The Expression of Active CD11b Monocytes in Blood and Disease Progression in Amyotrophic Lateral Sclerosis
Abstract
:1. Introduction
2. Results
2.1. Study Population
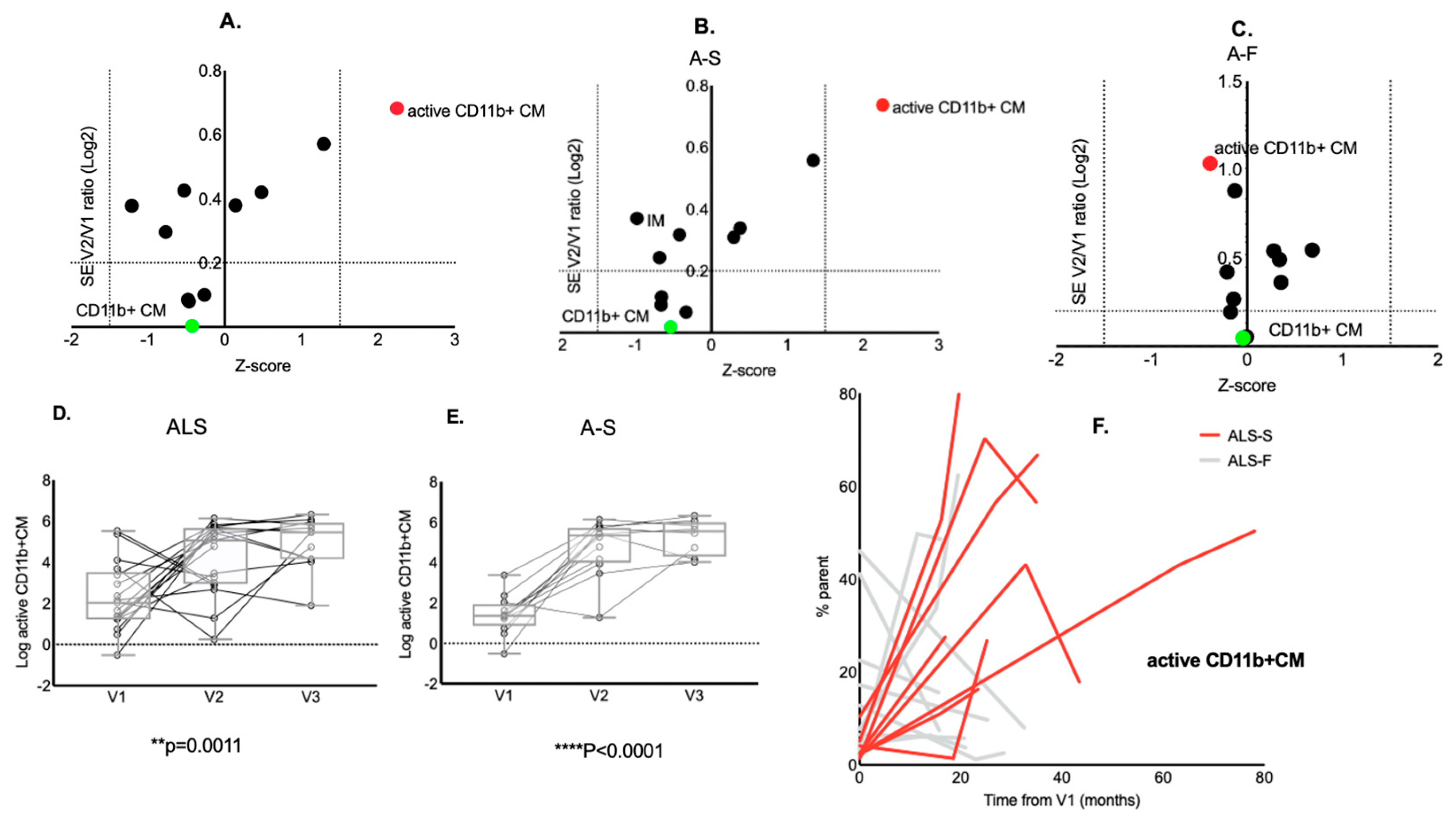
2.2. Baseline Monocyte Frequencies and Disease Progression
2.3. Multiple Variables Linear Regression and Survival Analysis
2.4. Baseline Monocyte Frequencies and Age
2.5. Longitudinal Analysis
2.6. Soluble CD11b and Monocyte Subsets Expression
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Peripheral Blood Mononuclear Cell (PBMC) Separation and Plasma Extraction
4.3. Staining and Data Analysis
4.4. Measurement of Plasma Neurofilament Light Chain (Nf-L) and Soluble CD11b (sCD11b)
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bede, P.; Bokde, A.; Elamin, M.; Byrne, S.; McLaughlin, R.L.; Jordan, N.; Hampel, H.; Gallagher, L.; Lynch, C.; Fagan, A.J.; et al. Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): A neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J. Neurol. Neurosurg. Psychiatry 2012, 84, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, M.; Jones, A.; Talbot, K.; Al-Chalabi, A.; Turner, M.R. The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Franchignoni, F.; Mandrioli, J.; Giordano, A.; Ferro, S.; ERRALS Group. A further Rasch study confirms that ALSFRS-R does not conform to fundamental measurement requirements. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Macdonald-Wallis, C.; Gray, E.; Pearce, N.; Petzold, A.; Norgren, N.; Giovannoni, G.; Fratta, P.; Sidle, K.; Fish, M.; et al. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015, 84, 2247–2257. [Google Scholar] [CrossRef]
- Mantovani, S.; Garbelli, S.; Pasini, A.; Alimonti, D.; Perotti, C.; Melazzini, M.; Bendotti, C.; Mora, G. Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. J. Neuroimmunol. 2009, 210, 73–79. [Google Scholar] [CrossRef]
- Murdock, B.J.; Bender, D.E.; Kashlan, S.R.; Figueroa-Romero, C.; Backus, C.; Callaghan, B.C.; Goutman, S.A.; Feldman, E.L. Increased ratio of circulating neutrophils to monocytes in amyotrophic lateral sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e242. [Google Scholar] [CrossRef] [PubMed]
- Murdock, B.J.; Zhou, T.; Kashlan, S.R.; Little, R.J.; Goutman, S.; Feldman, E.L. Correlation of Peripheral Immunity With Rapid Amyotrophic Lateral Sclerosis Progression. JAMA Neurol. 2017, 74, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- McGill, R.; Steyn, F.; Ngo, S.; Thorpe, K.A.; Heggie, S.; Ruitenberg, M.J.; Henderson, R.D.; McCombe, P.A.; Woodruff, T.M. Monocytes and neutrophils are associated with clinical features in amyotrophic lateral sclerosis. Brain Commun. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]
- Geissmann, F.; Manz, M.G.; Jung, J.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef]
- Graves, M.C.; Fiala, M.; Dinglasan, L.A.V.; Liu, N.Q.; Sayre, J.; Chiappelli, F.; van Kooten, C.; Vinters, H.V. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph. Lateral Scler. 2004, 5, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.S.; Engelhardt, J.I.; Siklós, L.; Simpson, E.P.; Kim, S.H.; Pan, T.; Goodman, J.C.; Siddique, T.; Beers, D.R.; Appel, S.H. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann. Neurol. 2003, 55, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Siddiqui, S.; Gabriely, G.; Lanser, A.J.; Dake, B.; Murugaiyan, G.; Doykan, C.E.; Wu, P.M.; Gali, R.R.; Iyer, L.; et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J. Clin. Investig. 2012, 122, 3063–3087. [Google Scholar] [CrossRef]
- Zondler, L.; Müller, K.; Khalaji, S.; Bliederhäuser, C.; Ruf, W.P.; Grozdanov, V.; Thiemann, M.; Fundel-Clemes, K.; Freischmidt, A.; Holzmann, K.; et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol. 2016, 132, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Beers, D.R.; Hooten, K.G.; Sieglaff, D.H.; Zhang, A.; Kalyana-Sundaram, S.; Traini, C.M.; Halsey, W.S.; Hughes, A.M.; Sathe, G.M.; et al. Characterization of Gene Expression Phenotype in Amyotrophic Lateral Sclerosis Monocytes. JAMA Neurol. 2017, 74, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Zondler, L.; Feiler, M.S.; Freischmidt, A.; Ruf, W.P.; Ludolph, A.C.; Danzer, K.M.; Weishaupt, J.H. Impaired activation of ALS monocytes by exosomes. Immunol. Cell Biol. 2017, 95, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Baufeld, C.; O’Loughlin, E.; Calcagno, N.; Madore, C.; Butovsky, O. Differential contribution of microglia and monocytes in neurodegenerative diseases. J. Neural Transm. 2017, 125, 809–826. [Google Scholar] [CrossRef]
- Miguel, L.I.; Almeida, C.B.; Traina, F.; Canalli, A.A.; Dominical, V.M.; Saad, S.T.O.; Costa, F.F.; Conran, N. Inhibition of phosphodiesterase 9A reduces cytokine-stimulated in vitro adhesion of neutrophils from sickle cell anemia individuals. Agents Actions 2011, 60, 633–642. [Google Scholar] [CrossRef]
- Nardo, G.; Pozzi, S.; Pignataro, M.; Lauranzano, E.; Spano, G.; Garbelli, S.; Mantovani, S.; Marinou, K.; Papetti, L.; Monteforte, M.; et al. Amyotrophic Lateral Sclerosis Multiprotein Biomarkers in Peripheral Blood Mononuclear Cells. PLoS ONE 2011, 6, e25545. [Google Scholar]
- Butovsky, O.; Jedrychowski, M.P.; Cialic, R.; Krasemann, S.; Murugaiyan, G.; Fanek, Z.; Greco, D.J.; Wu, P.M.; Doykan, C.E.; Kiner, O.; et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann. Neurol. 2014, 77, 75–99. [Google Scholar] [CrossRef]
- Quek, H.; Cuní-López, C.; Stewart, R.; Colletti, T.; Notaro, A.; Sun, Y.; Guo, C.C.; Lupton, M.K.; Nguyen, T.H.; Oikari, L.E.; et al. ALS monocyte-derived microglia reveal cytoplasmic TDP-43 accumulation, DNA damage, and cell-specific impairment of phagocytosis associated with disease progression. J. Neuroinflamm. 2022, 28, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Zhao, W.; Wang, J.; Zhang, X.; Wen, S.; Neal, D.; Thonhoff, J.R.; Alsuliman, A.S.; Shpall, E.J.; Rezvani, K.; et al. ALS patients’ regulatory T lymphocytes are dysfunctional, and correlate with disease progression rate and severity. JCI Insight 2017, 2, e89530. [Google Scholar] [CrossRef] [PubMed]
- Benatar, M.; Zhang, L.; Wang, L.; Granit, V.; Statland, J.; Barohn, R.; Swenson, A.; Ravits, J.; Jackson, C.; Burns, T.M.; et al. Validation of serum neurofilaments as prognostic and potential pharmacodynamic biomarkers for ALS. Neurology 2020, 95, e59–e69. [Google Scholar] [CrossRef] [PubMed]
- Benatar, M.; Wuu, J.; Lombardi, V.; Jeromin, A.; Bowser, R.; Andersen, P.M.; Malaspina, A. Neurofilaments in pre-symptomatic ALS and the impact of genotype. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 538–548. [Google Scholar] [CrossRef]
- Steinacker, P.; Verde, F.; Fang, L.; Feneberg, E.; Oeckl, P.; Roeber, S.; Anderl-Straub, S.; Danek, A.; Diehl-Schmid, J.; Fassbender, K.; et al. Chitotriosidase (CHIT1) is increased in microglia and macrophages in spinal cord of amyotrophic lateral sclerosis and cerebrospinal fluid levels correlate with disease severity and progression. J. Neurol. Neurosurg. Psychiatry 2017, 89, 239–247. [Google Scholar] [CrossRef]
- Shepheard, S.R.; Karnaros, V.; Benyamin, B.; Schultz, D.W.; Dubowsky, M.; Wuu, J.; Chataway, T.; Malaspina, A.; Benatar, M.; Rogers, M.L. Urinary neopterin: A novel biomarker of disease progression in amyotrophic lateral sclerosis. Eur. J. Neurol. 2022, 29, 990–999. [Google Scholar] [CrossRef]
- Zubiri, I.; Lombardi, V.; Bremang, M.; Mitra, V.; Nardo, G.; Adiutori, R.; Lu, C.-H.; Leoni, E.; Yip, P.; Yildiz, O.; et al. Tissue-enhanced plasma proteomic analysis for disease stratification in amyotrophic lateral sclerosis. Mol. Neurodegener. 2018, 13, 60. [Google Scholar] [CrossRef]
- Leoni, E.; Bremang, M.; Mitra, V.; Zubiri, I.; Jung, S.; Lu, C.H.; Adiutori, R.; Lombardi, V.; Russell, C.; Koncarevic, S.; et al. Combined tissue-fluid proteomics to unravel phenotypic variability in amyotrophic lateral sclerosis. Sci. Rep. 2019, 9, 4478. [Google Scholar] [CrossRef]
- Schmid, M.C.; Khan, S.Q.; Kaneda, M.M.; Pathria, P.; Shepard, R.; Louis, T.L.; Anand, S.; Woo, G.; Leem, C.; Faridi, M.H.; et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat. Commun. 2018, 9, 5379. [Google Scholar] [CrossRef]
- Varga, G.; Balkow, S.; Wild, M.K.; Stadtbaeumer, A.; Krummen, M.; Rothoeft, T.; Higuchi, T.; Beissert, S.; Wethmar, K.; Scharffetter-Kochanek, K.; et al. Active MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood 2006, 109, 661–669. [Google Scholar] [CrossRef]
- Ehirchiou, D.; Xiong, Y.; Xu, G.; Chen, W.; Shi, Y.; Zhang, L. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J. Exp. Med. 2007, 204, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, L.; Hilkens, C.M.; Morrison, V.L. β2 integrins as regulators of dendritic cell, monocyte, and macrophage function. Front. Immunol. 2017, 8, 1866. [Google Scholar] [CrossRef]
- Glass, J.D. Neuromuscular Disease: Protecting the nerve terminals. eLife 2018, 7, e35664. [Google Scholar] [CrossRef] [PubMed]
- Dadon-Nachum, M.; Melamed, E.; Offen, D. The “dying-back” phenomenon of motor neurons in ALS. J. Mol. Neurosci. 2011, 43, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Moloney, E.B.; De Winter, F.; Verhaagen, J. ALS as a distal axonopathy: Molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease. Front. Neurosci. 2014, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Chiot, A.; Lobsiger, C.S.; Boillée, S. New insights on the disease contribution of neuroinflammation in amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2019, 32, 764–770. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Mucke, L. Inflammation in Neurodegenerative Disease—A Double-Edged Sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Niccoli, T.; Partridge, L.; Isaacs, A.M. Ageing as a risk factor for ALS/FTD. Hum. Mol. Genet. 2017, 26, R105–R113. [Google Scholar] [CrossRef]
- Heaney, G.M.L.; David, W. Soluble receptors in human disease. J. Leukoc. Biol. 1998, 64, 135–146. [Google Scholar] [CrossRef]



| Clinical Characteristics | ALS (n = 38) | NNC (n = 20) |
|---|---|---|
| Age at baseline in years, median (IQR) | 66 (11.2) | 60.4 (10.8) |
| Female (%) | 50% | 50% |
| Site of disease onset: Bulbar (%) | 44.8% | N/a |
| Time to baseline in months, median (IQR) | 16.5 (14.8) | N/a |
| Baseline ALSFRS-R, mean (±SD) | 35.6 (±9.1) | N/a |
| Baseline ΔFRS (points/month), mean (±SD) | 0.8 (±0.7) | N/a |
| ALSFRS-R change (points/months), mean (±SD) | 0.8 (±0.6) | N/a |
| Survival from baseline in months, median (IQR) | 15.1 (12.2) | N/a |
| Nf-L at baseline in pg/mL, median (IQR) | 106.3 (174.6) | N/a |
| All monocytes (%), mean (±SD) | 2.7 (±2.7) | 3.5 (±3.4) |
| Covariates | * ΔFRS Estimates (95% CI) | p Value | ** Survival HR (95% CI) | p Value |
|---|---|---|---|---|
| Gender (male) | −0.103 (−0.41–0.20) | 0.79 | 0.83 (0.34–2.02) | 0.68 |
| Age at baseline | 0.012 (−0.003–0.02) | 0.15 | 1.06 (1.02–1.10) | 0.002 |
| ALSFRS-R at baseline | −0.033 (−0.05–0.01) | <0.001 | 0.93 (0.94–1.04) | 0.88 |
| Site of onset (Bulbar) | 0.17 (−0.21–0.55) | 0.53 | 0.94 (0.40–2.20) | 0.90 |
| ΔFRS | - | - | 1.059 (1.58–9.07) | 0.003 |
| NfL | 0.0002 (−0.0005) | 0.09 | 1.001 (1.0002–1.003) | 0.023 |
| Active CD11b+ CM | −0.003 (0.02–0.01) | 0.63 | −0.013 (0.94–1.03) | 0.57 |
| Active CD11b+ NCM | 0.005 (−0.018–0.028) | 0.66 | 0.008 (0.92–1.09) | 0.83 |
| CD11b+ NCM | 0.02 (0.01–0.03) | <0.001 | 1.05 (1.01–1.11) | 0.013 |
| NCM | 0.03 (0.01–0.04) | <0.001 | 0.25 (0.97–1.08) | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildiz, O.; Schroth, J.; Lombardi, V.; Pucino, V.; Bobeva, Y.; Yip, P.K.; Schmierer, K.; Mauro, C.; Tree, T.; Henson, S.M.; et al. The Expression of Active CD11b Monocytes in Blood and Disease Progression in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2022, 23, 3370. https://doi.org/10.3390/ijms23063370
Yildiz O, Schroth J, Lombardi V, Pucino V, Bobeva Y, Yip PK, Schmierer K, Mauro C, Tree T, Henson SM, et al. The Expression of Active CD11b Monocytes in Blood and Disease Progression in Amyotrophic Lateral Sclerosis. International Journal of Molecular Sciences. 2022; 23(6):3370. https://doi.org/10.3390/ijms23063370
Chicago/Turabian StyleYildiz, Ozlem, Johannes Schroth, Vittoria Lombardi, Valentina Pucino, Yoana Bobeva, Ping Kei Yip, Klaus Schmierer, Claudio Mauro, Timothy Tree, Sian Mari Henson, and et al. 2022. "The Expression of Active CD11b Monocytes in Blood and Disease Progression in Amyotrophic Lateral Sclerosis" International Journal of Molecular Sciences 23, no. 6: 3370. https://doi.org/10.3390/ijms23063370
APA StyleYildiz, O., Schroth, J., Lombardi, V., Pucino, V., Bobeva, Y., Yip, P. K., Schmierer, K., Mauro, C., Tree, T., Henson, S. M., & Malaspina, A. (2022). The Expression of Active CD11b Monocytes in Blood and Disease Progression in Amyotrophic Lateral Sclerosis. International Journal of Molecular Sciences, 23(6), 3370. https://doi.org/10.3390/ijms23063370







