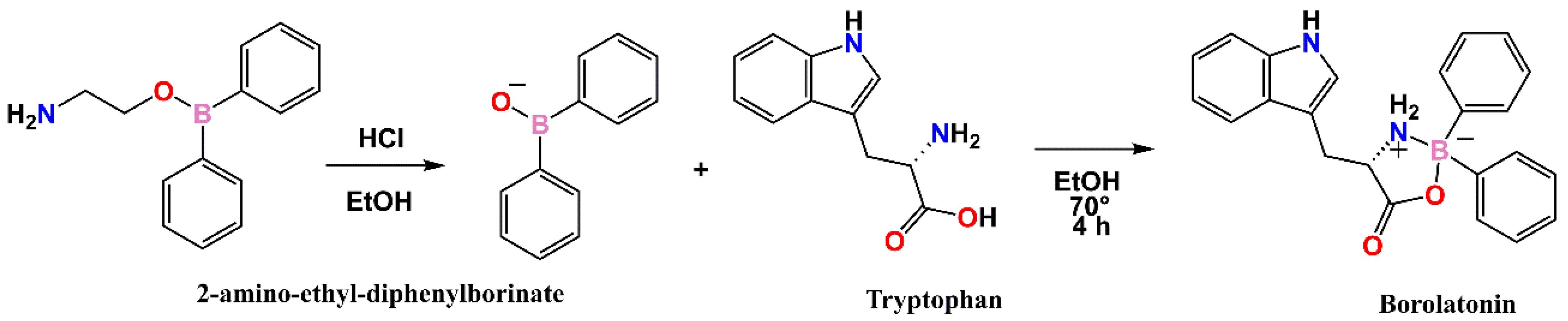
2.1. Chemistry
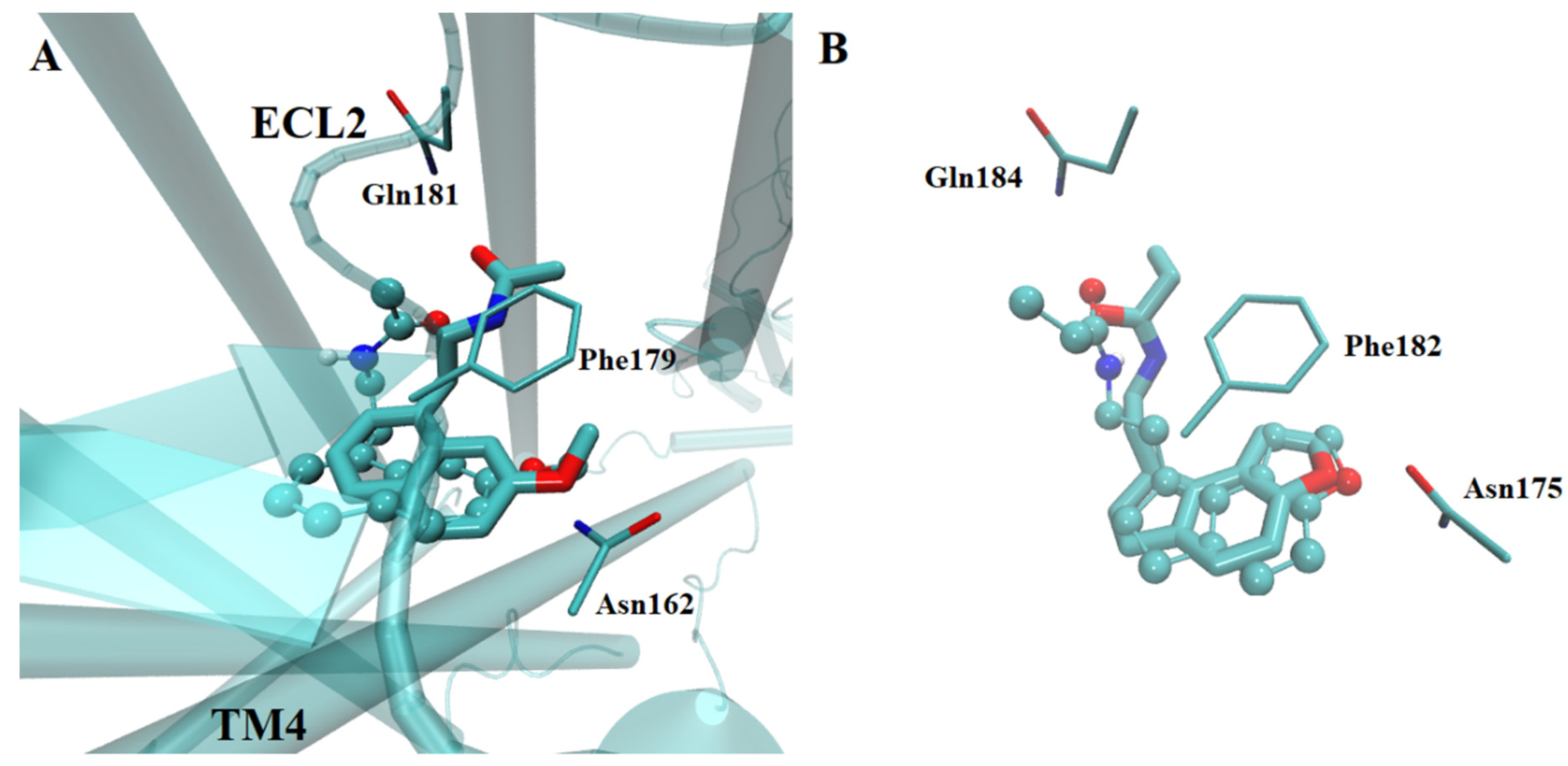
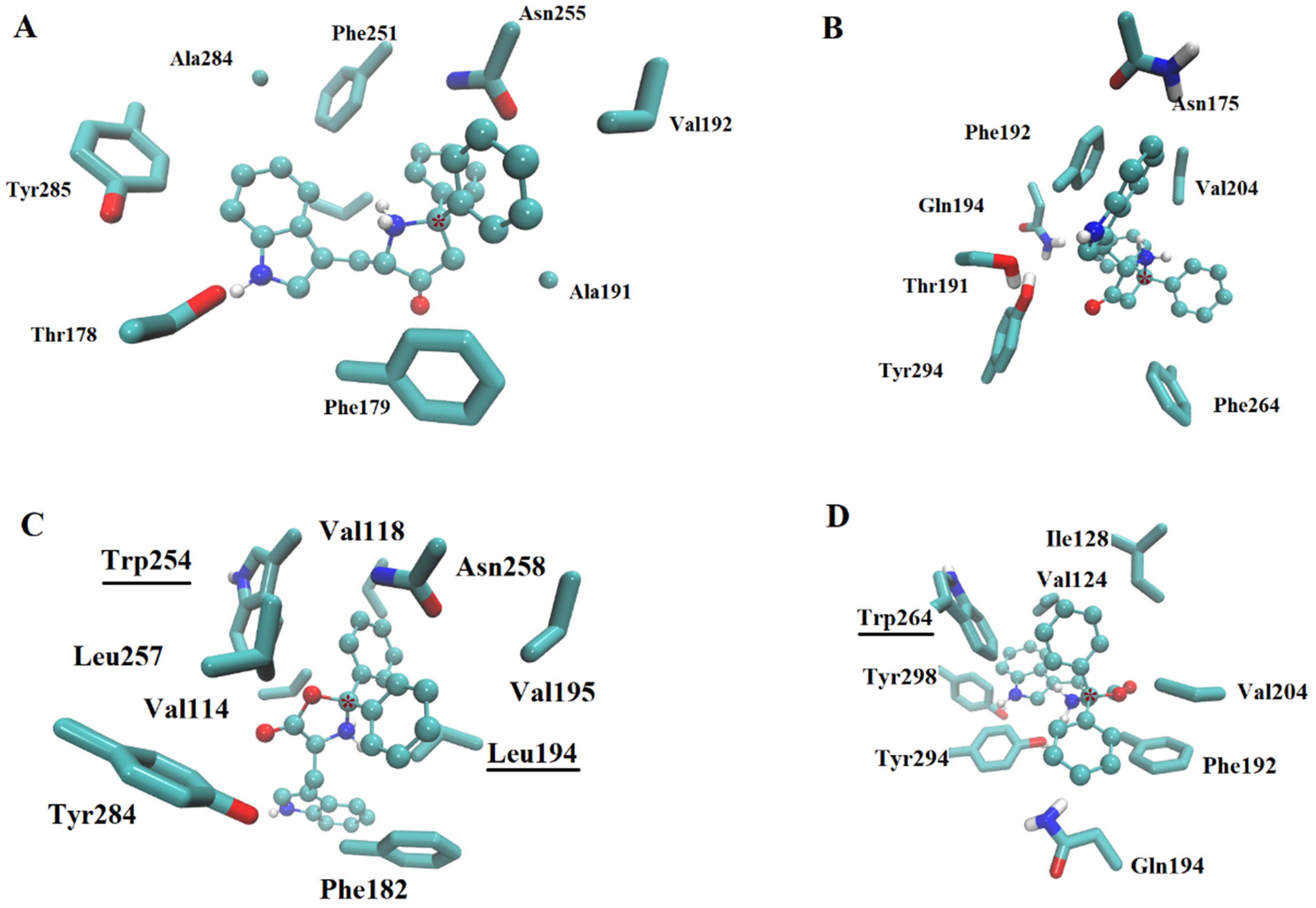
2.2. Molecular Modeling
2.2.1. Ligand Interactions with Melatonin Receptors
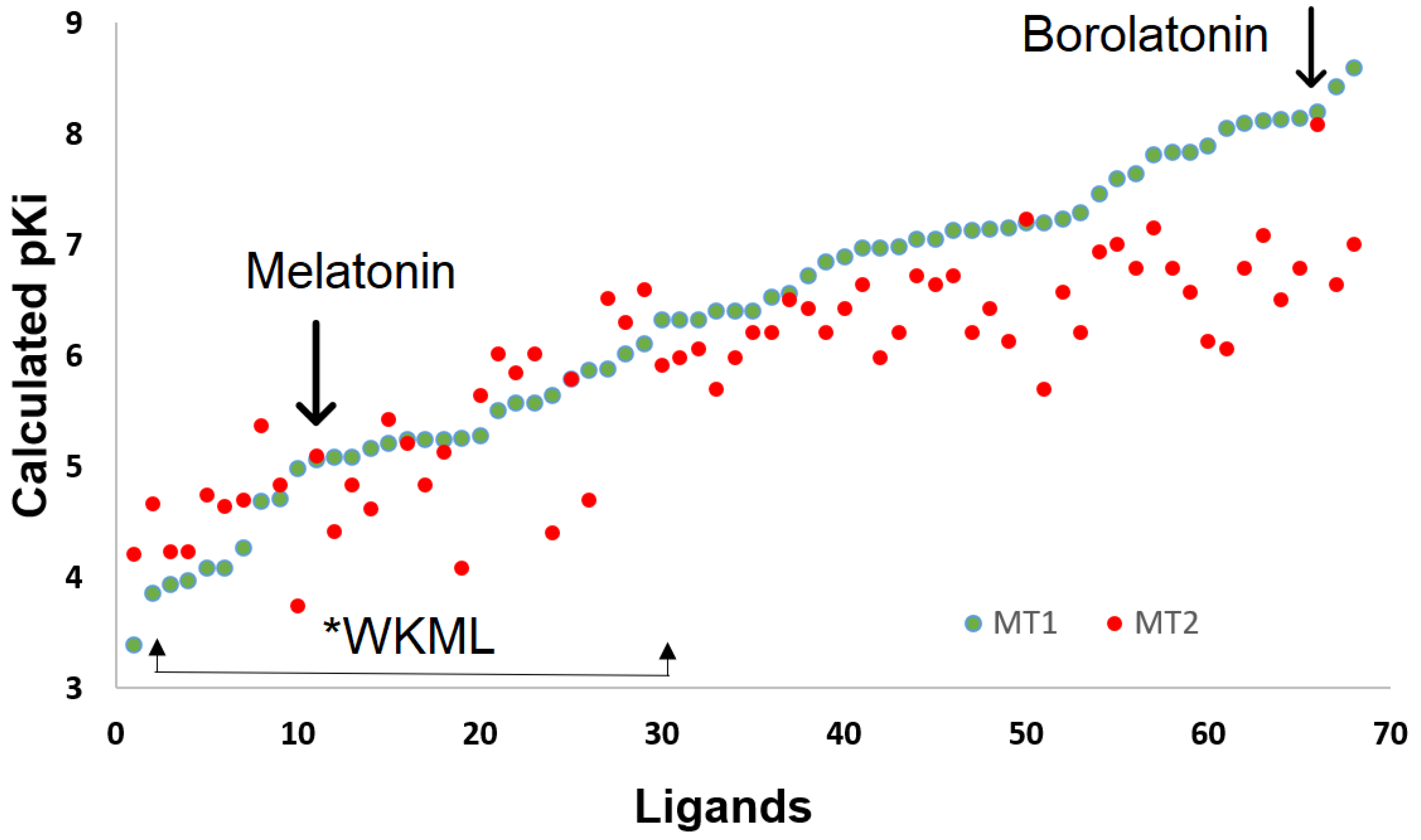
2.2.2. Affinity Estimation
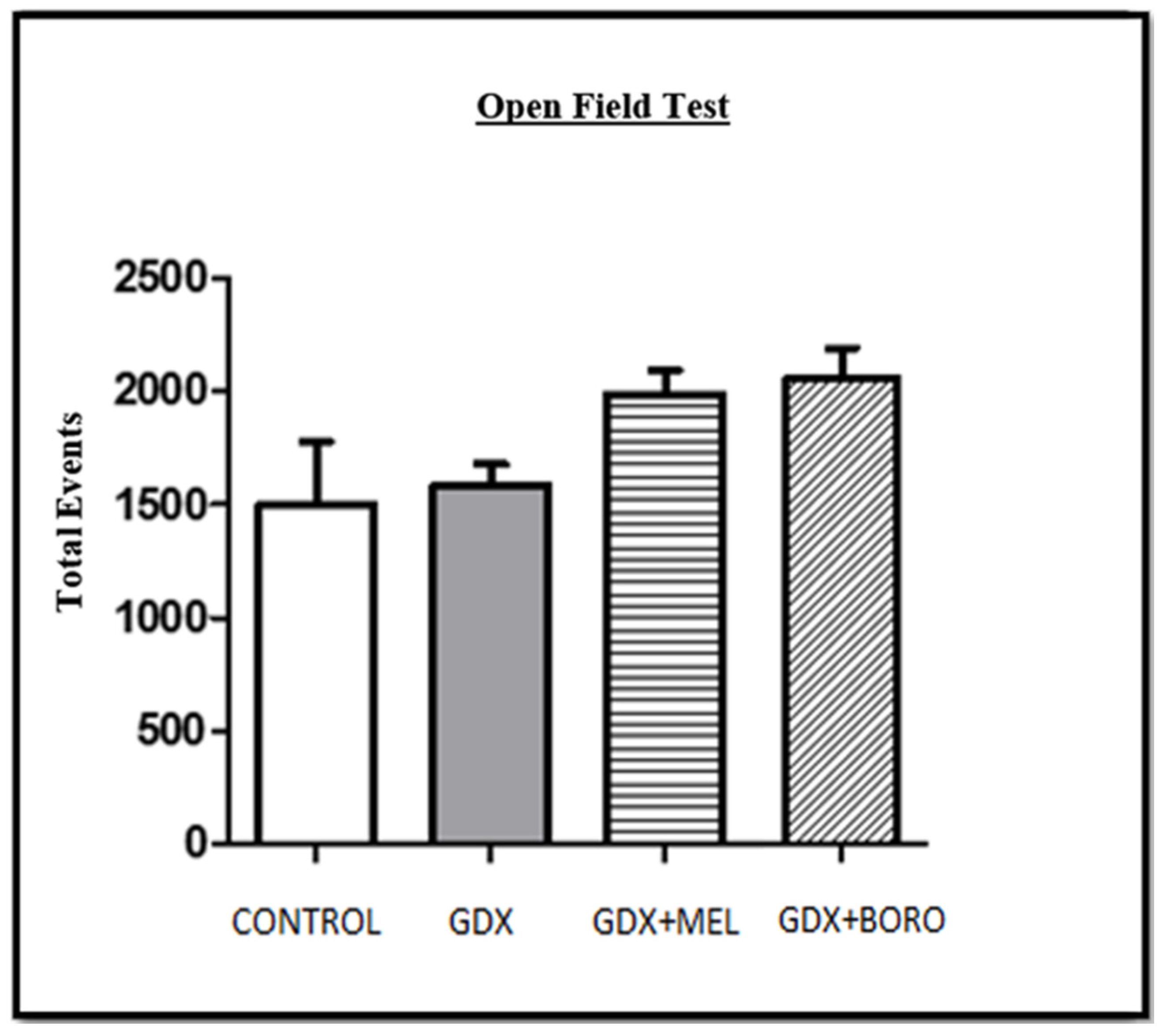
2.3. Behavioral Changes by Hormone Deprivation and the Effects of Melatonin or Borolatonin
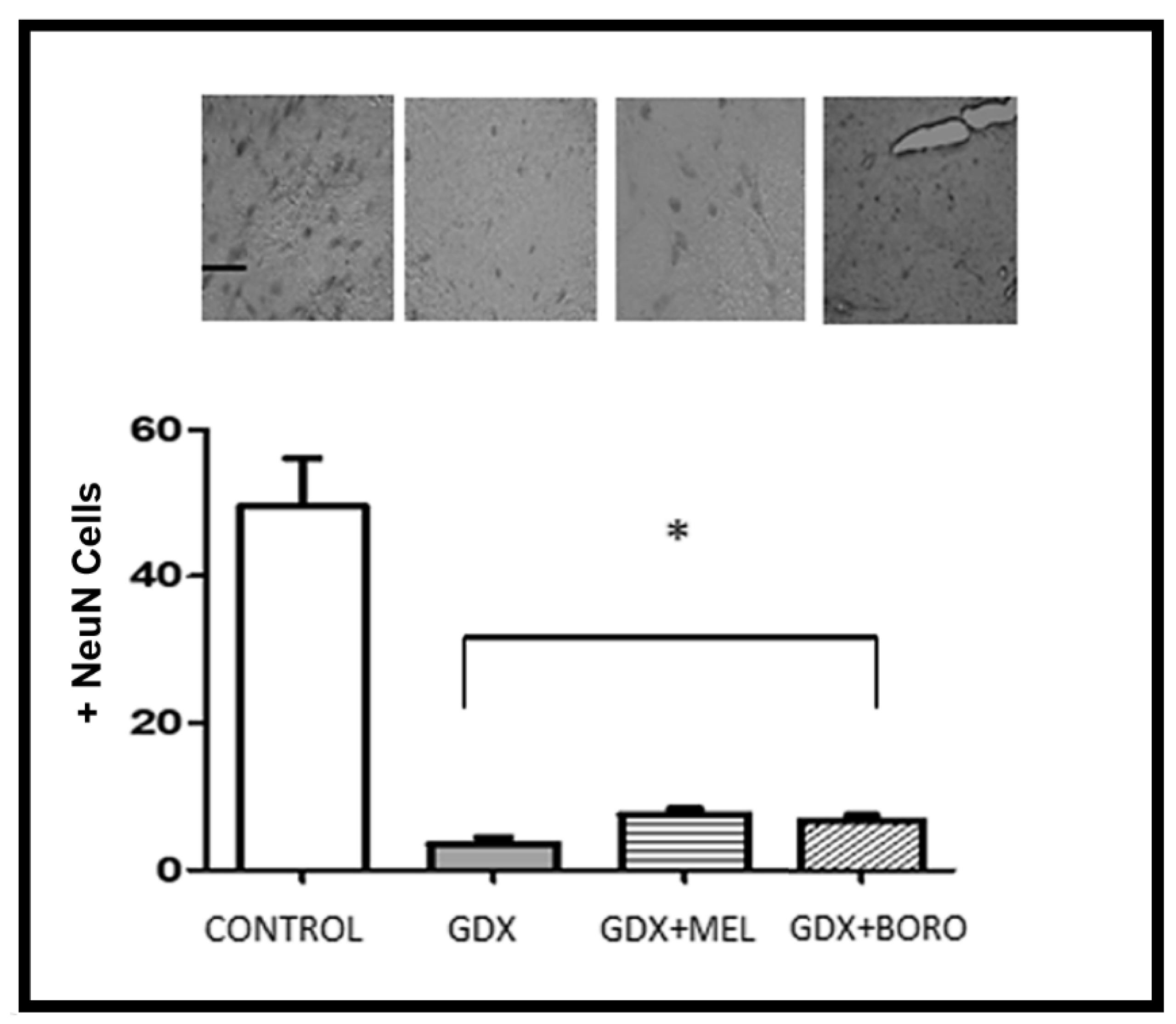
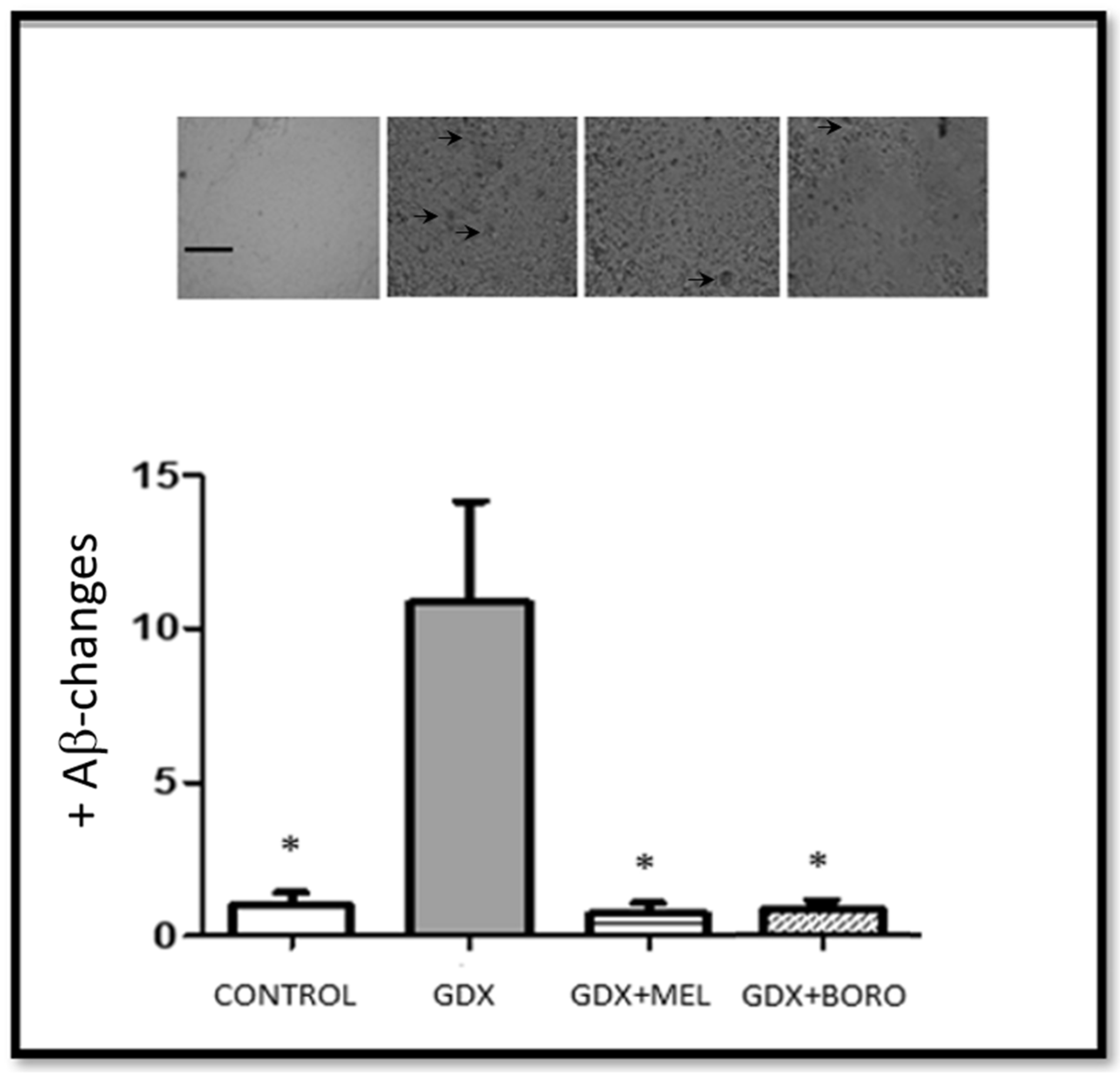
2.4. Effects on Neuronal Survival and Beta Amyloid Presence in Hippocampus from Immunohistochemistry Assays
3. Materials and Methods
3.1. In Silico Assays
3.1.1. Molecular Modeling
3.1.2. Ligand Retrieval
3.1.3. Docking Methodology
3.2. Chemistry
3.2.1. Chemicals
3.2.2. Synthesis of Borolatonin
3.3. Behavioral Evaluation
3.4. Immunohistochemistry Assays
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hoang, C.L.; Ha, G.H.; Pham, K.T.H.; Tran, B.X.; Latkin, C.A.; Ho, C.S.H.; Ho, R.C.M. Global Mapping of Interventions to Improve Quality of Life of Patients with Alzheimer’s Disease during 1990–2018. Dement. Geriatr. Cogn. Disord. 2019, 48, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Ashford, J.W. Treatment of Alzheimer’s disease: The legacy of the cholinergic hypothesis, neuroplasticity, and future directions. J. Alzheimer’s Dis. 2015, 47, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Majdi, A.; Sadigh-Eteghad, S.; Aghsan, S.R.; Farajdokht, F.; Vatandoust, S.M.; Namvaran, A.; Mahmoudi, J. Amyloid-β, tau, and the cholinergic system in Alzheimer’s disease: Seeking direction in a tangle of clues. Rev. Neurosci. 2020, 31, 391–413. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, A.A.D.T.; Deshapriya, R.D.U.S.; Udawatte, C. Alzheimer’s disease; a review of the pathophysiological basis and therapeutic interventions. Life Sci. 2020, 256, 117996. [Google Scholar] [CrossRef]
- Nous, A.; Engelborghs, S.; Smolders, I. Melatonin levels in the Alzheimer’s disease continuum: A systematic review. Alzheimers. Res. Ther. 2021, 13, 52. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wan, J.; Liu, A.; Sun, J. Melatonin regulates Aβ production/clearance balance and Aβ neurotoxicity: A potential therapeutic molecule for Alzheimer’s disease. Biomed. Pharmacother. 2020, 132, 110887. [Google Scholar] [CrossRef]
- Roy, J.; Tsui, K.C.; Ng, J.; Fung, M.-L.; Lim, L.W. Regulation of Melatonin and Neurotransmission in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 6841. [Google Scholar] [CrossRef]
- Farfán-García, E.D.; Márquez-Gómez, R.; Barrón-González, M.; Pérez-Capistran, T.; Rosales-Hernández, M.C.; Pinto-Almazán, R.; Soriano-Ursúa, M.A. Monoamines and their derivatives on GPCRs: Potential therapy for alzheimer’s disease. Curr. Alzheimer Res. 2019, 16, 871–894. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, melatonin, and the pro-and anti-inflammatory networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [Green Version]
- Nabavi, S.M.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Baldi, A.; Khan, H.; Daglia, M. Anti-inflammatory effects of Melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 2019, 59, S4–S16. [Google Scholar] [CrossRef]
- Song, S.; Gao, P.; Sun, L.; Kang, D.; Kongsted, J.; Poongavanam, V.; Zhan, P.; Liu, X. Recent developments in the medicinal chemistry of single boron atom-containing compounds. Acta Pharm. Sin. B 2021, 11, 3035–3059. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.S.; Denny, W.A.; Dos Santos, J.L. Boron in drug design: Recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Ursúa, M.A.; Farfán-García, E.D.; Geninatti-Crich, S. Turning Fear of Boron Toxicity into Boron-containing Drug Design. Curr. Med. Chem. 2019, 26, 5005–5018. [Google Scholar] [CrossRef] [PubMed]
- Coban, F.K.; Ince, S.; Kucukkurt, I.; Demirel, H.H.; Hazman, O. Boron attenuates malathion-induced oxidative stress and acetylcholinesterase inhibition in rats. Drug Chem. Toxicol. 2015, 38, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Ozansoy, M.; AltintaŞ, M.Ö.; Ozansoy, M.B.; GÜnay, N.; KiliÇ, E.; KiliÇ, Ü. Two boron-containing compounds affect the cellular viability of SH-SY5Y cells in an in vitro amyloid-beta toxicity model. Turk. J. Biol. 2020, 44, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aguilar, K.S.; Arciniega-Martínez, I.M.; Farfán-García, E.D.; Campos-Rodríguez, R.; Reséndiz-Albor, A.A.; Soriano-Ursúa, M.A. Effects of boron-containing compounds on immune responses: Review and patenting trends. Expert Opin. Ther. Pat. 2019, 29, 339–351. [Google Scholar] [CrossRef]
- Karademir, M.; Gönül, Y.; Şimşek, N.; Eser, O. The neuroprotective effects of 2-apb in rats with experimentally-induced severe acute pancreatitis. Bratisl. Med. J. 2018, 119, 752–756. [Google Scholar] [CrossRef]
- Sakuma, W.; Nakagawasai, O.; Nemoto, W.; Odaira, T.; Ogawa, T.; Ohta, K.; Endo, Y.; Tan-No, K. Antidepressant effect of BE360, a new selective estrogen receptor modulator, activated via CREB/BDNF, Bcl-2 signaling pathways in ovariectomized mice. Behav. Brain Res. 2020, 393, 112764. [Google Scholar] [CrossRef]
- Tang, J.; Zheng, X.T.; Xiao, K.; Wang, K.L.; Wang, J.; Wang, Y.X.; Wang, K.; Wang, W.; Lu, S.; Yang, K.L.; et al. Effect of Boric Acid Supplementation on the Expression of BDNF in African Ostrich Chick Brain. Biol. Trace Elem. Res. 2016, 170, 208–215. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Topcu, G.; Elmaci, İ. Boron’s neurophysiological effects and tumoricidal activity on glioblastoma cells with implications for clinical treatment. Int. J. Neurosci. 2019, 129, 963–977. [Google Scholar] [CrossRef]
- Abad-García, A.; Ocampo-Néstor, A.L.; Das, B.C.; Farfán-García, E.D.; Bello, M.; Trujillo-Ferrara, J.G.; Soriano-Ursúa, M.A. Interactions of a boron-containing levodopa derivative on D2 dopamine receptor and its effects in a Parkinson disease model. J. Biol. Inorg. Chem. 2022, 27, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Ritacca, A.G.; Ritacco, I.; Dabbish, E.; Russo, N.; Mazzone, G.; Sicilia, E. A Boron-Containing Compound Acting on Multiple Targets Against Alzheimer’s Disease. Insights from Ab Initio and Molecular Dynamics Simulations. J. Chem. Inf. Model. 2021, 61, 3397–3410. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aligaga, K.; Bermejo-Bescós, P.; Martín-Aragón, S.; Csákÿ, A.G. Discovery of alkenylboronic acids as neuroprotective agents affecting multiple biological targets involved in Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2013, 23, 426–429. [Google Scholar] [CrossRef]
- Lu, C.-J.; Hu, J.; Wang, Z.; Xie, S.; Pan, T.; Huang, L.; Li, X. Discovery of boron-containing compounds as Aβ aggregation inhibitors and antioxidants for the treatment of Alzheimer’s disease. Medchemcomm 2018, 9, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.; Manna, J.; Burch, Z.N.; Flaherty, D.B.; Larkin, J.D.; Dunbar, G.L. Ameliorative Properties of Boronic Compounds in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6664. [Google Scholar] [CrossRef]
- Ocampo-Néstor, A.L.; López-Mayorga, R.M.; Castillo-Henkel, E.F.; Padilla-Martínez, I.I.; Trujillo-Ferrara, J.G.; Soriano-Ursúa, M.A. Design, synthesis and in vitro evaluation of a Dopa-organoboron compound that acts as a bladder relaxant through non-catecholamine receptors. Mol. Divers. 2019, 23, 361–370. [Google Scholar] [CrossRef]
- Cecon, E.; Liu, L.; Jockers, R. Melatonin receptor structures shed new light on melatonin research. J. Pineal Res. 2019, 67, e12606. [Google Scholar] [CrossRef]
- Chan, K.H.; Lap, H.T.; Huang, X.; Wong, Y.H. Molecular basis defining the selectivity of substituted isoquinolinones for the melatonin MT2 receptor. Biochem. Pharmacol. 2020, 177, 114020. [Google Scholar] [CrossRef]
- Al Kury, L.T.; Zeb, A.; Abidin, Z.U.; Irshad, N.; Malik, I.; Alvi, A.M.; Khalil, A.A.K.; Ahmad, S.; Faheem, M.; Khan, A.-U. Neuroprotective effects of melatonin and celecoxib against ethanol-induced neurodegeneration: A computational and pharmacological approach. Drug Des. Devel. Ther. 2019, 13, 2715. [Google Scholar] [CrossRef] [Green Version]
- Alkozi, H.A.; Sanchez Montero, J.M.; Doadrio, A.L.; Pintor, J. Docking studies for melatonin receptors. Expert Opin. Drug Discov. 2018, 13, 241–248. [Google Scholar] [CrossRef]
- Soriano-Ursúa, M.A.; Bello, M.; Hernández-Martínez, C.F.; Santillán-Torres, I.; Guerrero-Ramírez, R.; Correa-Basurto, J.; Arias-Montaño, J.A.; Trujillo-Ferrara, J.G. Cell-based assays and molecular dynamics analysis of a boron-containing agonist with different profiles of binding to human and guinea pig beta2 adrenoceptors. Eur. Biophys. J. 2019, 48, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Delagrange, P.; Dubocovich, M.L.; Jockers, R.; Krause, D.N.; Markus, R.P.; Olcese, J.; Pintor, J.; Renault, N.; Sugden, D.; et al. Melatonin receptors (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guid. Pharmacol. CITE 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Azam, S.S.; Abbasi, S.W. Molecular docking studies for the identification of novel melatoninergic inhibitors for acetylserotonin-O-methyltransferase using different docking routines. Theor. Biol. Med. Model. 2013, 10, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciprés-Flores, F.J.; Farfán-García, E.D.; Andrade-Jorge, E.; Cuevas-Hernández, R.I.; Tamay-Cach, F.; Martínez-Archundia, M.; Trujillo-Ferrara, J.G.; Soriano-Ursúa, M.A. Identification of two arylimides as cholinesterase inhibitors and testing of propranolol addition on impaired rat memory. Drug Dev. Res. 2020, 81, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, J.; Soultanov, V.; Nikitina, T.; Roschin, V.; Ordyan, N.; Hritcu, L. Cognitive-enhancing activities of the polyprenol preparation Ropren® in gonadectomized β-amyloid (25–35) rat model of Alzheimer’s disease. Physiol. Behav. 2016, 157, 55–62. [Google Scholar] [CrossRef]
- Fiacco, S.; Walther, A.; Ehlert, U. Steroid secretion in healthy aging. Psychoneuroendocrinology 2019, 105, 64–78. [Google Scholar] [CrossRef]
- Resnick, S.M.; Matsumoto, A.M.; Stephens-Shields, A.J.; Ellenberg, S.S.; Gill, T.M.; Shumaker, S.A.; Pleasants, D.D.; Barrett-Connor, E.; Bhasin, S.; Cauley, J.A. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 2017, 317, 717–727. [Google Scholar] [CrossRef]
- Wagels, L.; Votinov, M.; Kellermann, T.; Konzok, J.; Jung, S.; Montag, C.; Schneider, F.; Eisert, A.; Beyer, C.; Habel, U. Exogenous testosterone and the monoamine-oxidase A polymorphism influence anger, aggression and neural responses to provocation in males. Neuropharmacology 2019, 156, 107491. [Google Scholar] [CrossRef]
- Long, T.; Yao, J.K.; Li, J.; Kirshner, Z.Z.; Nelson, D.; Dougherty, G.G.; Gibbs, R.B. Comparison of transitional vs surgical menopause on monoamine and amino acid levels in the rat brain. Mol. Cell. Endocrinol. 2018, 476, 139–147. [Google Scholar] [CrossRef]
- Tuzcu, M.; Baydas, G. Effect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. Eur. J. Pharmacol. 2006, 537, 106–110. [Google Scholar] [CrossRef]
- Labban, S.; Alghamdi, B.S.; Alshehri, F.S.; Kurdi, M. Effects of melatonin and resveratrol on recognition memory and passive avoidance performance in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2021, 402, 113100. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Ng, K.Y.; Koh, R.Y.; Chye, S.M. Pharmacological effects of melatonin as neuroprotectant in rodent model: A review on the current biological evidence. Cell. Mol. Neurobiol. 2020, 40, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.; Prast, H.; Philippu, A. Melatonin facilitates short-term memory. Eur. J. Pharmacol. 1998, 349, 159–162. [Google Scholar] [CrossRef]
- Labban, S.; Alshehri, F.S.; Kurdi, M.; Alatawi, Y.; Alghamdi, B.S. Melatonin Improves Short-Term Spatial Memory in a Mouse Model of Alzheimer’s Disease. Degener. Neurol. Neuromuscul. Dis. 2021, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Cheng, Y.; Zhang, J. Long-term effects of melatonin or 17β-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J. Pineal Res. 2004, 37, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Soumier, A.; Banasr, M.; Lortet, S.; Masmejean, F.; Bernard, N.; Kerkerian-Le-Goff, L.; Gabriel, C.; Millan, M.J.; Mocaer, E.; Daszuta, A. Mechanisms contributing to the phase-dependent regulation of neurogenesis by the novel antidepressant, agomelatine, in the adult rat hippocampus. Neuropsychopharmacology 2009, 34, 2390–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, P.; Ouyang, X.; Zhou, S.; Yin, W.; Tang, C.; Laudon, M.; Tian, S. A novel melatonin agonist Neu-P11 facilitates memory performance and improves cognitive impairment in a rat model of Alzheimer’disease. Horm. Behav. 2013, 64, 1–7. [Google Scholar] [CrossRef]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef]
- Taheri, P.; Mogheiseh, A.; Tabrizi, A.S.; Nazifi, S.; Salavati, S.; Koohi, F. Changes in thyroid hormones, leptin, ghrelin and, galanin following oral melatonin administration in intact and castrated dogs: A preliminary study. BMC Vet. Res. 2019, 15, 145. [Google Scholar] [CrossRef] [Green Version]
- Blask, D.E. Potential Sites of Action of Pineal Hormones within the Neuroendocrine-Reproductive Axis. In The Pineal Gland; CRC Press: Boca Raton, FL, USA, 2020; pp. 189–216. ISBN 0429280939. [Google Scholar]
- Nikmahzar, E.; Jahanshahi, M.; Elyasi, L.; Saeidi, M.; Babakordi, F.; Bahlakeh, G. Human chorionic gonadotropin attenuates amyloid-β plaques induced by streptozotocin in the rat brain by affecting cytochrome c-ir neuron density. Iran. J. Basic Med. Sci. 2019, 22, 166. [Google Scholar]
- Cheboub, A.; Regouat, N.; Djidjik, R.; Slimani, A.; Hadj-Bekkouche, F. Short-term aromatase inhibition induces prostatic alterations in adult wistar rat: A biochemical, histopathological and immunohistochemical study. Acta Histochem. 2019, 121, 151441. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, G.D.; Miller, M.C.; Messier, A.A.; Majmudar, S.; Machan, J.T.; Donahue, J.E.; Stopa, E.G.; Johanson, C.E. Amyloid deposition and influx transporter expression at the blood-brain barrier increase in normal aging. J. Neuropathol. Exp. Neurol. 2010, 69, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciprés-Flores, F.J.; Segura-Uribe, J.J.; Orozco-Suárez, S.; Guerra-Araiza, C.; Guevara-Salazar, J.A.; Castillo-García, E.L.; Soriano-Ursúa, M.A.; Farfán-García, E.D. Beta-blockers and salbutamol limited emotional memory disturbance and damage induced by orchiectomy in the rat hippocampus. Life Sci. 2019, 224, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Kaur, C.; Pandi-Perumal, S.; Brown, G.M.; Cardinali, D.P. Melatonin and its agonist ramelteon in Alzheimer’s disease: Possible therapeutic value. Int. J. Alzheimer’s Dis. 2011, 2011, 741974. [Google Scholar] [CrossRef] [Green Version]
- Rosalez, M.N.; Estevez-Fregoso, E.; Alatorre, A.; Abad-García, A.; A Soriano-Ursúa, M. 2-Aminoethyldiphenyl Borinate: A Multitarget Compound with Potential as a Drug Precursor. Curr. Mol. Pharmacol. 2019, 13, 57–75. [Google Scholar] [CrossRef]
- Lu, Y.; Ho, C.S.; McIntyre, R.S.; Wang, W.; Ho, R.C. Agomelatine-induced modulation of brain-derived neurotrophic factor (BDNF) in the rat hippocampus. Life Sci. 2018, 210, 177–184. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Bourne, Y.; Grassi, J.; Bougis, P.E.; Marchot, P. Conformational flexibility of the acetylcholinesterase tetramer suggested by x-ray crystallography. J. Biol. Chem. 1999, 274, 30370–30376. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Huey, R.; Linkstrom, W.; Sanner, M.; Belew, R.; Goodsell, D. Olson AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09 Revision D.01. In Gaussian 09 Revis. C.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Meng, E.C.; Shoichet, B.K.; Kuntz, I.D. Automated docking with grid-based energy evaluation. J. Comput. Chem. 1992, 13, 505–524. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Weinstein, H. Integrated methods for the Construction of Three-Dimensional Models and Computational Probing of Structure-Function Relations in G Protein-Coupled Receptors. In Methods in Neurosciences; Elsevier: Amsterdam, The Netherlands, 1995; Volume 25, pp. 366–428. ISBN 1043-9471. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Joseph, E.; Villalobos-Acosta, D.M.Á.; Torres-Ramos, M.A.; Farfán-García, E.D.; Gómez-López, M.; Miliar-García, Á.; Fragoso-Vázquez, M.J.; García-Marín, I.D.; Correa-Basurto, J.; Rosales-Hernández, M.C. Neuroprotective Effects of Apocynin and Galantamine During the Chronic Administration of Scopolamine in an Alzheimer’s Disease Model. J. Mol. Neurosci. 2020, 70, 180–193. [Google Scholar] [CrossRef] [PubMed]
- El Mrabet Fatima, Z.; Lagbouri, I.; Mesfioui, A.; El Hessni, A.; Ouichou, A. The Influence of gonadectomy on anxiolytic and antidepressant effects of melatonin in male and female Wistar rats: A possible implication of sex hormones. Neurosci. Med. 2012, 3, 162–173. [Google Scholar]
- Lamtai, M.; Zghari, O.; Azirar, S.; Ouakki, S.; Mesfioui, A.; El Hessni, A.; Berkiks, I.; Marmouzi, I.; Ouichou, A. Melatonin modulates copper-induced anxiety-like, depression-like and memory impairments by acting on hippocampal oxidative stress in rat. Drug Chem. Toxicol. 2021, 1–9. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrón-González, M.; Rosales-Hernández, M.C.; Abad-García, A.; Ocampo-Néstor, A.L.; Santiago-Quintana, J.M.; Pérez-Capistran, T.; Trujillo-Ferrara, J.G.; Padilla-Martínez, I.I.; Farfán-García, E.D.; Soriano-Ursúa, M.A. Synthesis, In Silico, and Biological Evaluation of a Borinic Tryptophan-Derivative That Induces Melatonin-like Amelioration of Cognitive Deficit in Male Rat. Int. J. Mol. Sci. 2022, 23, 3229. https://doi.org/10.3390/ijms23063229
Barrón-González M, Rosales-Hernández MC, Abad-García A, Ocampo-Néstor AL, Santiago-Quintana JM, Pérez-Capistran T, Trujillo-Ferrara JG, Padilla-Martínez II, Farfán-García ED, Soriano-Ursúa MA. Synthesis, In Silico, and Biological Evaluation of a Borinic Tryptophan-Derivative That Induces Melatonin-like Amelioration of Cognitive Deficit in Male Rat. International Journal of Molecular Sciences. 2022; 23(6):3229. https://doi.org/10.3390/ijms23063229
Chicago/Turabian StyleBarrón-González, Mónica, Martha C. Rosales-Hernández, Antonio Abad-García, Ana L. Ocampo-Néstor, José M. Santiago-Quintana, Teresa Pérez-Capistran, José G. Trujillo-Ferrara, Itzia I. Padilla-Martínez, Eunice D. Farfán-García, and Marvin A. Soriano-Ursúa. 2022. "Synthesis, In Silico, and Biological Evaluation of a Borinic Tryptophan-Derivative That Induces Melatonin-like Amelioration of Cognitive Deficit in Male Rat" International Journal of Molecular Sciences 23, no. 6: 3229. https://doi.org/10.3390/ijms23063229
APA StyleBarrón-González, M., Rosales-Hernández, M. C., Abad-García, A., Ocampo-Néstor, A. L., Santiago-Quintana, J. M., Pérez-Capistran, T., Trujillo-Ferrara, J. G., Padilla-Martínez, I. I., Farfán-García, E. D., & Soriano-Ursúa, M. A. (2022). Synthesis, In Silico, and Biological Evaluation of a Borinic Tryptophan-Derivative That Induces Melatonin-like Amelioration of Cognitive Deficit in Male Rat. International Journal of Molecular Sciences, 23(6), 3229. https://doi.org/10.3390/ijms23063229







