Chloroplast Thylakoidal Ascorbate Peroxidase, PtotAPX, Has Enhanced Resistance to Oxidative Stress in Populus tomentosa
Abstract
:1. Introduction
2. Results
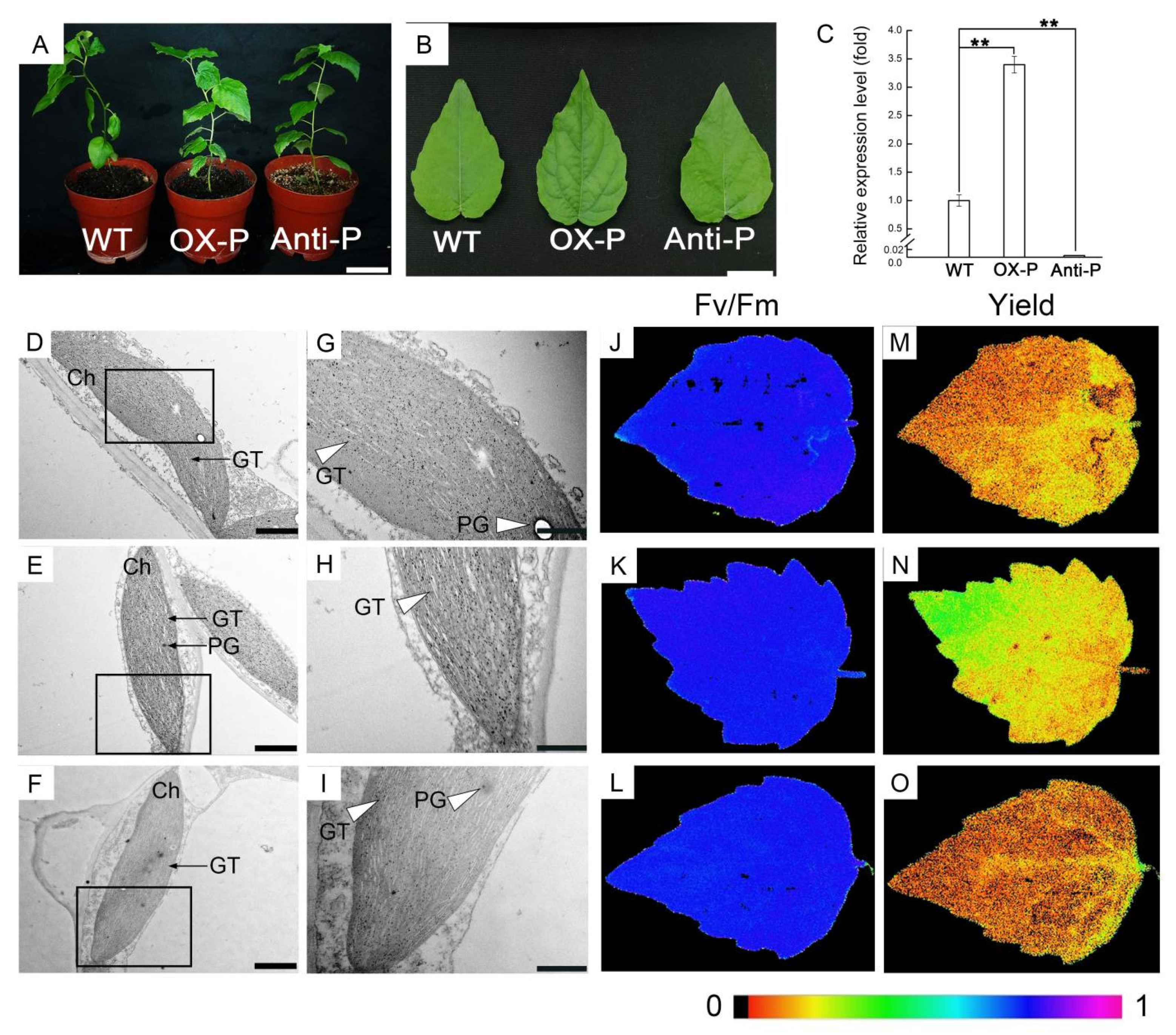
2.1. Upregulation or Downregulation of PtotAPX in Transgenic Populus tomentosa Plants Results in Altered Plant Growth
2.2. Decreased PtotAPX Content in Antisense Transgenic Plants Leads to More Chloroplastic Structure Damage in MV-Treated Leaves
2.3. Decreased PtotAPX Content in Antisense Transgenic Plants Leads to the Change in Oxidant or Reduced Content in MV-Treated Leaves, Resulted in Chloroplast Structure Damage
2.4. Enzymatic Properties of Chloroplast PtotAPX
3. Discussion
3.1. Chloroplast PtotAPX, Acting as One of the Components in Antioxidant Defense Systems Genes, Can Be Complemented by Other Antioxidant Defense Systems
3.2. PtotAPX Is a Key Enzyme in Chloroplasts That Scavenges H2O2 under Oxidative Stress, Resisting Oxidative Stress Damage
3.3. The Absence of AsA Affects the Function of PtotAPX
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Molecular Cloning and Plasmid Construction
4.3. qRT-PCR
4.4. Transmission Electron Microscopy (TEM)
4.5. Stem Histochemistry
4.6. Measurement of Net Photosynthetic Rate (Pn) and Maximal Photochemical Efficiency of PSII (Fv/Fm)
4.7. Determination of H2O2 and Malondialdehyde (MDA) Contents, Reduced AsA/Total AsA, NADP+/NADPH, and ADP/ATP Ratios
4.8. Enzyme-Activity Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Redox regulation of plant development. Antioxid. Redox Signal. 2014, 21, 1305–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davletova, S.; Rizhsky, L.; Liang, H.; Shengqiang, Z.; Oliver, D.J.; Coutu, J.; Shulaev, V.; Schlauch, K.; Mittler, R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 2005, 17, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, M.; Feng, H.L.; Wang, L.Y.; Li, D.; Meng, Q.W. Overexpression of thylakoidal ascorbate peroxidase shows enhanced resistance to chilling stress in tomato. J. Plant Physiol. 2012, 169, 867–877. [Google Scholar] [CrossRef]
- Kitajima, S. Hydrogen peroxide-mediated inactivation of two chloroplastic peroxidases, ascorbate peroxidase and 2-cys peroxiredoxin. Photochem. Photobiol. 2008, 84, 1404–1409. [Google Scholar] [CrossRef]
- Yabuta, Y.; Motoki, T.; Yoshimura, K.; Takeda, T.; Ishikawa, T.; Shigeoka, S. Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidative systems under photo-oxidative stress. Plant. J. 2002, 32, 915–925. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Takanori, M.; Shigeru, S.; Takahiro, I. Diversity and Evolution of Ascorbate Peroxidase Functions in Chloroplasts: More Than Just a Classical Antioxidant Enzyme? Plant Cell Physiol. 2016, 7, 1377–1386. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.P.; Moller, A.L.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T. P Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [Green Version]
- Murgia, I.; Tarantino, D.; Vannini, C.; Bracale, M.; Carravieri, S.; Soave, C. Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to Paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J. 2004, 38, 940–953. [Google Scholar] [CrossRef]
- Maruta, T.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2010, 51, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Du, X.; Tu, L.; Tang, W.; Wang, P.; Wang, M.; Liu, Z.; Zhang, X. Fibre elongation requires normal redox homeostasis modulated by cytosolic ascorbate peroxidase in cotton (Gossypium hirsutum). J. Exp. Bot. 2016, 67, 3289–3301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liszkay, A.; van der Zalm, E.; Schopfer, P. Production of reactive oxygen intermediates O2−, H2O2, and .OH by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004, 136, 3114–3123, discussion 3001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, C.; Jin, X.; Zhu, L.; Xie, Q.; Wang, X.; Li, H. Genome-wide investigation and expression profiling of APX gene family in Gossypium hirsutum provide new insights in redox homeostasis maintenance during different fiber development stages. Mol. Genet Genom. 2018, 293, 685–697. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Liu, Y.; Liu, X.; Mai, K.K.K.; Li, J.; Guo, X.; Zhang, C.; Li, H.; Kang, B.H.; Hwang, I.; et al. Chloroplast thylakoid ascorbate peroxidase PtotAPX plays a key role in chloroplast development by decreasing hydrogen peroxide in Populus tomentosa. J. Exp. Bot. 2021, 72, 4333–4354. [Google Scholar] [CrossRef]
- Shigeto, J.; Itoh, Y.; Tsutsumi, Y.; Kondo, R. Identification of Tyr74 and Tyr177 as substrate oxidation sites in cationic cell wall-bound peroxidase from Populus alba L. FEBS J. 2012, 279, 348–357. [Google Scholar] [CrossRef]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torresk, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jonesk, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Caverzan, A.; Bonifacio, A.; Carvalho, F.E.; Andrade, C.M.; Passaia, G.; Schunemann, M.; Maraschin Fdos, S.; Martins, M.O.; Teixeira, F.K.; Rauber, R.; et al. The knockdown of chloroplastic ascorbate peroxidases reveals its regulatory role in the photosynthesis and protection under photo-oxidative stress in rice. Plant Sci. 2014, 214, 74–87. [Google Scholar] [CrossRef]
- Maruta, T.; Noshi, M.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. H2O2-triggered Retrograde Signaling from Chloroplasts to Nucleus Plays Specific Role in Response to Stress. J. Biol. Chem. 2012, 287, 11717–11729. [Google Scholar] [CrossRef] [Green Version]
- Leple, J.C.; Dauwe, R.; Morreel, K.; Storme, V.; Lapierre, C.; Pollet, B.; Naumann, A.; Kang, K.Y.; Kim, H.; Ruel, K.; et al. Downregulation of cinnamoyl-coenzyme A reductase in poplar: Multiple-level phenotyping reveals effects on cell wall polymer metabolism and structure. Plant Cell 2007, 19, 3669–3691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myouga, F.; Hosoda, C.; Umezawa, T.; Iizumi, H.; Kuromori, T.; Motohashi, R.; Shono, Y.; Nagata, N.; Ikeuchi, M.; Shinozaki, K. A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell 2008, 20, 3148–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, X.; Liu, Y.; Fu, Q.; Tian, C.; Wu, C.; Shi, H.; Yuan, Z.; Tan, S.; Liu, S.; et al. Transcriptome analysis reveals enrichment of genes associated with auditory system in swimbladder of channel catfish. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 27, 30–39. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Guo, Z.; Lu, S. Differential Responses of Polyamines and Antioxidants to Drought in a Centipedegrass Mutant in Comparison to Its Wild Type Plants. Front. Plant Sci. 2017, 8, 792. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Niu, M.X.; Feng, C.H.; Li, H.G.; Su, Y.; Su, W.L.; Pang, H.; Yang, Y.; Yu, X.; Wang, H.L.; et al. PeSTZ1 confers salt stress tolerance by scavenging the accumulation of ROS through regulating the expression of PeZAT12 and PeAPX2 in Populus. Tree Physiol. 2020, 40, 1292–1311. [Google Scholar] [CrossRef]
- Jiang, G.; Yin, D.; Zhao, J.; Chen, H.; Guo, L.; Zhu, L.; Zhai, W. The rice thylakoid membrane-bound ascorbate peroxidase OsAPX8 functions in tolerance to bacterial blight. Sci. Rep. 2016, 6, 26104. [Google Scholar] [CrossRef] [Green Version]
- Yin, B.; Zhang, J.; Liu, Y.; Pan, X.; Zhao, Z.; Li, H.; Zhang, C.; Li, C.; Du, X.; Li, Y.; et al. PtomtAPX, a mitochondrial ascorbate peroxidase, plays an important role in maintaining the redox balance of Populus tomentosa Carr. Sci. Rep. 2019, 9, 19541. [Google Scholar] [CrossRef]
- Giacomelli, L.; Masi, A.; Ripoll, D.R.; Lee, M.J.; van Wijk, K.J. Arabidopsis thaliana deficient in two chloroplast ascorbate peroxidases shows accelerated light-induced necrosis when levels of cellular ascorbate are low. Plant Mol. Biol. 2007, 65, 627–644. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, D.; Lv, X.; Wang, Y.; Xun, Z.; Liu, Z.; Li, F.; Lu, H. The cysteine protease CEP1, a key executor involved in tapetal programmed cell death, regulates pollen development in Arabidopsis. Plant Cell 2014, 26, 2939–2961. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Li, H.; Yin, B.; Zhang, Y.; Liu, Y.; Cheng, Z.; Liu, D.; Lu, H. The papain-like cysteine protease CEP1 is involved in programmed cell death and secondary wall thickening during xylem development in Arabidopsis. J. Exp. Bot. 2019, 70, 205–215. [Google Scholar] [CrossRef] [PubMed]



| APX Isoforms | Substrate | Km (mM) | Vmax (mM min–1 mg–1) |
|---|---|---|---|
| PtotAPX | AsA | 0.66 ± 0.27 | 25.12 ± 5.98 |
| H2O2 | 0.02 ± 0.002 | 14.77 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, J.; Du, X.; Zhang, J.; Zou, Y.; Liu, Y.; Li, Y.; Lin, H.; Li, H.; Liu, D.; et al. Chloroplast Thylakoidal Ascorbate Peroxidase, PtotAPX, Has Enhanced Resistance to Oxidative Stress in Populus tomentosa. Int. J. Mol. Sci. 2022, 23, 3340. https://doi.org/10.3390/ijms23063340
Li C, Li J, Du X, Zhang J, Zou Y, Liu Y, Li Y, Lin H, Li H, Liu D, et al. Chloroplast Thylakoidal Ascorbate Peroxidase, PtotAPX, Has Enhanced Resistance to Oxidative Stress in Populus tomentosa. International Journal of Molecular Sciences. 2022; 23(6):3340. https://doi.org/10.3390/ijms23063340
Chicago/Turabian StyleLi, Conghui, Jiaxin Li, Xihua Du, Jiaxue Zhang, Yirong Zou, Yadi Liu, Ying Li, Hongyan Lin, Hui Li, Di Liu, and et al. 2022. "Chloroplast Thylakoidal Ascorbate Peroxidase, PtotAPX, Has Enhanced Resistance to Oxidative Stress in Populus tomentosa" International Journal of Molecular Sciences 23, no. 6: 3340. https://doi.org/10.3390/ijms23063340
APA StyleLi, C., Li, J., Du, X., Zhang, J., Zou, Y., Liu, Y., Li, Y., Lin, H., Li, H., Liu, D., & Lu, H. (2022). Chloroplast Thylakoidal Ascorbate Peroxidase, PtotAPX, Has Enhanced Resistance to Oxidative Stress in Populus tomentosa. International Journal of Molecular Sciences, 23(6), 3340. https://doi.org/10.3390/ijms23063340






