BcSOC1 Promotes Bolting and Stem Elongation in Flowering Chinese Cabbage
Abstract
:1. Introduction
2. Results
2.1. Expression and Subcellular Localization of BcSOC1 in Flowering Chinese Cabbage
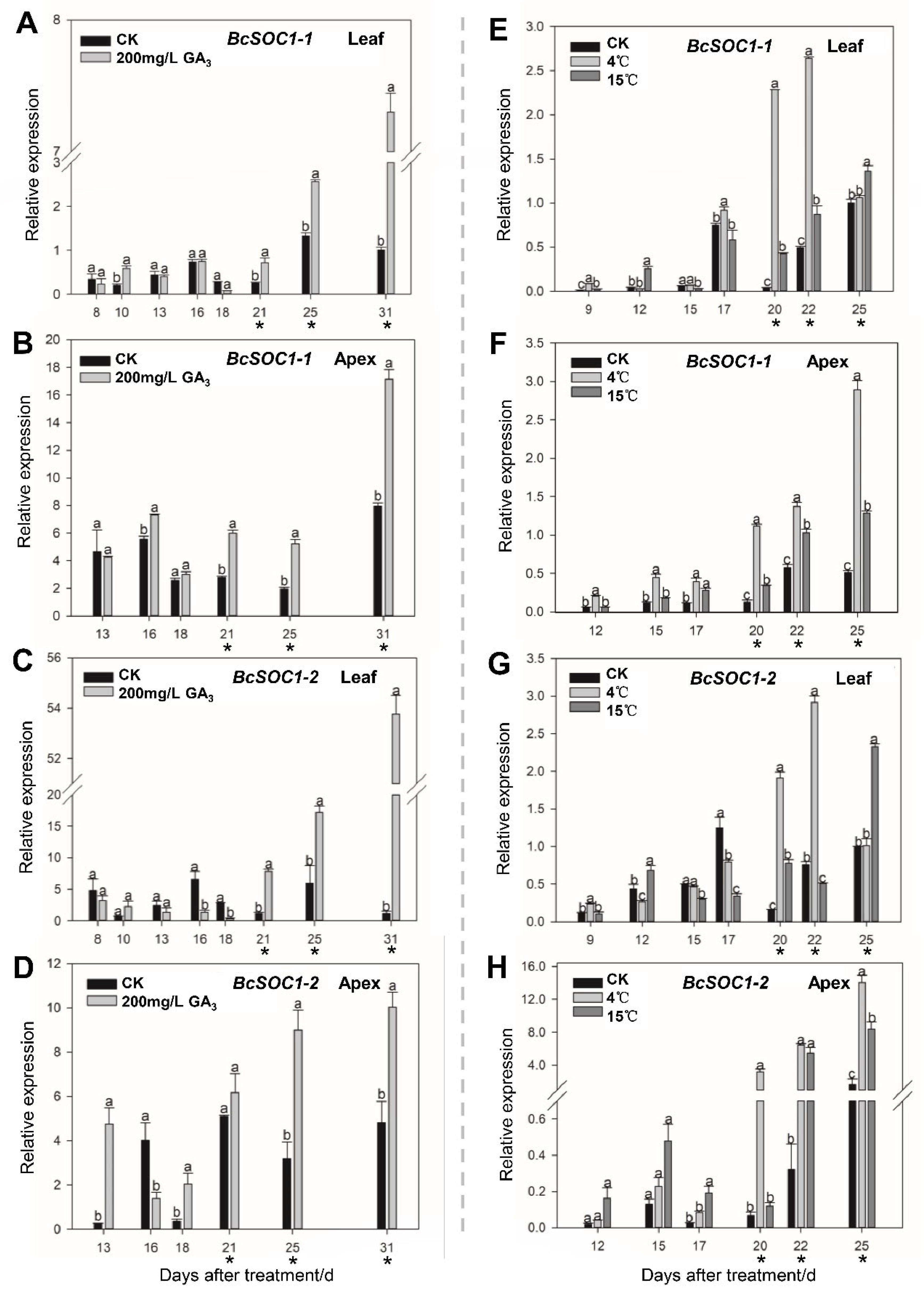
2.2. GA3 and Low-Temperature Treatments Affect Bolting and BcSOC1 Expression in Flowering Chinese Cabbage
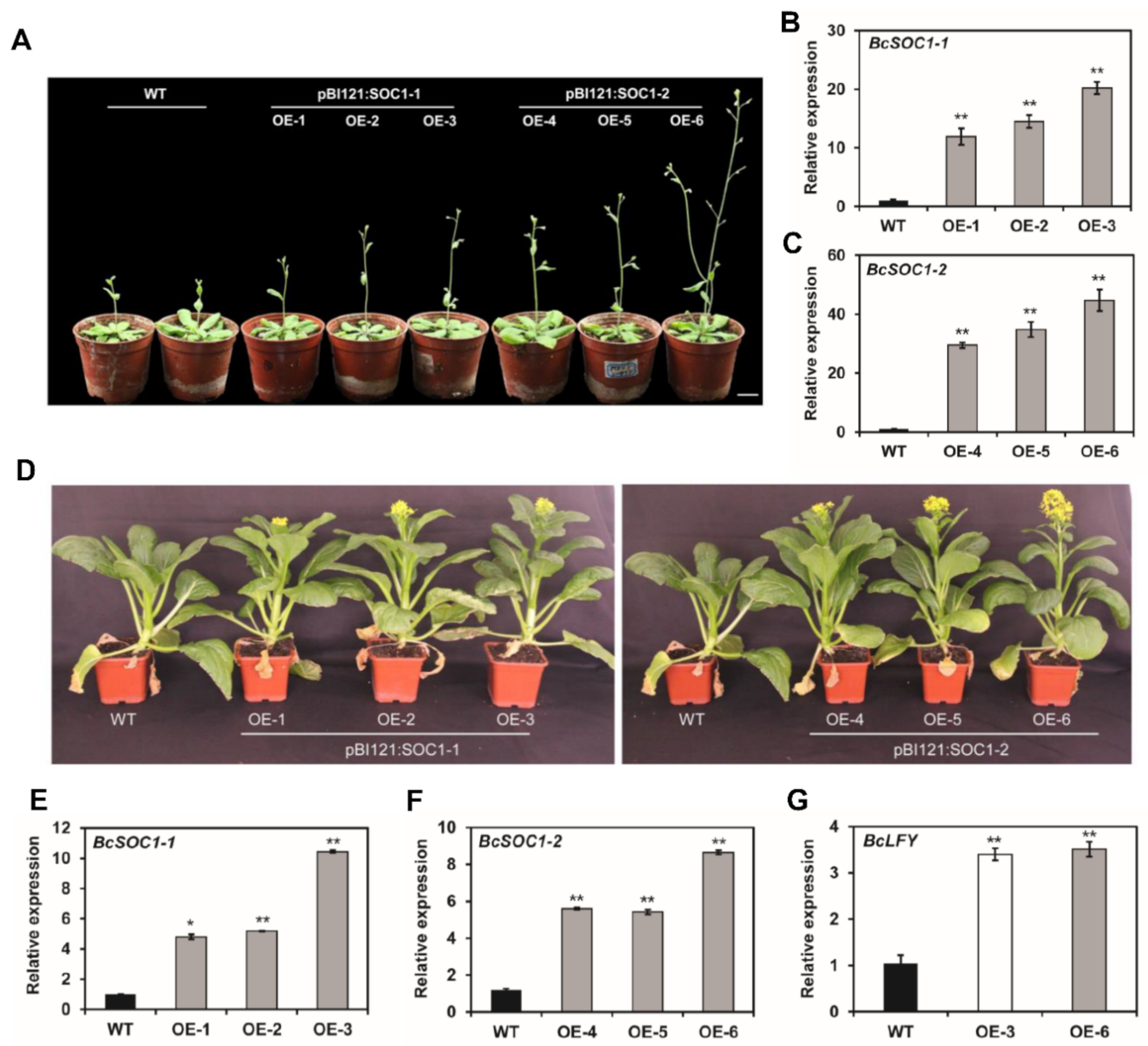
2.3. BcSOC1 Overexpression Accelerates Stem Elongation and Flowering in Arabidopsis and Flowering Chinese Cabbage
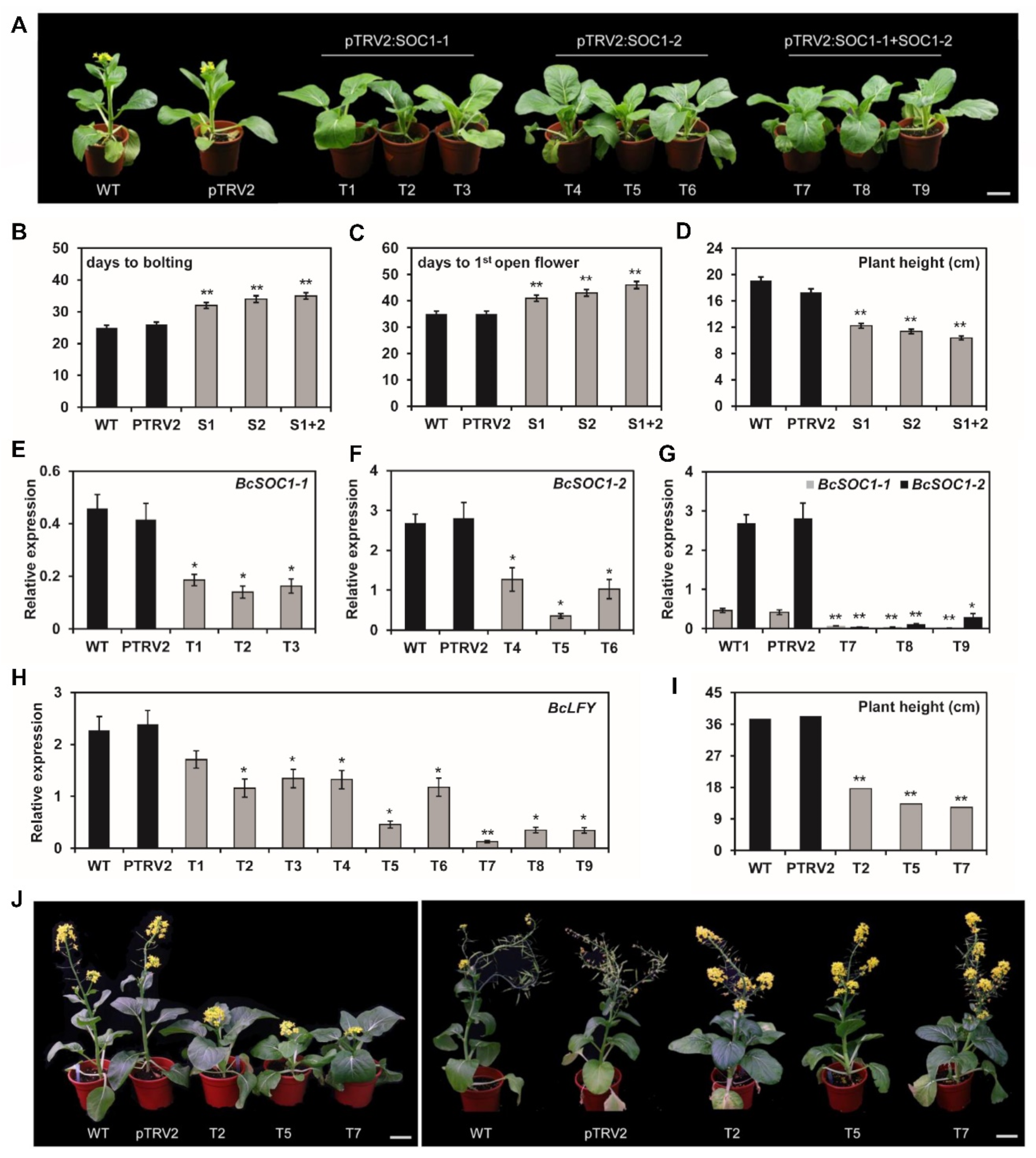
2.4. BcSOC1 Silencing Delays Bolting and Stem Elongation in Flowering Chinese Cabbage
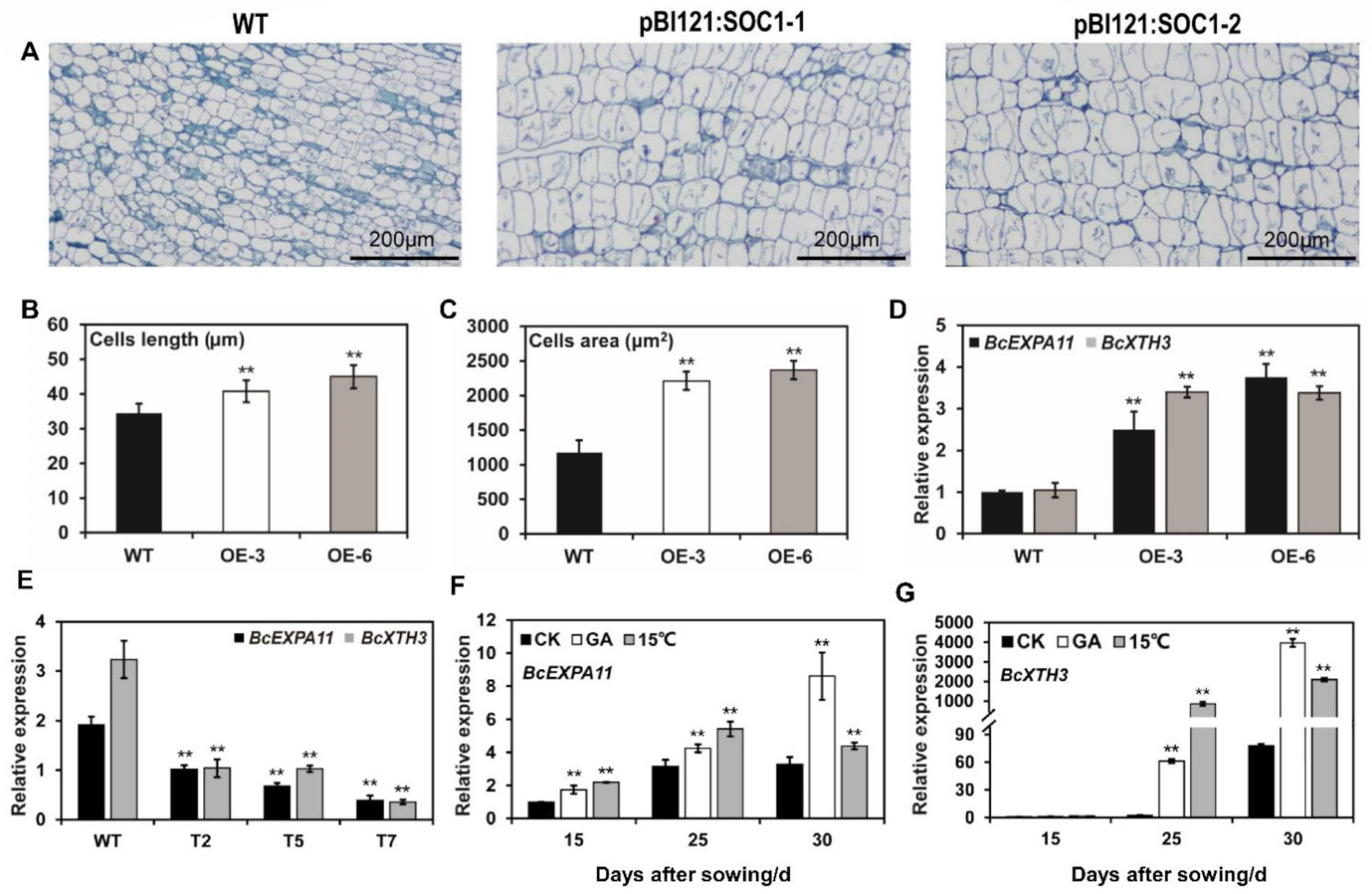
2.5. BcSOC1 Overexpression Probably Induces Cell Expansion by Upregulating BcEXPA11 and BcXTH3
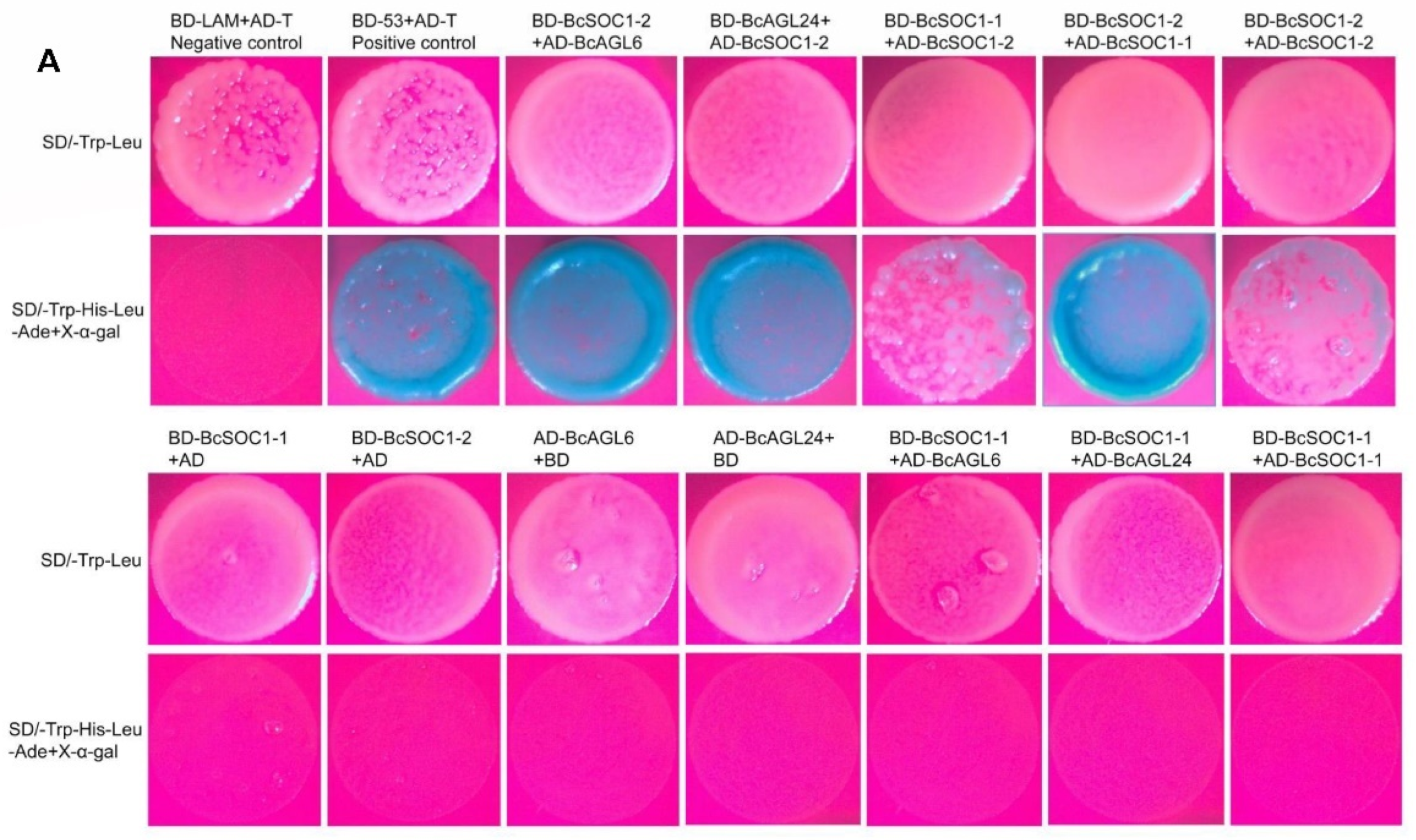
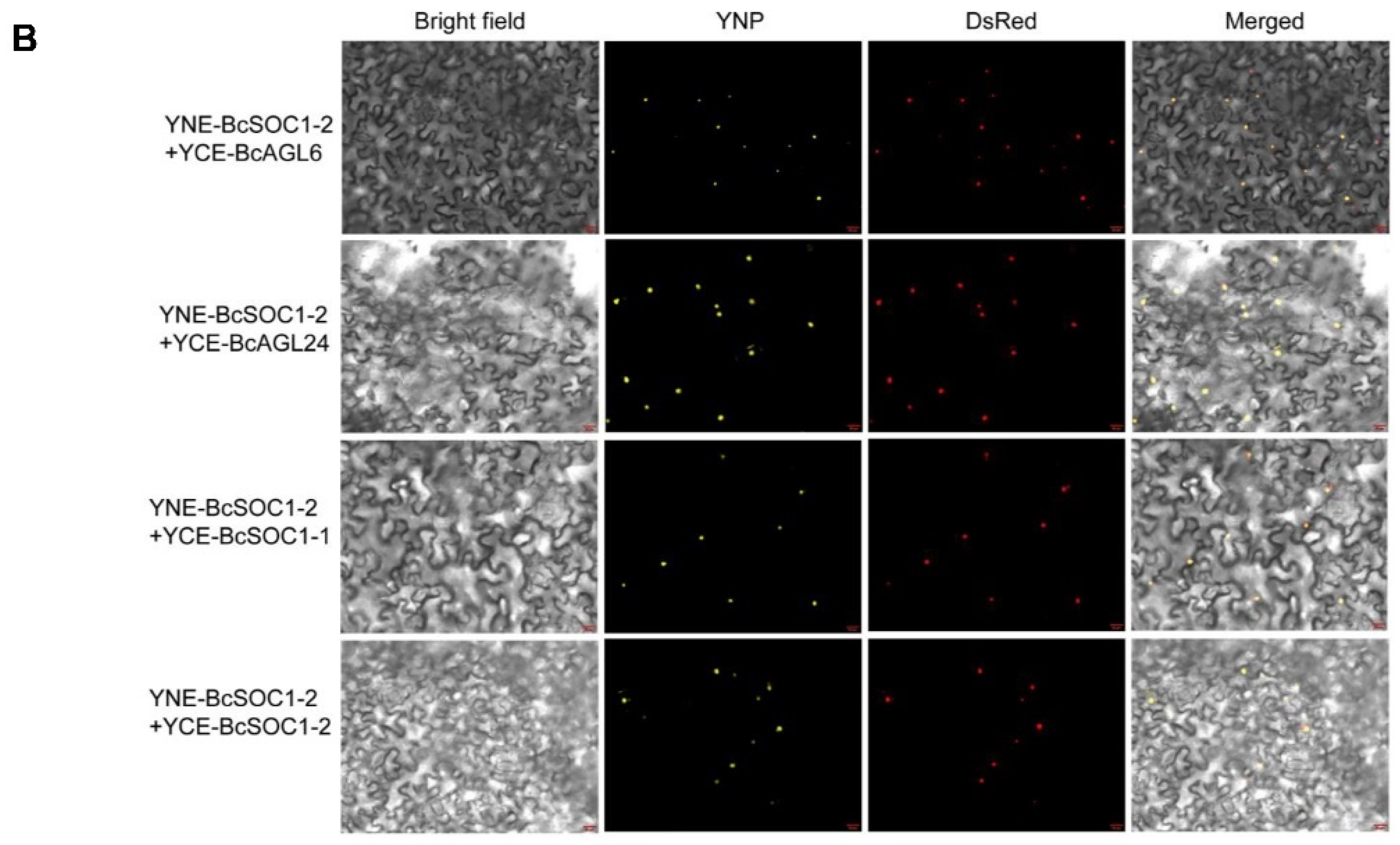
2.6. BcSOC1 Interacts with BcAGL6, BcAGL24, and BcSOC1
3. Discussion
3.1. SOC1 Expression Pattern Correlates with Bolting in Flowering Chinese Cabbage
3.2. GA3 and Low-Temperature Treatments Accelerate Bolting and Flowering in Flowering Chinese Cabbage by Mediating BcSOC1 Expression
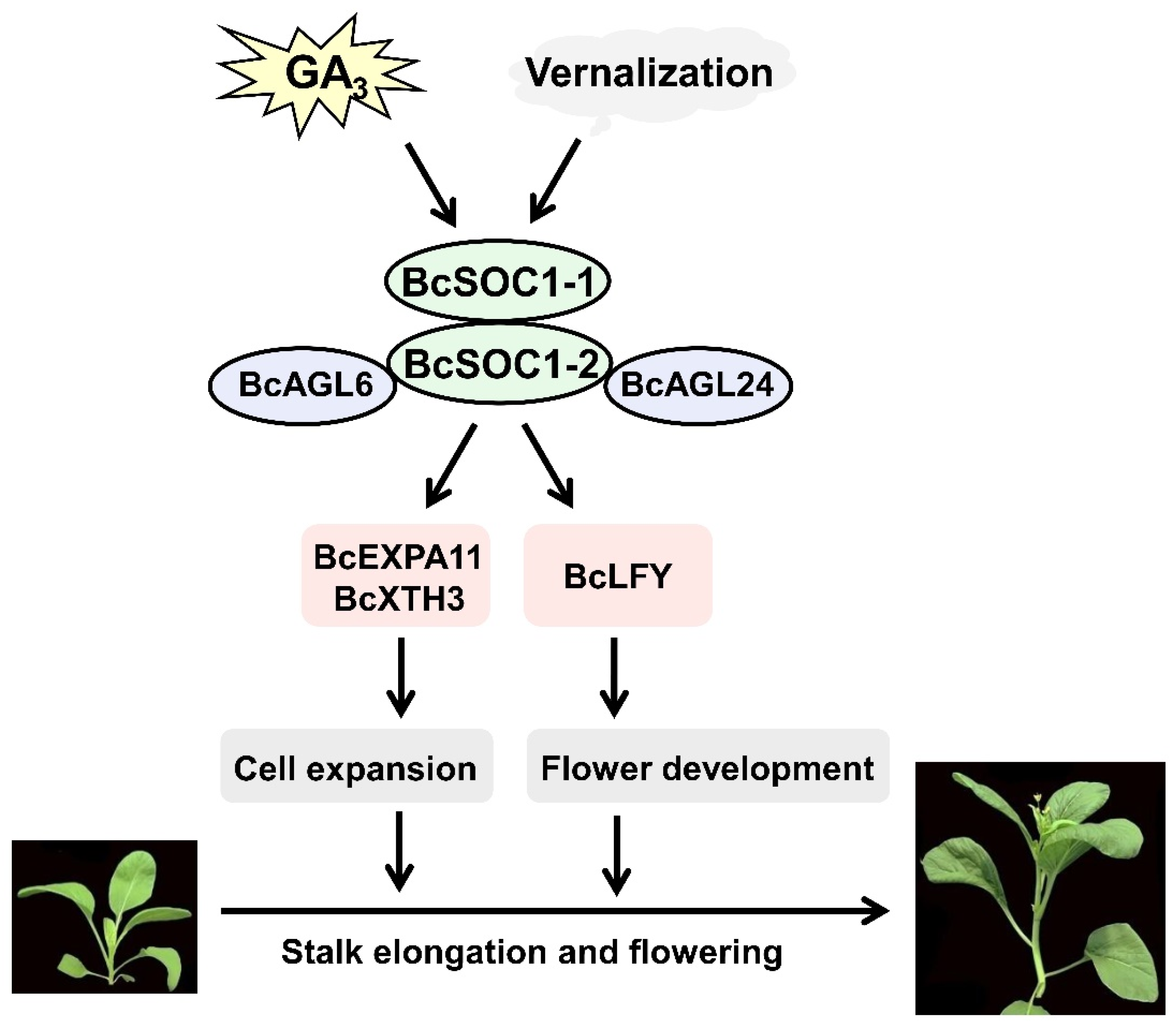
3.3. BcSOC1 Plays a Vital Role in Regulating Bolting and Stem Elongation in Flowering Chinese Cabbage
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Gene Cloning
4.3. Phylogenetic Analyses of SOC1 Homologs
4.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.5. Subcellular Localization
4.6. GA3 and Low-Temperature Treatments
4.7. Ectopic Expression of BcSOC1 in Arabidopsis
4.8. Virus-Induced Gene Silencing (VIGS) Assay
4.9. Agrobacterium-Mediated Transformation in Flowering Chinese Cabbage
4.10. Yeast Two-Hybrid Assay
4.11. Bimolecular Fluorescence Complementation Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Dekker, M. Glucosinolates in Brassica vegetables: The infuence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219–S265. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, J.J.; Wang, X.W.; Zhao, J.J.; Wang, X.W.; Deng, B.; Lou, P.; Wu, J.; Sun, R.F.; Xu, Z.Y.; et al. Genetic relationships within Brassica rapa as inferred from AFLP fingerprints. Teor. Appl. Genet. 2005, 110, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wu, J.; Wang, X. Genome triplication drove the diversification of Brassica plants. Hortic. Res. 2014, 1, 14024. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Zhou, G.F.; Cai, Y.F.; Yang, J.P. Transcriptome analysis of stem development in the tumourous stem mustard Brassica juncea var. tumida Tsen et Lee by RNA sequencing. BMC Plant Biol. 2012, 12, 53. [Google Scholar]
- Cheng, F.; Sun, R.F.; Hou, X.L.; Zheng, H.K. Subgenome parallel selection is associated with morphotype diversification and convergent crop domestication in Brassica rapa and Brassica oleracea. Nat. Genet. 2016, 48, 1218. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Phenolic component profiles of mustard greens, yu choy, and 15 other Brassica vegetables. J. Agr. Food Chem. 2010, 58, 6850. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Cai, X.; Wu, J.; Liu, M.; Grob, S.; Cheng, F.; Liang, J.; Cai, C.C.; Liu, Z.Y.; Liu, B. Improved Brassica rapa reference genome by single-molecule sequencing and chromosome conformation capture technologies. Hortic. Res. 2018, 5, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Manchali, S.; Murthy, K.N.C.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Xiao, X.; Wu, C.; Xu, Z.; Yang, Y.; Fan, S.; Wang, H. Molecular cloning, characterization and expression analysis of bolting-associated genes in flowering Chinese cabbage. Genes Genom. 2015, 37, 357–363. [Google Scholar] [CrossRef]
- Liu, C.; Thong, Z.; Yu, H. Coming into bloom: The specification of floral meristems. Development 2009, 136, 3379–3391. [Google Scholar] [CrossRef] [Green Version]
- Fornara, F.; de Montaigu, A.; Coupland, G. SnapShot: Control of flowering in Arabidopsis. Cell 2010, 141, 550–550.e2. [Google Scholar] [CrossRef] [Green Version]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell. Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, L.; Giakountis, A.; Farrona, S.; Gissot, L. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef] [Green Version]
- Gregis, V.; Sessa, A.; Dorca-Fornell, C.; Kater, M.M. The Arabidopsis floral meristem identity genes AP1, AGL24 and SVP directly repress class B and C floral homeotic genes. Plant J. 2009, 60, 626–637. [Google Scholar] [CrossRef]
- Wigge, P.A.; Kim, M.C.; Jaeger, K.E.; Busch, W.; Schmid, M.; Lohmann, J.U.; Weigel, D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309, 1056–1059. [Google Scholar] [CrossRef]
- Lee, J.; Oh, M.; Park, H.; Lee, I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates leafy. Plant J. 2008, 55, 832–843. [Google Scholar] [CrossRef]
- Hepworth, S.R.; Valverde, F.; Ravenscroft, D.; Mouradov, A.; Coupland, G. Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 2002, 21, 4327–4337. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Ying, H.; Helliwell, C.A.; Taylor, J.M.; Peacock, W.J.; Dennis, E.S. FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc. Natl Acad. Sci. USA 2011, 108, 6680–6685. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Suh, S.S.; Lee, H.; Choi, K.R.; Hong, C.B.; Paek, N.C.; Kim, S.G.; Lee, I. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003, 35, 613–623. [Google Scholar] [CrossRef]
- Ferrario, S.; Busscher, J.; Franken, J. Ectopic Expression of the Petunia MADS Box Gene UNSHAVEN Accelerates Flowering and Confers Leaf-Like Characteristics to Floral Organs in a Dominant-Negative Manner. Plant Cell 2004, 16, 1490–1505. [Google Scholar] [CrossRef] [Green Version]
- Mouhu, K.; Kurokura, T.; Koskela, E.A.; Albert, V.A.; Elomaa, H. The Fragaria vesca homolog of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 represses flowering and promotes vegetative growth. Plant Cell 2013, 25, 3296–3310. [Google Scholar] [CrossRef] [Green Version]
- Voogd, C.; Wang, T.; Varkonyi-Gasic, E. Functional and expression analyses of kiwifruit SOC1-like genes suggest that they may not have a role in the transition to flowering but may affect the duration of dormancy. J. Exp. Bot. 2015, 66, 4699–4710. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Lei, Y.; Huang, X.; Su, W.; Chen, R.; Hao, Y. Crosstalk of cold and gibberellin effects on bolting and flowering in flowering Chinese cabbage. J. Integr. Agric. 2019, 18, 992–1000. [Google Scholar] [CrossRef]
- Huang, X.; Lei, Y.; Guan, H.; Hao, Y.; Liu, H.; Sun, G.; Chen, R.; Song, S. Transcriptomic analysis of the regulation of stalk development in flowering Chinese cabbage (Brassica campestris) by RNA sequencing. Sci. Rep. 2017, 7, 15517. [Google Scholar] [CrossRef] [Green Version]
- Huang, X. Transcriptomic and Metabolomic Analysis of the Regulation of Stalk Development in Flowering Chinese Cabbage. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2018. [Google Scholar]
- Immink, R.G.H.; Pose, D.; Ferrario, S. Characterization of SOC1’s central role in flowering by the identification of its upstream and downstream regulators. Plant Physiol. 2012, 160, 433–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borner, R.; Kampmann, G.; Chandler, J.; Gleissner, R.; Melzer, S. A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J. 2000, 24, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Samach, A.; Onouchi, H.; Gold, S.E.; Ditta, G.S.; Schwarz-Sommer, Z.; Yanofsky, M.F.; Coupland, G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 2000, 288, 1613–1616. [Google Scholar] [CrossRef] [Green Version]
- Searle, I.; He, Y.; Turck, F. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev. 2006, 20, 898–912. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Qi, S.; Yang, L.; Dai, Y.; Dai, S. Characterization of Chrysanthemum ClSOC1-1, and ClSOC1-2, homologous genes of SOC1. Plant Mol. Biol. Rep. 2014, 32, 740–749. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Han, J.J.; Han, M.J.; An, G. Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in rice. Plant J. 2004, 38, 754–764. [Google Scholar] [CrossRef]
- Shitsukawa, N.; Ikari, C.; Mitsuya, T.; Sakiyama, T.; Ishikawa, A.; Takumi, S.; Murai, K. Wheat SOC1 functions independently of WAP1/VRN1, an integrator of vernalization and photoperiod flowering promotion pathways. Physiol. Plant 2007, 130, 627–636. [Google Scholar] [CrossRef]
- Lei, H.J.; Yuan, H.Z.; Liu, Y.; Guo, X.W.; Liao, X.; Liu, L.L.; Wang, Q.; Li, T.H. Identification and characterization of FaSOC1, a homolog of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 from strawberry. Gene 2013, 531, 158–167. [Google Scholar] [CrossRef]
- Zhong, X.; Dai, X.; Xv, J.; Wu, H.; Liu, B.; Li, H. Cloning and expression analysis of GmGAL1, SOC1 homolog gene in soybean. Mol. Biol. Rep. 2012, 39, 6967–6974. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.; Ge, D.; Han, H.; Ning, K.; Luo, C.; Wang, S.; Liu, R.; Zhang, X. LCM-seq reveals the crucial role of LsSOC1 in heat-promoted bolting of lettuce (Lactuca sativa L.). Plant J. 2018, 95, 516–528. [Google Scholar] [CrossRef] [Green Version]
- Liu, C. Regulation of floral patterning by flowering time genes. Dev. Cell. 2009, 16, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Dorca-Fornell, C.; Gregis, V.; Grandi, V.; Coupland, G.; Colombo, L.; Kater, M.M. The Arabidopsis SOC1-like genes AGL42, AGL71 and AGL72 promote flowering in the shoot apical and axillary meristems. Plant J. 2011, 67, 1006–1017. [Google Scholar] [CrossRef]
- Lee, H.; Suh, S.S.; Park, E. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 2000, 14, 2366–2376. [Google Scholar] [CrossRef] [Green Version]
- Mauren, J.; Lulu, Z.; Chong, C.; Li, G.F.; Tang, Y.H.; Wen, J.Q.; Mysore, K.S.; Putterill, J. A SOC1-like gene MtSOC1a promotes flowering and primary stem elongation in Medicago. J. Exp. Bot. 2018, 69, 4867–4880. [Google Scholar]
- Zhao, S.; Zhang, M.L.; Ma, T.L.; Wang, Y. Phosphorylation of ARF2 relieves its repression of transcription of the K+ transporter gene HAK5 in response to low potassium stress. Plant Cell 2016, 28, 3005–3019. [Google Scholar] [CrossRef] [Green Version]
- Nayar, S.; Kapoor, M.; Kapoor, S. Post-translational regulation of rice MADS29 function: Homodimerization or binary interactions with other seed-expressed MADS proteins modulate its translocation into the nucleus. J. Exp. Bot. 2014, 65, 5339–5350. [Google Scholar] [CrossRef] [Green Version]
- Severing, E.I.; van Dijk, A.D.; Morabito, G.; Busscher-Lange, J.; Immink, R.G.; van Ham, R.C. Predicting the Impact of Alternative Splicing on Plant MADS Domain Protein Function. PLoS ONE 2012, 7, e30524. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Wang, L.L.; Sun, L.T.; Dong, L.L.; Liu, B.; Chen, L.P. Cell division and endoreduplication play important roles in stem swelling of tuber mustard (Brassica juncea Coss. var. tumida Tsen et Lee). Plant Biol. 2012, 14, 956–963. [Google Scholar] [CrossRef]
- Kou, E.; Huang, X.; Zhu, Y.; Su, W.; Liu, H.; Sun, G.; Chen, R.; Song, S. Crosstalk between auxin and gibberellin during stalk elongation in flowering chinese cabbage. Sci. Rep. 2021, 11, 3976. [Google Scholar] [CrossRef]
- Marowa, P.; Ding, A.; Kong, Y. Expansions: Roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 2016, 35, 949–965. [Google Scholar] [CrossRef] [Green Version]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.B.; Jiang, C.; Chen, X.Y.; Zhang, T.; Ding, L.; Song, W.B.; Luo, H.B.; Lai, J.S.; Chen, H.B.; Liu, R.Y.; et al. Maize LAZY1 Mediates Shoot Gravitropism and Inflorescence Development through Regulating Auxin Transport, Auxin Signaling, and Light Response. Plant Physiol. 2013, 163, 1306–1322. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, G.; Hao, X.; Cao, B.; Chen, Q.; Liu, S.; Lei, J. CaMF2, an anther-specific lipid transfer protein (LTP) gene, affects pollen development in Capsicum annuum L. Plant Sci. 2011, 181, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Liang, W. Establishment and Optimization of a High-efficiency Regeneration and Genetic Transformation System of Flowering Chinese Cabbage. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2020. [Google Scholar]
- Ding, L.; Yan, S.; Jiang, L.; Zhao, W.; Ning, K.; Zhao, J.; Liu, X.; Zhang, J.; Wang, Q.; Zhang, X. HANABA TARANU (HAN) Bridges Meristem and Organ Primordia Boundaries through PINHEAD, JAGGED, BLADE-ON-PETIOLE2 and CYTOKININ OXIDASE 3 during Flower Development in Arabidopsis. PLoS Genet. 2015, 11, e1005479. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.; Chaban, C.; Schutze, K.; Batistic, O.; Weckermann, K.; Näke, C.; Blazevic, D.; Grefen, C.; Schumacher, K.; Oecking, C.; et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 2004, 40, 428–438. [Google Scholar] [CrossRef]

| Species | Lines | Days to Flowering (d) | Days to Bolting (d) | Plant Height (cm) |
|---|---|---|---|---|
| WT | 31.58 ± 0.96 a | / | 5.11 ± 0.86 c | |
| Arabidopsis | pBI121:SOC1-1 | 27.72 ± 0.74 b | / | 9.83 ± 1.33 b |
| pBI121:SOC1-2 | 25.77 ± 1.22 c | / | 16.84 ± 3.96 a | |
| WT | 38.92 ± 1.08 a | 30.08 ± 0.90 a | 19.03 ± 1.05 c | |
| B. campestris | pBI121:SOC1-1 | 32.92 ± 1.51 b | 24.92 ± 1.24 b | 25.80 ± 1.21 b |
| pBI121:SOC1-2 | 29.83 ± 1.47 c | 23.17 ± 1.19 c | 28.06 ± 1.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Huang, X.; Huang, X.; Su, W.; Hao, Y.; Liu, H.; Chen, R.; Song, S. BcSOC1 Promotes Bolting and Stem Elongation in Flowering Chinese Cabbage. Int. J. Mol. Sci. 2022, 23, 3459. https://doi.org/10.3390/ijms23073459
Wang Y, Huang X, Huang X, Su W, Hao Y, Liu H, Chen R, Song S. BcSOC1 Promotes Bolting and Stem Elongation in Flowering Chinese Cabbage. International Journal of Molecular Sciences. 2022; 23(7):3459. https://doi.org/10.3390/ijms23073459
Chicago/Turabian StyleWang, Yudan, Xiu Huang, Xinmin Huang, Wei Su, Yanwei Hao, Houcheng Liu, Riyuan Chen, and Shiwei Song. 2022. "BcSOC1 Promotes Bolting and Stem Elongation in Flowering Chinese Cabbage" International Journal of Molecular Sciences 23, no. 7: 3459. https://doi.org/10.3390/ijms23073459






