Neurohormonal Changes in the Gut–Brain Axis and Underlying Neuroendocrine Mechanisms following Bariatric Surgery
Abstract
1. Introduction
2. The Role of the Nervous System in Appetite and Energy Regulation
2.1. Central Nervous System Related Mechanisms
2.2. Autonomous-Nervous-System-Related Mechanisms
3. Effects of Bariatric Surgery on Nervous System
3.1. Central Nervous System and Neuropeptide Changes after Bariatric Surgery
3.2. Autonomous Nervous System Changes after Bariatric Surgery
4. Enteroendocrine Effects of Bariatric Surgery on the Gut–Brain Axis
4.1. Ghrelin
4.2. Cholecystokinin (CCK)
4.3. Peptide Tyrosine–Tyrosine (PYY)
4.4. Glucagon-like Peptide 1 (GLP-1)
4.5. Oxyntomodulin (OXM) and Glicentin
4.6. Neurotensin
4.7. Gastric Inhibitory Polypeptide, Glucose-Dependent Insulinotropic Polypeptide (GIP)
4.8. Gastrin
4.9. Fibroblast Growth Factors (FGFs)
4.10. Bile Acids
4.11. Secretin
4.12. Nesfatin
4.13. Gustducin
4.14. Uroguanylin
4.15. Obestatin
4.16. Glucagon-like Peptide-2 (GLP-2)
4.17. Leptin
4.18. Insulin
5. Gut Microbiota
Gut Microbiota and Bariatric Surgery
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Vasathi, S.M.; Walter, C.W.; Frank, B.H. Nearly a decade on—Trends, risk factors and policy implications in global obesity. Nat. Rev. Endocrinol. 2020, 16, 615–616. [Google Scholar]
- Prevalence of Obesity. Available online: https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity (accessed on 12 February 2022).
- Organisation, W.H. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 12 February 2022).
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and Severe Obesity Forecasts Through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, E984–E1010. [Google Scholar] [CrossRef] [PubMed]
- Doumouras, A.G.; Lee, Y.; Paterson, J.M.; Gerstein, H.C.; Shah, B.R.; Sivapathasundaram, B.; Tarride, J.E.; Anvari, M.; Hong, D. Association Between Bariatric Surgery and Major Adverse Diabetes Outcomes in Patients With Diabetes and Obesity. JAMA Netw. Open 2021, 4, e216820. [Google Scholar] [CrossRef] [PubMed]
- Al-Najim, W.; Docherty, N.G.; le Roux, C.W. Food Intake and Eating Behavior After Bariatric Surgery. Physiol. Rev. 2018, 98, 1113–1141. [Google Scholar] [CrossRef] [PubMed]
- Petroni, M.L.; Caletti, M.T.; Calugi, S.; Dalle Grave, R.; Marchesini, G. Long-term treatment of severe obesity: Are lifestyle interventions still an option? Expert Rev. Endocrinol. Metab. 2017, 12, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, N.; Antoniou, S.A.; Batterham, R.L.; Busetto, L.; Godoroja, D.; Iossa, A.; Carrano, F.M.; Agresta, F.; Alarcon, I.; Azran, C.; et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: Update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg. Endosc. 2020, 34, 2332–2358. [Google Scholar] [CrossRef] [PubMed]
- Courcoulas, A.P.; Yanovski, S.Z.; Bonds, D.; Eggerman, T.L.; Horlick, M.; Staten, M.A.; Arterburn, D.E. Long-term outcomes of bariatric surgery: A national institutes of health symposium. JAMA Surg. 2014, 149, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Nathan, D.M.; Eckel, R.H.; Schauer, P.R.; Alberti, K.G.M.; Zimmet, P.Z.; Prato, S.D.; Ji, L.; Sadikot, S.M.; Herman, W.H.; et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: A joint statement by international diabetes organizations. Diabetes Care 2016, 39, 861–877. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. 1), S113–S124. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Arterburn, D.E.; Westbrook, E.O.; Kuzma, J.N.; Stewart, S.D.; Chan, C.P.; Bock, S.N.; Landers, J.T.; Kratz, M.; Foster-Scubert, K.E.; et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: The CROSSROADS randomised controlled trial. Diabetologia 2016, 59, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.M.S.; Peltonen, M.; Ahlin, S.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Jacobson, P.; Lonroth, H.; Maglio, C.; Naslund, I.; et al. Bariatric Surgery and Prevention of Type 2 Diabetes in Swedish Obese Subjects. N. Engl. J. Med. 2012, 367, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Shikora, S.; Petry, T.; Caravatto, P.P.; Le Roux, C. The Diabetes Surgery Summit II Guidelines: A Disease-Based Clinical Recommendation. Obes. Surg. 2016, 26, 1989–1991. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Aminian, A.; Pothier, C.E.; Kin, E.S.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. NEJM 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.M.S.; Sjöholm, K.; Jacobson, P.; Andersson-Assarsson, J.C.; Svensson, P.-A.; Taube, M.; Carlsson, B.; Peltonen, M. Life Expectancy after Bariatric Surgery in the Swedish Obese Subjects Study. N. Engl. J. Med. 2020, 383, 1535. [Google Scholar] [CrossRef] [PubMed]
- Syn, N.L.; Cummings, D.E.; Wang, L.Z.; Lin, D.J.; Zhao, J.J.; Loh, M.; Koh, Z.J.; Chew, C.A.; Loo, Y.E.; Tai, B.C.; et al. Association of metabolic–bariatric surgery with long-term survival in adults with and without diabetes: A one-stage meta-analysis of matched cohort and prospective controlled studies with 174,772 participants. Lancet 2021, 397, 1830–1841. [Google Scholar] [CrossRef]
- Morris, A. Life expectancy: Benefits of bariatric surgery clarified. Nat. Rev. Endocrinol. 2021, 17, 4–5. [Google Scholar] [CrossRef]
- Nuzzo, A.; Czernichow, S.; Hertig, A.; Ledoux, S.; Poghosyan, T.; Quilliot, D.; Le Gall, M.; Bado, A.; Joly, F. Prevention and treatment of nutritional complications after bariatric surgery. Lancet Gastroenterol. Hepatol. 2021, 6, 238–251. [Google Scholar] [CrossRef]
- Ochner, C.N.; Gibson, C.; Shanik, M.; Goel, V.; Geliebter, A. Changes in neurohormonal gut peptides following bariatric surgery. Int. J. Obes. 2011, 35, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Khatri, I.A. An overview of complications affecting the central nervous system following bariatric surgery. Neurosciences 2018, 23, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Angelidi, A.M.; Belanger, M.J.; Kokkinos, A.; Koliaki, C.C.; Mantzoros, C.S. Novel Noninvasive Approaches to the Treatment of Obesity: From Pharmacotherapy to Gene Therapy. Endocr. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C.; Samsell, L.; Racusen, L.; Gladfelter, W. Hypothalamic lesions induce obesity and sex-dependent glomerular damage and increases in blood pressure in rats. Hypertension 1996, 27, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Dallman, M.F. Hypothalamic obesity: Multiple routes mediated by loss of function in medial cell groups. Endocrinology 1999, 140, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, M.; Gomis, R.; Claret, M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J. Endocrinol. 2014, 220, T25–T46. [Google Scholar] [CrossRef]
- Farr, O.M.; Li, C.S.R.; Mantzoros, C.S. Central nervous system regulation of eating: Insights from human brain imaging. Metab. Clin. Exp. 2016, 65, 699–713. [Google Scholar] [CrossRef]
- Parker, J.A.; Bloom, S.R. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 2012, 63, 18–30. [Google Scholar] [CrossRef]
- Yang, L.; Scott, K.A.; Hyun, J.; Tamashiro, K.L.; Tray, N.; Moran, T.H.; Bi, S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J. Neurosci. 2009, 29, 179–190. [Google Scholar] [CrossRef]
- Graham, M.; Shutter, J.R.; Sarmiento, U.; Sarosi, L.; Stark, K.L. Overexpression of agrt leads to obesity in transgenic mice. Nat. Genet. 1997, 17, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Small, C.J.; Liu, Y.L.; Stanley, S.A.; Connoley, I.P.; Kennedy, A.; Stock, M.J.; Bloom, S.R. Chronic CNS administration of Agouti-related protein (AgRP) reduces energy expenditure. Int. J. Obes. 2003, 27, 530–533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef]
- De Vrind, V.A.J.; Rozeboom, A.; Wolterink-Donselaar, I.G.; Luijendijk-Berg, M.C.M.; Adan, R.A.H. Effects of GABA and Leptin Receptor-Expressing Neurons in the Lateral Hypothalamus on Feeding, Locomotion, and Thermogenesis. Obesity 2019, 27, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Boyle, M.P.; Palmiter, R.D. Loss of GABAergic Signaling by AgRP Neurons to the Parabrachial Nucleus Leads to Starvation. Cell 2009, 137, 1225–1234. [Google Scholar] [CrossRef]
- Suzuki, K.; Simpson, K.A.; Minnion, J.S.; Shillito, J.C.; Bloom, S.R. The role of gut hormones and the hypothalamus in appetite regulation. Endocr. J. 2010, 57, 359–372. [Google Scholar] [CrossRef]
- Savontaus, E.; Breen, T.L.; Kim, A.; Yang, L.M.; Chua, S.C.; Wardlaw, S.L. Metabolic effects of transgenic melanocyte-stimulating hormone overexpression in lean and obese mice. Endocrinology 2004, 145, 3881–3891. [Google Scholar] [CrossRef]
- Mercer, A.J.; Hentges, S.T.; Meshul, C.K.; Low, M.J. Unraveling the central proopiomelanocortin neural circuits. Front. Neurosci. 2013, 7, 19. [Google Scholar] [CrossRef]
- Butler, A.A.; Cone, R.D. The melanocortin receptors: Lessons from knockout models. Neuropeptides 2002, 36, 77–84. [Google Scholar] [CrossRef]
- Chen, A.S.; Marsh, D.J.; Trumbauer, M.E.; Frazier, E.G.; Guan, X.M.; Yu, H.; Rosenblum, C.I.; Vongs, A.; Feng, Y.; Cao, L.; et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000, 26, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.S.; Metzger, J.M.; Trumbauer, M.E.; Guan, X.M.; Yu, H.; Frazier, E.G.; Marsh, D.J.; Forrest, M.J.; Gopal-Truter, S.; Fisher, J.; et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000, 9, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Vrang, N. Anatomy of hypothalamic CART neurons. Peptides 2006, 27, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.M.; Stanley, S.; Gardiner, J.; Abbott, C.; Murphy, K.; Seth, A.; Connoley, I.; Ghatei, M.; Stephens, D.; Bloom, S. A role for arcuate cocaine and amphetamine-regulated transcript in hyperphagia, thermogenesis, and cold adaptation. FASEB J. 2003, 17, 1688–1690. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Models Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Balthasar, N.; Dalgaard, L.T.; Lee, C.E.; Yu, J.; Funahashi, H.; Williams, T.; Ferreira, M.; Tang, V.; McGovern, R.A.; Kenny, C.D.; et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005, 123, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.A.; Martin, N.M.; Bloom, S.R. Regulação hipotalâmica da ingestão alimentar e suas aplicações terapêuticas clínicas. Arq. Bras. Endocrinol. Metabol. 2009, 53, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Goulding, E.H.; Zang, K.; Cepoi, D.; Cone, R.D.; Jones, K.R.; Tecott, L.H.; Reichardt, L.F. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003, 6, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.A.; Martin, N.M.; Bloom, S.R. Hypothalamic regulation of appetite. Expert Rev. Endocrinol. Metab. 2008, 3, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, T.C. Endocannabinoids in the regulation of appetite and body weight. Behav. Pharmacol. 2005, 16, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, P.; Bellocchio, L.; Clark, S.; Cannich, A.; Klugmann, M.; Lutz, B.; Marsicano, G.; Cota, D. Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology 2012, 153, 4136–4143. [Google Scholar] [CrossRef]
- Moran, T.H. Unraveling the obesity of OLETF rats. Physiol. Behav. 2008, 94, 71–78. [Google Scholar] [CrossRef]
- De la Serre, C.B.; Kim, Y.J.; Moran, T.H.; Bi, S. Dorsomedial hypothalamic NPY affects cholecystokinin-induced satiety via modulation of brain stem catecholamine neuronal signaling. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 311, R930–R939. [Google Scholar] [CrossRef] [PubMed]
- Milam, K.M.; Keesey, R.E.; Stern, J.S. Body composition and adiposity in LH-lesioned and pair-fed obese Zucker rats. Am. J. Physiol.-Endocrinol. Metab. 1982, 242, E437–E444. [Google Scholar] [CrossRef]
- Milam, K.M.; Stern, J.S.; Storlien, L.H.; Keesey, R.E. Effect of lateral hypothalamic lesions on regulation of body weight and adiposity in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1980, 239, R337–R343. [Google Scholar] [CrossRef]
- Valenzano, A.; Tartaglia, N.; Ambrosi, A.; Tafuri, D.; Monda, M.; Messina, A.; Sessa, F.; Campanozzi, A.; Monda, V.; Cibelli, G.; et al. The metabolic rearrangements of bariatric surgery: Focus on orexin-a and the adiponectin system. J. Clin. Med. 2020, 9, 3327. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Ishii, Y.; Halford, J.C.G.; Blundell, J.E. Orexins and appetite regulation. Neuropeptides 2002, 36, 303–325. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Tritos, N.A.; Mastaitis, J.W.; Kulkarni, R.; Kokkotou, E.; Elmquist, J.; Lowell, B.; Flier, J.; Flier, E.M. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J. Clin. Investig. 2001, 107, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, C.; Hsu, C.K.; Zhang, Q.; Bi, C.; Asnicar, M.; Hsiung, H.M.; Fox, N.; Slieker, L.J.; Yang, D.D.; et al. Targeted disruption of the melanin-concentrating hormone receptor-1 results in hyperphagia and resistance to diet-induced obesity. Endocrinology 2002, 143, 2469–2477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Russo, B.; Menduni, M.; Borboni, P.; Picconi, F.; Frontoni, S. Autonomic nervous system in obesity and insulin-resistance—The complex interplay between leptin and central nervous system. Int. J. Mol. Sci. 2021, 22, 5187. [Google Scholar] [CrossRef]
- Leiria, L.O.; Tseng, Y.H. Lipidomics of brown and white adipose tissue: Implications for energy metabolism. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158788. [Google Scholar] [CrossRef]
- Guarino, D.; Nannipieri, M.; Iervasi, G.; Taddei, S.; Bruno, R.M. The role of the autonomic nervous system in the pathophysiology of obesity. Front. Physiol. 2017, 8, 665. [Google Scholar] [CrossRef]
- Garretson, J.T.; Szymanski, L.A.; Schwartz, G.J.; Xue, B.; Ryu, V.; Bartness, T.J. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol. Metab. 2016, 5, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Navegantes, L.C.C.; Sjöstrand, M.; Gudbjörnsdottir, S.; Strindberg, L.; Elam, M.; Lönnroth, P. Regulation and Counterregulation of Lipolysis in Vivo: Different Roles of Sympathetic Activation and Insulin. J. Clin. Endocrinol. Metab. 2003, 88, 5515–5520. [Google Scholar] [CrossRef] [PubMed]
- Youngstrom, T.G.; Bartness, T.J. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1998, 275, R1488–R1493. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Shrestha, Y.B.; Vaughan, C.H.; Schwartz, G.J.; Song, C.K. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol. Cell. Endocrinol. 2010, 318, 34. [Google Scholar] [CrossRef]
- Bartness, T.J.; Liu, Y.; Shrestha, Y.B.; Ryu, V. Neural Innervation of White Adipose Tissue and the Control of Lipolysis. Front. Neuroendocrinol. 2014, 35, 473. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013, 123, 3404–3408. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Jbilo, O.; Ravinet-Trillou, C.; Arnone, M.; Buisson, I.; Bribes, E.; Péleraux, A.; Pénarier, G.; Soubrié, P.; Le Fur, G.; Galiegue, S.; et al. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J. 2005, 19, 1567–1569. [Google Scholar] [CrossRef]
- Perwitz, N.; Fasshauer, M.; Klein, J. Cannabinoid receptor signaling directly inhibits thermogenesis and alters expression of adiponectin and visfatin. Horm. Metab. Res. 2006, 38, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Bellocchio, L.; Cervino, C.; Pasquali, R.; Pagotto, U. The Endocannabinoid System and Energy Metabolism. J. Neuroendocrinol. 2008, 20, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Cork, S.C. The role of the vagus nerve in appetite control: Implications for the pathogenesis of obesity. J. Neuroendocrinol. 2018, 30, e12643. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Shin, A.C.; Zheng, H. Obesity surgery and gut-brain communication. Physiol. Behav. 2011, 105, 106–119. [Google Scholar] [CrossRef]
- Gil, K.; Bugajski, A.; Kurnik, M.; Thor, P. Electrical chronic vagus nerve stimulation activates the hypothalamic-pituitary-adrenal axis in rats fed high-fat diet. Neuroendocrinol. Lett. 2013, 34, 314–321. [Google Scholar]
- Bugajski, A.J.; Gil, K.; Ziomber, A.; Zurowski, D.; Zaraska, W.; Thor, P.J. Effect of long-term vagal stimulation on food intake and body weight during diet induced obesity in rats. J. Physiol. Pharmacol. 2007, 58, 5–12. [Google Scholar] [PubMed]
- Gil, K.; Bugajski, A.; Kurnik, M.; Thor, P. Chronic vagus nerve stimulation reduces body fat, blood cholesterol and triglyceride levels in rats fed a high-fat diet. Folia Med. Cracov. 2012, 52, 79–96. [Google Scholar]
- Almeida, P.P.; Valdetaro, L.; Thomasi, B.B.D.M.; Stockler-Pinto, M.B.; Tavares-Gomes, A.L. High-fat diets on the enteric nervous system: Possible interactions and mechanisms underlying dysmotility. Obes. Rev. 2021, 23, e13404. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut–Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.; Wemelle, E.; Cani, P.D.; Knauf, C. Interactions between the microbiota and enteric nervous system during gut-brain disorders. Neuropharmacology 2021, 197, 108721. [Google Scholar] [CrossRef] [PubMed]
- Romanova, I.V.; Ramos, E.J.B.; Xu, Y.; Quinn, R.; Chen, C.; George, Z.M.; Inui, A.; Das, U.; Meguid, M. Neurobiologic changes in the hypothalamus associated with weight loss after gastric bypass. J. Am. Coll. Surg. 2004, 199, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Barkholt, P.; Pedersen, P.J.; Hay-Schmidt, A.; Jelsing, J.; Hansen, H.H.; Vrang, N. Alterations in hypothalamic gene expression following Roux-en-Y gastric bypass. Mol. Metab. 2016, 5, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, I.J.; Stylopoulos, N.; Vanhoose, A.M.; Boyd, K.L.; Yin, D.P.; Ellacott, K.L.J.; Ma, L.L.; Blaszcyk, K.; Keogh, J.M.; Cone, R.D.; et al. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J. Clin. Endocrinol. Metab. 2012, 97, E1023–E1031. [Google Scholar] [CrossRef] [PubMed]
- Mirshahi, U.L.; Still, C.D.; Masker, K.K.; Gerhard, G.S.; Carey, D.J.; Mirshahi, T. The MC4R(I251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J. Clin. Endocrinol. Metab. 2011, 96, E:2088–E:2096. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.; Pucci, A.; Batterham, R.L. Roux-en-Y gastric bypass: Effects on feeding behavior and underlying mechanisms. J. Clin. Investig. 2015, 125, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Rodríguez, J.R.; Rodríguez-Cano, T.; Polo, F.; Sáenz-Mateos, L.; Agarrado, A.; Segura, E.; Casas, E.; Martin-Fernandez, J.; Beato-Fernandez, L.; Salas, E.; et al. The Neuroendocrine and Metabolic Outcomes of Bariatric Surgery Depend on Presurgical Control over Eating. Neuroendocrinology 2020, 110, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Shugart, Y.Y.; Chen, L.; Day, I.N.M.; Lewis, S.J.; Timpson, N.J.; Yuan, W.; Abdollahi, M.R.; Ring, S.M.; Ebrahim, S.; Golding, J.; et al. Two British women studies replicated the association between the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) and BMI. Eur. J. Hum. Genet. 2009, 17, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Peña, E.; Caixàs, A.; Arenas, C.; Pareja, R.; León-Mengíbar, J.; Rigla, M.; Powell, T.R.; Cardoner, N.; Rosa, A. Influence of the BDNF Val66Met polymorphism on weight loss after bariatric surgery: A 24-month follow-up. Surg. Obes. Relat. Dis. 2021, 17, 185–192. [Google Scholar] [CrossRef]
- Ciudin, A.; Fidilio, E.; Gutiérrez-Carrasquilla, L.; Caixàs, A.; Vilarrasa, N.; Pellitero, S.; Simo-Servat, A.; Vilallonga, R.; Ruiz, A.; Vilarrasa, N.; et al. A clinical-genetic score for predicting weight loss after bariatric surgery: The obegen study. J. Pers. Med. 2021, 11, 1040. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Ramos, E.J.B.; Goncalves, C.G.; Chen, C.; Meguid, M.M. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery 2005, 138, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Xanthakos, S. Bariatric surgery for extreme adolescent obesity: Indications, outcomes, and physiologic effects on the gut–brain axis. Pathophysiol 2008, 15, 135–146. [Google Scholar] [CrossRef][Green Version]
- Mumphrey, M.B.; Patterson, L.M.; Zheng, H.; Berthoud, H.R. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol. Motil. 2013, 25, e70–e79. [Google Scholar] [CrossRef]
- Gupta, A.; Miegueu, P.; Lapointe, M.; Poirier, P.; Martin, J.; Bastien, M.; Tiwari, S.; Cianflone, K. Acute post-bariatric surgery increase in orexin levels associates with preferential lipid profile improvement. PLoS ONE 2014, 9, e84803. [Google Scholar] [CrossRef]
- Cigdem Arica, P.; Kocael, A.; Tabak, O.; Taskin, M.; Zengin, K.; Uzun, H. Plasma ghrelin, leptin, and orexin-A levels and insulin resistance after laparoscopic gastric band applications in morbidly obese patients. Minerva Med. 2013, 104, 309–316. [Google Scholar]
- Ahmed, K.; Penney, N.; Darzi, A.; Purkayastha, S. Taste Changes after Bariatric Surgery: A Systematic Reivew. Obes. Surg. 2018, 28, 3321–3332. [Google Scholar] [CrossRef] [PubMed]
- Sewaybricker, L.E.; Schur, E.A. Is Bariatric Surgery Brain Surgery? Diabetes 2021, 70, 1244–1246. [Google Scholar] [CrossRef]
- De Lima-Júnior, J.C.; Velloso, L.A.; Geloneze, B. The Obese Brain—Effects of Bariatric Surgery on Energy Balance Neurocircuitry. Curr. Atheroscler. Rep. 2015, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Wilms, B.; Veit, R.; Ernst, B.; Thurnheer, M.; Kullmann, S.; Fritsche, A.; Birbaumer, N.; Preissl, H.; Schultes, B. Altered brain activity in severely obese women may recover after Roux-en y gastric bypass surgery. Int. J. Obes. 2014, 38, 341–348. [Google Scholar] [CrossRef]
- Van De Sande-Lee, S.; Pereira, F.R.S.; Cintra, D.E.; Fernandes, P.T.; Cardoso, A.R.; Garlipp, C.R.; Chaim, E.A.; Pareja, J.C.; Geloneze, B.; Li, L.M.; et al. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes 2011, 60, 1699–1704. [Google Scholar] [CrossRef]
- Zeighami, Y.; Iceta, S.; Dadar, M.; Pelletier, M.; Nadeau, M.; Biertho, L.; Lafortune, A.; Tchernof, A.; Fulton, S.; Evans, A.; et al. Spontaneous neural activity changes after bariatric surgery: A resting-state fMRI study. Neuroimage 2021, 241, 118419. [Google Scholar] [CrossRef]
- Ochner, C.N.; Stice, E.; Hutchins, E.; Afifi, L.; Geliebter, A.; Hirsch, J.; Teixeira, L. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience 2012, 209, 128–135. [Google Scholar] [CrossRef]
- Ochner, C.N.; Kwok, Y.; Conceição, E.; Pantazatos, S.P.; Puma, L.M.; Carnell, S.; Teixeira, J.; Hirsch, J.; Geliebter, A. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann. Surg. 2011, 253, 502–507. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Wang, H.; Mokadem, M. Role of the Autonomic Nervous System in Mechanism of Energy and Glucose Regulation Post Bariatric Surgery. Front. Neurosci. 2021, 15, 770690. [Google Scholar] [CrossRef] [PubMed]
- Hankir, M.; Bueter, M.; Gsell, W.; Seyfried, F.; Khalil, M.; Smith, K.L.; Bloom, S.R.; Bell, J.D.; le Roux, C. Increased energy expenditure in gastric bypass rats is not caused by activated brown adipose tissue. Obes. Facts 2012, 5, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Baraboi, E.D.; Li, W.; Labbé, S.M.; Roy, M.C.; Samson, P.; Hould, F.S.; Level, S.; Marceau, S.; Biertho, L.; Richard, D. Metabolic changes induced by the biliopancreatic diversion in diet-induced obesity in male rats: The contributions of sleeve gastrectomy and duodenal switch. Endocrinology 2015, 156, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Curry, T.B.; Somaraju, M.; Hines, C.N.; Groenewald, C.B.; Miles, J.M.; Joyner, M.J.; Charkoudian, N. Sympathetic support of energy expenditure and sympathetic nervous system activity after gastric bypass surgery. Obesity 2013, 21, 480–485. [Google Scholar] [CrossRef]
- Chu, Y.; Tian, L.; Herz, H.; Linden, B.; Morgan, D.A.; Naber, M.C.; Potthoff, M.; Rahmouni, K.; Mokadem, M. Gastric Bypass Sensitizes Sympathetic and Thermogenic Activity of Brown Adipose Tissue to Cold Exposure. Obes. Surg. 2021, 31, 4653–4656. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Abu El Haija, M.; Morgan, D.A.; Guo, D.; Song, Y.; Frank, A.; Tian, L.; Riedl, R.A.; Burnett, C.M.L.; Gao, Z.; et al. Endocannabinoid Receptor-1 and Sympathetic Nervous System Mediate the Beneficial Metabolic Effects of Gastric Bypass. Cell Rep. 2020, 33, 108270. [Google Scholar] [CrossRef]
- Stefanidis, A.; Oldfield, B.J. Neuroendocrine mechanisms underlying bariatric surgery: Insights from human studies and animal models. J. Neuroendocrinol. 2017, 29, e12534. [Google Scholar] [CrossRef] [PubMed]
- Ballsmider, L.A.; Vaughn, A.C.; David, M.; Hajnal, A.; Di Lorenzo, P.M.; Czaja, K. Sleeve gastrectomy and roux-en-y gastric bypass alter the gut-brain communication. Neural Plast. 2015, 2015, 601985. [Google Scholar] [CrossRef] [PubMed]
- Ionut, V.; Burch, M.; Youdim, A.; Bergman, R.N. Gastrointestinal hormones and bariatric surgery-induced weight loss. Obesity 2013, 21, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Prehn, K.; Profitlich, T.; Rangus, I.; Heßler, S.; Witte, A.V.; Grittner, U.; Ordemann, J.; Floel, A. Bariatric surgery and brain health—A longitudinal observational study investigating the effect of surgery on cognitive function and gray matter volume. Nutrients 2020, 12, 127. [Google Scholar] [CrossRef]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Psichas, A.; Reimann, F.; Gribble, F.M. Gut chemosensing mechanisms Find the latest version: Gut chemosensing mechanisms. J. Clin. Investig. 2015, 125, 908–917. [Google Scholar] [CrossRef]
- Bauer, P.V.; Hamr, S.C.; Duca, F.A. Regulation of energy balance by a gut-brain axis and involvement of the gut microbiota. Cell. Mol. Life Sci. 2016, 73, 737–755. [Google Scholar] [CrossRef]
- Dockray, G.J. Enteroendocrine cell signalling via the vagus nerve. Curr. Opin. Pharmacol. 2013, 13, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Cinci, L.; Rotondo, A.; Serio, R.; Faussone-Pellegrini, M.S.; Vannucchi, M.G.; Mule, F. Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol. Motil. 2010, 22, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Sayegh, A.I.; Covasa, M.; Ritter, R.C. Intestinal infusions of oleate and glucose activate distinct enteric neurons in the rat. Auton. Neurosci. 2004, 115, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.S.B.; Wilding, J.P.H.; Pinkney, J.H. Gut peptides and the regulation of appetite. Obes. Rev. 2006, 7, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Hayashida, T.; Kuroiwa, T.; Nakahara, K.; Ida, T.; Mondal, M.S.; Nakazato, M.; Kojima, M.; Kangawa, K. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J. Endocrinol. 2002, 174, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Callahan, H.S.; Cummings, D.E.; Pepe, M.S.; Breen, P.A.; Matthys, C.C.; Weigle, D.S. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J. Clin. Endocrinol. Metab. 2004, 89, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Bencivenga, R.; Longobardi, N.; Serritella, C.; Maj, M. Differential Responses of Circulating Ghrelin to High-Fat or High-Carbohydrate Meal in Healthy Women. J. Clin. Endocrinol. Metab. 2003, 88, 5510–5514. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Neary, N.M.; Halsey, T.J.; Small, C.J.; Martinez-Isla, A.M.; Ghatei, M.A.; Theodorou, N.A.; Bloom, S.R. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J. Clin. Endocrinol. Metab. 2005, 90, 4521–4524. [Google Scholar] [CrossRef]
- Le Roux, C.W.; Patterson, M.; Vincent, R.P.; Hunt, C.; Ghatei, M.A.; Bloom, S.R. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J. Clin. Endocrinol. Metab. 2005, 90, 1068–1071. [Google Scholar] [CrossRef]
- Tuero, C.; Valenti, V.; Rotellar, F.; Landecho, M.F.; Cienfuegos, J.A.; Frühbeck, G. Revisiting the Ghrelin Changes Following Bariatric and Metabolic Surgery. Obes. Surg. 2020, 30, 2763–2780. [Google Scholar] [CrossRef]
- Langer, F.B.; Hoda, M.A.R.; Bohdjalian, A.; Felberbauer, F.X.; Zacherl, J.; Wenzl, E.; Schindler, K.; Luger, A.; Ludvik, B.; Prager, G. Sleeve gastrectomy and gastric banding: Effects on plasma ghrelin levels. Obes. Surg. 2005, 15, 1024–1029. [Google Scholar] [CrossRef]
- Stoeckli, R.; Clianda, R.; Langer, I.; Keller, U. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes. Res. 2004, 12, 346–350. [Google Scholar] [CrossRef]
- Hady, H.R.; Dadan, J.; Gołaszewski, P. 100 obese patients after laparoscopic adjustable gastric banding—The influence on BMI, gherlin and insulin concentration, parameters of lipid balance and co-morbidities. Adv. Med. Sci. 2012, 57, 58–64. [Google Scholar] [CrossRef]
- Nijhuis, J.; Van Dielen, F.M.H.; Buurman, W.A.; Greve, J.W.M. Ghrelin, leptin and insulin levels after restrictive surgery: A 2-year follow-up study. Obes. Surg. 2004, 14, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Gelisgen, R.; Zengin, K.; Kocael, A.; Baysal, B.; Kocael, P.; Erman, H.; Taskin, M.; Uzun, H. Effects of laparoscopic gastric band applications on plasma and fundic acylated ghrelin levels in morbidly obese patients. Obes. Surg. 2012, 22, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Hanusch-Enserer, U.; Cauza, E.; Brabant, G.; Dunky, A.; Rosen, H.; Pacini, G.; Tuchler, H.; Prager, R.; Roden, M. Plasma ghrelin in obesity before and after weight loss after laparoscopical adjustable gastric banding. J. Clin. Endocrinol. Metab. 2004, 89, 3352–3358. [Google Scholar] [CrossRef][Green Version]
- Leonetti, F.; Silecchia, G.; Iacobellis, G.; Ribaudo, M.C.; Zappaterreno, A.; Tiberti, C.; Iannucci, C.V.; Perrotta, N.; Bacci, V.; Basso, M.S.; et al. Different plasma ghrelin levels after laparoscopic gastric bypass and adjustable gastric banding in morbid obese subjects. J. Clin. Endocrinol. Metab. 2003, 88, 4227–4231. [Google Scholar] [CrossRef]
- Cummings, D.E.; Purnell, J.Q.; Frayo, S.R. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Schindler, K.; Prager, G.; Ballaban, T.; Kretschmer, S.; Riener, R.; Buranyi, B.; Maier, C.; Luger, A.; Ludvik, B. Impact of laparoscopic adjustable gastric banding on plasma ghrelin, eating behaviour and body weight. Eur. J. Clin. Investig. 2004, 34, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Tsouristakis, A.I.; Febres, G.; McMahon, D.J.; Tchang, B.; Conwell, I.M.; Tsang, A.J.; Ahmed, L.; Bessler, M.; Korner, J. Long-Term Modulation of Appetitive Hormones and Sweet Cravings After Adjustable Gastric Banding and Roux-en-Y Gastric Bypass. Obes. Surg. 2019, 29, 3698–3705. [Google Scholar] [CrossRef]
- Adami, G.F.; Cordera, R.; Marinari, G.; Lamerini, G.; Andraghetti, G.; Scopinaro, N. Plasma Ghrelin Concentratin in the Short-Term following Biliopancreatic Diversion. Obes. Surg. 2003, 13, 889–892. [Google Scholar] [CrossRef]
- Tsoli, M.; Chronaiou, A.; Kehagias, I.; Kalfarentzos, F.; Alexandrides, T.K. Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: A comparative prospective study. Surg. Obes. Relat. Dis. 2013, 9, 667–677. [Google Scholar] [CrossRef]
- Garcia-Fuentes, E.; Garrido-Sanchez, L.; Garcia-Almeida, J.M.; Garcia-Arnes, J.; Gallego-Perales, J.L.; Rivas-Marin, J.; Morcillo, S.; Cardona, I.; Soriguer, C.F. Different effect of laparoscopic Roux-en-Y gastric bypass and open biliopancreatic diversion of Scopinaro on serum PYY and ghrelin levels. Obes. Surg. 2008, 18, 1424–1429. [Google Scholar] [CrossRef]
- García-Unzueta, M.T.; Fernández-Santiago, R.; Domínguez-Díez, A.; Vazquez-Salví, L.; Fernández-Escalante, J.C.; Amado, J.A. Fasting plasma ghrelin levels increase progressively after billiopancreatic diversion: One-year follow-up. Obes. Surg. 2005, 15, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Diez-Caballero, A.; Gil, M.J.; Montero, I.; Gómez-Ambrosi, J.; Salvador, J.; Cienfuegos, J.A. The decrease in plasma ghrelin concentrations following bariatric surgery depends on the functional integrity of the fundus. Obes. Surg. 2004, 14, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Kotidis, E.V.; Koliakos, G.; Papavramidis, T.S.; Papavramidis, S.T. The effect of biliopancreatic diversion with pylorus-preserving sleeve gastrectomy and duodenal switch on fasting serum ghrelin, leptin and adiponectin levels: Is there a hormonal contribution to the weight-reducing effect of this procedure? Obes. Surg. 2006, 16, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Yousseif, A.; Emmanuel, J.; Karra, E.; Millet, Q.; Elkalaawy, M.; Jenkinson, A.D.; Hashemi, M.; Adamo, M.; Finer, N.; Fiennes, A.G.; et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes. Surg. 2014, 24, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Ramón, J.M.; Salvans, S.; Crous, X.; Puig, S.; Goday, A.; Benaiges, D.; Trillo, L.; Pera, M.; Grander, L. Effect of Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy on Glucose and Gut Hormones: A Prospective Randomised Trial. J. Gastrointest. Surg. 2012, 16, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Daskalakis, M.; Kampa, M.; Peppe, A.; Papadakis, J.A.; Melissas, J. Alterations in gut hormones after laparoscopic sleeve gastrectomy: A prospective clinical and laboratory investigational study. Ann. Surg. 2013, 257, 647–654. [Google Scholar] [CrossRef]
- Karamanakos, S.N.; Vagenas, K.; Kalfarentzos, F.; Alexandrides, T.K. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-yy levels after roux-en-y gastric bypass and sleeve gastrectomy a prospective, double blind study. Ann. Surg. 2008, 247, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Bohdjalian, A.; Langer, F.B.; Shakeri-Leidenmühler, S.; Gfrerer, L.; Ludvik, B.; Zacherl, J.; Prager, G. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-Year results for weight loss and ghrelin. Obes. Surg. 2010, 20, 535–540. [Google Scholar] [CrossRef]
- Alamuddin, N.; Vetter, M.L.; Ahima, R.S.; Hesson, L.; Ritter, S.; Minnick, A.; Faulconbridge, L.F.; Allison, K.C.; Sarwer, D.B.; Chittams, J.; et al. Changes in Fasting and Prandial Gut and Adiposity Hormones Following Vertical Sleeve Gastrectomy or Roux-en-Y-Gastric Bypass: An 18-Month Prospective Study. Obes. Surg. 2017, 27, 1563–1572. [Google Scholar] [CrossRef]
- Cummings, D.E.; Shannon, M.H. Ghrelin and gastric bypass: Is there a hormonal contribution to surgical weight loss? J. Clin. Endocrinol. Metab. 2003, 88, 2999–3002. [Google Scholar] [CrossRef]
- Tritos, N.A.; Mun, E.; Bertkau, A.; Grayson, R.; Maratos-Flier, E.; Goldfine, A. Serum ghrelin levels in response to glucose load in obese subjects post-gastric bypass surgery. Obes. Res. 2003, 11, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Peterli, R.; Steinert, R.E.; Woelnerhanssen, B.; Peters, T.; Christoffel-Courtin, C.; Gass, M.; Kern, B.; von Fluee, M.; Beglinger, C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: A randomized, prospective trial. Obes. Surg. 2012, 22, 740–748. [Google Scholar] [CrossRef]
- Lin, E.; Gletsu, N.; Fugate, K.; McClusky, D.; Gu, L.H.; Zhu, J.L.; Ramshaw, B.L.; Papanicolaou, D.A.; Ziegler, T.R.; Smith, D. The effects of gastric surgery on systemic ghrelin levels in the morbidly obese. Arch. Surg. 2004, 139, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Kruljac, I.; Mirošević, G.; Kirigin, L.S.; Nikolić, M.; Ljubičić, N.; Budimir, I.; Beslin, B.M.; Vrkljan, M. Changes in metabolic hormones after bariatric surgery and their predictive impact on weight loss. Clin. Endocrinol. 2016, 85, 852–860. [Google Scholar] [CrossRef]
- Korner, J.; Bessler, M.; Cirilo, L.J.; Conwell, I.M.; Daud, A.; Restuccia, N.L.; Wardlaw, S.L. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide, Y.Y.; and insulin. J. Clin. Endocrinol. Metab. 2005, 90, 359–365. [Google Scholar] [CrossRef]
- Barazzoni, R.; Zanetti, M.; Nagliati, C.; Cattin, M.R.; Ferreira, C.; Giuricin, M.; Palmisano, S.; Edalucci, E.; Dore, F.; Guarnieri, G.; et al. Gastric Bypass Does Not Normalize Obesity-Related Changes in Ghrelin Profile and Leads to Higher Acylated Ghrelin Fraction. Obesity 2012, 21, 718–722. [Google Scholar] [CrossRef]
- Sundbom, M.; Holdstock, C.; Engström, B.E.; Karlsson, F.A. Early changes in ghrelin following Roux-en-Y gastric bypass: Influence of vagal nerve functionality? Obes. Surg. 2007, 17, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Faraj, M.; Havel, P.J.; Phélis, S.; Blank, D.; Sniderman, A.D.; Cianflone, K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J. Clin. Endocrinol. Metab. 2003, 88, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.K.; Randeva, M.S.; Miras, A.D. Potential Hormone Mechanisms of Bariatric Surgery. Curr. Obes. Rep. 2017, 6, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Dockray, G.J. Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 8–12. [Google Scholar] [CrossRef]
- Cheung, G.W.C.; Kokorovic, A.; Lam, C.K.L.; Chari, M.; Lam, T.K.T. Intestinal Cholecystokinin Controls Glucose Production through a Neuronal Network. Cell Metab. 2009, 10, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Buhmann, H.; Le Roux, C.W.; Bueter, M. The gutebrain axis in obesity. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 559–571. [Google Scholar] [CrossRef]
- Bliss, E.S.; Whiteside, E. The gut-brain axis, the human gut microbiota and their integration in the development of obesity. Front. Physiol. 2018, 9, 900. [Google Scholar] [CrossRef]
- Rehfeld, J.F. Cholecystokinin-From local gut hormone to ubiquitous messenger. Front. Endocrinol. 2017, 8, 47. [Google Scholar] [CrossRef]
- Kopin, A.S.; Mathes, W.F.; McBride, E.W.; Nguyen, M.; Al-Haider, W.; Schmitz, F.; Bonner-Weir, S.; Kanarek, R.; Beinborn, M. The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J. Clin. Investig. 1999, 103, 383–391. [Google Scholar] [CrossRef]
- West, D.B.; Fey, D.; Woods, S.C. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1984, 15, 776–787. [Google Scholar] [CrossRef]
- Fong, T.M. Advances in anti-obesity therapeutics. Expert Opin. Investig. Drugs 2005, 14, 243–250. [Google Scholar] [CrossRef]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gourcerol, G.; Yuan, P.Q.; Wu, S.V.; Million, M.; Larauche, M.; Tache, Y. Peripheral peptide YY inhibits propulsive colonic motor function through Y2 receptor in conscious mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G45–G56. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.B.; Grybäck, P.; Holst, J.J.; Hilsted, L.; Hellström, P.M.; Jacobsson, H.; Schmidt, P.T. Differential effect of PYY1-36 and PYY3-36 on gastric emptying in man. Regul. Pept. 2009, 158, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Seeley, R.J.; Sandoval, D.A. Signalling from the periphery to the brain that regulates energy homeostasis. Nat. Rev. Neurosci. 2018, 19, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Batterham, R.L.; Aylwin, S.J.B.; Patterson, M.; Borg, C.M.; Wynne, K.J.; Kent, A.; Vincent, R.P.; Gardiner, J.; Ghateir, M.A.; et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 2006, 147, 3–8. [Google Scholar] [CrossRef]
- Dirksen, C.; Bojsen-Møller, K.N.; Jørgensen, N.B.; Jacobsen, S.H.; Kristiansen, V.B.; Naver, L.S.; Hansen, D.L.; Worm, D.; Holst, J.J.; Madsbad, S. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia 2013, 56, 2679–2687. [Google Scholar] [CrossRef]
- Arakawa, R.; Febres, G.; Cheng, B.; Krikhely, A.; Bessler, M.; Korner, J. Prospective study of gut hormone and metabolic changes after laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. PLoS ONE 2020, 15, e0236133. [Google Scholar] [CrossRef]
- Gu, L.; Lin, K.; Du, N.; Ng, D.M.; Lou, D.; Chen, P. Differences in the effects of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass on gut hormones: Systematic and meta-analysis. Surg. Obes. Relat. Dis. 2021, 17, 444–455. [Google Scholar] [CrossRef]
- Drucker, D.J. The biology of incretin hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Meek, C.L.; Lewis, H.B.; Reimann, F.; Gribble, F.M.; Park, A.J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 2016, 77, 28–37. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Larsen, P.J.; Tang-Christensen, M.; Jessop, D.S. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology 1997, 138, 4445–4455. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.R.; Monteiro, M.; Small, C.J.; Sajedi, A.; Smith, K.L.; Parkinson, J.R.C.; Ghatei, M.A.; Bloom, S.R. The inhibitory effects of peripheral administration of peptide YY 3-36 and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005, 1044, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Dickson, S.L.; Shirazi, R.H.; Hansson, C.; Bergquist, F.; Nissbrandt, H.; Skibicka, K.P. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: A new role for mesolimbic GLP-1 receptors. J. Neurosci. 2012, 32, 4812–4820. [Google Scholar] [CrossRef] [PubMed]
- Skibicka, K.P. The central GLP-1: Implications for food and drug reward. Front. Neurosci. 2013, 7, 181. [Google Scholar] [CrossRef]
- Frühbeck, G. Aquaporin enters the picture. Nature 2005, 438, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Giménez, L.; Becerril, S.; Camões, S.P.; Da Silva, I.V.; Rodrigues, C.; Moncada, R.; Valenti, V.; Catalan, V.; Gomez-Ambrosi, J.; Miranda, J.P.; et al. Role of aquaporin-7 in ghrelin- and GLP-1-induced improvement of pancreatic β-cell function after sleeve gastrectomy in obese rats. Int. J. Obes. 2017, 41, 1394–1402. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Rössner, S.; Van Gaal, L.; Rissanen, A.; Niskanen, L.; Al Hakim, M.; Madsen, J.; Rasmussen, M.F.; Lean, M.E.; NN8022-1808 Study Group; et al. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009, 374, 1606–1616. [Google Scholar] [CrossRef]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jespen, C.H.; Kabisch, M.; Wilding, J.P. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef]
- McCarty, T.R.; Jirapinyo, P.; Thompson, C.C. Effect of Sleeve Gastrectomy on Ghrelin, GLP-1, PYY, and GIP Gut Hormones: A Systematic Review and Meta-analysis. Ann. Surg. 2020, 272, 72–80. [Google Scholar] [CrossRef]
- Svane, M.S.; Bojsen-Møller, K.N.; Martinussen, C.; Dirksen, C.; Madsen, J.L.; Reitelseder, S.; Holm, L.; Rehdeld, J.F.; Kristiansen, V.B.; van Hall, G.; et al. Postprandial Nutrient Handling and Gastrointestinal Hormone Secretion After Roux-en-Y Gastric Bypass vs. Sleeve Gastrectomy. Gastroenterology 2019, 156, 1627–1641.e1. [Google Scholar] [CrossRef]
- Papamargaritis, D.; Le Roux, C.W.; Sioka, E.; Koukoulis, G.; Tzovaras, G.; Zacharoulis, D. Changes in gut hormone profile and glucose homeostasis after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2013, 9, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Kokkinos, A.; Peradze, N.; Tentolouris, N.; Ghaly, W.; Pilitsi, E.; Upadhyay, J.; Alexandrou, A.; Mantzoros, C.S. Circulating levels of gastrointestinal hormones in response to the most common types of bariatric surgery and predictive value for weight loss over one year: Evidence from two independent trials. Metabolism 2019, 101, 153997. [Google Scholar] [CrossRef]
- Ribeiro-Parenti, L.; Jarry, A.C.; Cavin, J.B.; Willemetz, A.; Le Beyec, J.; Sannier, A.; Benadda, S.; Pelletier, A.L.; Hourseau, M.; Leger, T.; et al. Bariatric surgery induces a new gastric mucosa phenotype with increased functional glucagon-like peptide-1 expressing cells. Nat. Commun. 2021, 12, 110. [Google Scholar] [CrossRef]
- Dirksen, C.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Kielgast, U.; Jacobsen, S.H.; Clausen, T.R.; Worm, D.; Hartmann, B.; Rehfeld, J.F.; Damgaard, M.; et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int. J. Obes. 2013, 37, 1452–1459. [Google Scholar] [CrossRef]
- Dar, M.S.; Chapman, W.H.; Pender, J.R.; Drake, A.J.; O’Brien, K.; Tanenberg, R.J.; Dohm, G.L.; Pories, W.J. GLP-1 response to a mixed meal: What happens 10 years after Roux-en-Y Gastric Bypass (RYGB)? Obes. Surg. 2012, 22, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; MacHineni, S.; Oliván, B.; Teixeira, J.; McGinty, J.J.; Bawa, B.; Koshy, N.; Colarusso, A.; Laferrere, B. Superior Appetite Hormone Profile after Equivalent Weight Loss by Gastric Bypass Compared to Gastric Banding. Obesity 2010, 18, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Usinger, L.; Hansen, K.B.; Kristiansen, V.B.; Larsen, S.; Holst, J.J.; Knop, F.K. Gastric emptying of orally administered glucose solutions and incretin hormone responses are unaffected by laparoscopic adjustable gastric banding. Obes. Surg. 2011, 21, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Orskov, C.; Holst, J.J.; Knuhtsen, S.; Baldissera, F.G.A.; Poulsen, S.S.; Nielsen, V. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 1986, 119, 1467–1475. [Google Scholar] [CrossRef]
- Sinclair, E.M.; Drucker, D.J. Proglucagon-derived peptides: Mechanisms of action and therapeutic potential. Physiology 2005, 20, 357–365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jarrousse, C.; Bataille, D.; Jeanrenaud, B. A pure enteroglucagon, oxyntomodulin (glucagon 37), stimulates insulin release in perfused rat pancreas. Endocrinology 1984, 115, 102–105. [Google Scholar] [CrossRef]
- Jarrousse, C.; Audousset-Puech, M.P.; Dubrasquet, M.; Niel, H.; Martinez, J.; Bataille, D. Oxyntomodulin (glucagon-37) and its C-terminal octapeptide inhibit gastric acid secretion. FEBS Lett. 1985, 188, 81–84. [Google Scholar] [CrossRef][Green Version]
- Schjoldager, B.; Mortensen, P.E.; Myhre, J.; Christiansen, J.; Holst, J.J. Oxyntomodulin from distal gut—Role in regulation of gastric and pancreatic functions. Dig. Dis. Sci. 1989, 34, 1411–1419. [Google Scholar] [CrossRef]
- Baldissera, F.G.A.; Holst, J.J.; Knuhtsen, S.; Hilsted, L.; Nielsen, O.V. Oxyntomodulin (glicentin-(33-69)): Pharmacokinetics, binding to liver cell membranes, effects on isolated perfused pig pancreas, and secretion from isolated perfused lower small intestine of pigs. Regul. Pept. 1988, 21, 151–166. [Google Scholar] [CrossRef]
- Maida, A.; Lovshin, J.A.; Baggio, L.L.; Drucker, D.J. The glucagon-like peptide-1 receptor agonist oxyntomodulin enhances β-cell function but does not inhibit gastric emptying in mice. Endocrinology 2008, 149, 5670–5678. [Google Scholar] [CrossRef]
- Cohen, M.A.; Ellis, S.M.; Le Roux, C.W.; Batterham, R.L.; Park, A.; Patterson, M.; Frost, G.S.; Ghateir, M.A.; Bloom, S.R. Oxyntomodulin Suppresses Appetite and Reduces Food Intake in Humans. J. Clin. Endocrinol. Metab. 2003, 88, 4696–4701. [Google Scholar] [CrossRef]
- Baggio, L.L.; Huang, Q.; Brown, T.J.; Drucker, D.J. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 2004, 127, 546–558. [Google Scholar] [CrossRef]
- Pocai, A. Unraveling oxyntomodulin, GLP1’s enigmatic brother. J. Endocrinol. 2012, 215, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Wynne, K.; Park, A.J.; Small, C.J.; Patterson, M.; Ellis, S.M.; Murphy, K.G.; Wren, A.M.; Frost, G.S.; Meeran, K.; Ghateir, M.A.; et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: A double-blind, randomized, controlled trial. Diabetes 2005, 54, 2390–2395. [Google Scholar] [CrossRef]
- Wynne, K.; Park, A.J.; Small, C.J.; Meeran, K.; Ghatei, M.A.; Frost, G.S.; Bloom, S.R. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: A randomised controlled trial. Int. J. Obes. 2006, 30, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Alexiadou, K.; Cuenco, J.; Howard, J.; Wewer Albrechtsen, N.J.; Ilesanmi, I.; Kamocka, A.; Tharakan, G.; Behary, P.; Bech, P.R.; Ahmed, A.R.; et al. Proglucagon peptide secretion profiles in type 2 diabetes before and after bariatric surgery: 1-year prospective study. BMJ Open Diabetes Res. Care 2020, 8, e001076. [Google Scholar] [CrossRef] [PubMed]
- Laferrère, B.; Heshka, S.; Wang, K.; Khan, Y.; McGinty, J.; Teixeira, J.; Hart, A.B.; Olivan, B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007, 30, 1709–1716. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Ritz, C.; Albrechtsen, N.J.W.; Holst, J.J.; le Roux, C.W.; Sjödin, A. Oxyntomodulin and glicentin may predict the effect of bariatric surgery on food preferences and weight loss. J. Clin. Endocrinol. Metab. 2020, 105, dgaa061. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, R.A.; O’Harte, F.P.M.; Irwin, N.; Gault, V.A.; Flatt, P.R. Proglucagon-Derived Peptides as Therapeutics. Front. Endocrinol. 2021, 12, 585. [Google Scholar] [CrossRef]
- Price, S.L.; Minnion, J.S.; Bloom, S.R. Increased food intake with oxyntomodulin analogues. Peptides 2015, 73, 95–100. [Google Scholar] [CrossRef]
- Kirkegaard, P.; Moody, A.J.; Holst, J.J. Glicentin inhibits gastric acid secretion in the rat. Nature 1982, 297, 156–157. [Google Scholar] [CrossRef]
- Shibata, C.; Naito, H.; Jin, X.L.; Ueno, T.; Funayama, Y.; Fukushima, K.; Hashimoto, A.; Matsuno, S.; Sasaki, I. Effect of glucagon, glicentin, glucagon-like peptide-1 and -2 on interdigestive gastroduodenal motility in dogs with a vagally denervated gastric pouch. Scand. J. Gastroenterol. 2001, 36, 1049–1055. [Google Scholar] [PubMed]
- Ohneda, A.; Ohneda, K.; Nagsaki, T.; Sasaki, K. Insulinotropic action of human glicentin in dogs. Metabolism 1995, 44, 47–51. [Google Scholar] [CrossRef]
- Myojo, S.; Tsujikawa, T.; Sasaki, M.; Fujiyama, Y.; Bamba, T. Trophic effects of glicentin on rat small-intestinal mucosa in vivo and in vitro. J. Gastroenterol. 1997, 32, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Mantzoros, C.S. The role of glicentin and oxyntomodulin in human metabolism: New evidence and new directions. J. Clin. Endocrinol. Metab. 2020, 105, e3003–e3005. [Google Scholar] [CrossRef]
- Geneviève, R.; Magous, R.; Mochizuki, T.; Le Nguyen, D.; Martinez, J.; Bali, J.P.; Bataille, D.; Jarrousse, C. Glicentin and oxyntomodulin modulate both the phosphoinositide and cyclic adenosine monophosphate signaling pathways in gastric myocytes. Endocrinology 1999, 140, 22–28. [Google Scholar] [CrossRef]
- Poitou, C.; Bouaziz-Amar, E.; Genser, L.; Oppert, J.M.; Lacorte, J.M.; Le Beyec, J. Fasting levels of glicentin are higher in Roux-en-Y gastric bypass patients exhibiting postprandial hypoglycemia during a meal test. Surg. Obes. Relat. Dis. 2018, 14, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Raffort, J.; Panaïa-Ferrari, P.; Lareyre, F.; Bayer, P.; Staccini, P.; Fénichel, P.; Chinetti, G. Fasting Circulating Glicentin Increases After Bariatric Surgery. Obes. Surg. 2017, 27, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Kalafatakis, K.; Triantafyllou, K. Contribution of neurotensin in the immune and neuroendocrine modulation of normal and abnormal enteric function. Regul. Pept. 2011, 170, 7–17. [Google Scholar] [CrossRef]
- Feifel, D.; Goldenberg, J.; Melendez, G.; Shilling, P.D. The acute and subchronic effects of a brain-penetrating, neurotensin-1 receptor agonist on feeding, body weight and temperature. Neuropharmacology 2010, 58, 195–198. [Google Scholar] [CrossRef]
- Boules, M.; Cusack, B.; Zhao, L.; Fauq, A.; McCormick, D.J.; Richelson, E. A novel neurotensin peptide analog given extracranially decreases food intake and weight in rodents. Brain Res. 2000, 865, 35–44. [Google Scholar] [CrossRef]
- Cingöz, G.; Özyurt, G.; Uzun, H.; Doruk, Ö.G.; Küme, T.; Dündar, B.N.; Catli, G. High serum neurotensin level in obese adolescents is not associated with metabolic parameters, hyperphagia or food preference. J. Pediatr. Endocrinol. Metab. 2021, 34, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Eiken, A.; Fuglsang, S.; Eiken, M.; Svane, M.S.; Kuhre, R.E.; Wewer Albrechtsen, N.J.; Hansen, S.H.; Trammell, S.A.J.; Svenningsen, J.S.; Rehfeld, J.F.; et al. Bilio-enteric flow and plasma concentrations of bile acids after gastric bypass and sleeve gastrectomy. Int. J. Obes. 2020, 44, 1872–1883. [Google Scholar] [CrossRef]
- Svane, M.S.; Øhrstrøm, C.C.; Plamboeck, A.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Dirksen, C.; Matrinussen, C.; Vilsbøll, T.; Hartmann, B.; Deacon, C.F.; et al. Neurotensin secretion after Roux-en-Y gastric bypass, sleeve gastrectomy, and truncal vagotomy with pyloroplasty. Neurogastroenterol. Motil. 2021, 34, e14210. [Google Scholar] [CrossRef]
- McIntosh, C.H.S.; Widenmaier, S.; Kim, S.J. Chapter 15 Glucose-Dependent Insulinotropic Polypeptide (Gastric Inhibitory Polypeptide; GIP). Vitam. Horm. 2009, 80, 409–471. [Google Scholar] [PubMed]
- Kim, S.J.; Nian, C.; McIntosh, C.H.S. Activation of lipoprotein lipase by glucose-dependent insulinotropic polypeptide in adipocytes: A role for a protein kinase, B.; LKB1, and AMP-activated protein kinase cascade. J. Biol. Chem. 2007, 282, 8557–8567. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. The Role of GIP Receptor in the CNS for the Pathogenesis of Obesity. Diabetes 2021, 70, 1929–1937. [Google Scholar] [CrossRef]
- Miyawaki, K.; Yamada, Y.; Ban, N.; Ihara, Y.; Tsukiyama, K.; Zhou, H.; Fujimoto, S.; Oku, A.; Tsuda, K.; Toyokuni, S.; et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med. 2002, 8, 738–742. [Google Scholar] [CrossRef]
- Killion, E.A.; Lu, S.; Fort, M. Glucose-dependent insulinotropic polypeptide receptor therapies for the treatment of obesity, do agonists = antagonists? Endocr. Rev. 2020, 41, bnz002. [Google Scholar] [CrossRef]
- Adriaenssens, A.E.; Biggs, E.K.; Darwish, T.; Tadross, J.; Sukthankar, T.; Girish, M.; Polex-Wolf, J.; Lam, B.Y.; Zvetkova, I.; Pan, W.; et al. Glucose-Dependent Insulinotropic Polypeptide Receptor-Expressing Cells in the Hypothalamus Regulate Food Intake. Cell Metab. 2019, 30, 987–996.e6. [Google Scholar] [CrossRef]
- Zhang, Q.; Delessa, C.T.; Augustin, R.; Bakhti, M.; Colldén, G.; Drucker, D.J.; Feuchtinger, A.; Caceres, C.G.; Grandl, G.; Harger, A.; et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab. 2021, 33, 833–844.e5. [Google Scholar] [CrossRef]
- Bunt, J.C.; Blackstone, R.; Thearle, M.S.; Vinales, K.L.; Votruba, S.; Krakoff, J. Changes in glycemia, insulin and gut hormone responses to a slowly ingested solid low-carbohydrate mixed meal after laparoscopic gastric bypass or band surgery. Int. J. Obes. 2017, 41, 706–713. [Google Scholar] [CrossRef]
- Wallenius, V.; Dirinck, E.; Fändriks, L.; Maleckas, A.; le Roux, C.W.; Thorell, A. Glycemic Control after Sleeve Gastrectomy and Roux-En-Y Gastric Bypass in Obese Subjects with Type 2 Diabetes Mellitus. Obes. Surg. 2018, 28, 1461–1472. [Google Scholar] [CrossRef]
- Falkén, Y.; Hellström, P.M.; Holst, J.J.; Näslund, E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: Role of gut peptides. J. Clin. Endocrinol. Metab. 2011, 96, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Shak, J.R.; Roper, J.; Perez-Perez, G.I.; Tseng, C.H.; Francois, F.; Gamagaris, Z.; Patterson, C.; Weinshel, E.; Fielding, G.A.; Ren, C.; et al. The effect of laparoscopic gastric banding surgery on plasma levels of appetite-control, insulinotropic, and digestive hormones. Obes. Surg. 2008, 18, 1089–1096. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacobsen, S.H.; Olesen, S.C.; Dirksen, C.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Kielgast, U.; Worm, K.; Almdal, T.; Naver, L.S.; Hvolris, L.E.; et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes. Surg. 2012, 22, 1084–1096. [Google Scholar] [CrossRef]
- Grong, E.; Græslie, H.; Munkvold, B.; Arbo, I.B.; Kulseng, B.E.; Waldum, H.L.; Marvik, R. Gastrin Secretion After Bariatric Surgery—Response to a Protein-Rich Mixed Meal Following Roux-En-Y Gastric Bypass and Sleeve Gastrectomy: A Pilot Study in Normoglycemic Women. Obes. Surg. 2016, 26, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Stenström, B.; Zhao, C.M.; Tømmerås, K.; Arum, C.J.; Chen, D. Is gastrin partially responsible for body weight reduction after gastric bypass? Eur. Surg. Res. 2006, 38, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.M.; Mangelsdorf, D.J.; Kliewer, S.A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 2015, 26, 22–29. [Google Scholar] [CrossRef]
- Tomlinson, E.; Fu, L.; John, L.; Hultgren, B.; Huang, X.; Renz, M.; Stephan, J.P.; Tsai, S.P.; Powell-Braxton, L.; French, D.; et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002, 143, 1741–1747. [Google Scholar] [CrossRef]
- Holt, J.A.; Luo, G.; Billin, A.N.; Bisi, J.; McNeill, Y.Y.; Kozarsky, K.F.; Donahee, M.; Wang, Y.; Mansfield, T.A.; Kliewer, S.A.; et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003, 17, 1581–1591. [Google Scholar] [CrossRef]
- Ryan, K.K.; Tremaroli, V.; Clemmensen, C.; Kovatcheva-Datchary, P.; Myronovych, A.; Karns, R.; Wilson-Perez, H.E.; Sandoval, D.A.; Kohli, R.; Backhed, F.; et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014, 509, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, F.; Groen, A.K. FXR: The key to benefits in bariatric surgery? Nat. Med. 2014, 20, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Gallego-Escuredo, J.M.; Catalán, V.; Rodríguez, A.; Domingo, P.; Moncada, R.; Valenti, V.; Salvador, J.; Giralt, M.; Villarroya, F.; et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin. Nutr. 2017, 36, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, J.; Nemati, R.; Plank, L.D.; Murphy, R. Acute Changes of Bile Acids and FGF19 After Sleeve Gastrectomy and Roux-en-Y Gastric Bypass. Obes. Surg. 2019, 29, 3605–3621. [Google Scholar] [CrossRef] [PubMed]
- Martinez de la Escalera, L.; Kyrou, I.; Vrbikova, J.; Hainer, V.; Sramkova, P.; Fried, M.; Piya, M.; Kumar, S.; Tripathi, G.; McTermsn, P.G. Impact of gut hormone FGF-19 on type-2 diabetes and mitochondrial recovery in a prospective study of obese diabetic women undergoing bariatric surgery. BMC Med. 2017, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Nemati, R.; Lu, J.; Dokpuang, D.; Booth, M.; Plank, L.D.; Murphy, R. Increased Bile Acids and FGF19 After Sleeve Gastrectomy and Roux-en-Y Gastric Bypass Correlate with Improvement in Type 2 Diabetes in a Randomized Trial. Obes. Surg. 2018, 28, 2672–2686. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Wang, Q.; Billington, C.; Connett, J.; Ahmed, L.; Inabnet, W.; Chua, S.; Ikramuddin, S.; Korner, J. FGF 19 and Bile Acids Increase Following Roux-en-Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. Obes. Surg. 2016, 26, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Escalona, A.; Muñoz, R.; Irribarra, V.; Solari, S.; Allende, F.; Francisco Miquel, J. Bile acids synthesis decreases after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2016, 12, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Thöni, V.; Pfister, A.; Melmer, A.; Enrich, B.; Salzmann, K.; Kaser, S.; Lamina, C.; Ebenbichler, C.F.; Hackle, H.; Tilg, H.; et al. Dynamics of bile acid profiles, GLP-1, and FGF19 after laparoscopic gastric banding. J. Clin. Endocrinol. Metab. 2017, 102, 2974–2984. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.M.; Hayward, N.E.; Sless, R.T.; Garwood, P.; Rahmani, J. Effect of bariatric surgery on circulating FGF-19: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13038. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.M.; Ding, X.; Morgan, D.A.; Coate, K.C.; Bookout, A.L.; Rahmouni, K.; Kliewer, S.A.; Mangelsdorf, D.J. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014, 20, 670–677. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Kliewer, S.A.; Mangelsdorf, D.J. Endocrine fibroblast growth factors 15/19 and 21: From feast to famine. Genes Dev. 2012, 26, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Bookout, A.L.; De Groot, M.H.M.; Owen, B.M.; Lee, S.; Gautron, L.; Lawrence, H.L.; Ding, X.; Elmquist, J.K.; Takahashi, J.S.; Mangelsdorf, D.J.; et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 2013, 19, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Morgan, D.A.; Rahmouni, K.; Sonoda, J.; Fu, X.; Burgess, S.C.; Holland, W.L.; Kliewer, S.A.; Manglesdorf, D.J. FGF19, FGF21, and an FGFR1/β-Klotho-Activating Antibody Act on the Nervous System to Regulate Body Weight and Glycemia. Cell Metab. 2017, 26, 709–718.e3. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Düfer, M.; Hörth, K.; Wagner, R.; Schittenhelm, B.; Prowald, S.; Wagner, T.F.J.; Oberwinkler, J.; Lukowski, R.; Gonzalez, F.J.; Krippeit-Drews, P.; et al. Bile acids acutely stimulate insulin secretion of mouse β-cells via farnesoid X receptor activation and K ATP channel inhibition. Diabetes 2012, 61, 1479–1489. [Google Scholar] [CrossRef]
- Lundåsen, T.; Gälman, C.; Angelin, B.; Rudling, M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J. Intern. Med. 2006, 260, 530–536. [Google Scholar] [CrossRef]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C.L.; McDonald, J.G.; Luo, G.; Jones, S.A.; Goodwin, B.; Richardson, J.A.; et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005, 2, 217–225. [Google Scholar] [CrossRef]
- Inagaki, T.; Moschetta, A.; Lee, Y.K.; Peng, L.; Zhao, G.; Downes, M.; Yu, R.T.; Shelton, J.M.; Richardson, J.A.; Repa, J.J.; et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 3920–3925. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.G.M.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef]
- Mertens, K.L.; Kalsbeek, A.; Soeters, M.R.; Eggink, H.M. Bile acid signaling pathways from the enterohepatic circulation to the central nervous system. Front. Neurosci. 2017, 11, 617. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Quinn, M.; Divan, A.; Grant, S.; Patel, N.; Newell-Rogers, K.; DeMorrow, S. Suppression of the HPA axis during cholestasis can be attributed to hypothalamic bile acid signaling. Mol. Endocrinol. 2015, 29, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Schubring, S.R.; Fleischer, W.; Lin, J.S.; Haas, H.L.; Sergeeva, O.A. The bile steroid chenodeoxycholate is a potent antagonist at NMDA and GABA A receptors. Neurosci. Lett. 2012, 506, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Jahansouz, C.; Xu, H.; Hertzel, A.V.; Serrot, F.J.; Kvalheim, N.; Cole, A.; Abraham, A.; Luthra, G.; Ewing, K.; Lesile, D.B.; et al. Bile acids increase independently from hypocaloric restriction after bariatric surgery. Ann. Surg. 2016, 264, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Peterli, R.; Keller, S.; Meyer-Gerspach, A.C.; Drewe, J.; Peters, T.; Beglinger, C. Bile acids and gut peptide secretion after bariatric surgery: A 1-year prospective randomized pilot trial. Obesity 2013, 21, E660–E668. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Houten, S.M.; Bianco, A.C.; Bernier, R.; Larsen, P.R.; Holst, J.J.; Badman, M.K.; Marator-Flier, E.; Mun, E.C.; Pihlajamaki, J.; et al. Serum bile acids are higher in humans with prior gastric bypass: Potential contribution to improved glucose and lipid metabolism. Obesity 2009, 17, 1671–1677. [Google Scholar] [CrossRef]
- Browning, M.G.; Pessoa, B.M.; Khoraki, J.; Campos, G.M. Changes in Bile Acid Metabolism, Transport, and Signaling as Central Drivers for Metabolic Improvements After Bariatric Surgery. Curr. Obes. Rep. 2019, 8, 175–184. [Google Scholar] [CrossRef]
- Cole, A.J.; Teigen, L.M.; Jahansouz, C.; Earthman, C.P.; Sibley, S.D. The Influence of Bariatric Surgery on Serum Bile Acids in Humans and Potential Metabolic and Hormonal Implications: A Systematic Review. Curr. Obes. Rep. 2015, 4, 441–450. [Google Scholar] [CrossRef]
- Whitmore, T.E.; Holloway, J.L.; Lofton-Day, C.E.; Maurer, M.F.; Chen, L.; Quinton, T.J.; Vincent, J.B.; Scherer, S.W.; Lok, S. Human secretin (SCT): Gene structure, chromosome location, and distribution of mRNA. Cytogenet. Cell Genet. 2000, 90, 47–52. [Google Scholar] [CrossRef]
- Sekar, R.; Chow, B.K. Lipolytic actions of secretin in mouse adipocytes. J. Lipid Res. 2014, 55, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.Y.; Chu, J.Y.S.; Chow, B.K.C. Central and peripheral administration of secretin inhibits food intake in mice through the activation of the melanocortin system. Neuropsychopharmacology 2011, 36, 459–471. [Google Scholar] [CrossRef]
- Li, Y.; Schnabl, K.; Gabler, S.M.; Willershäuser, M.; Reber, J.; Karlas, A.; Laurila, S.; Lahesmaa, M.; Din, M.U.; Bast-Habesbrunner, A.; et al. Secretin-Activated Brown Fat Mediates Prandial Thermogenesis to Induce Satiation. Cell 2018, 175, 1561–1574.e12. [Google Scholar] [CrossRef]
- Nergård, B.J.; Lindqvist, A.; Gislason, H.G.; Groop, L.; Ekelund, M.; Wierup, N.; Hedenbro, J.L. Mucosal glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide cell numbers in the super-obese human foregut after gastric bypass. Surg. Obes. Relat. Dis. 2015, 11, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Rhee, N.A.; Wahlgren, C.D.; Pedersen, J.; Mortensen, B.; Langholz, E.; Wandall, E.P.; Friis, S.U.; Vilmann, P.; Paulsen, S.J.; Kristiansen, V.B.; et al. Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia 2015, 58, 2254–2258. [Google Scholar] [CrossRef]
- Chen, X.; Shu, X.; Cong, Z.K.; Jiang, Z.Y.; Jiang, H. Nesfatin-1 acts on the dopaminergic reward pathway to inhibit food intake. Neuropeptides 2015, 53, 45–50. [Google Scholar] [CrossRef]
- Anwar, G.M.; Yamamah, G.; Ibrahim, A.; El-Lebedy, D.; Farid, T.M.; Mahmoud, R. Nesfatin-1 in childhood and adolescent obesity and its association with food intake, body composition and insulin resistance. Regul. Pept. 2014, 188, 21–24. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, J.; Tang, Y.; Bi, F.; Liu, J.N. The novel function of nesfatin-1: Anti-hyperglycemia. Biochem. Biophys. Res. Commun. 2010, 391, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, D.H.; Martin, J.; Shimizu, H.; Tagaya, Y.; Tsuchiya, T.; Marceau, S.; Biertho, L.; Bastien, M.; Caron-Cantin, S.M.; Simard, S.; et al. Association between nesfatin-1 levels and metabolic improvements in severely obese patients who underwent biliopancreatic derivation with duodenal switch. Peptides 2016, 86, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Majorczyk, M.; Staszkiewicz, M.; Szklarczyk, J.; Major, P.; Pisarska, M.; Wysocki, M.; Stefura, T.; Kacprzyk, A.; Dros, J.; Holda, M.K.; et al. The influence of bariatric surgery on serum levels of irisin and nesfatin-1. Acta Chir. Belg. 2019, 119, 363–369. [Google Scholar] [CrossRef]
- Lee, W.-J.; Chen, C.-Y.; Ser, K.-H.; Chong, K.; Chen, S.-C.; Lee, P.-C.; Liao, Y.-D.; Lee, S.-D. Differential Influences of Gastric Bypass and Sleeve Gastrectomy on Plasma Nesfatin-1 and Obestatin Levels in Patients with Type 2 Diabetes Mellitus. Curr. Pharm. Des. 2013, 19, 5830–5835. [Google Scholar] [CrossRef] [PubMed]
- Margolskee, R.F. Molecular mechanisms of bitter and sweet taste transduction. J. Biol. Chem. 2002, 277, 1–4. [Google Scholar] [CrossRef]
- Steensels, S.; Lannoo, M.; Avau, B.; Laermans, J.; Vancleef, L.; Farre, R.; Verveke, K.; Depoortere, I. The role of nutrient sensing in the metabolic changes after gastric bypass surgery. J. Endocrinol. 2017, 232, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Mokadem, M.; Zechner, J.F.; Margolskee, R.F.; Drucker, D.J.; Aguirre, V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol. Metab. 2014, 3, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G. Gastrointestinal hormones: Uroguanylin-a new gut-derived weapon against obesity? Nat. Rev. Endocrinol. 2012, 8, 5–6. [Google Scholar] [CrossRef]
- Currie, M.G.; Fok, K.F.; Kato, J.; Moore, R.J.; Hamra, F.K.; Duffin, K.L.; Smith, C.E. Guanylin: An endogenous activator of intestinal guanylate cyclase. Proc. Natl. Acad. Sci. USA 1992, 89, 947–951. [Google Scholar] [CrossRef]
- Valentino, M.A.; Lin, J.E.; Snook, A.E.; Li, P.; Kim, G.W.; Marszalowicz, G.; Magee, M.S.; Hyslop, T.; Schulz, S.; Waldman, S.A. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J. Clin. Investig. 2011, 121, 3578–3588. [Google Scholar] [CrossRef]
- Di Guglielmo, M.D.; Tonb, D.; He, Z.; Adeyemi, A.; Van Golen, K.L. Pilot Study Measuring the Novel Satiety Hormone, Pro-Uroguanylin, in Adolescents with and Without Obesity. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 489–495. [Google Scholar] [CrossRef]
- Torquati, A.; Shantavasinkul, P.C.; Omotosho, P.; Corsino, L.; Spagnoli, A. Perioperative changes in prouroguanylin hormone response in severely obese subjects after bariatric surgery. Surgery 2019, 166, 456–459. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Ibanez, P.; Vila, N.; Margall, M.A.; Moncada, R.; et al. Guanylin and uroguanylin stimulate lipolysis in human visceral adipocytes. Int. J. Obes. 2016, 40, 1405–1415. [Google Scholar] [CrossRef]
- Gargantini, E.; Grande, C.; Trovato, L.; Ghigo, E.; Granata, R. The role of obestatin in glucose and lipid metabolism. Horm. Metab. Res. 2013, 45, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Kobelt, P.; Wisser, A.S.; Stengel, A.; Goebel, M.; Bannert, N.; Gourcerol, G.; Inhoff, T.; Noetzel, S.; Wiedenmann, B.; Klapp, B.F.; et al. Peripheral obestatin has no effect on feeding behavior and brain Fos expression in rodents. Peptides 2008, 29, 1018–1027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gourcerol, G.; St-Pierre, D.H.; Taché, Y. Lack of obestatin effects on food intake: Should obestatin be renamed ghrelin-associated peptide (GAP)? Regul. Pept. 2007, 141, 1–7. [Google Scholar] [CrossRef]
- Bassil, A.K.; Häglund, Y.; Brown, J.; Rudholm, T.; Hellström, P.M.; Näslund, E.; Lee, K.; Sanger, G.J. Little or no ability of obestatin to interact with ghrelin or modify motility in the rat gastrointestinal tract. Br. J. Pharmacol. 2007, 150, 58–64. [Google Scholar] [CrossRef]
- Nakahara, T.; Harada, T.; Yasuhara, D.; Shimada, N.; Amitani, H.; Sakoguchi, T.; Kamiji, M.M.; Asakawa, A.; Inui, A. Plasma Obestatin Concentrations Are Negatively Correlated with Body Mass Index, Insulin Resistance Index, and Plasma Leptin Concentrations in Obesity and Anorexia Nervosa. Biol. Psychiatry 2008, 64, 252–255. [Google Scholar] [CrossRef]
- Roth, C.L.; Reinehr, T.; Schernthaner, G.H.; Kopp, H.P.; Kriwanek, S.; Schernthaner, G. Ghrelin and obestatin levels in severely obese women before and after weight loss after Roux-en-Y gastric bypass surgery. Obes. Surg. 2009, 19, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Estall, J.L.; Drucker, D.J. Dual Regulation of Cell Proliferation and Survival via Activation of Glucagon-Like Peptide-2 Receptor Signaling. J. Nutr. 2003, 133, 3708–3711. [Google Scholar] [CrossRef][Green Version]
- Martin, G.R.; Wallace, L.E.; Hartmann, B.; Holst, J.J.; Demchyshyn, L.; Toney, K.; Sigalet, D.L. Nutrient-stimulated GLP-2 release and crypt cell proliferation in experimental short bowel syndrome. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288, 431–439. [Google Scholar] [CrossRef]
- De Heer, J.; Pedersen, J.; Ørskov, C.; Holst, J.J. The alpha cell expresses glucagon-like peptide-2 receptors and glucagon-like peptide-2 stimulates glucagon secretion from the rat pancreas. Diabetologia 2007, 50, 2135–2142. [Google Scholar] [CrossRef]
- Guan, X.; Karpen, H.E.; Stephens, J.; Bukowski, J.T.; Niu, S.; Zhang, G.; Stoll, B.; Finegold, M.J.; Holst, J.J.; Hadsell, D.; et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology 2006, 130, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, X. GLP-2 potentiates L-type Ca 2+ channel activity associated with stimulated glucose uptake in hippocampal neurons. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E156–E166. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Sharp, J.W.; Brownfield, M.S.; Raybould, H.E.; Ney, D.M. Localization and activation of glucagon-like peptide-2 receptors on vagal afferents in the rat. Endocrinology 2007, 148, 1954–1962. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. The CNS glucagon-like peptide-2 receptor in the control of energy balance and glucose homeostasis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, R585–R596. [Google Scholar] [CrossRef]
- Sun, H.; Meng, K.; Hou, L.; Shang, L.; Yan, J. Melanocortin receptor-4 mediates the anorectic effect induced by the nucleus tractus solitarius injection of glucagon-like Peptide-2 in fasted rats. Eur. J. Pharmacol. 2021, 901, 174072. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Borg, C.; Wallis, K.; Vincent, R.P.; Bueter, M.; Goodlad, R.; Ghatei, M.A.; Patel, A.; Bloom, S.R.; Aylwin, S.J. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann. Surg. 2010, 252, 50–56. [Google Scholar] [CrossRef]
- Cazzo, E.; Pareja, J.C.; Chaim, E.A.; Geloneze, B.; Barreto, M.R.L.; Magro, D.O. GLP-1 and GLP-2 Levels are Correlated with Satiety Regulation After Roux-en-Y Gastric Bypass: Results of an Exploratory Prospective Study. Obes. Surg. 2017, 27, 703–708. [Google Scholar] [CrossRef]
- Romero, F.; Nicolau, J.; Flores, L.; Casamitjana, R.; Ibarzabal, A.; Lacy, A.; Vidal, J. Comparable early changes in gastrointestinal hormones after Sleeve gastrectomy and Roux-en-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg. Endosc. 2012, 26, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B.S. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta-Mol. Basis Dis. 2014, 1842, 414–423. [Google Scholar] [CrossRef]
- Sinha, M.K.; Ohannesian, J.P.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Magosin, S.; Marco, S.; Caro, J.F. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J. Clin. Investig. 1996, 97, 1344–1347. [Google Scholar] [CrossRef]
- Caron, A.; Lemko, H.M.D.; Castorena, C.M.; Fujikawa, T.; Lee, S.; Lord, C.C.; Ahmed, N.; Lee, C.E.; Holland, W.L.; Liu, C.; et al. POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. Elife 2018, 7, e33710. [Google Scholar] [CrossRef]
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; de la Higuera, M.; Frühbeck, G. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef]
- Procaccini, C.; Jirillo, E.; Matarese, G. Leptin as an immunomodulator. Mol. Aspects Med. 2012, 33, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Kim, K.W.; Kim, M.S. Leptin signalling pathways in hypothalamic neurons. Cell. Mol. Life Sci. 2016, 73, 1457–1477. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.A.; do Carmo, J.M.; Hall, J.E. CNS Regulation of Glucose Homeostasis: Role of the Leptin-Melanocortin System. Curr. Diabetes Rep. 2020, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Margalet, V.; Fernández-Riejos, P.; Najib, S.; Santos-Alvarez, J.; Martín-Romero, C.; Pérez-Pérez, A.; Gonzalez-Yanes, C.; Sanchez-Margalet, V. Role of leptin in the activation of immune cells. Mediat. Inflamm. 2010, 2010, 568343. [Google Scholar]
- Molina, A.; Vendrell, J.; Gutiérrez, C.; Simón, I.; Masdevall, C.; Soler, J.; Gomez, J.M. Insulin resistance, leptin and TNF-α system in morbidly obese women after gastric bypass. Obes. Surg. 2003, 13, 615–621. [Google Scholar] [CrossRef]
- Uzun, H.; Zengin, K.; Taskin, M.; Aydin, S.; Simsek, G.; Dariyerli, N. Changes in leptin, plasminogen activator factor and oxidative stress in morbidly obese patients following open and laparoscopic Swedish adjustable gastric banding. Obes. Surg. 2004, 14, 659–665. [Google Scholar] [CrossRef]
- Holdstock, C.; Engström, B.E.; Öhrvall, M.; Lind, L.; Sundbom, M.; Karlsson, F.A. Ghrelin and adipose tissue regulatory peptides: Effect of gastric bypass surgery in obese humans. J. Clin. Endocrinol. Metab. 2003, 88, 3177–3183. [Google Scholar] [CrossRef]
- Edwards, C.; Hindle, A.K.; Fu, S.; Brody, F. Downregulation of leptin and resistin expression in blood following bariatric surgery. Surg. Endosc. 2011, 25, 1962–1968. [Google Scholar] [CrossRef]
- Faramia, J.; Ostinelli, G.; Drolet-Labelle, V.; Picard, F.; Tchernof, A. Metabolic adaptations after bariatric surgery: Adipokines, myokines and hepatokines. Curr. Opin. Pharmacol. 2020, 52, 67–74. [Google Scholar] [CrossRef]
- Sebunova, N.; Stsepetova, J.; Kullisaar, T.; Suija, K.; Ratsep, A.; Junkin, I.; Soeorg, H.; Lember, M.; Sillakivi, T.; Mandar, R. Changes in adipokine levels and metabolic profiles following bariatric surgery. BMC Endocr. Disord. 2022, 22, 33. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Gómez-Ambrosi, J. Rationale for the existence of additional adipostatic hormones. FASEB J. 2001, 15, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, V.L.; MacDonald, P.E.; Klip, A. The cell biology of systemic insulin function. J. Cell Biol. 2018, 217, 2273–2289. [Google Scholar] [CrossRef] [PubMed]
- Ramnanan, C.J.; Edgerton, D.S.; Rivera, N.; Irimia-Dominguez, J.; Farmer, B.; Neal, D.W.; Lautz, M.; Donahue, P.; Meyer, C.M.; Roach, P.J.; et al. Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes 2010, 59, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Kleinridders, A.; Small, D.M.; Fritsche, A.; Häring, H.U.; Preissl, H.; Heni, M. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 2020, 8, 524–534. [Google Scholar] [CrossRef]
- Meistas, M.T.; Margolis, S.; Kowarski, A.A. Hyperinsulinemia of obesity is due to decreased clearance of insulin. Am. J. Physiol.-Endocrinol. Metab. 1983, 215, E155–E159. [Google Scholar] [CrossRef] [PubMed]
- Anderwald, C.H.; Tura, A.; Promintzer-Schifferl, M.; Prager, G.; Stadler, M.; Ludvik, B.; Esterbauer, H.; Bischof, M.G.; Luger, A.; Pacini, G.; et al. Alterations in gastrointestinal, endocrine, and metabolic processes after bariatric Roux-en-Y gastric bypass surgery. Diabetes Care 2012, 35, 2580–2587. [Google Scholar] [CrossRef]
- Salehi, M.; Prigeon, R.L.; D’Alessio, D.A. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 2011, 60, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, E.; Rask, E.; Szabo, E.; Naslund, I.; Ottosson, J. The Effect of Laparoscopic Gastric Bypass Surgery on Insulin Resistance and Glycosylated Hemoglobin A1c: A 2-Year Follow-up Study. Obes. Surg. 2020, 30, 3489–3495. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Cummings, D.E. Changes of insulin sensitivity and secretion after bariatric/metabolic surgery. Surg. Obes. Relat. Dis. 2016, 12, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Gagner, M.; Gentileschi, P.; Kini, S.; Fukuyama, S.; Feng, J.; Diamond, E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann. Surg. 2004, 240, 236–242. [Google Scholar] [CrossRef]
- Salinari, S.; Carr, R.D.; Guidone, C.; Bertuzzi, A.; Cercone, S.; Riccioni, M.E.; Manto, A.; Ghirlanda, G.; Mingrone, G. Nutrient infusion bypassing duodenum-jejunum improves insulin sensitivity in glucose-tolerant and diabetic obese subjects. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E59–E66. [Google Scholar] [CrossRef]
- Öztürk Özkan, G. Effects of Nesfatin-1 on Food Intake and Hyperglycemia. J. Am. Coll. Nutr. 2020, 39, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, 1556–1573. [Google Scholar] [CrossRef]
- Marchesi, J.R. Human distal gut microbiome. Environ. Microbiol. 2011, 13, 3088–3102. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Cani, P.D. Gutmicrobiota and obesity: Lessons from the microbiome. Brief. Funct. Genom. 2013, 12, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Cardinelli, C.S.; Sala, P.C.; Alves, C.C.; Torrinhas, R.S.; Waitzberg, D.L. Influence of Intestinal Microbiota on Body Weight Gain: A Narrative Review of the Literature. Obes. Surg. 2015, 25, 346–353. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Villanueva-Millán, M.J.; Pérez-Matute, P.; Oteo, J.A. Gut microbiota: A key player in health and disease. A review focused on obesity. J. Physiol. Biochem. 2015, 71, 509–525. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.D.; Wang, Y.D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Wu, W.; Qin, Y.; Xue, W.; Li, J.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Gou, Y.K.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-Induced Obesity Is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Neslon, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.P.; Shanahan, F. Gut microbiota and obesity: Role in aetiology and potential therapeutic target. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; de Preter, V.; Windey, K.; Verbeke, K. Functional analysis of colonic bacterial metabolism: Relevant to health? Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G1. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.J.; Zhou, J.; McCutcheon, K.L.; Raggio, A.M.; Bateman, H.G.; Todd, E.; Jones, C.K.; Tulley, R.T.; Melton, S.; Martin, R.J.; et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity 2006, 14, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.D.; Chen, F.Q.; Wu, T.X.; Tang, H.G.; Zhao, Z.Y. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J. Zhejiang Univ. Sci. B 2009, 10, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Bomhof, M.R.; Saha, D.C.; Reid, D.T.; Paul, H.A.; Reimer, R.A. Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity 2014, 22, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Tsujimoto, G.; Kimura, I. Regulation of energy homeostasis by GPR41. Front. Endocrinol. 2014, 5, 81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bindels, L.B.; Dewulf, E.M.; Delzenne, N.M. GPR43/FFA2: Physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 2013, 34, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Egerod, K.L.; Christiansen, S.H.; Gille, A.; Offermanns, S.; Schwartz, T.W.; Moller, M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015, 290, 126–137. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van De Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef]
- Hur, K.Y.; Lee, M.S. Gut microbiota and metabolic disorders. Diabetes Metab. J. 2015, 39, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Compare, D.; Rocco, A.; Sanduzzi Zamparelli, M.; Nardone, G. The Gut Bacteria-Driven Obesity Development. Dig. Dis. 2016, 34, 221–229. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Screnzel, J.; Francois, P.; Cani, P. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Zuo, D.C.; Choi, S.; Shahi, P.K.; Kim, M.Y.; Park, C.G.; Kim, Y.D.; Lee, J.; Chang, I.Y.; So, I.; Jun, J.Y. Inhibition of pacemaker activity in interstitial cells of Cajal by LPS via NF-kB and MAP kinase. World J. Gastroenterol. 2013, 19, 1210–1218. [Google Scholar] [CrossRef]
- Stasi, C.; Sadalla, S.; Milani, S. The Relationship Between the Serotonin Metabolism, Gut-Microbiota and the Gut-Brain Axis. Curr. Drug Metab. 2019, 20, 646–655. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Sanchez, M.; Darimont, C.; Panahi, S.; Drapeau, V.; Marette, A.; Taylor, V.H.; Dore, J.; Tremblay, A. Effects of a diet-based weight-reducing program with probiotic supplementation on satiety efficiency, eating behaviour traits, and psychosocial behaviours in obese individuals. Nutrients 2017, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Darimont, C.; Drapeau, V.; Emady-Azar, S.; Lepage, M.; Rezzonico, E.; Ngom-Bru, C.; Berge, B.; Philippe, L.; Ammon-Zuffrey, C.; et al. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br. J. Nutr. 2014, 111, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Evrensel, A.; Ünsalver, B.Ö.; Ceylan, M.E. Immune-kynurenine pathways and the gut microbiota-brain axis in anxiety disorders. Adv. Exp. Med. Biol. 2020, 1191, 155–167. [Google Scholar] [PubMed]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan metabolism and gut-brain homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.P.; Paziuk, M.; Luevano, J.M.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra41. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fandriks, L.; le Roux, C.W.; Nielsen, J.; Backhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef]
- Dalmas, E.; Rouault, C.; Abdennour, M.; Rovere, C.; Rizkalla, S.; Bar-Hen, A.; Nahon, J.-L.; Bouillot, J.-L.; Guerre-Millo, M.; Clement, K.; et al. Variations in circulating inflammatory factors are related to changes in calorie and carbohydrate intakes early in the course of surgery-induced weight reduction1-3. Am. J. Clin. Nutr. 2011, 94, 450–458. [Google Scholar] [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jorgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Luijten, J.C.; Vugts, G.; Nieuwenhuijzen, G.A.; Luyer, M.D. The Importance of the Microbiome in Bariatric Surgery: A Systematic Review. Obes. Surg. 2019, 29, 2338–2349. [Google Scholar] [CrossRef] [PubMed]
- Debédat, J.; Clément, K.; Aron-Wisnewsky, J. Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery. Curr. Obes. Rep. 2019, 8, 229–242. [Google Scholar] [CrossRef]
- Anhê, F.F.; Varin, T.V.; Schertzer, J.D.; Marette, A. The Gut Microbiota as a Mediator of Metabolic Benefits after Bariatric Surgery. Can. J. Diabetes 2017, 41, 439–447. [Google Scholar] [CrossRef]
- Martinez, K.B.; Pierre, J.F.; Chang, E.B. The Gut Microbiota: The Gateway to Improved Metabolism. Gastroenterol. Clin. N. Am. 2016, 45, 601–614. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.M.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Francois, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef]
- Yan, M.; Song, M.-M.; Bai, R.-X.; Cheng, S.; Yan, W.-M. Effect of Roux-en-Y gastric bypass surgery on intestinal Akkermansia muciniphila. World J. Gastrointest. Surg. 2016, 8, 301. [Google Scholar] [CrossRef]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Dore, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Sioka, E.; Chatedaki, C.; Zacharoulis, D. Impact of Bariatric Surgery on Metabolic and Gut Microbiota Profile: A Systematic Review and Meta-analysis. Obes. Surg. 2017, 27, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Tadross, J.A.; Le Roux, C.W. The mechanisms of weight loss after bariatric surgery. Int. J. Obes. 2009, 33, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, Z.E.; Dibaise, J.K.; Isern, N.G.; Hoyt, D.W.; Marcus, A.K.; Kang, D.W.; Crowell, M.D.; Rittmann, B.E.; Krajmalnik-Brown, R. Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. ISME J. 2017, 11, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.A.; Pedreros, J.P.; Turiel, D.; Quezada, N.; Pimentel, F.; Escalona, A.; Garrido, D. Distinct patterns in the gut microbiota after surgical or medical therapy in obese patients. PeerJ 2017, 5, e3443. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Le May, C.; Dastugue, A.; Ayer, A.; Blanchard, C.; Martin, J.C.; de Barros, J.-P.; Delaby, P.; Le Bourgot, C.; Ledoux, S.; et al. The tryptophan/kynurenine pathway: A novel cross-talk between nutritional obesity, bariatric surgery and taste of fat. Nutrients 2021, 13, 1366. [Google Scholar] [CrossRef] [PubMed]
- De-Oliveira, G.J.M.; Schieferdecker, M.E.M.; Campos, A.C.L. Are enterotypes in obese modified by bariatric surgery, the use of probiotic supplements and food habits? Arq. Bras. Cir. Dig. 2021, 34, 1601. [Google Scholar] [CrossRef]
- Kapoor, N.; Al Najim, W.; Menezes, C.; Price, R.K.; O’boyle, C.; Bodnar, Z.; Spector, A.C.; Docherty, N.G.; le Roux, C.W. A comparison of total food intake at a personalised buffet in people with obesity, before and 24 months after roux-en-Y-gastric bypass surgery. Nutrients 2021, 13, 3873. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Bradley, D.; Eagon, J.C.; Sullivan, S.; Abumrad, N.A.; Klein, S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity 2014, 22, E13–E20. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Moreno-Indias, I.; Tinahones, F.J. Shifts in gut microbiota and their metabolites induced by bariatric surgery. Impact of factors shaping gut microbiota on bariatric surgery outcomes. Rev. Endocr. Metab. Disord. 2021, 22, 1137–1156. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivery, K.L.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Deledda, A.; Pintus, S.; Loviselli, A.; Fosci, M.; Fantola, G.; Velluzzi, F. Nutritional management in bariatric surgery patients. Int. J. Environ. Res. Public Health 2021, 18, 12049. [Google Scholar] [CrossRef] [PubMed]
- Quilliot, D.; Coupaye, M.; Ciangura, C.; Czernichow, S.; Sallé, A.; Gaborit, B.; Alligier, M.; Nguyen-Thi, P.-L.; Dargent, J.; Msika, S.; et al. Recommendations for nutritional care after bariatric surgery: Recommendations for best practice and SOFFCO-MM/AFERO/SFNCM/expert consensus. J. Visc. Surg. 2021, 158, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Pareja, I.; Clemente-Postigo, M.; Tinahones, F.J. Metabolic and Endocrine Consequences of Bariatric Surgery. Front. Endocrinol. 2019, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Brethauer, S.A.; Kirwan, J.P.; Kashyap, S.R.; Burguera, B.; Schauer, P. How safe is metabolic/diabetes surgery? Diabetes Obes. Metab. 2015, 17, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.M.; Khoraki, J.; Browning, M.G.; Pessoa, B.M.; Mazzini, G.S.; Wolfe, L. Changes in utilization of bariatric surgery in the United States from 1993 to 2016. Ann. Surg. 2020, 271, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.S.; Irish, W.; Pories, W.J.; Shah, A.; DeMaria, E.J. Examining the Rates of Obesity and Bariatric Surgery in the United States. Obes. Surg. 2021, 31, 4754–4760. [Google Scholar] [CrossRef] [PubMed]
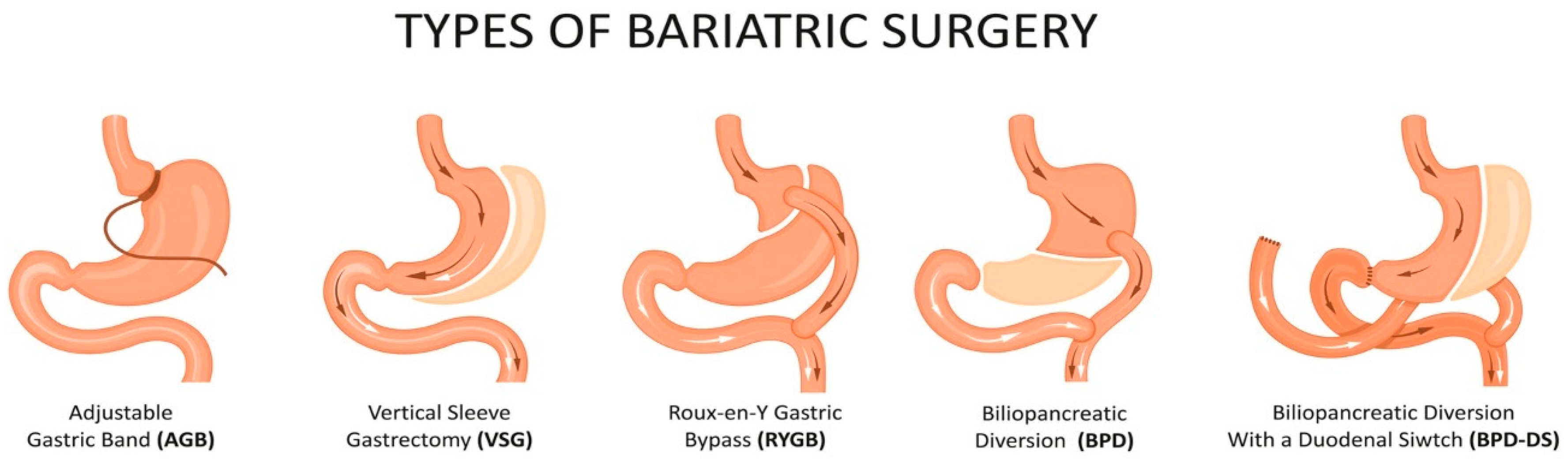
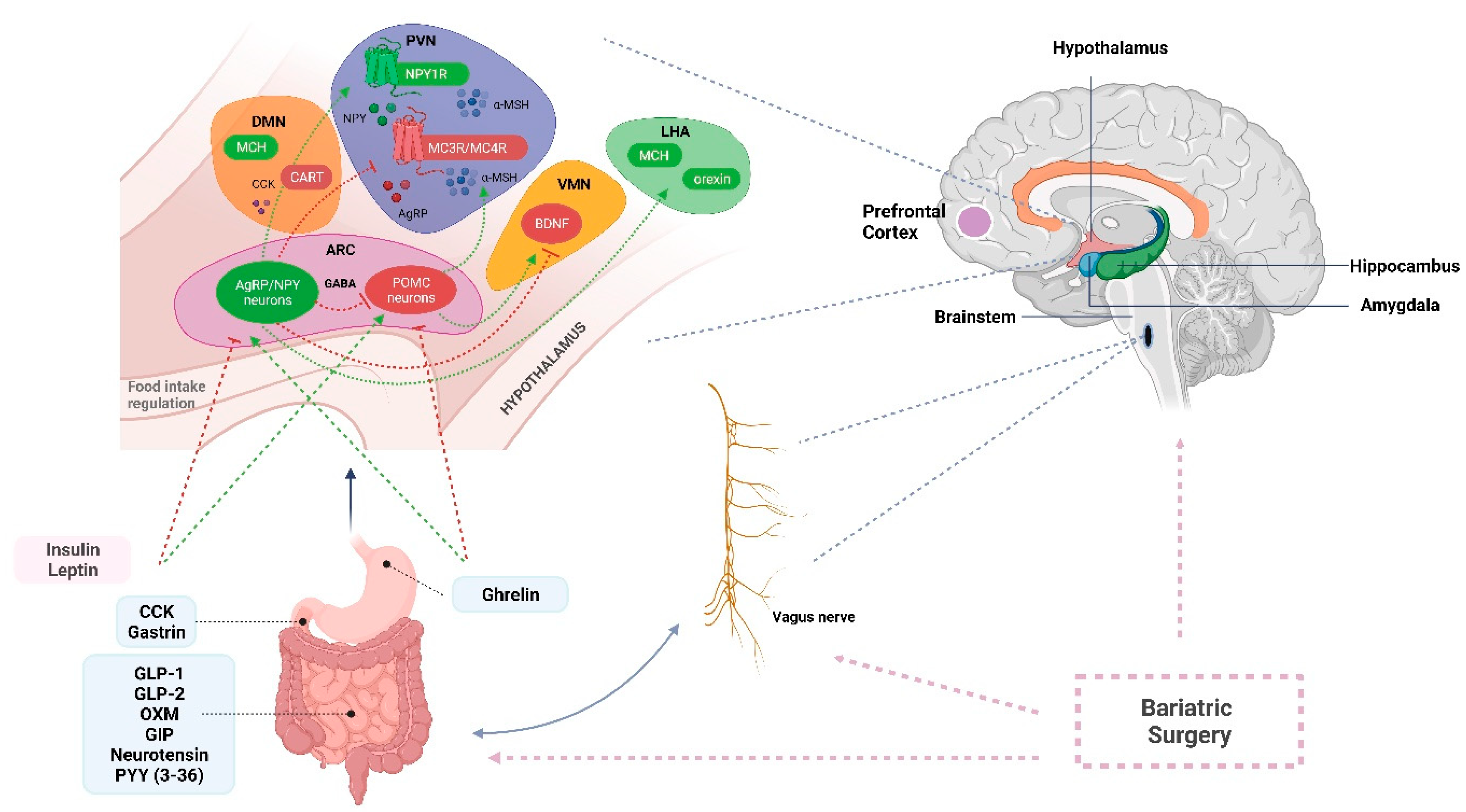
| Peptide | Site of Secretion | Effect on Food Intake | Main Functions | Modulation after BS | References |
|---|---|---|---|---|---|
| Ghrelin | P/D1 cells (gastric fundus) | ↑ | -↑ food intake | ↑ AGB ↓ BPD-DS+VSG (-) RYGB | [127,128,129,130,132,133,135] |
| Gastrin | G cells (pyloric antrum of stomach, duodenum and pancreas) | ↓ | -↑ HCl production -↑ gastric motility | ↓ RYGB (-) VSG | [152,163,184,247] |
| Leptin | Adipose tissue and gastric EECs | ↓ | -↓ glucose production and steatosis in the liver -↑ glucose uptake and fatty acid oxidation in muscles -↓ insulin and glucagon secretion -↑ sympathetic nervous system tone -↑ thyroid hormones-modulates immunity and fertility | (-) RYGB, VSG | [218,323,324,328] |
| Obestatin | Stomach | ? | -controversial role in food intake -regulates cell proliferation and survival -regulates glucose and lipid metabolism | (-) RYGB | [303,304,305,306,307] |
| Nesfatin | EECs (stomach and small intestine), pancreatic cells | ↓ | -↓ appetite -stimulates glucagon and insulin secretion -improves insulin sensitivities | ↓ RYGB ↓ VSG ↓ BPD-DS | [288,289,290,291,292,345] |
| Gustducin | EECs (stomach and intestine) | ? | -stimulates GLP-1 secretion -promotes absorption of oral glucose | ? | [294,295,296] |
| CCK | I cells (duodenum) | ↓ | -stimulates secretion of digestive enzymes from pancreas -stimulates release of bile from gallbladder -slows down gastric emptying -induces satiety | ↑ RYGB ↑ VSG | [152,158,166,174] |
| Secretin | S cells (duodenum) | ? | -inhibits secretion of gastric acid -stimulates production of bicarbonate -stimulates bile production | (-)/↓ RYGB | [282,286,287] |
| Uroguanylin | EECs (duodenum) | ↓ | -induces satiety -↓ water and sodium permeability and chloride secretion in the gut | ↑ RYGB | [297,298,300,302] |
| GIP | K cells (duodenum and jejunum) | ? | -stimulates insulin release -promotes triglyceride storage in the adipocytes | (-) RYGB (-) VSG (-) AGB | [199,218,243,244] |
| GLP-1 | L cells (distal ileum and colon) | ↓ | -stimulates post-prandial insulin secretion -delays gastric emptying -inhibits glucagon secretion -↓ appetite centrally | ↑ RYGB ↑ VSG | [150,158,183,185,196] |
| GLP-2 | EECs in small intestine | ↓ | -stimulates gut hypertrophy -↓ apoptosis | ↓ RYGB ↓ VSG | [309,310,317,318,319] |
| Glicentin | L cells (ileum) | ? | -may stimulate insulin secretion, gastrointestinal motility and gut growth | ↑ RYGB | [217,219,226] |
| Neurotensin | N cells (ileum and CNS) | ↓ | -inhibits intestinal and gastric motility -↑ fat absorption by increase in pancreas and bile acid secretion | ↑ RYGB ↑ VSG | [180,230,231,232,233,234,235] |
| OXM | L cells (ileum) | ↓ | -↓ food intake -↓ glucose levels -↓ gastric acid secretion and delays gastric emptying -↑ energy expenditure | ↑ RYGB | [184,211,213,217,219,226] |
| PYY(3-36) | L cells (distal ileum and colon) | ↓ | -delays gastric emptying -↓ appetite centrally -↓ post-prandial insulin production -alters colonic motility | ↑ RYGB ↑ VSG ↑ AGB | [161,175,176,180] |
| FGF15/19 | Ileum, gallbladder and brain | ↓ | -suppresses bile acid synthesis and gluconeogenesis -promotes glycogen and protein synthesis -↑ energy expenditure | ↑ RYGB ↑ VSG (-) AGB | [255,257,258,259,261,271] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinou, E.; Stefanova, I.; Iosif, E.; Angelidi, A.M. Neurohormonal Changes in the Gut–Brain Axis and Underlying Neuroendocrine Mechanisms following Bariatric Surgery. Int. J. Mol. Sci. 2022, 23, 3339. https://doi.org/10.3390/ijms23063339
Martinou E, Stefanova I, Iosif E, Angelidi AM. Neurohormonal Changes in the Gut–Brain Axis and Underlying Neuroendocrine Mechanisms following Bariatric Surgery. International Journal of Molecular Sciences. 2022; 23(6):3339. https://doi.org/10.3390/ijms23063339
Chicago/Turabian StyleMartinou, Eirini, Irena Stefanova, Evangelia Iosif, and Angeliki M. Angelidi. 2022. "Neurohormonal Changes in the Gut–Brain Axis and Underlying Neuroendocrine Mechanisms following Bariatric Surgery" International Journal of Molecular Sciences 23, no. 6: 3339. https://doi.org/10.3390/ijms23063339
APA StyleMartinou, E., Stefanova, I., Iosif, E., & Angelidi, A. M. (2022). Neurohormonal Changes in the Gut–Brain Axis and Underlying Neuroendocrine Mechanisms following Bariatric Surgery. International Journal of Molecular Sciences, 23(6), 3339. https://doi.org/10.3390/ijms23063339







