Elongation and Contraction of Scallop Sarcoplasmic Reticulum (SR): ATP Stabilizes Ca2+-ATPase Crystalline Array Elongation of SR Vesicles
Abstract
:1. Introduction
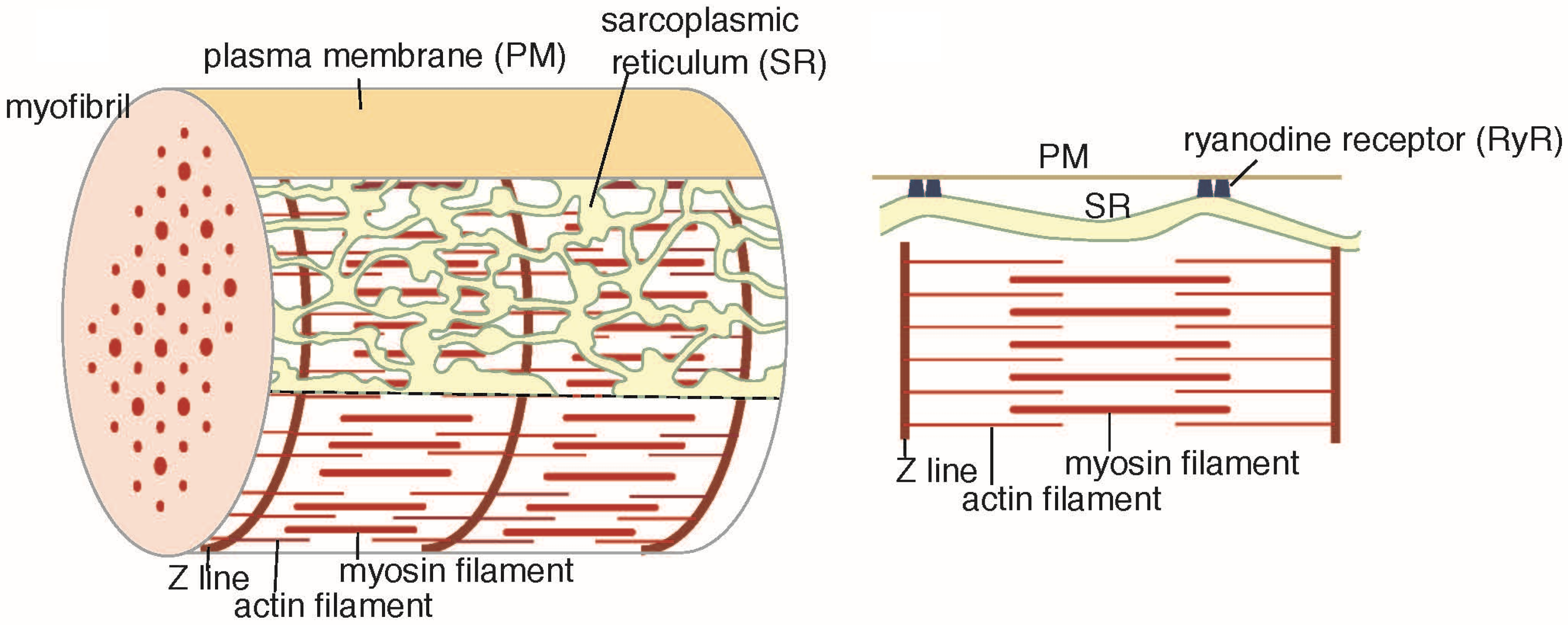
2. Results
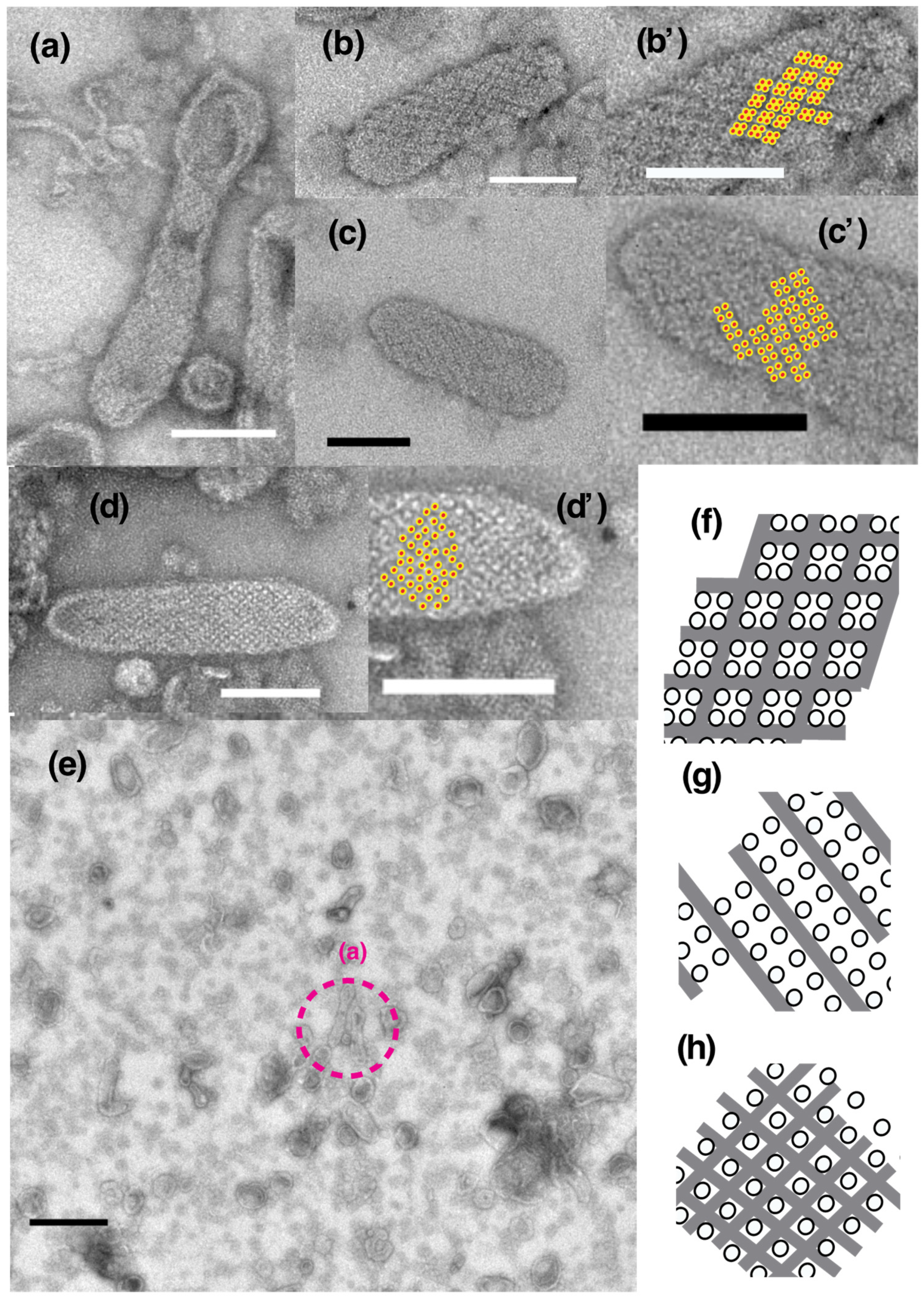
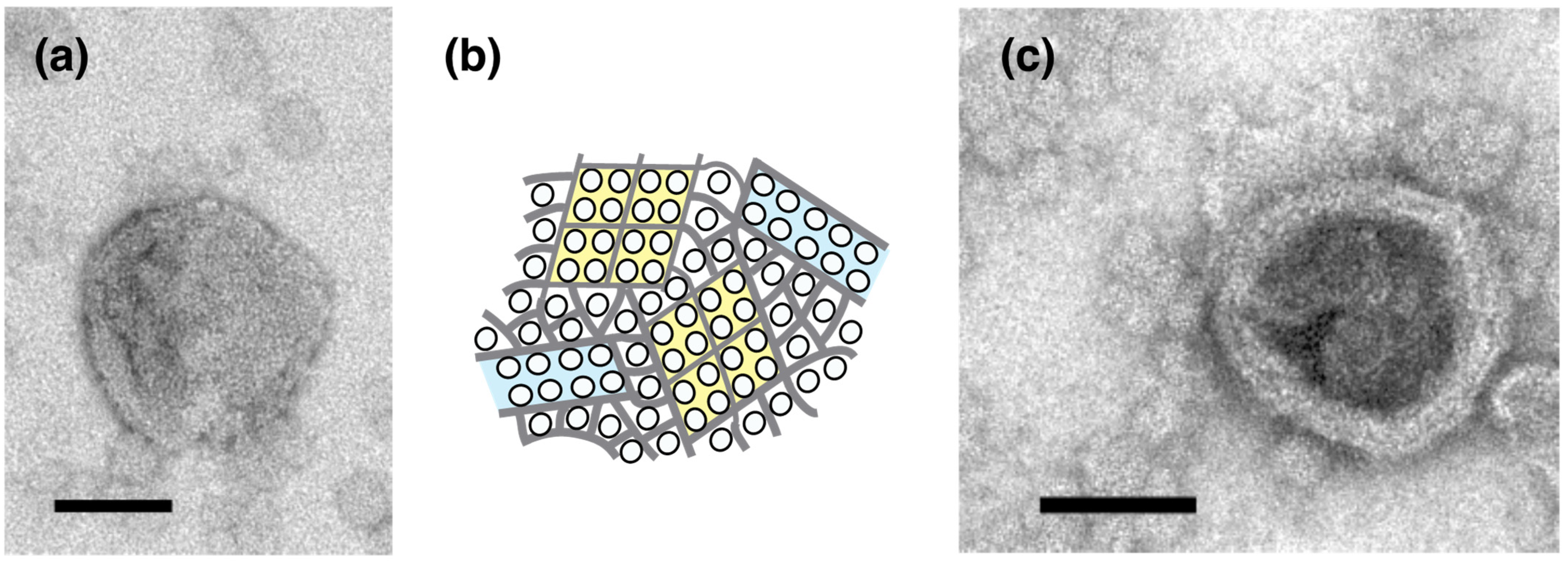
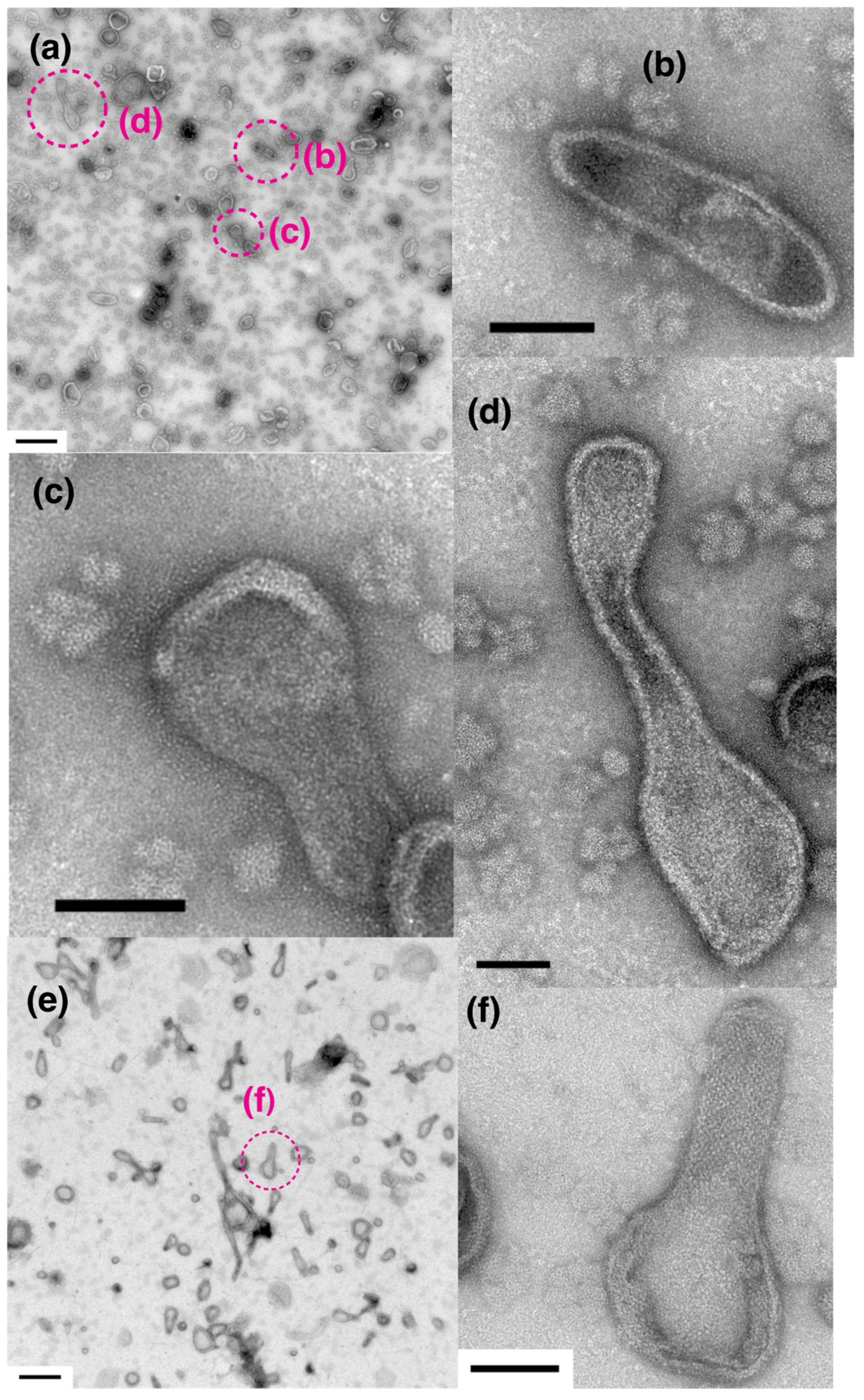
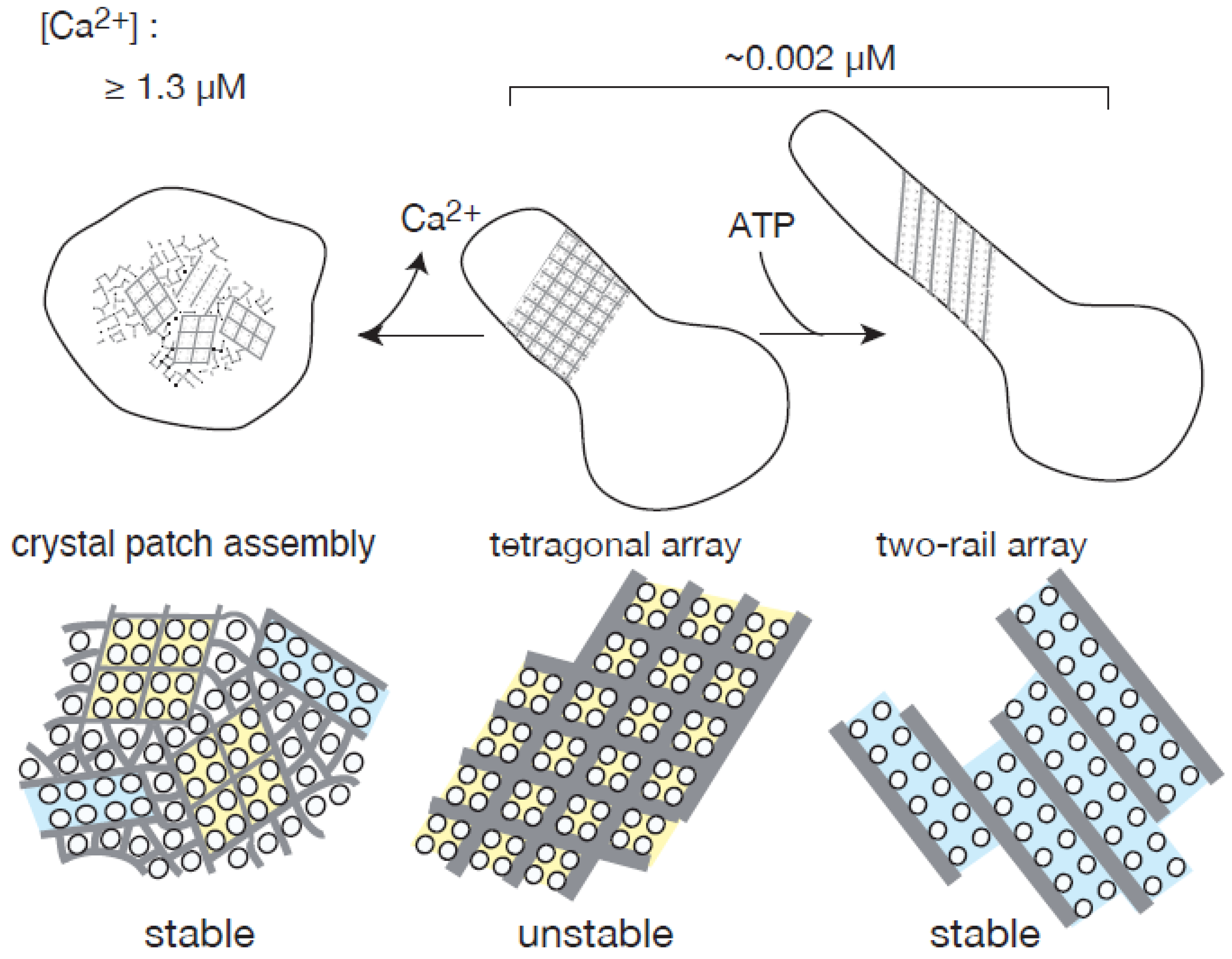
2.1. Effect of ATP on Crystallization of ATPase Surface Particles of SR
2.2. Thapsigargin Inhibits ATPase from Crystallization and Restrains SR Vesicles from Elongation
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SR | sarcoplasmic reticulum |
| EGTA | ethylenebis (oxyethylenenitrilo) tetraacetic acid |
| TG | thapsigargin |
| DMSO | dimethylsulfoxide |
| TEM | Transmission electron microscopy/microscope |
References
- De Meis, L.; Inesi, G. The transport of calcium by sarcoplasmic reticulum and various microsomal preparations. In Membrane Transport of Calcium; Carafoli, E., Ed.; Academic Press, Inc.: Orland, FL, USA, 1982; pp. 141–186. [Google Scholar]
- MacLennan, D.H.; Brandl, C.J.; Korczak, B.; Green, N.M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complimentary DNA sequence. Nature 1985, 316, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Brandl, C.J.; Green, N.M.; Korczak, B.; MacLennan, D.H. Two Ca2+ ATPase genes: Homologies and mechanistic implications of deduced amino acid sequences. Cell 1986, 44, 597–607. [Google Scholar] [CrossRef]
- Hasselbach, W. Relaxing factor and the relaxation of muscle. In Progress in Biophysics and Molecular Biology; Bupler, J.A.V., Huxley, H.E., Eds.; Pergamon Press: London, UK, 1964; pp. 167–222. [Google Scholar]
- Ebashi, S.; Endo, M. Calcium ion and muscle contraction. In Progress in Biophysics and Molecular Biology; Bupler, J.A.V., Huxley, H.E., Eds.; Pergamon Press: London, UK, 1968; pp. 123–183. [Google Scholar]
- Nakamura, J.; Maruyama, Y.; Tajima, G.; Suwa, M.; Sato, C. Ca2+-ATPase molecules as a calcium-sensitive membrane-endoskeleton of sarcoplasmic reticulum. Int. J. Mol. Sci. 2021, 22, 2624. [Google Scholar] [CrossRef] [PubMed]
- Castellani, L.; Hardwicke, P.M.D. Crystalline structure of sarcoplasmic reticulum from scallop. J. Cell Biol. 1983, 97, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellani, L.; Hardwicke, P.M.D.; Franzini-Armstrong, C. Effect of Ca2+ on the dimeric structure of scallop sarcoplasmic reticulum. J. Cell Biol. 1989, 108, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Sanger, J.W.; Sanger, J.M. Sarcoplasmic reticulum in the adductor muscle of a Bermuda scallop: Comparison of smooth versus cross-striated portions. Biol. Bull. 1985, 168, 447–460. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C. Studies of the triad. I. Structure of the junction in frog twitch fibers. J. Cell Biol. 1970, 47, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Inui, M.; Saito, A.; Fleischer, S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J. Biol. Chem. 1987, 262, 1740–1747. [Google Scholar] [CrossRef]
- Lai, F.A.; Erickson, H.P.; Pousseau, E.; Liu, Q.Y.; Meissner, G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature 1988, 331, 315–319. [Google Scholar]
- Meissner, G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim. Biophys. Acta 1975, 389, 51–68. [Google Scholar] [CrossRef]
- Abe, M.; Shirakata, Y.; Sato, D.; Konishi, K.; Watanabe, T.; Nakamura, J. Isolation and characteristics of scallop sarcoplasmic reticulum with calcium transport activity. J. Biochem. 1992, 112, 822–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, D.; Takahashi, T.; Tajima, G.; Sato, C.; Nagata, Y.; Yamamoto, T.; Nakamura, J. The Ca2+-ATPase of the scallop sarcoplasmic reticulum is of a cold-adapted type. J. Membr. Biol. 2003, 196, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Loesser, K.E.; Castellani, L.; Franzini-Armstrong, C. Dispositions of junctional feet in muscles of invertebrates. J. Muscle Res. Cell Motil. 1992, 13, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.E.; Castellani, L.; Ondrias, K.; Ehrlich, B.E. Characterization of the ryanodine receptor/channel of invertebrate muscle. Am. J. Physiol. 1998, 274, R494–R502. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.R.; Wu, F.; Liu, Y.; Anderson, D.M.; McAnally, J.; Lin, W.; Cannon, S.C.; Bassel-Duby, R.; Olson, E.N. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. USA 2013, 110, 11881–11886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellani, L.; Hardwicke, P.M.D.; Vibert, P. Dimer ribbons in the three-dimensional structure of sarcoplasmic reticulum. J. Mol. Biol. 1985, 185, 579–594. [Google Scholar] [CrossRef]
- Shi, X.; Chen, M.; Huvos, P.E.; Hardwicke, P.M.D. Amino acid sequence of a Ca2+-transporting ATPase from the sarcoplasmic reticulum of the cross-striated part of the adductor muscle of the deep sea scallop: Comparison to cerca enzymes of other animals. Comp. Biochem. Physiol. 1998, B120, 359–374. [Google Scholar] [CrossRef]
- Møller, J.V.; Lind, K.E.; Andersen, J.P. Enzyme kinetics and substrate stabilization of detergent-solubilized and membranous (Ca2+ + Mg2+)-activated ATPase from sarcoplasmic reticulum. Effect of protein-protein interactions. J. Biol. Chem. 1980, 255, 1912–1920. [Google Scholar] [CrossRef]
- Kalabokis, V.N.; Bozzola, J.J.; Castellani, L.; Hardwicke, P.M.D. A possible role for the dimer ribbon state of scallop sarcoplasmic reticulum. Dimer ribbons are associated with stabilization of the Ca2+-free, Ca-ATPase. J. Biol. Chem. 1991, 266, 22044–22050. [Google Scholar] [CrossRef]
- Sagara, Y.; Wade, J.B.; Inesi, G. A conformational mechanism for formation of a dead-end complex by the sarcoplasmic reticulum ATPase with thapsigargin. J. Biol. Chem. 1992, 267, 1286–1292. [Google Scholar] [CrossRef]
- Patel, A.; Malinovska, L.; Saha, S.; Wang, J.; Alberti, S.; Krishnan, Y.; Hyman, A.A. ATP as a biological hydrotrope. Science 2017, 356, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Dux, L.; Martonosi, A. Two-dimensional arrays of proteins in sarcoplasmic reticulum and purified Ca2+-ATPase vesicles treated with vanadate. J. Biol. Chem. 1983, 258, 2599–2603. [Google Scholar] [CrossRef]
- Varga, S.; Csermely, P.; Martonosi, A. The binding of vanadium (V) oligomer to sarcoplasmic reticulum. Eur. J. Biochem. 1985, 148, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokes, D.L.; Lacapère, J.-J. Conformation of Ca2+-ATPase in two crystal forms. Effect of Ca2+, thapsigargin, adenosine 5′-(β, γ-methylene)triphosphate, and chromium(III)-ATP on crystallization. J. Biol. Chem. 1994, 269, 11606–11613. [Google Scholar] [CrossRef]
- Nagata, Y.; Yamamoto, T.; Ema, M.; Mimura, J.; Fujii-Kuriyama, Y.; Suzuki, T.; Furukohri, T.; Konishi, K.; Sato, D.; Tajima, G.; et al. cDNA cloning and predicted primary structure of scallop sarcoplasmic reticulum Ca2+-ATPase. Comp. Biochem. Physiol. 1998, B119, 777–785. [Google Scholar] [CrossRef]
- Inesi, G.; Kurzmack, M.; Coan, C.; Lewis, D.E. Cooperative calcium binding and ATPase activation in sarcoplasmic reticulum vesicles. J. Biol. Chem. 1980, 255, 3025–3031. [Google Scholar] [CrossRef]
- Nakamura, J.; Tajima, G.; Sato, C.; Furukohri, T.; Konishi, K. Substrate regulation of calcium binding in Ca2+-ATPase molecules of the sarcoplasmic reticulum. I. Effect of ATP. J. Biol. Chem. 2002, 277, 24180–24190. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, J.; Tajima, G.; Sato, C. Substrate regulation of calcium binding in Ca2+-ATPase molecules of the sarcoplasmic reticulum. II. Effect of CTP, GTP, ITP, and UTP. J. Biol. Chem. 2002, 277, 24191–24196. [Google Scholar] [CrossRef] [Green Version]
- Harafuji, H.; Ogawa, Y. Re-examination of the apparent binding constant of ethylene glycol bis(β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid with calcium around neutral pH. J. Biochem. 1980, 87, 130. [Google Scholar] [CrossRef]
- Khan, M.M.T.; Martell, A.E. Thermodynamic quantities associated with the interaction of adenosine triphosphate with metal ions. J. Am. Chem. Soc. 1966, 88, 668–671. [Google Scholar] [CrossRef]
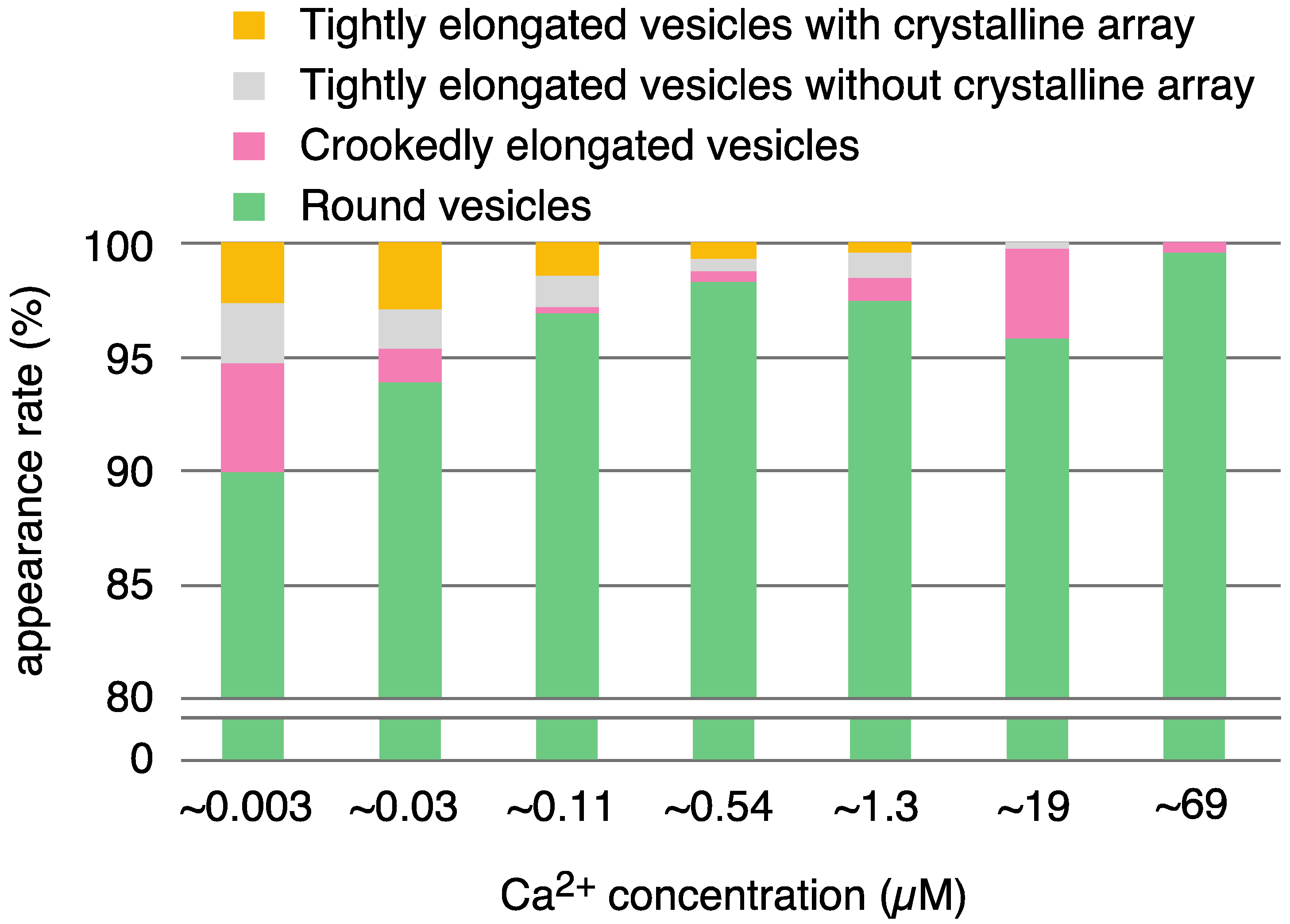

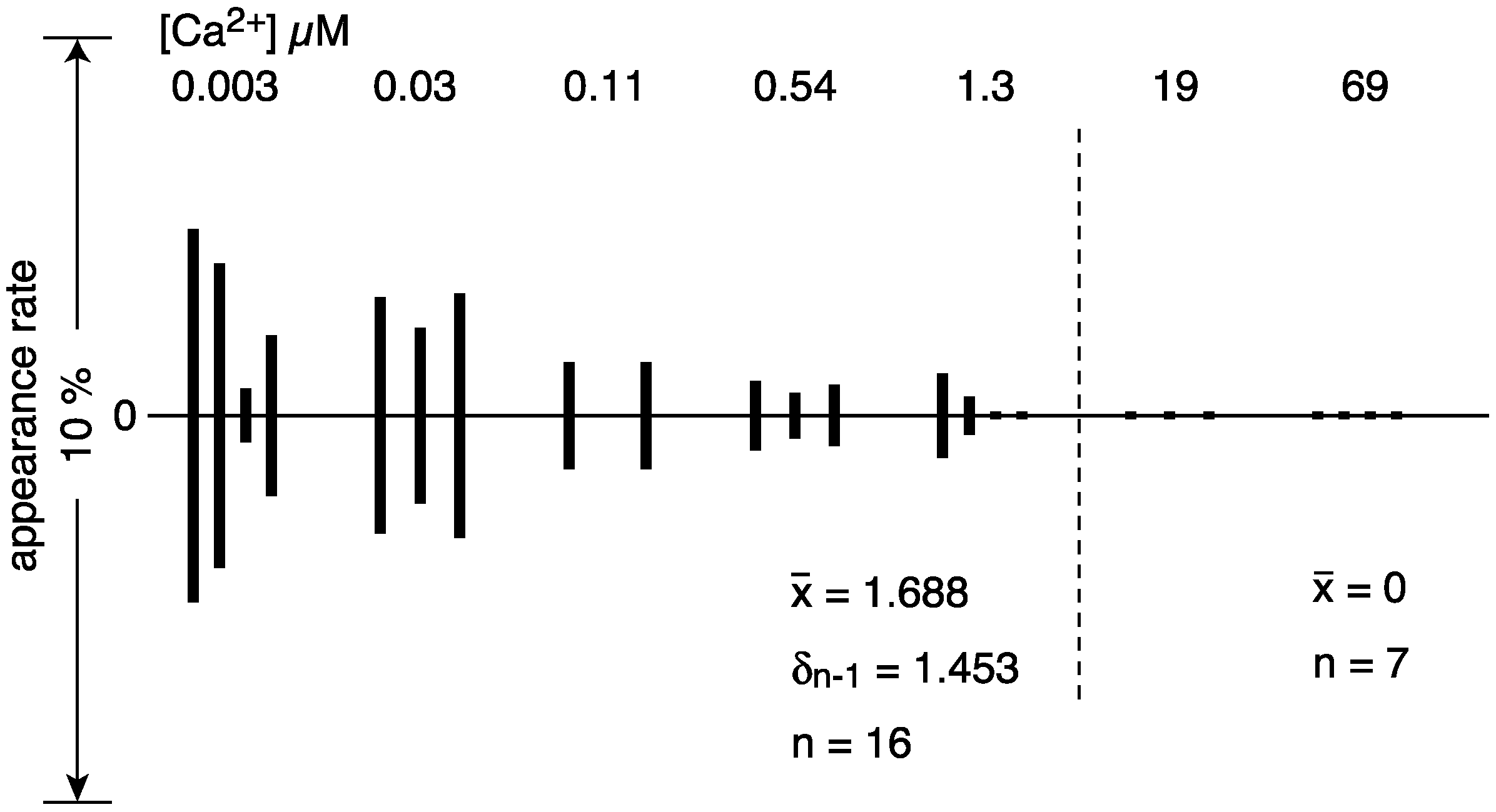



| Calcium Concentration (µM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.003 | 0.03 | 0.11 | 0.54 | 1.3 | 19 | 69 | |||
| elongated vesicles | 47 (10.0%) | 36 (6.1%) | 9 (3.1%) | 7 (1.7%) | 12 (2.5%) | 18 (4.2%) | 1 (0.4%) | ||
| tightly elongated | 25 (5.3) | 27 (4.6) | 8 (2.8) | 5 (1.2) | 7 (1.5) | 2 (0.5) | 0 | ||
| with crystal-array | 12 (2.6) | 17 (2.9) | 4 (1.4) | 3 (0.7) | 2 (0.4) | 0 | 0 | ||
| without crystal-array | 13 (2.8) | 10 (1.7) | 4 (1.4) | 2 (0.5) | 5 (1.0) | 2 (0.5) | 0 | ||
| crookedly elongated | 22 (4.7) | 9 (1.5) | 1 (0.3) | 2 (0.5) | 5 (1.0) | 17 (4.0) | 1 (0.4) | ||
| round vesicles | 421 (90.0) | 557 (93.9) | 279 (96.9) | 394 (98.3) | 465 (97.5) | 407 (95.5) | 284 (99.6) | ||
| total | 486 | 593 | 288 | 401 | 477 | 426 | 285 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, J.; Maruyama, Y.; Tajima, G.; Suwa, M.; Sato, C. Elongation and Contraction of Scallop Sarcoplasmic Reticulum (SR): ATP Stabilizes Ca2+-ATPase Crystalline Array Elongation of SR Vesicles. Int. J. Mol. Sci. 2022, 23, 3311. https://doi.org/10.3390/ijms23063311
Nakamura J, Maruyama Y, Tajima G, Suwa M, Sato C. Elongation and Contraction of Scallop Sarcoplasmic Reticulum (SR): ATP Stabilizes Ca2+-ATPase Crystalline Array Elongation of SR Vesicles. International Journal of Molecular Sciences. 2022; 23(6):3311. https://doi.org/10.3390/ijms23063311
Chicago/Turabian StyleNakamura, Jun, Yuusuke Maruyama, Genichi Tajima, Makiko Suwa, and Chikara Sato. 2022. "Elongation and Contraction of Scallop Sarcoplasmic Reticulum (SR): ATP Stabilizes Ca2+-ATPase Crystalline Array Elongation of SR Vesicles" International Journal of Molecular Sciences 23, no. 6: 3311. https://doi.org/10.3390/ijms23063311






