Graphene Oxide Framework Structures and Coatings: Impact on Cell Adhesion and Pre-Vascularization Processes for Bone Grafts
Abstract
:1. Introduction
2. Results
2.1. Impact of F-GO and F-rGO on Mesenchymal Stem Cell Adhesion and Proliferation
2.2. Influence of F-GO and F-rGO on VEGF Levels and on VEGF Binding Rates
2.3. Influence of F-GO and F-rGO on the Formation of Prevascular Structures Using Co-Culture Models
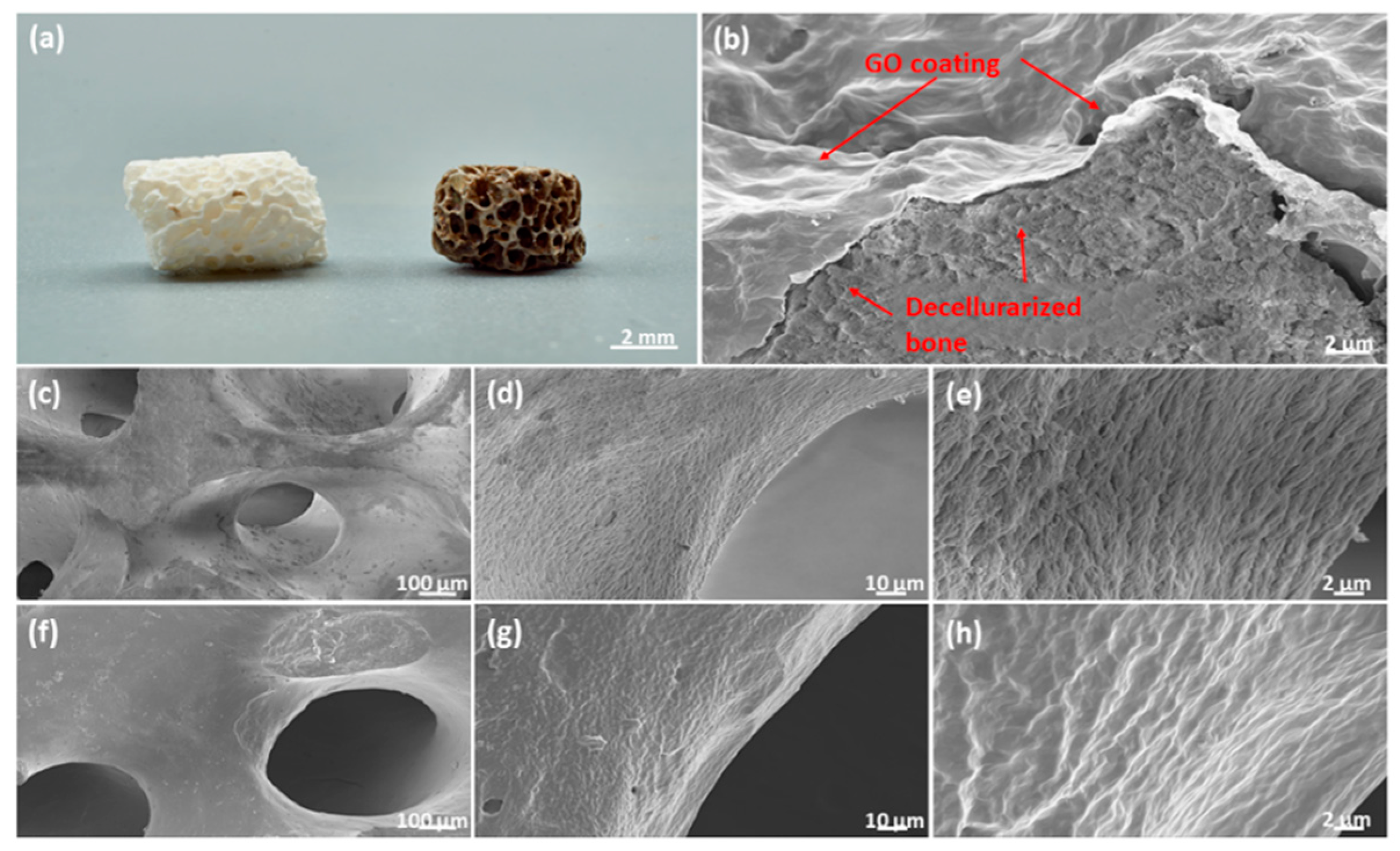
2.4. Graphene Oxide Coatings for Clinically Relevant Implant Materials
2.5. Impact of GO Coated Bio-Oss® on MSC Function
2.6. Impact of GO Coated Bio-Oss® on Vascularization Processes
2.7. Quantitative Assessment of Cellular Markers and Vascularization Associated Factors on GO Coated Bio-Oss®
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Human Mesenchymal Stem Cells (MSCs)
4.2. Isolation and Culture of Human Outgrowth Endothelial Cells (OECs)
4.3. Fabrication of Graphene Oxide and Reduced Graphene Oxide Framework Structures
4.4. GO Coating of Hydroxyapatite Xenografts
4.5. Cell Seeding on the Scaffolds
4.5.1. GO/rGO Framework Structures
4.5.2. Bio-Oss® and GO-Coated Bio-Oss®
4.6. Immunofluorescence Staining and Visualization
4.7. Scanning Electron Microscopy (SEM)
4.8. Quantification of DNA Content
4.9. Gene Expression Analysis
4.10. Quantification of Osteogenic Activity
4.11. Enzyme Linked Immunosorbent Assay (ELISA)
4.12. VEGF Binding Capacity of F-GO and F-rGO or GO Coated Bio-Oss®
4.13. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannoudis, P.V.; Chris Arts, J.J.; Schmidmaier, G.; Larsson, S. What should be the characteristics of the ideal bone graft substitute? Injury 2011, 42, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Boyce, T.; Edwards, J.; Scarborough, N. Allograft bone: The influence of processing on safety and performance. Orthop. Clin. N. Am. 1999, 30, 571–581. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Brink, O. The choice between allograft or demineralized bone matrix is not unambiguous in trauma surgery. Injury 2020, 11, S23–S28. [Google Scholar] [CrossRef]
- Hurzeler, M.B.; Quinones, C.R.; Kirsch, A.; Gloker, C.; Schupbach, P.; Strub, J.R.; Caffesse, R.G. Maxillary sinus augmentation using different grafting materials and dental implants in monkeys. Part I. Evaluation of anorganic bovine-derived bone matrix. Clin. Oral Implant. Res. 1997, 8, 476–486. [Google Scholar] [CrossRef]
- Rasch, A.; Naujokat, H.; Wang, F.; Seekamp, A.; Fuchs, S.; Kluter, T. Evaluation of bone allograft processing methods: Impact on decellularization efficacy, biocompatibility and mesenchymal stem cell functionality. PLoS ONE 2019, 14, e0218404. [Google Scholar] [CrossRef] [Green Version]
- Larochette, N.; El-Hafci, H.; Potier, E.; Setterblad, N.; Bensidhoum, M.; Petite, H.; Logeart-Avramoglou, D. Osteogenic-differentiated mesenchymal stem cell-secreted extracellular matrix as a bone morphogenetic protein-2 delivery system for ectopic bone formation. Acta Biomater. 2020, 116, 186–200. [Google Scholar] [CrossRef]
- Lammens, J.; Maréchal, M.; Delport, H.; Geris, L.; Oppermann, H.; Vukicevic, S.; Luyten, F.P. A cell-based combination product for the repair of large bone defects. Bone 2020, 138, 115511. [Google Scholar] [CrossRef]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the road to smart biomaterials for bone research: Definitions, concepts, advances, and outlook. Bone Res. 2021, 9, 12. [Google Scholar] [CrossRef]
- Stahl, A.; Yang, Y.P. Regenerative Approaches for the Treatment of Large Bone Defects. Tissue Eng. Part B Rev. 2021, 27, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Oreffo, R.O. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cell Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Owen, H.C.; Vanhees, I.; Solie, L.; Roberts, S.J.; Wauters, A.; Luyten, F.P.; Van Cromphaut, S.; Van den Berghe, G. Critical illness-related bone loss is associated with osteoclastic and angiogenic abnormalities. J. Bone Miner. Res. 2012, 27, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Loundagin, L.L.; Edwards, W.B. Stressed volume around vascular canals explains compressive fatigue life variation of secondary osteonal bone but not plexiform bone. J. Mech. Behav. Biomed. Mater. 2020, 111, 104002. [Google Scholar] [CrossRef] [PubMed]
- Klenke, F.M.; Liu, Y.; Yuan, H.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W. Impact of pore size on the vascularization and osseointegration of ceramic bone substitutes in vivo. J. Biomed. Mater. Res. Part A 2008, 85, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Sicchieri, L.G.; Crippa, G.E.; de Oliveira, P.T.; Beloti, M.M.; Rosa, A.L. Pore size regulates cell and tissue interactions with PLGA-CaP scaffolds used for bone engineering. J. Tissue Eng. Regen. Med. 2012, 6, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, S.; Li, J.; Guo, K.; Yuan, Q.; Zhong, A.; Yang, J.; Wang, J.; Sun, J.; Wang, Z. Collagen Functionalized With Graphene Oxide Enhanced Biomimetic Mineralization and in Situ Bone Defect Repair. ACS Appl. Mater. Interfaces 2018, 10, 44080–44091. [Google Scholar] [CrossRef]
- La, W.G.; Jin, M.; Park, S.; Yoon, H.H.; Jeong, G.J.; Bhang, S.H.; Park, H.; Char, K.; Kim, B.S. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014, 9 (Suppl. S1), 107–116. [Google Scholar]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells on graphene oxide-incorporated electrospun poly(lactic-co-glycolic acid) nanofibrous mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef]
- Wang, C.; Hu, H.; Li, Z.; Shen, Y.; Xu, Y.; Zhang, G.; Zeng, X.; Deng, J.; Zhao, S.; Ren, T.; et al. Enhanced Osseointegration of Titanium Alloy Implants with Laser Microgrooved Surfaces and Graphene Oxide Coating. ACS Appl. Mater. Interfaces 2019, 11, 39470–39483. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, H.; Pan, Q.; Gao, C.; Wang, Y.; Yang, S.; Zan, X.; Guan, Y. Graphene Oxide and Lysozyme Ultrathin Films with Strong Antibacterial and Enhanced Osteogenesis. Langmuir 2019, 35, 6752–6761. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Lamberti, A.; Genchi, G.G.; Roppolo, I.; Canavese, G.; Vitale-Brovarone, C.; Ciofani, G.; Cauda, V. Graphene Oxide Finely Tunes the Bioactivity and Drug Delivery of Mesoporous ZnO Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Schütt, F.; Signetti, S.; Krüger, H.; Röder, S.; Smazna, D.; Kaps, S.; Gorb, S.N.; Mishra, Y.K.; Pugno, N.M.; Adelung, R. Hierarchical self-entangled carbon nanotube tube networks. Nat. Commun. 2017, 8, 1215. [Google Scholar] [CrossRef]
- Fuchs, S.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. Outgrowth endothelial cells isolated and expanded from human peripheral blood progenitor cells as a potential source of autologous cells for endothelialization of silk fibroin biomaterials. Biomaterials 2006, 27, 5399–5408. [Google Scholar] [CrossRef]
- Kolbe, M.; Dohle, E.; Katerla, D.; Kirkpatrick, C.J.; Fuchs, S. Enrichment of outgrowth endothelial cells in high and low colony-forming cultures from peripheral blood progenitors. Tissue Eng. Part C Methods 2010, 16, 877–886. [Google Scholar] [CrossRef] [Green Version]
- Lai, P.X.; Chen, C.W.; Wei, S.C.; Lin, T.Y.; Jian, H.J.; Lai, I.P.; Mao, J.Y.; Hsu, P.H.; Lin, H.J.; Tzou, W.S.; et al. Ultrastrong trapping of VEGF by graphene oxide: Anti-angiogenesis application. Biomaterials 2016, 109, 12–22. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.-t.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, K.; Feng, L.; Liu, Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon 2011, 49, 4040–4049. [Google Scholar] [CrossRef]
- Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. Graphene oxide touches blood: In vivo interactions of bio-coronated 2D materials. Nanoscale Horiz. 2019, 4, 273–290. [Google Scholar] [CrossRef]
- Eckhart, K.E.; Holt, B.D.; Laurencin, M.G.; Sydlik, S.A. Covalent conjugation of bioactive peptides to graphene oxide for biomedical applications. Biomater. Sci. 2019, 7, 3876–3885. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Wang, J.; Li, J.; Lin, Y. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Lacey, S.D.; Xu, L.; Xie, H.; Li, T.; Danner, V.A.; Hu, L. Reduced graphene oxide film with record-high conductivity and mobility. Mater. Today 2018, 21, 186–192. [Google Scholar] [CrossRef]
- Kolanthai, E.; Sindu, P.A.; Khajuria, D.K.; Veerla, S.C.; Kuppuswamy, D.; Catalani, L.H.; Mahapatra, D.R. Graphene Oxide-A Tool for the Preparation of Chemically Crosslinking Free Alginate-Chitosan-Collagen Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2018, 10, 12441–12452. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, Y.C.; Lee, S.M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.M.; Huh, J.B.; Han, D.W. Enhanced Osteogenesis by Reduced Graphene Oxide/Hydroxyapatite Nanocomposites. Sci. Rep. 2015, 5, 18833. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, X.; Cai, H.; Chen, Z.; Wang, T.; Jia, L.; Wang, J.; Wan, Q.; Pei, X. Osteogenic activity and antibacterial effect of zinc oxide/carboxylated graphene oxide nanocomposites: Preparation and in vitro evaluation. Colloids Surf. B Biointerfaces 2016, 147, 397–407. [Google Scholar] [CrossRef]
- Paz, E.; Ballesteros, Y.; Abenojar, J.; Del Real, J.C.; Dunne, N.J. Graphene Oxide and Graphene Reinforced PMMA Bone Cements: Evaluation of Thermal Properties and Biocompatibility. Materials 2019, 12, 3146. [Google Scholar] [CrossRef] [Green Version]
- Paz, E.; Ballesteros, Y.; Forriol, F.; Dunne, N.J.; Del Real, J.C. Graphene and graphene oxide functionalisation with silanes for advanced dispersion and reinforcement of PMMA-based bone cements. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109946. [Google Scholar] [CrossRef]
- Taale, M.; Schütt, F.; Zheng, K.; Mishra, Y.K.; Boccaccini, A.R.; Adelung, R.; Selhuber-Unkel, C. Bioactive Carbon-Based Hybrid 3D Scaffolds for Osteoblast Growth. ACS Appl. Mater. Interfaces 2018, 10, 43874–43886. [Google Scholar] [CrossRef] [Green Version]
- Barbeck, M.; Udeabor, S.E.; Lorenz, J.; Kubesch, A.; Choukroun, J.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Induction of multinucleated giant cells in response to small sized bovine bone substitute (Bio-Oss) results in an enhanced early implantation bed vascularization. Ann. Maxillofac. Surg. 2014, 4, 150–157. [Google Scholar]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes--comparison with human bone. J. Biomed. Mater. Res. B Appl Biomater. 2010, 92, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.L.; Da Rosa, W.L.O.; Cuevas-Suarez, C.E.; Carreno, N.L.V.; da Silva, A.F.; Guim, T.N.; Dellagostin, O.A.; Piva, E. Histological Evaluation of Bone Repair with Hydroxyapatite: A Systematic Review. Calcif. Tissue Int. 2017, 101, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Jiang, X.; Gotman, I.; Makarov, C.; Schmidt, H.; Gutmanas, E.Y.; Kirkpatrick, C.J. Influence of polymer content in Ca-deficient hydroxyapatite-polycaprolactone nanocomposites on the formation of microvessel-like structures. Acta Biomater. 2010, 6, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, M.; Xiang, Z.; Dohle, E.; Tonak, M.; Kirkpatrick, C.J.; Fuchs, S. Paracrine effects influenced by cell culture medium and consequences on microvessel-like structures in cocultures of mesenchymal stem cells and outgrowth endothelial cells. Tissue Eng. Part A 2011, 17, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Ghanaati, S.; Orth, C.; Barbeck, M.; Kolbe, M.; Hofmann, A.; Eblenkamp, M.; Gomes, M.; Reis, R.L.; Kirkpatrick, C.J. Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials 2009, 30, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Schmidt, H.; Pavleska, D.; Wermann, T.; Seekamp, A.; Fuchs, S. Crude Fucoidan Extracts Impair Angiogenesis in Models Relevant for Bone Regeneration and Osteosarcoma via Reduction of VEGF and SDF-1. Mar. Drugs 2017, 15, 186. [Google Scholar] [CrossRef] [Green Version]
- Asti, A.; Visai, L.; Dorati, R.; Conti, B.; Saino, E.; Sbarra, S.; Gastaldi, G.; Benazzo, F. Improved cell growth by Bio-Oss/PLA scaffolds for use as a bone substitute. Technol. Health Care 2008, 16, 401–413. [Google Scholar] [CrossRef]
- Mayer, Y.; Ginesin, O.; Khutaba, A.; Machtei, E.E.; Zigdon Giladi, H. Biocompatibility and osteoconductivity of PLCL coated and noncoated xenografts: An in vitro and preclinical trial. Clin. Implant Dent. Relat. Res. 2018, 20, 294–299. [Google Scholar] [CrossRef]
- Rasch, F.; Schütt, F.; Saure, L.M.; Kaps, S.; Strobel, J.; Polonskyi, O.; Nia, A.S.; Lohe, M.R.; Mishra, Y.K.; Faupel, F.; et al. Wet-Chemical Assembly of 2D Nanomaterials into Lightweight, Microtube-Shaped, and Macroscopic 3D Networks. ACS Appl. Mater. Interfaces 2019, 11, 44652–44663. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Materials Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Kaps, S.; Schuchardt, A.; Paulowicz, I.; Jin, X.; Gedamu, D.; Freitag, S.; Claus, M.; Wille, S.; Kovalev, A.; et al. Fabrication of Macroscopically Flexible and Highly Porous 3D Semiconductor Networks from Interpenetrating Nanostructures by a Simple Flame Transport Approach. Part. Part. Syst. Charact. 2013, 30, 775–783. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Kaps, S.; Schuchardt, A.; Paulowicz, I.; Jin, X.; Gedamu, D.; Wille, S.; Lupan, O.; Adelung, R. Versatile Fabrication of Complex Shaped Metal Oxide Nano-Microstructures and Their Interconnected Networks for Multifunctional Applications. KONA Powder Part. J. 2014, 31, 92–110. [Google Scholar] [CrossRef] [Green Version]
- Mishra, Y.K.; Modi, G.; Cretu, V.; Postica, V.; Lupan, O.; Reimer, T.; Paulowicz, I.; Hrkac, V.; Benecke, W.; Kienle, L.; et al. Direct Growth of Freestanding ZnO Tetrapod Networks for Multifunctional Applications in Photocatalysis, UV Photodetection, and Gas Sensing. ACS Appl. Mater. Interfaces 2015, 7, 14303–14316. [Google Scholar] [CrossRef]
- Shaygan Nia, A.; Rana, S.; Döhler, D.; Noirfalise, X.; Belfiore, A.; Binder, W.H. Click chemistry promoted by graphene supported copper nanomaterials. Chem. Commun. 2014, 50, 15374–15377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaygan Nia, A.; Rana, S.; Döhler, D.; Jirsa, F.; Meister, A.; Guadagno, L.; Koslowski, E.; Bron, M.; Binder, W.H. Carbon-Supported Copper Nanomaterials: Recyclable Catalysts for Huisgen [3+2] Cycloaddition Reactions. Chem. A Eur. J. 2015, 21, 10763–10770. [Google Scholar] [CrossRef]







| Gene Name | Primer Assay | Catalogue Number |
|---|---|---|
| ALP | Hs_ALPL_1_SG QuantiTect Primer Assay | QT00012957 |
| CD31 | Hs_PECAM1_1_SG QuantiTect Primer Assay | QT00081172 |
| Osteocalcin | Hs_BGLAP_1_SG QuantiTect Primer Assay | QT00232771 |
| Angiopoietin-1 | Hs_ANGPT1_1_SG QuantiTect Primer Assay | QT00046865 |
| Angiopoietin-2 | Hs_ANGPT2_1_SG QuantiTect Primer Assay | QT00100947 |
| Collagen type I | Hs_COL1A1_1_SG QuantiTect Primer Assay | QT00037793 |
| SDF-1 | Hs_CXCL12_1_SG QuantiTect Primer Assay | QT00087591 |
| VE-Cadherin | Hs_CDH5_1_SG QuantiTect Primer Assay | QT00013244 |
| VEGF | Hs_VEGFA_2_SG QuantiTech Primer Assay | QT01036861 |
| vwf | Hs_VWF_1_SG QuantiTect Primer Assay | QT00051975 |
| Integrin β1 | Hs_ITGB1_1_SG QuantiTect Primer Assay | QT00068124 |
| RPL13A | Hs_RPL13A_1_SG QuantiTect Primer Assay | QT00089915 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Saure, L.M.; Schütt, F.; Lorich, F.; Rasch, F.; Nia, A.S.; Feng, X.; Seekamp, A.; Klüter, T.; Naujokat, H.; et al. Graphene Oxide Framework Structures and Coatings: Impact on Cell Adhesion and Pre-Vascularization Processes for Bone Grafts. Int. J. Mol. Sci. 2022, 23, 3379. https://doi.org/10.3390/ijms23063379
Wang F, Saure LM, Schütt F, Lorich F, Rasch F, Nia AS, Feng X, Seekamp A, Klüter T, Naujokat H, et al. Graphene Oxide Framework Structures and Coatings: Impact on Cell Adhesion and Pre-Vascularization Processes for Bone Grafts. International Journal of Molecular Sciences. 2022; 23(6):3379. https://doi.org/10.3390/ijms23063379
Chicago/Turabian StyleWang, Fanlu, Lena Marie Saure, Fabian Schütt, Felix Lorich, Florian Rasch, Ali Shaygan Nia, Xinliang Feng, Andreas Seekamp, Tim Klüter, Hendrik Naujokat, and et al. 2022. "Graphene Oxide Framework Structures and Coatings: Impact on Cell Adhesion and Pre-Vascularization Processes for Bone Grafts" International Journal of Molecular Sciences 23, no. 6: 3379. https://doi.org/10.3390/ijms23063379






