Current Advances in 3D Bioprinting for Cancer Modeling and Personalized Medicine
Abstract
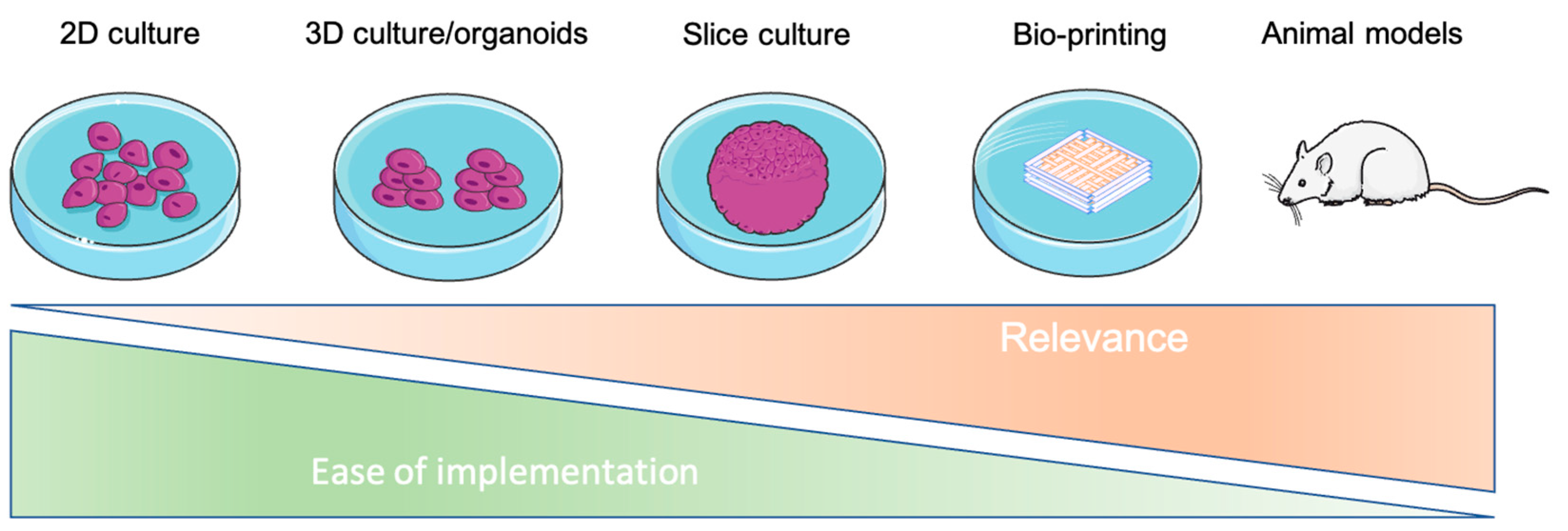
:1. 3D Bioprinting at a Glance
1.1. Introduction
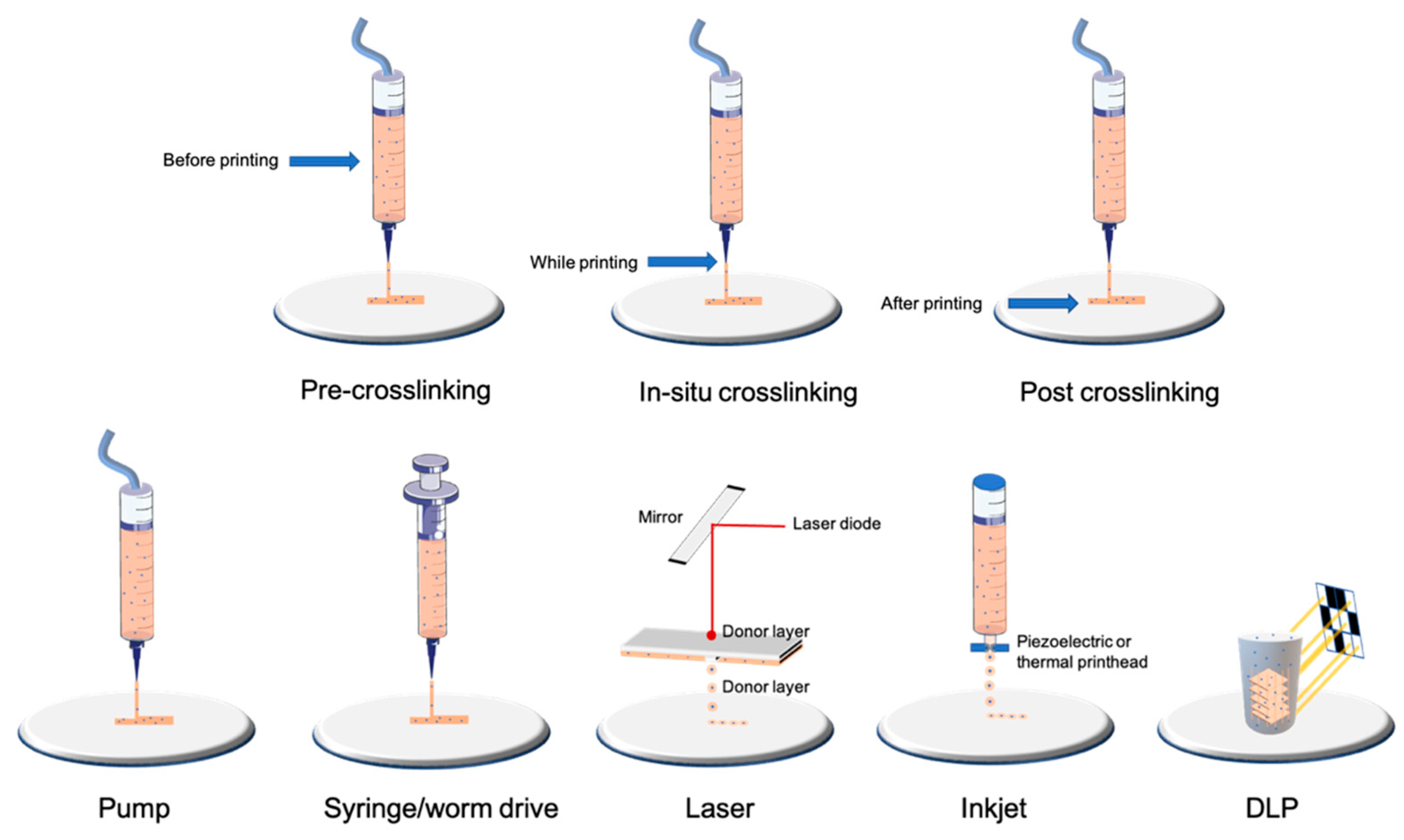
1.2. Bioprinting Technologies
1.3. Bioinks
1.4. Crosslinking
| Type of Technology | Example of Printing Method | Advantages | Disadvantages | Cell Density | Average Cell Viability | Crosslinking | References |
|---|---|---|---|---|---|---|---|
| Droplet-based | Laser | Very high accuracy and resolution Low shear stress Very expensive | Only low-viscosity bioinks Only 2D patterns (limited high) | Low (less than 10 million per mL) | High | Depends on biomaterial used | [19,20] |
| Inkjet | High accuracy Low shear stress | [17,18] | |||||
| Filament-based | Worm drive Pneumatic Syringe/piston | Large panel of bioinks available Low cost Highly tunable | Higher shear stress and lower cell viability than other bioprinting technologies | High (more than 10 million per mL) | Medium/high depending on nozzle and pressure | Depends on biomaterial used | [13,14,15,33] |
| Plane-based/Volumetric | DLP/SLA | Fast for large and complex 3D models Very high accuracy | Few bioinks available Waste of bioink due to its conception | High (more than 10 million per mL) | High | Photocurable by DLP/SLA technology | [23,24,25,26,27] |
| Material | Type of Bioink | Bioprinting Technology | Tissue Engineering Model | Cancer Models | Advantages | Drawbacks | Type of Crosslinking | References | |
|---|---|---|---|---|---|---|---|---|---|
| Bioink derived from natural biomaterials | Alginate-based | Natural polysaccharide (brown algae) | Drop-based Filament-based | Vascular, cartilage, bone, neural tissue, fibroblast, and many more | Drug delivery Cancer stem cell research Breast cancer, melanoma, and many more cancers Tumor spheroids | Low cost Good printability Excellent bio-compatibility | Poor cell adhesion Fast degradation | Ionic | [34,35,36,37,38,39,40] |
| Gelatin-based | Natural protein (bovine skin and tendon) | Drop-based Filament-based Plane-based | Vascular, cartilage, bone, muscle, fibroblast, and many more | Cholangiocarcinoma, bladder cancer, and many more cancers Tumor spheroids | Excellent bio-compatibility Low-cost High cellular adhesion | Low viscosity at room or higher temperatures Need a temperature-controlled (cooled printhead) and a cooled printbed Low mechanical strength (higher if blended with methacrylate) | Chemical Thermal UV Covalent Enzymatic | [38,41,42,43,44,45,46] | |
| Cellulose and nanocellulose-based | Natural polysaccharide obtained from the biosynthesis of plants or bacteria | Filament-based | Cartilage and bone | Drug delivery Gastric, cervical, pancreatic, and many more cancers | Great similarity with ECM Excellent bio-compatibility | Low viscosity for cellulose nanocrystals Mainly used mixed with other natural biomaterials | Enzymatic UV | [47,48,49,50,51] | |
| Matrigel | Solubilized basement membrane matrix secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells | Filament-based Drop-based | Vascular, liver, bone, lung, and many more | Tumor spheroids Many types of cancer | Most used material in cancer research Excellent bio-compatibility Very well characterized for organoid/spheroid formation | Cannot be used alone due to its complex rheological behavior and low mechanical properties Limited use in vivo due to its mouse tumor origin Expensive High batch variability | Thermal | [52,53,54,55,56] | |
| Collagen-I-based | Natural protein (rat tail or bovine skin and tendon) | Drop-based Filament-based | Hard tissues (bone, osteochondral, cartilage) Skin, cardiovascular, and liver tissues; nervous system; and cornea | Tumor spheroids Neuroblastoma, breast cancer | Excellent bio-compatibility High cellular adhesion Minimal immunogenicity Excellent printability Enzymatically degradableMechanical and structural properties close to native tissue | Low shape fidelity | pH Thermal | [57,58,59,60] | |
| Hyaluronic-acid-based | Natural polysaccharide (bacterial fermentation or animal products) | Filament-based | Hard tissues (bone, osteochondral, cartilage) | Tumor spheroids Melanoma, breast cancer | Excellent bio-compatibility Highly tunable (wide variety and high degree of potential chemical modifications) Interact with cell receptors | Poor mechanical strength Mainly used mixed with other natural biomaterials | Depends on the other biomaterial/chemical modifications Physical or covalent | [61,62,63,64] | |
| Agarose-based | Natural polysaccharide derived from red seaweed | Filament-based | Bone, vascular, neural, and adipose tissue | Leukemia | Good biocompatibility Great similarity with ECM Thermo-reversible gelling | Poor cell survival if not blended with another biomaterial Poor printability (needs high temperature for dispensing (70 °C) and gels at low temperatures) | Thermal Ionic | [53,65,66] | |
| Fibrin-based | Natural protein (human plasma) | Filament-based Drop-based | Muscular, neural, skin, and adipose tissue, wound healing model | Drug release Glioblastoma | High shape fidelity (depending on fibrinogen–thrombin concentration) Excellent biocompatibility Enzymatically degradable | Medium cell adhesion Low mechanical properties | Enzymatic (fibrinogen–thrombin) | [67,68,69] | |
| Silk-derived | Natural protein (bombyx mory) | Filament-based | Hard tissues (bone, osteochondral, cartilage), vascular tissue | Drug delivery | High shape fidelity Low Cost Good biocompatibility | Lacks cell-binding domains Medium cell viability Needs other supportive material for cell proliferation (alginate, gelatin, etc.) Poor printability performance | Enzymatic Physical | [70,71,72,73] | |
| Gellan gum | Natural polysaccharide | Filament-based | Hard tissues (bone, osteochondral, cartilage), brain-like structures | Drug delivery | Excellent biocompatibility Low cost Rapid gelation | Poor printability performance | Thermal | [74,75,76] | |
| Chitosan | Natural polysaccharide produced by deacetylation of chitin (extract from shrimps) | Filament-based Drop-based Plane-based | Hard tissues (bone, osteochondral, cartilage), vascular, skin, and hepatic tissues | Drug delivery | Good biocompatibility Medium to high cell viability | Medium cell adhesion Low shape fidelity Low mechanical properties | Ionic UV | [77,78,79] | |
| Polypeptides | Corning (PuraMatrix) | Filament-based Droplet-based Plane-based | Liver, neural | Ovarian cancer | Self-assembly Adapted for soft-tissue applications and in conjunction with other materials | Low pH leading to low cell viability | Ionic-complementary self-assembly | [80,81] | |
| De-cellularized matrix-based (dECM) | Natural matrix | Filament-based | Adipose, hepatic, and heart tissues; MSCs; cancer models | Many tumor models depending on dECM | Renders natural ECM Tissue-specific | Low mechanical properties Protein denaturation during fabrication processes Poor printability if not mixed with another biomaterial Long procedure | Depends on the other biomaterial/chemical modifications | [82,83,84,85] | |
| Bioink derived from synthetic biomaterials | AM (acrylamide) | Polyacrylamide | Filament-based Plane-based Droplet-based | Different stiffness models | Melanoma, breast cancer | Wide range of elasticity Most standardized protocol | Suitable for 2D culture only or necessary to couple it with another material | UV | [86,87] |
| PCL/PLGA | Poly(caprolactone)/Poly(lactic–glycolic acid) | Filament-based Drop-based | Hard tissues (bone, osteochondral, cartilage) | Mainly depends on the natural biomaterial used | Good mechanical strength Controllable rate of degradation | Mainly used as a scaffold (melting temperature around 60 °C not compatible with cell viability) Needs other supportive material for cell proliferation (alginate, gelatin, etc.) | Depends on the natural biomaterial used | [88,89,90] | |
| PEG | Polymer of ethylene oxide | Filament-based | Vascular and bone tissue | Highly tunable (mechanical properties, polymerization, chemical composition) | Needs chemical modification to be printed Requires the addition of bioactive molecules to allow cellular interaction (high hydrophobicity) | UV if mixed with a photoinitiator Condensation Michael-type addition Click chemistry Native chemical ligation Enzymatic reaction | [91,92,93] | ||
| Pluronic | Triblock copolymer of poly(ethylene glycol)-poly(propylene oxide)-poly(propylene glycol) | Filament-based | Cartilage | High shape fidelity Good printability | Lacks cell-binding domains Low cell viability Poor mechanical strength | Covalent | [94,95] | ||
| PU | Polyurethane | Filament-based | Cartilage Neural stem cells | Good biocompatibility and biodegradability High mechanical strength | Needs other supportive material for cell proliferation (alginate, gelatin, etc.) | Depends on the natural biomaterial used | [96,97] |
2. Characterization of Cells after Bioprinting
2.1. In Situ Characterization of Cells
2.1.1. Light Microscopy
2.1.2. Fluorescence Microscopy
2.1.3. Electronic Microscopy
2.1.4. Colorimetric and Fluorimetric Methods
2.1.5. Metabolic Fluxes Analysis
2.2. Characterization of Cells after Isolation or Lysis
2.2.1. Molecular Biology
2.2.2. Flow Cytometry
| Methods | Description | Pros and Cons | Markers | REF | |
|---|---|---|---|---|---|
| Microscopy | |||||
| Light | Phase contrast | Monitoring of proliferation and morphology of cells | +: • Nondestructive • No markers are added • Low cost • Easy with transparent gels (GelMA, matrigel) −: • No possibility to identify subcellular structures • Difficult with opaque or non-transparent gels (e.g.,: alginate with nanocellulose) | Not suitable | [100,101,102] |
| Bright field | The transmission of light is more or less attenuated depending on the density or marking of the sample | +: • Suitable for large samples −: • Requires histological staining • Preparation of sample • Quantification of thick sample | Hematoxylin–eosin Masson’s trichrome Trypan blue | [101,102,103] | |
| Fluorescence | LSM Epifluorescence Confocal | The use of a fluorescent marker is necessary to highlight a subcellular structure; possibility of monitoring structures over time (if vital markers) | +: • Monitoring of many possible structures −: • Requires cutting for oversized constructions for epi and confocal microscopy • Need to fix for certain markers • Important autofluorescence for chitosan or alginate/cellulose hydrogels in UV | Live/dead staining Or calcein AM/propidium iodide Or ethidium homodimer Active-caspase3/7 green Hoechst 33342 HIF1-α, Ki67 | [108,109,110,111,144] |
| Electronic | Scanning | Surface is scanned with a beam of electrons, emitted signal provides images | +: • High resolution −: • The preparation procedure is tedious • Frequent preparation artifacts (collapse) | Not suitable | [102] |
| Transmission | The part of beam of electrons is transmitted into specimens allowed to obtain images | Not suitable | [102,115] | ||
| Flow cytometry | |||||
| Flow cytometry | Analysis of physical parameters (size and granularity) for each cell but also the level of fluorescence | +: • Quantitative analysis −: • Disaggregation can be a problem • Necessity to have a large cell number due to loss of cells during dissociation | 7-AAD CFSE | [102,139] | |
| Spectroscopy | |||||
| Spectrometry or fluorimetry | Production or utilization of a fluorescent or chromatic compound | +: • Well-described for 2D culture and frequently used • Can be used for kinetic monitoring −: • Ensure that the efficiency is adapted for 3D | ACP, LDH, prestoblue, alamar blue, DNA content | [112,119,120,121] | |
| Molecular biology | |||||
| RTqPCR Western blot | Quantification of gene expression at mRNA or protein level | +: • Quantitative analysis • Easier by using the enzymatic method on natural inks (e.g., collagenase for GelMA or ColMA, hyaluronidase for hyaluronic acid) −: •Adaptation of the homogenization and extraction protocol to obtain an adequate quantity and quality of RNA/proteins for analyses | Bax/Bcl2 HIF1-α, Ki67 | [103,115,118] | |
| Metabolism | |||||
| GC–MS (Gas chromatography–mass spectrometry) | Detection of molecules of interest according to their mass/charge ratio after ionization | +: • Considerably less cellular material compared to NMR, high sensitivity, −: • Use of radioisotopes, complex sample preparation, high cost | 13C-Glucose | [129,132] | |
| NMR (nuclear magnetic resonance) spectroscopy | Determination of the composition of a sample by applying a magnetic field via the orientation of the nuclear spins of the atoms | +: • High reproducibility, sample can be analyzed directly, low cost −: • Use of radioisotopes, low sensitivity | [130,131] | ||
| PET scan (positron emission tomography) | Injection of a radiographic tracer and monitoring by imaging to detect localization of [18F]FDG | +: • Classically used in medicine, monitoring over time −: • Low resolution (1.5 mm) | [18F]FDG | [120,125] | |
| Seahorse | Quantification of the oxygen consumption rate (OCR) and the extracellular acidification rate (ECAR) | +: • High sensitivity (from 5000 cells, theoretically), possibility to test many conditions in parallel −: • Difficulties in normalizing results, limited number of injections, limited sample thickness | Not suitable | [126,128] | |
2.3. Recapitulate Cancer’s Relation to the Microenvironment
2.3.1. Cells–ECM Interaction
2.3.2. Neoangiogenesis
2.3.3. Migration and Invasion
2.3.4. Enrichment in Cancer Stem Cells
2.4. Mechanical Environment
2.4.1. Mechanotransduction
2.4.2. The Link between Extracellular Stiffness and Cell Metabolism
Amino Acid, Glucose, and Lipid Metabolism
Nucleus and Cell Cycle
Mitochondria
3. The Link between Stiffness, Cancer, and Resistance to Anticancer Therapies
3D Bioprinting for Drug Delivery and Screening
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, S.H.; Liu, P.; Mokasdar, A.; Hou, L. Additive Manufacturing and Its Societal Impact: A Literature Review. Int. J. Adv. Manuf. Technol. 2013, 67, 1191–1203. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Ng, S.H.; Yoon, Y.-J. A Review on 3D Printed Bioimplants. Int. J. Precis. Eng. Man. 2015, 16, 1035–1046. [Google Scholar] [CrossRef]
- Diment, L.E.; Thompson, M.S.; Bergmann, J.H.M. Clinical Efficacy and Effectiveness of 3D Printing: A Systematic Review. BMJ Open 2017, 7, e016891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belhouideg, S. Impact of 3D Printed Medical Equipment on the Management of the Covid19 Pandemic. Int. J. Health Plan. Manag. 2020, 35, 1014–1022. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Tan, H.W.; Patel, D.C.; Choong, W.T.N.; Chen, C.-H.; Low, H.Y.; Tan, M.J.; Patel, C.D.; Chua, C.K. The Global Rise of 3D Printing during the COVID-19 Pandemic. Nat. Rev. Mater. 2020, 5, 637–639. [Google Scholar] [CrossRef]
- Tino, R.; Moore, R.; Antoline, S.; Ravi, P.; Wake, N.; Ionita, C.N.; Morris, J.M.; Decker, S.J.; Sheikh, A.; Rybicki, F.J.; et al. COVID-19 and the Role of 3D Printing in Medicine. 3D Print. Med. 2020, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Nuseir, A.; Hatamleh, M.M.; Alnazzawi, A.; Al-Rabab’ah, M.; Kamel, B.; Jaradat, E. Direct 3D Printing of Flexible Nasal Prosthesis: Optimized Digital Workflow from Scan to Fit. J. Prosthodont. 2019, 28, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Faglin, P.; Gradwohl, M.; Depoortere, C.; Germain, N.; Drucbert, A.-S.; Brun, S.; Nahon, C.; Dekiouk, S.; Rech, A.; Azaroual, N.; et al. Rationale for the Design of 3D-Printable Bioresorbable Tissue-Engineering Chambers to Promote the Growth of Adipose Tissue. Sci. Rep. 2020, 10, 11779. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Wei, Y.; Hou, W.; Tong, H.; Bai, S. 3D Bioprinting Technologies for Hard Tissue and Organ Engineering. Materials 2016, 9, 802. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.-W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A Definition of Bioinks and Their Distinction from Biomaterial Inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Coppi, P.D.; Atala, A. Opportunities and Challenges of Translational 3D Bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Boland, T.; Mironov, V.; Gutowska, A.; Roth, E.A.; Markwald, R.R. Cell and Organ Printing 2: Fusion of Cell Aggregates in Three-dimensional Gels. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 272, 497–502. [Google Scholar] [CrossRef]
- Ramesh, S.; Zhang, Y.; Cormier, D.R.; Rivero, I.V.; Harrysson, O.L.A.; Rao, P.K.; Tamayol, A.; Tamayol, A. Extrusion Bioprinting: Recent Progress, Challenges, and Future Opportunities. Bioprinting 2020, 21, e00116. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Haghiashtiani, G.; Hübscher, T.; Kelly, D.J.; Lee, J.M.; Lutolf, M.; McAlpine, M.C.; Yeong, W.Y.; Zenobi-Wong, M.; Malda, J. 3D Extrusion Bioprinting. Nat. Rev. Methods Primers 2021, 1, 75. [Google Scholar] [CrossRef]
- Gudapati, H.; Dey, M.; Ozbolat, I. A Comprehensive Review on Droplet-Based Bioprinting: Past, Present and Future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10596–10636. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ebbens, S.; Zhao, X. Inkjet Printing of Mammalian Cells—Theory and Applications. Bioprinting 2021, 23, e00157. [Google Scholar] [CrossRef]
- Ventura, R.D. An Overview of Laser-Assisted Bioprinting (LAB) in Tissue Engineering Applications. Med. Lasers 2021, 10, 76–81. [Google Scholar] [CrossRef]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and Its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Jentsch, S.; Nasehi, R.; Kuckelkorn, C.; Gundert, B.; Aveic, S.; Fischer, H. Multiscale 3D Bioprinting by Nozzle-Free Acoustic Droplet Ejection. Small Methods 2021, 5, 2000971. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Lee, J.M.; Yeong, W.Y.; Naing, M.W. Microvalve-Based Bioprinting—Process, Bio-Inks and Applications. Biomater. Sci. 2017, 5, 632–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Eglin, D.; Alini, M.; Richards, G.R.; Qin, L.; Lai, Y. Visible Light-Induced 3D Bioprinting Technologies and Corresponding Bioink Materials for Tissue Engineering: A Review. Engineering 2020, 7, 966–978. [Google Scholar] [CrossRef]
- Kilian, D.; Ahlfeld, T.; Akkineni, A.R.; Lode, A.; Gelinsky, M. Three-Dimensional Bioprinting of Volumetric Tissues and Organs. Mrs. Bull. 2017, 42, 585–592. [Google Scholar] [CrossRef]
- Wang, M.; Li, W.; Mille, L.S.; Ching, T.; Luo, Z.; Tang, G.; Garciamendez, C.E.; Lesha, A.; Hashimoto, M.; Zhang, Y.S. Digital Light Processing Based Bioprinting with Composable Gradients. Adv. Mater. 2022, 34, 2107038. [Google Scholar] [CrossRef]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, e1904209. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Parra-Cantu, C.; Wang, Z.; Zhang, Y.S. Improving Bioprinted Volumetric Tumor Microenvironments In Vitro. Trends Cancer 2020, 6, 745–756. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Basu, B. An Overview of Hydrogel-Based Bioinks for 3D Bioprinting of Soft Tissues. J. Indian Inst. Sci. 2019, 99, 405–428. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels. Small 2020, 16, 2002931. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Healthc. Mater. 2020, 9, 1901648. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [Green Version]
- Schmid, R.; Schmidt, S.K.; Hazur, J.; Detsch, R.; Maurer, E.; Boccaccini, A.R.; Hauptstein, J.; Teßmar, J.; Blunk, T.; Schrüfer, S.; et al. Comparison of Hydrogels for the Development of Well-Defined 3D Cancer Models of Breast Cancer and Melanoma. Cancers 2020, 12, 2320. [Google Scholar] [CrossRef]
- Qiao, S.; Zhao, Y.; Li, C.; Yin, Y.; Meng, Q.; Lin, F.-H.; Liu, Y.; Hou, X.; Guo, K.; Chen, X.; et al. An Alginate-Based Platform for Cancer Stem Cell Research. Acta Biomater. 2016, 37, 83–92. [Google Scholar] [CrossRef]
- Piras, C.C.; Smith, D.K. Multicomponent Polysaccharide Alginate-Based Bioinks. J. Mater. Chem. B 2020, 8, 8171–8188. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Gu, K.; Bavoux, M.M.; Flores-Torres, S.; Kort-Mascort, J.; Grant, J.; Vijayakumar, S.; Leon-Rodriguez, A.D.; Ehrlicher, A.J.; et al. Engineering Bioprintable Alginate/Gelatin Composite Hydrogels with Tunable Mechanical and Cell Adhesive Properties to Modulate Tumor Spheroid Growth Kinetics. Biofabrication 2020, 12, 015024. [Google Scholar] [CrossRef]
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-Based Hydrogels for Cancer Therapy and Research. Int. J. Biol. Macromol. 2020, 170, 424–436. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-Based Hydrogels as Drug Delivery Vehicles in Cancer Treatment and Their Applications in Wound Dressing and 3D Bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Ying, G.; Jiang, N.; Yu, C.; Zhang, Y.S. Three-Dimensional Bioprinting of Gelatin Methacryloyl (GelMA). Bio-Des. Manuf. 2018, 1, 215–224. [Google Scholar] [CrossRef]
- He, H.; Li, D.; Lin, Z.; Peng, L.; Yang, J.; Wu, M.; Cheng, D.; Pan, H.; Ruan, C. Temperature-Programmable and Enzymatically Solidifiable Gelatin-Based Bioinks Enable Facile Extrusion Bioprinting. Biofabrication 2020, 12, 045003. [Google Scholar] [CrossRef]
- Rajabi, N.; Rezaei, A.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Luo, H.; RamaKrishna, S.; Berto, F. Recent Advances on Bioprinted Gelatin Methacrylate-Based Hydrogels for Tissue Repair. Tissue Eng. Part A 2021, 27, 679–702. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Santiago, G.T.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; He, J.; Zhao, Y.; Liu, T.; Xie, F.; Yang, H.; Mao, Y.; Pang, Y.; Sun, W. Bioprinting of Patient-Derived in Vitro Intrahepatic Cholangiocarcinoma Tumor Model: Establishment, Evaluation and Anti-Cancer Drug Testing. Biofabrication 2020, 12, 045014. [Google Scholar] [CrossRef]
- Miranda, M.A.; Marcato, P.D.; Mondal, A.; Chowdhury, N.; Gebeyehu, A.; Surapaneni, S.K.; Bentley, M.V.L.B.; Amaral, R.; Pan, C.-X.; Singh, M. Cytotoxic and Chemosensitizing Effects of Glycoalkaloidic Extract on 2D and 3D Models Using RT4 and Patient Derived Xenografts Bladder Cancer Cells. Mater. Sci. Eng. C 2021, 119, 111460. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.C.; Fernández-Prieto, S.; Borggraeve, W.M.D. Nanocellulosic Materials as Bioinks for 3D Bioprinting. Biomater. Sci. 2017, 5, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Xu, C. Nanocellulose-Based Inks for 3D Bioprinting: Key Aspects in Research Development and Challenging Perspectives in Applications—A Mini Review. Bioengineering 2020, 7, 40. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.; Cho, D.-W. Controlling Cancer Cell Behavior by Improving the Stiffness of Gastric Tissue-Decellularized ECM Bioink With Cellulose Nanoparticles. Front. Bioeng. Biotechnol. 2021, 9, 605819. [Google Scholar] [CrossRef]
- Gospodinova, A.; Nankov, V.; Tomov, S.; Redzheb, M.; Petrov, P.D. Extrusion Bioprinting of Hydroxyethylcellulose-Based Bioink for Cervical Tumor Model. Carbohyd. Polym. 2021, 260, 117793. [Google Scholar] [CrossRef]
- Olmos-Juste, R.; Alonso-Lerma, B.; Pérez-Jiménez, R.; Gabilondo, N.; Eceiza, A. 3D Printed Alginate-Cellulose Nanofibers Based Patches for Local Curcumin Administration. Carbohyd. Polym. 2021, 264, 118026. [Google Scholar] [CrossRef] [PubMed]
- Stefano, P.D.; Briatico-Vangosa, F.; Bianchi, E.; Pellegata, A.F.; de Hartungen, A.H.; Corti, P.; Dubini, G. Bioprinting of Matrigel Scaffolds for Cancer Research. Polymers 2021, 13, 2026. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Piou, M.; Darling, E.; Cormier, D.; Sun, J.; Wan, J. Bio-Printing Cell-Laden Matrigel–Agarose Constructs. J. Biomater. Appl. 2016, 31, 684–692. [Google Scholar] [CrossRef]
- Snyder, J.E.; Hamid, Q.; Wang, C.; Chang, R.; Emami, K.; Wu, H.; Sun, W. Bioprinting Cell-Laden Matrigel for Radioprotection Study of Liver by pro-Drug Conversion in a Dual-Tissue Microfluidic Chip. Biofabrication 2011, 3, 034112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horváth, L.; Umehara, Y.; Jud, C.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Engineering an in Vitro Air-Blood Barrier by 3D Bioprinting. Sci. Rep. 2015, 5, 7974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benton, G.; Kleinman, H.K.; George, J.; Arnaoutova, I. Multiple Uses of Basement Membrane-like Matrix (BME/Matrigel) in Vitro and in Vivo with Cancer Cells. Int. J. Cancer 2011, 128, 1751–1757. [Google Scholar] [CrossRef]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as Bioink for Bioprinting: A Comprehensive Review. Int. J. Bioprint. 2020, 6, 270. [Google Scholar] [CrossRef]
- Swaminathan, S.; Hamid, Q.; Sun, W.; Clyne, A.M. Bioprinting of 3D Breast Epithelial Spheroids for Human Cancer Models. Biofabrication 2019, 11, 025003. [Google Scholar] [CrossRef]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-Based Bioinks for Hard Tissue Engineering Applications: A Comprehensive Review. J. Mater. Sci. Mater. Med. 2019, 30, 32. [Google Scholar] [CrossRef]
- Campos, D.F.D.; Marquez, A.B.; O’Seanain, C.; Fischer, H.; Blaeser, A.; Vogt, M.; Corallo, D.; Aveic, S. Exploring Cancer Cell Behavior In Vitro in Three-Dimensional Multicellular Bioprintable Collagen-Based Hydrogels. Cancers 2019, 11, 180. [Google Scholar] [CrossRef] [Green Version]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D Printable Hyaluronic Acid-Based Hydrogel for Its Potential Application as a Bioink in Tissue Engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, R.; Schmidt, S.K.; Detsch, R.; Horder, H.; Blunk, T.; Schrüfer, S.; Schubert, D.W.; Fischer, L.; Thievessen, I.; Heltmann-Meyer, S.; et al. A New Printable Alginate/Hyaluronic Acid/Gelatin Hydrogel Suitable for Biofabrication of In Vitro and In Vivo Metastatic Melanoma Models. Adv. Funct. Mater. 2021, 32, 2107993. [Google Scholar] [CrossRef]
- Petta, D.; DAmora, U.; Ambrosio, L.; Grijpma, D.W.; Eglin, D.; DEste, M. Hyaluronic Acid as a Bioink for Extrusion-Based 3D Printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef] [PubMed]
- Horder, H.; Lasheras, M.G.; Grummel, N.; Nadernezhad, A.; Herbig, J.; Ergün, S.; Teßmar, J.; Groll, J.; Fabry, B.; Bauer-Kreisel, P.; et al. Bioprinting and Differentiation of Adipose-Derived Stromal Cell Spheroids for a 3D Breast Cancer-Adipose Tissue Model. Cells 2021, 10, 803. [Google Scholar] [CrossRef]
- Lόpez-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; García, J.M.; O’Connell, G.D. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, S.H.; Jung, Y. Current Status of Three-Dimensional Printing Inks for Soft Tissue Regeneration. Tissue Eng. Regen. Med. 2016, 13, 636–646. [Google Scholar] [CrossRef]
- Abelseth, E.; Abelseth, L.; la Vega, L.D.; Beyer, S.T.; Wadsworth, S.J.; Willerth, S.M. 3D Printing of Neural Tissues Derived from Human Induced Pluripotent Stem Cells Using a Fibrin-Based Bioink. ACS Biomater. Sci. Eng. 2019, 5, 234–243. [Google Scholar] [CrossRef]
- Lee, C.; Abelseth, E.; de la Vega, L.; Willerth, S.M. Bioprinting a Novel Glioblastoma Tumor Model Using a Fibrin-Based Bioink for Drug Screening. Mater. Today Chem. 2019, 12, 78–84. [Google Scholar] [CrossRef]
- Sharma, R.; Smits, I.P.M.; Vega, L.D.L.; Lee, C.; Willerth, S.M. 3D Bioprinting Pluripotent Stem Cell Derived Neural Tissues Using a Novel Fibrin Bioink Containing Drug Releasing Microspheres. Front. Bioeng. Biotechnol. 2020, 8, 57. [Google Scholar] [CrossRef]
- Chawla, S.; Midha, S.; Sharma, A.; Ghosh, S. Silk-Based Bioinks for 3D Bioprinting. Adv. Healthc. Mater. 2018, 7, 1701204. [Google Scholar] [CrossRef] [PubMed]
- Gangrade, A.; Mandal, B.B. Drug Delivery of Anticancer Drugs from Injectable 3D Porous Silk Scaffold for Prevention of Gastric Cancer Growth and Recurrence. ACS Biomater. Sci. Eng. 2020, 6, 6195–6206. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.-Y.; Song, Y.; Yan, X.; Dong, L.; Xue, J.; Xu, Y.; Wang, B.; Cao, B.; Hou, Q.; Peng, W.; et al. Injectable Ferrimagnetic Silk Fibroin Hydrogel for Magnetic Hyperthermia Ablation of Deep Tumor. Biomaterials 2020, 259, 120299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, G.; Yan, S.; Zhang, Q. 3D Printing of Silk Fibroin for Biomedical Applications. Materials 2019, 12, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitarresi, G.; Martorana, A.; Palumbo, F.S.; Fiorica, C.; Giammona, G. New Gellan Gum-Graft-Poly(d,l-Lactide-co-Glycolide) Copolymers as Promising Bioinks: Synthesis and Characterization. Int. J. Biol. Macromol. 2020, 162, 1653–1667. [Google Scholar] [CrossRef]
- Zhu, S.; Yao, L.; Pan, C.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. 3D Printed Gellan Gum/Graphene Oxide Scaffold for Tumor Therapy and Bone Reconstruction. Compos. Sci. Technol. 2021, 208, 108763. [Google Scholar] [CrossRef]
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R.; Stewart, E.M.; in het Panhuis, M.; Romero-Ortega, M.; Wallace, G.G. 3D Printing of Layered Brain-like Structures Using Peptide Modified Gellan Gum Substrates. Biomaterials 2015, 67, 264–273. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Carmagnola, I.; Chiappone, A.; Feng, Z.; Ciardelli, G.; Hakkarainen, M.; Sangermano, M. Photocurable Chitosan as Bioink for Cellularized Therapies towards Personalized Scaffold Architecture. Bioprinting 2020, 18, e00082. [Google Scholar] [CrossRef]
- Babu, A.; Ramesh, R. Multifaceted Applications of Chitosan in Cancer Drug Delivery and Therapy. Mar. Drugs 2017, 15, 96. [Google Scholar] [CrossRef] [Green Version]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A Critical Review on Three Dimensional-Printed Chitosan Hydrogels for Development of Tissue Engineering. Bioprinting 2020, 17, e00063. [Google Scholar] [CrossRef]
- Roth, A.D.; Lama, P.; Dunn, S.; Hong, S.; Lee, M.-Y. Polymer Coating on a Micropillar Chip for Robust Attachment of PuraMatrix Peptide Hydrogel for 3D Hepatic Cell Culture. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 634–644. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, H.; Zhao, X. Designer Self-Assembling Peptide Hydrogels to Engineer 3D Cell Microenvironments for Cell Constructs Formation and Precise Oncology Remodeling in Ovarian Cancer. Adv. Sci. 2020, 7, 1903718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pati, F.; Jang, J.; Ha, D.-H.; Kim, S.W.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing Three-Dimensional Tissue Analogues with Decellularized Extracellular Matrix Bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.P.; Gaspar, V.M.; Mano, J.F. Decellularized Extracellular Matrix for Bioengineering Physiomimetic 3D in Vitro Tumor Models. Trends Biotechnol. 2020, 38, 1397–1414. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D Printed Complex Tissue Construct Using Stem Cell-Laden Decellularized Extracellular Matrix Bioinks for Cardiac Repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; San, B.H.; Turner, N.J.; White, L.J.; Faulk, D.M.; Badylak, S.F.; Li, Y.; Yu, S.M. Molecular Assessment of Collagen Denaturation in Decellularized Tissues Using a Collagen Hybridizing Peptide. Acta Biomater. 2017, 53, 268–278. [Google Scholar] [CrossRef]
- He, Y.; Wang, F.; Wang, X.; Zhang, J.; Wang, D.; Huang, X. A Photocurable Hybrid Chitosan/Acrylamide Bioink for DLP Based 3D Bioprinting. Mater. Des. 2021, 202, 109588. [Google Scholar] [CrossRef]
- Poellmann, M.J.; Johnson, A.J.W. Multimaterial Polyacrylamide: Fabrication with Electrohydrodynamic Jet Printing, Applications, and Modeling. Biofabrication 2014, 6, 035018. [Google Scholar] [CrossRef]
- Poh, P.S.P.; Hutmacher, D.W.; Stevens, M.M.; Woodruff, M.A. Fabrication and in Vitro Characterization of Bioactive Glass Composite Scaffolds for Bone Regeneration. Biofabrication 2013, 5, 045005. [Google Scholar] [CrossRef] [Green Version]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An Additive Manufacturing-Based PCL-Alginate-Chondrocyte Bioprinted Scaffold for Cartilage Tissue Engineering. J. Tissue Eng. Regen. Med. 2013, 9, 1286–1297. [Google Scholar] [CrossRef]
- Zamani, Y.; Mohammadi, J.; Amoabediny, G.; Helder, M.N.; Zandieh-Doulabi, B.; Klein-Nulend, J. Bioprinting of Alginate-Encapsulated Pre-Osteoblasts in PLGA/β-TCP Scaffolds Enhances Cell Retention but Impairs Osteogenic Differentiation Compared to Cell Seeding after 3D-Printing. Regen. Eng. Transl. Med. 2021, 7, 485–493. [Google Scholar] [CrossRef]
- Abelardo, E. 3D Bioprinting for Reconstructive Surgery; Woodhead Publishing Series in Biomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 137–144. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive Modification of Poly(Ethylene Glycol) Hydrogels for Tissue Engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Adv. Mater. 2015, 27, 4035–4040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic Hydrogels as Bioinks for 3D Bioprinting. Biofabrication 2015, 7, 035006. [Google Scholar] [CrossRef] [PubMed]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 Hydrogel Characterization and Biofabrication in Cellularized Constructs for Tissue Engineering Applications. Proc. Cirp. 2016, 49, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, F.-Y.; Lin, H.-H.; Hsu, S. 3D Bioprinting of Neural Stem Cell-Laden Thermoresponsive Biodegradable Polyurethane Hydrogel and Potential in Central Nervous System Repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef]
- Hung, K.; Tseng, C.; Hsu, S. Synthesis and 3D Printing of Biodegradable Polyurethane Elastomer by a Water-Based Process for Cartilage Tissue Engineering Applications. Adv. Healthc. Mater. 2014, 3, 1578–1587. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-Based Drug Screen: Considerations and Practical Approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D Tumor Spheroid Models for in Vitro Therapeutic Screening: A Systematic Approach to Enhance the Biological Relevance of Data Obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, Q.; Han, S.; Wu, Y.; Tomshine, J.C.; Wang, D.; Gan, Y.; Zou, G.; Liang, X.-J. Multicellular Tumor Spheroids as an in Vivo–Like Tumor Model for Three-Dimensional Imaging of Chemotherapeutic and Nano Material Cellular Penetration. Mol. Imaging 2012, 11, 7290.2012.00012. [Google Scholar] [CrossRef]
- Longati, P.; Jia, X.; Eimer, J.; Wagman, A.; Witt, M.-R.; Rehnmark, S.; Verbeke, C.; Toftgård, R.; Löhr, M.; Heuchel, R.L. 3D Pancreatic Carcinoma Spheroids Induce a Matrix-Rich, Chemoresistant Phenotype Offering a Better Model for Drug Testing. BMC Cancer 2013, 13, 95. [Google Scholar] [CrossRef] [Green Version]
- Loebsack, A.B.; Halberstadt, C.R.; Holder, W.D.; Culberson, C.R.; Beiler, R.J.; Greene, K.G.; Roland, W.D.; Burg, K.J.L. The Development of an Embedding Technique for Polylactide Sponges. J. Biomed. Mater. Res. 1999, 48, 504–510. [Google Scholar] [CrossRef]
- Ruan, J.-L.; Tulloch, N.L.; Muskheli, V.; Genova, E.E.; Mariner, P.D.; Anseth, K.S.; Murry, C.E. An Improved Cryosection Method for Polyethylene Glycol Hydrogels Used in Tissue Engineering. Tissue Eng. Part C Methods 2013, 19, 794–801. [Google Scholar] [CrossRef] [Green Version]
- James, R.; Jenkins, L.; Ellis, S.E.; Burg, K.J.L. Histological Processing of Hydrogel Scaffolds for Tissue-Engineering Applications. J. Histotechnol. 2004, 27, 133–139. [Google Scholar] [CrossRef]
- Béduer, A.; Piacentini, N.; Aeberli, L.; Silva, A.D.; Verheyen, C.A.; Bonini, F.; Rochat, A.; Filippova, A.; Serex, L.; Renaud, P.; et al. Additive Manufacturing of Hierarchical Injectable Scaffolds for Tissue Engineering. Acta Biomater. 2018, 76, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Huisken, J.; Swoger, J.; Bene, F.D.; Wittbrodt, J.; Stelzer, E.H.K. Optical Sectioning Deep Inside Live Embryos by Selective Plane Illumination Microscopy. Science 2004, 305, 1007–1009. [Google Scholar] [CrossRef] [Green Version]
- Schneckenburger, H.; Weber, P.; Wagner, M.; Schickinger, S.; Richter, V.; Bruns, T.; Strauss, W.S.l.; Wittig, R. Light Exposure and Cell Viability in Fluorescence Microscopy. J. Microsc. 2012, 245, 311–318. [Google Scholar] [CrossRef]
- Smyrek, I.; Stelzer, E.H.K. Quantitative Three-Dimensional Evaluation of Immunofluorescence Staining for Large Whole Mount Spheroids with Light Sheet Microscopy. Biomed. Opt. Express 2017, 8, 484–499. [Google Scholar] [CrossRef] [Green Version]
- Lazzari, G.; Vinciguerra, D.; Balasso, A.; Nicolas, V.; Goudin, N.; Garfa-Traore, M.; Fehér, A.; Dinnyés, A.; Nicolas, J.; Couvreur, P.; et al. Light Sheet Fluorescence Microscopy versus Confocal Microscopy: In Quest of a Suitable Tool to Assess Drug and Nanomedicine Penetration into Multicellular Tumor Spheroids. Eur. J. Pharm. Biopharm. 2019, 142, 195–203. [Google Scholar] [CrossRef]
- Yu, F.; Han, X.; Zhang, K.; Dai, B.; Shen, S.; Gao, X.; Teng, H.; Wang, X.; Li, L.; Ju, H.; et al. Evaluation of a Polyvinyl Alcohol-Alginate Based Hydrogel for Precise 3D Bioprinting. J. Biomed. Mater. Res. A 2018, 106, 2944–2954. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D Culture of Chondrocytes in Gelatin Hydrogels with Different Stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cambria, E.; Brunner, S.; Heusser, S.; Fisch, P.; Hitzl, W.; Ferguson, S.J.; Wuertz-Kozak, K. Cell-Laden Agarose-Collagen Composite Hydrogels for Mechanotransduction Studies. Front. Bioeng. Biotechnol. 2020, 8, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norberg, K.J.; Liu, X.; Moro, C.F.; Strell, C.; Nania, S.; Blümel, M.; Balboni, A.; Bozóky, B.; Heuchel, R.L.; Löhr, J.M. A Novel Pancreatic Tumour and Stellate Cell 3D Co-Culture Spheroid Model. BMC Cancer 2020, 20, 475. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Dong, Y.; Wang, W. Encapsulation and 3D Culture of Human Adipose-Derived Stem Cells in an in-Situ Crosslinked Hybrid Hydrogel Composed of PEG-Based Hyperbranched Copolymer and Hyaluronic Acid. Stem Cell Res. Ther. 2013, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Fei, F.; Li, X.; Nie, Z.; Zhou, D.; Liu, L.; Zhang, J.; Zhang, H.; Fei, Z.; Xu, T. A Facile, Versatile Hydrogel Bioink for 3D Bioprinting Benefits Long-Term Subaqueous Fidelity, Cell Viability and Proliferation. Regen. Biomater. 2021, 8, rbab026. [Google Scholar] [CrossRef]
- Sbrana, F.V.; Pinos, R.; Barbaglio, F.; Ribezzi, D.; Scagnoli, F.; Scarfò, L.; Redwan, I.N.; Martinez, H.; Farè, S.; Ghia, P.; et al. 3D Bioprinting Allows the Establishment of Long-Term 3D Culture Model for Chronic Lymphocytic Leukemia Cells. Front. Immunol. 2021, 12, 639572. [Google Scholar] [CrossRef]
- Khattak, S.F.; Spatara, M.; Roberts, L.; Roberts, S.C. Application of Colorimetric Assays to Assess Viability, Growth and Metabolism of Hydrogel-Encapsulated Cells. Biotechnol. Lett. 2006, 28, 1361–1370. [Google Scholar] [CrossRef]
- Polley, C.; Mau, R.; Lieberwirth, C.; Stenzel, J.; Vollmar, B.; Seitz, H. Bioprinting of Three Dimensional Tumor Models: A Preliminary Study Using a Low Cost 3D Printer. Curr. Dir. Biomed. Eng. 2017, 3, 135–138. [Google Scholar] [CrossRef]
- Ho, W.Y.; Yeap, S.K.; Ho, C.L.; Rahim, R.A.; Alitheen, N.B. Development of Multicellular Tumor Spheroid (MCTS) Culture from Breast Cancer Cell and a High Throughput Screening Method Using the MTT Assay. PLoS ONE 2012, 7, e44640. [Google Scholar] [CrossRef] [Green Version]
- Maloney, E.; Clark, C.; Sivakumar, H.; Yoo, K.; Aleman, J.; Rajan, S.A.P.; Forsythe, S.; Mazzocchi, A.; Laxton, A.W.; Tatter, S.B.; et al. Immersion Bioprinting of Tumor Organoids in Multi-Well Plates for Increasing Chemotherapy Screening Throughput. Micromachines 2020, 11, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germain, N.; Dhayer, M.; Boileau, M.; Fovez, Q.; Kluza, J.; Marchetti, P. Lipid Metabolism and Resistance to Anticancer Treatment. Biology 2020, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Guerreschi, P.; Mortier, L.; Kluza, J. Integration of Mitochondrial Targeting for Molecular Cancer Therapeutics. Int. J. Cell Biol. 2015, 2015, 283145. [Google Scholar] [CrossRef] [Green Version]
- Tondera, C.; Hauser, S.; Krüger-Genge, A.; Jung, F.; Neffe, A.T.; Lendlein, A.; Klopfleisch, R.; Steinbach, J.; Neuber, C.; Pietzsch, J. Gelatin-Based Hydrogel Degradation and Tissue Interaction in Vivo: Insights from Multimodal Preclinical Imaging in Immunocompetent Nude Mice. Theranostics 2016, 6, 2114–2128. [Google Scholar] [CrossRef] [PubMed]
- Noel, P.; Muñoz, R.; Rogers, G.W.; Neilson, A.; Hoff, D.D.V.; Han, H. Preparation and Metabolic Assay of 3-Dimensional Spheroid Co-Cultures of Pancreatic Cancer Cells and Fibroblasts. J. Vis. Exp. Jove. 2017, 126, e56081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, F.-Y.; Tao, L.; Wei, Y.; Hsu, S. A Novel Biodegradable Self-Healing Hydrogel to Induce Blood Capillary Formation. NPG Asia Mater. 2017, 9, e363. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, P.; Fovez, Q.; Germain, N.; Khamari, R.; Kluza, J. Mitochondrial Spare Respiratory Capacity: Mechanisms, Regulation, and Significance in Non-Transformed and Cancer Cells. FASEB J. 2020, 34, 13106–13124. [Google Scholar] [CrossRef]
- Hunnewell, M.; Forbes, N.S. Active and Inactive Metabolic Pathways in Tumor Spheroids: Determination by GC-MS. Biotechnol. Prog. 2010, 26, 789–796. [Google Scholar] [CrossRef]
- Forbes, N.S.; Meadows, A.L.; Clark, D.S.; Blanch, H.W. Estradiol Stimulates the Biosynthetic Pathways of Breast Cancer Cells: Detection by Metabolic Flux Analysis. Metab. Eng. 2006, 8, 639–652. [Google Scholar] [CrossRef]
- Kim, B.; Forbes, N.S. Flux Analysis Shows That Hypoxia-Inducible-Factor-1-Alpha Minimally Affects Intracellular Metabolism in Tumor Spheroids. Biotechnol. Bioeng. 2007, 96, 1167–1182. [Google Scholar] [CrossRef]
- Klapa, M.I.; Aon, J.-C.; Stephanopoulos, G. Ion-Trap Mass Spectrometry Used in Combination with Gas Chromatography for High-Resolution Metabolic Flux Determination. Biotechniques 2003, 34, 832–849. [Google Scholar] [CrossRef] [PubMed]
- Ewa-Choy, Y.W.; Pingguan-Murphy, B.; Abdul-Ghani, N.A.; Jahendran, J.; Chua, K.H. Effect of Alginate Concentration on Chondrogenesis of Co-Cultured Human Adipose-Derived Stem Cells and Nasal Chondrocytes: A Biological Study. Biomater. Res. 2017, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Kawazoe, N.; Chen, G. Influence of Microporous Gelatin Hydrogels on Chondrocyte Functions. J. Mater. Chem. B 2017, 5, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Meinert, C.; Schrobback, K.; Hutmacher, D.W.; Klein, T.J. A Novel Bioreactor System for Biaxial Mechanical Loading Enhances the Properties of Tissue-Engineered Human Cartilage. Sci. Rep. 2017, 7, 16997. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Mikos, A.G. Injectable Biodegradable Hydrogel Composites for Rabbit Marrow Mesenchymal Stem Cell and Growth Factor Delivery for Cartilage Tissue Engineering. Biomaterials 2007, 28, 3217–3227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugh, M.G.; Meyer, E.G.; Thorpe, S.D.; Vinardell, T.; Duffy, G.P.; Kelly, D.J. Temporal and Spatial Changes in Cartilage-Matrix-Specific Gene Expression in Mesenchymal Stem Cells in Response to Dynamic Compression. Tissue Eng. Part A 2011, 17, 3085–3093. [Google Scholar] [CrossRef]
- Gauch, S.; Hermann, R.; Feuser, P.; Oelmüller, U.; Bastian, H. Isolation of Total RNA Using Silica-Gel Based Membranes. In Molecular Tools for Screening Biodiversity: Plants and Animals; Karp, I.A., Ingram, P.G., David, S., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 67–70. [Google Scholar] [CrossRef]
- Köster, N.; Schmiermund, A.; Grubelnig, S.; Leber, J.; Ehlicke, F.; Czermak, P.; Salzig, D. Single-Step RNA Extraction from Different Hydrogel-Embedded Mesenchymal Stem Cells for Quantitative Reverse Transcription–Polymerase Chain Reaction Analysis. Tissue Eng. Part C Methods 2016, 22, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Bougault, C.; Paumier, A.; Aubert-Foucher, E.; Mallein-Gerin, F. Investigating Conversion of Mechanical Force into Biochemical Signaling in Three-Dimensional Chondrocyte Cultures. Nat. Protoc. 2009, 4, 928–938. [Google Scholar] [CrossRef]
- Shin, D.-S.; You, J.; Rahimian, A.; Vu, T.; Siltanen, C.; Ehsanipour, A.; Stybayeva, G.; Sutcliffe, J.; Revzin, A. Photodegradable Hydrogels for Capture, Detection, and Release of Live Cells. Angew. Chem. Int. Ed. 2014, 53, 8221–8224. [Google Scholar] [CrossRef]
- Friedrich, J.; Eder, W.; Castaneda, J.; Doss, M.; Huber, E.; Ebner, R.; Kunz-Schughart, L.A. A Reliable Tool to Determine Cell Viability in Complex 3-D Culture: The Acid Phosphatase Assay. J. Biomol. Screen. 2007, 12, 925–937. [Google Scholar] [CrossRef] [Green Version]
- Beaumont, K.A.; Anfosso, A.; Ahmed, F.; Weninger, W.; Haass, N.K. Imaging- and Flow Cytometry-Based Analysis of Cell Position and the Cell Cycle in 3D Melanoma Spheroids. J. Vis. Exp. 2015, 106, 53486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Z.; Zhang, H.; Xiong, Q.; Zhang, Y.; Zhao, H.; Wang, G. A Fluorescent Chitosan Hydrogel Detection Platform for the Sensitive and Selective Determination of Trace Mercury(II) in Water. J. Mater. Chem. A 2015, 3, 19455–19460. [Google Scholar] [CrossRef] [Green Version]
- Muir, A.; Danai, L.V.; Heiden, M.G.V. Microenvironmental Regulation of Cancer Cell Metabolism: Implications for Experimental Design and Translational Studies. Dis. Model. Mech. 2018, 11, dmm035758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, M.M.; Fusenig, N.E. Friends or Foes—Bipolar Effects of the Tumour Stroma in Cancer. Nat. Rev. Cancer 2004, 4, 839–849. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Duong, M.N.; Geneste, A.; Fallone, F.; Li, X.; Dumontet, C.; Muller, C. The Fat and the Bad: Mature Adipocytes, Key Actors in Tumor Progression and Resistance. Oncotarget 2017, 8, 57622–57641. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Wu, M.; Zeng, N.; Xiong, M.; Hu, W.; Lv, W.; Yi, Y.; Zhang, Q.; Wu, Y. Cancer-Associated Adipocytes: Emerging Supporters in Breast Cancer. J. Exp. Clin. Cancer Res. 2020, 39, 156. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Fiori, M.E.; Franco, S.D.; Villanova, L.; Bianca, P.; Stassi, G.; Maria, R.D. Cancer-Associated Fibroblasts as Abettors of Tumor Progression at the Crossroads of EMT and Therapy Resistance. Mol. Cancer 2019, 18, 70. [Google Scholar] [CrossRef] [Green Version]
- Hanley, C.J.; Thomas, G.J. T-Cell Tumour Exclusion and Immunotherapy Resistance: A Role for CAF Targeting. Br. J. Cancer 2020, 123, 1353–1355. [Google Scholar] [CrossRef]
- Mondal, A.; Gebeyehu, A.; Miranda, M.; Bahadur, D.; Patel, N.; Ramakrishnan, S.; Rishi, A.K.; Singh, M. Characterization and Printability of Sodium Alginate -Gelatin Hydrogel for Bioprinting NSCLC Co-Culture. Sci Rep. 2019, 9, 19914. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Dey, M.; Ataie, Z.; Unutmaz, D.; Ozbolat, I.T. 3D Bioprinting for Reconstituting the Cancer Microenvironment. NPJ Precis. Oncol. 2020, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wei, X.; Huang, P. 3D Bioprinting of Hydrogel-based Biomimetic Microenvironments. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Jafari, S.; Sepand, M.R.; Alaei, L.; Malvajerd, S.S.; Jaymand, M.; Ghobadinezhad, F.; Jahanshahi, F.; Hamblin, M.R.; Derakhshankhah, H.; et al. 3D Bioprinting Technology to Mimic the Tumor Microenvironment: Tumor-on-a-Chip Concept. Mater. Today Adv. 2021, 12, 100160. [Google Scholar] [CrossRef]
- Albritton, J.L.; Miller, J.S. 3D Bioprinting: Improving in Vitro Models of Metastasis with Heterogeneous Tumor Microenvironments. Dis. Model. Mech. 2017, 10, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Richards, D.; Jia, J.; Yost, M.; Markwald, R.; Mei, Y. 3D Bioprinting for Vascularized Tissue Fabrication. Ann. Biomed. Eng. 2017, 45, 132–147. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Liao, D.; Johnson, R.S. Hypoxia: A Key Regulator of Angiogenesis in Cancer. Cancer Metast. Rev. 2007, 26, 281–290. [Google Scholar] [CrossRef]
- Zervantonakis, I.K.; Hughes-Alford, S.K.; Charest, J.L.; Condeelis, J.S.; Gertler, F.B.; Kamm, R.D. Three-Dimensional Microfluidic Model for Tumor Cell Intravasation and Endothelial Barrier Function. Proc. Natl. Acad. Sci. USA 2012, 109, 13515–13520. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Meyer, C.M.; Joung, D.; Vallera, D.A.; McAlpine, M.C.; Panoskaltsis-Mortari, A. 3D Bioprinted In Vitro Metastatic Models via Reconstruction of Tumor Microenvironments. Adv. Mater. 2019, 31, 1806899. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.-B.; Espenel, S.; Vallard, A.; Battiston-Montagne, P.; Wozny, A.-S.; Ardail, D.; Alphonse, G.; Rancoule, C.; Rodriguez-Lafrasse, C.; Magne, N. Evaluation of the Cell Invasion and Migration Process: A Comparison of the Video Microscope-Based Scratch Wound Assay and the Boyden Chamber Assay. J. Vis. Exp. 2017, 129, e56337. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Skhinas, J.N.; Du, E.Y.; Tolentino, M.A.K.; Utama, R.H.; Engel, M.; Volkerling, A.; Sexton, A.; O’Mahony, A.P.; Ribeiro, J.C.C.; et al. A High-Throughput 3D Bioprinted Cancer Cell Migration and Invasion Model with Versatile and Broad Biological Applicability. Biorxiv 2021. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Ajani, J.A.; Song, S. Drug Resistance and Cancer Stem Cells. Cell Commun. Signal. 2021, 19, 19. [Google Scholar] [CrossRef]
- Seo, E.J.; Kim, D.K.; Jang, I.H.; Choi, E.J.; Shin, S.H.; Lee, S.I.; Kwon, S.-M.; Kim, K.-H.; Suh, D.-S.; Kim, J.H. Hypoxia-NOTCH1-SOX2 Signaling Is Important for Maintaining Cancer Stem Cells in Ovarian Cancer. Oncotarget 2016, 7, 55624–55638. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived Spheroids: Relevance to Cancer Stem Cells and Clinical Applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Chen, Y.; Ji, W.; Chen, X.; Li, C.; Ge, R. Enrichment of Cancer Stem Cells by Agarose Multi-Well Dishes and 3D Spheroid Culture. Cell Tissue Res. 2019, 375, 397–408. [Google Scholar] [CrossRef]
- Suzuka, J.; Tsuda, M.; Wang, L.; Kohsaka, S.; Kishida, K.; Semba, S.; Sugino, H.; Aburatani, S.; Frauenlob, M.; Kurokawa, T.; et al. Rapid Reprogramming of Tumour Cells into Cancer Stem Cells on Double-Network Hydrogels. Nat. Biomed. Eng. 2021, 5, 914–925. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Liu, Y.; Yang, L.; Li, N.; Guo, X.; Sun, G.; Ma, X. Enrichment of Cancer Stem Cell-like Cells by Culture in Alginate Gel Beads. J. Biotechnol. 2014, 177, 1–12. [Google Scholar] [CrossRef]
- Moore, C.A.; Siddiqui, Z.; Carney, G.J.; Naaldijk, Y.; Guiro, K.; Ferrer, A.I.; Sherman, L.S.; Guvendiren, M.; Kumar, V.A.; Rameshwar, P. A 3D Bioprinted Material That Recapitulates the Perivascular Bone Marrow Structure for Sustained Hematopoietic and Cancer Models. Polymers 2021, 13, 480. [Google Scholar] [CrossRef]
- Florczyk, S.J.; Kievit, F.M.; Wang, K.; Erickson, A.E.; Ellenbogen, R.G.; Zhang, M. 3D Porous Chitosan–Alginate Scaffolds Promote Proliferation and Enrichment of Cancer Stem-like Cells. J. Mater. Chem. B 2016, 4, 6326–6334. [Google Scholar] [CrossRef]
- Kievit, F.M.; Florczyk, S.J.; Leung, M.C.; Wang, K.; Wu, J.D.; Silber, J.R.; Ellenbogen, R.G.; Lee, J.S.H.; Zhang, M. Proliferation and Enrichment of CD133+ Glioblastoma Cancer Stem Cells on 3D Chitosan-Alginate Scaffolds. Biomaterials 2014, 35, 9137–9143. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xiao, Z.; Meng, Y.; Zhao, Y.; Han, J.; Su, G.; Chen, B.; Dai, J. The Enhancement of Cancer Stem Cell Properties of MCF-7 Cells in 3D Collagen Scaffolds for Modeling of Cancer and Anti-Cancer Drugs. Biomaterials 2012, 33, 1437–1444. [Google Scholar] [CrossRef]
- Wang, X.; Dai, X.; Zhang, X.; Li, X.; Xu, T.; Lan, Q. Enrichment of Glioma Stem Cell-like Cells on 3D Porous Scaffolds Composed of Different Extracellular Matrix. Biochem. Biophys. Res. Commun. 2018, 498, 1052–1057. [Google Scholar] [CrossRef]
- Feng, S.; Duan, X.; Lo, P.-K.; Liu, S.; Liu, X.; Chen, H.; Wang, Q. Expansion of Breast Cancer Stem Cells with Fibrous Scaffolds. Integr. Biol. 2013, 5, 768–777. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.S. Mechanotransduction—a Field Pulling Together? J. Cell Sci. 2008, 121, 3285–3292. [Google Scholar] [CrossRef] [Green Version]
- Kulangara, K.; Leong, K.W. Substrate Topography Shapes Cell Function. Soft Matter 2009, 5, 4072–4076. [Google Scholar] [CrossRef]
- Ghibaudo, M.; Saez, A.; Trichet, L.; Xayaphoummine, A.; Browaeys, J.; Silberzan, P.; Buguin, A.; Ladoux, B. Traction Forces and Rigidity Sensing Regulate Cell Functions. Soft Matter 2008, 4, 1836–1843. [Google Scholar] [CrossRef]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of Mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental Sensing through Focal Adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, F. Stiffness—an Unknown World of Mechanical Science? Injury 2000, 31, 14–84. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Tian, M.; Pei, Q.; Tan, F.; Pei, H. Extracellular Matrix Stiffness: New Areas Affecting Cell Metabolism. Front. Oncol. 2021, 11, 631991. [Google Scholar] [CrossRef] [PubMed]
- Strickaert, A.; Saiselet, M.; Dom, G.; Deken, X.D.; Dumont, J.E.; Feron, O.; Sonveaux, P.; Maenhaut, C. Cancer Heterogeneity Is Not Compatible with One Unique Cancer Cell Metabolic Map. Oncogene 2017, 36, 2637–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A Tense Situation: Forcing Tumour Progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Cox, A.G.; Tsomides, A.; Yimlamai, D.; Hwang, K.L.; Miesfeld, J.; Galli, G.G.; Fowl, B.H.; Fort, M.; Ma, K.Y.; Sullivan, M.R.; et al. Yap Regulates Glucose Utilization and Sustains Nucleotide Synthesis to Enable Organ Growth. EMBO J. 2018, 37. [Google Scholar] [CrossRef]
- Liu, Q.; Song, G. Matrix Stiffness Promotes Hepatoma Cell Glycolysis and Migration Through YAP-Mediated Mechanotransduction. Mol. Cell. Biomech. 2019, 16, 127. [Google Scholar] [CrossRef]
- Bays, J.L.; Campbell, H.K.; Heidema, C.; Sebbagh, M.; DeMali, K.A. Linking E-Cadherin Mechanotransduction to Cell Metabolism through Force-Mediated Activation of AMPK. Nat. Cell Biol. 2017, 19, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Du, W.; Wu, M. Regulation of the Pentose Phosphate Pathway in Cancer. Protein Cell 2014, 5, 592–602. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Cui, C.; Zhang, K.; Wang, J.; Wang, Y.; Lu, Y.; Chen, K.; Yuan, J.; Xiao, G.; Tang, B.; et al. Kindlin-2 Links Mechano-Environment to Proline Synthesis and Tumor Growth. Nat. Commun. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Guo, L.; Wu, C. How Signaling Pathways Link Extracellular Mechano-environment to Proline Biosynthesis: A Hypothesis. Bioessays 2021, 43, 2100116. [Google Scholar] [CrossRef] [PubMed]
- Bogatikov, E.; Munoz, C.; Pakladok, T.; Alesutan, I.; Shojaiefard, M.; Seebohm, G.; Föller, M.; Palmada, M.; Böhmer, C.; Bröer, S.; et al. Up-Regulation of Amino Acid Transporter SLC6A19 Activity and Surface Protein Abundance by PKB/Akt and PIKfyve. Cell. Physiol. Biochem. 2012, 30, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.G.; Ng, Y.L.D.; Lam, W.-L.M.; Plouffe, S.W.; Guan, K.-L. The Hippo Pathway Effectors YAP and TAZ Promote Cell Growth by Modulating Amino Acid Signaling to MTORC1. Cell Res. 2015, 25, 1299–1313. [Google Scholar] [CrossRef]
- Romani, P.; Brian, I.; Santinon, G.; Pocaterra, A.; Audano, M.; Pedretti, S.; Mathieu, S.; Forcato, M.; Bicciato, S.; Manneville, J.-B.; et al. Extracellular Matrix Mechanical Cues Regulate Lipid Metabolism through Lipin-1 and SREBP. Nat. Cell Biol. 2019, 21, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Luiken, J.J.F.P.; Coort, S.L.M.; Willems, J.; Coumans, W.A.; Bonen, A.; van der Vusse, G.J.; Glatz, J.F.C. Contraction-Induced Fatty Acid Translocase/CD36 Translocation in Rat Cardiac Myocytes Is Mediated Through AMP-Activated Protein Kinase Signaling. Diabetes 2003, 52, 1627–1634. [Google Scholar] [CrossRef] [Green Version]
- Edinger, A.L.; Thompson, C.B. Akt Maintains Cell Size and Survival by Increasing MTOR-Dependent Nutrient Uptake. Mol. Biol. Cell 2002, 13, 2276–2288. [Google Scholar] [CrossRef] [Green Version]
- Tilghman, R.W.; Blais, E.M.; Cowan, C.R.; Sherman, N.E.; Grigera, P.R.; Jeffery, E.D.; Fox, J.W.; Blackman, B.R.; Tschumperlin, D.J.; Papin, J.A.; et al. Matrix Rigidity Regulates Cancer Cell Growth by Modulating Cellular Metabolism and Protein Synthesis. PLoS ONE 2012, 7, e37231. [Google Scholar] [CrossRef] [Green Version]
- Tilghman, R.W.; Cowan, C.R.; Mih, J.D.; Koryakina, Y.; Gioeli, D.; Slack-Davis, J.K.; Blackman, B.R.; Tschumperlin, D.J.; Parsons, J.T. Matrix Rigidity Regulates Cancer Cell Growth and Cellular Phenotype. PLoS ONE 2010, 5, e12905. [Google Scholar] [CrossRef] [Green Version]
- Uhler, C.; Shivashankar, G.V. Regulation of Genome Organization and Gene Expression by Nuclear Mechanotransduction. Nat. Rev. Mol. Cell Biol. 2017, 18, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Lyra-Leite, D.M.; Andres, A.M.; Petersen, A.P.; Ariyasinghe, N.R.; Cho, N.; Lee, J.A.; Gottlieb, R.A.; McCain, M.L. Mitochondrial Function in Engineered Cardiac Tissues Is Regulated by Extracellular Matrix Elasticity and Tissue Alignment. Am. J. Physiol.-Heart C 2017, 313, H757–H767. [Google Scholar] [CrossRef] [PubMed]
- Bartolák-Suki, E.; Imsirovic, J.; Nishibori, Y.; Krishnan, R.; Suki, B. Regulation of Mitochondrial Structure and Dynamics by the Cytoskeleton and Mechanical Factors. Int. J. Mol. Sci. 2017, 18, 1812. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, Y.; Deng, X.; Guo, L.; Wu, C. Extracellular Matrix Stiffness Regulates Mitochondrial Dynamics through PINCH-1- and Kindlin-2-Mediated Signalling. Curr. Res. Cell Biol. 2021, 2, 100008. [Google Scholar] [CrossRef]
- Westermann, B. Bioenergetic Role of Mitochondrial Fusion and Fission. Biochim. Biophys. Acta BBA—Bioenerg. 2012, 1817, 1833–1838. [Google Scholar] [CrossRef] [Green Version]
- Gkretsi, V.; Stylianopoulos, T. Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef]
- Fattet, L.; Jung, H.-Y.; Matsumoto, M.W.; Aubol, B.E.; Kumar, A.; Adams, J.A.; Chen, A.C.; Sah, R.L.; Engler, A.J.; Pasquale, E.B.; et al. Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis via a Mechanoresponsive EPHA2/LYN Complex. Dev. Cell 2020, 54, 302–316.e7. [Google Scholar] [CrossRef]
- Ros, M.; Sala, M.; Saltel, F. Linking Matrix Rigidity with EMT and Cancer Invasion. Dev. Cell 2020, 54, 293–295. [Google Scholar] [CrossRef]
- Bordeleau, F.; Mason, B.N.; Lollis, E.M.; Mazzola, M.; Zanotelli, M.R.; Somasegar, S.; Califano, J.P.; Montague, C.; LaValley, D.J.; Huynh, J.; et al. Matrix Stiffening Promotes a Tumor Vasculature Phenotype. Proc. Natl. Acad. Sci. USA 2017, 114, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Rice, A.J.; Cortes, E.; Lachowski, D.; Cheung, B.C.H.; Karim, S.A.; Morton, J.P.; del Rio Hernández, A. Matrix Stiffness Induces Epithelial–Mesenchymal Transition and Promotes Chemoresistance in Pancreatic Cancer Cells. Oncogenesis 2017, 6, e352. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Lv, X.; Li, P.; Yang, R.; Xia, Q.; Chen, Y.; Peng, Y.; Li, L.; Li, S.; Li, T.; et al. Matrix Stiffness Modulates ILK-Mediated YAP Activation to Control the Drug Resistance of Breast Cancer Cells. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2020, 1866, 165625. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Pelissier, F.A.; Zhang, H.; Lakins, J.; Weaver, V.M.; Park, C.; LaBarge, M.A. Microenvironment Rigidity Modulates Responses to the HER2 Receptor Tyrosine Kinase Inhibitor Lapatinib via YAP and TAZ Transcription Factors. Mol. Biol. Cell 2015, 26, 3946–3953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, F.E.; Kelly, D.J. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzucu, M.; Vera, G.; Beaumont, M.; Fischer, S.; Wei, P.; Shastri, V.P.; Forget, A. Extrusion-Based 3D Bioprinting of Gradients of Stiffness, Cell Density, and Immobilized Peptide Using Thermogelling Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 2192–2197. [Google Scholar] [CrossRef]
- Monferrer, E.; Martín-Vañó, S.; Carretero, A.; García-Lizarribar, A.; Burgos-Panadero, R.; Navarro, S.; Samitier, J.; Noguera, R. A Three-Dimensional Bioprinted Model to Evaluate the Effect of Stiffness on Neuroblastoma Cell Cluster Dynamics and Behavior. Sci. Rep. 2020, 10, 6370. [Google Scholar] [CrossRef] [Green Version]
- Mazzocchi, A.; Soker, S.; Skardal, A. 3D Bioprinting for High-Throughput Screening: Drug Screening, Disease Modeling, and Precision Medicine Applications. Appl. Phys. Rev. 2019, 6, 011302. [Google Scholar] [CrossRef]
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D Bioprinting of Functional Tissue Models for Personalized Drug Screening and in Vitro Disease Modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef]
- Sharifi, M.; Bai, Q.; Babadaei, M.M.N.; Chowdhury, F.; Hasan, M.; Taghizadeh, A.; Derakhshankhah, H.; Khan, S.; Hasan, A.; Falahati, M. 3D Bioprinting of Engineered Breast Cancer Constructs for Personalized and Targeted Cancer Therapy. J. Control. Release 2021, 333, 91–106. [Google Scholar] [CrossRef]
- Lee, J.; Unnithan, A.R.; Park, C.H.; Kim, C.S. Woodhead Publishing Series in Biomaterials, 1st ed.; Woodhead Publishing Series in Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–72. [Google Scholar] [CrossRef]
- Bom, S.; Martins, A.M.; Ribeiro, H.M.; Marto, J. Diving into 3D (Bio)Printing: A Revolutionary Tool to Customize the Production of Drug and Cell-Based Systems for Skin Delivery. Int. J. Pharm. 2021, 605, 120794. [Google Scholar] [CrossRef]
- Grottkau, B.E.; Hui, Z.; Pang, Y. A Novel 3D Bioprinter Using Direct-Volumetric Drop-On-Demand Technology for Fabricating Micro-Tissues and Drug-Delivery. Int. J. Mol. Sci. 2020, 21, 3482. [Google Scholar] [CrossRef]
- Botti, G.; Bonito, M.D.; Cantile, M. Organoid Biobanks as a New Tool for Pre-Clinical Validation of Candidate Drug Efficacy and Safety. Int. J. Physiol. Pathophysiol. Pharmacol. 2021, 13, 17–21. [Google Scholar] [PubMed]
- Ravanbakhsh, H.; Luo, Z.; Zhang, X.; Maharjan, S.; Mirkarimi, H.S.; Tang, G.; Chávez-Madero, C.; Mongeau, L.; Zhang, Y.S. Freeform Cell-Laden Cryobioprinting for Shelf-Ready Tissue Fabrication and Storage. Matter 2022, 5, 573–593. [Google Scholar] [CrossRef]
- Miri, A.K.; Khalilpour, A.; Cecen, B.; Maharjan, S.; Shin, S.R.; Khademhosseini, A. Multiscale Bioprinting of Vascularized Models. Biomaterials 2019, 198, 204–216. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germain, N.; Dhayer, M.; Dekiouk, S.; Marchetti, P. Current Advances in 3D Bioprinting for Cancer Modeling and Personalized Medicine. Int. J. Mol. Sci. 2022, 23, 3432. https://doi.org/10.3390/ijms23073432
Germain N, Dhayer M, Dekiouk S, Marchetti P. Current Advances in 3D Bioprinting for Cancer Modeling and Personalized Medicine. International Journal of Molecular Sciences. 2022; 23(7):3432. https://doi.org/10.3390/ijms23073432
Chicago/Turabian StyleGermain, Nicolas, Melanie Dhayer, Salim Dekiouk, and Philippe Marchetti. 2022. "Current Advances in 3D Bioprinting for Cancer Modeling and Personalized Medicine" International Journal of Molecular Sciences 23, no. 7: 3432. https://doi.org/10.3390/ijms23073432
APA StyleGermain, N., Dhayer, M., Dekiouk, S., & Marchetti, P. (2022). Current Advances in 3D Bioprinting for Cancer Modeling and Personalized Medicine. International Journal of Molecular Sciences, 23(7), 3432. https://doi.org/10.3390/ijms23073432






