Laccases and Tyrosinases in Organic Synthesis
Abstract
:1. Introduction

2. Laccase-Catalyzed Reactions
2.1. Homocoupling
2.2. Heterocoupling
2.3. Other Oxidation Reactions
2.4. Sources of Laccases
3. Tyrosinase-Catalyzed Reactions
3.1. Ortho-Hydroxylation of Phenols
3.2. Sources of Tyrosinases
4. Major Challenges and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Chang, C.W.; Liao, J.Y.; Lu, K.T. Syntheses and characteristics of urushiol-based waterborne UV-cured wood coatings. Polymers 2021, 13, 4005. [Google Scholar] [CrossRef]
- Pezzella, C.; Guarino, L.; Piscitelli, A. How to enjoy laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef]
- Uyama, H. Functional polymers from renewable plant oils. Polym. J. 2018, 50, 1003–1011. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Swiderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkolazka, A.; Paszczynski, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.K.S.; Gupta, R.K.; Kim, S.Y.; Kim, I.W.; Kalia, V.C.; Lee, J.K. Rhus vernicifera laccase immobilization on magnetic nanoparticles to improve stability and its potential application in bisphenol A degradation. Indian J. Microbiol. 2021, 61, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Muschiol, J.; Meyer, A.S.; Kepp, K.P. A structural-chemical explanation of fungal laccase activity. Sci. Rep. 2018, 8, 17285. [Google Scholar] [CrossRef]
- Agrawal, K.; Verma, P. Multicopper oxidase laccases with distinguished spectral properties: A new outlook. Heliyon 2020, 6, e03972. [Google Scholar] [CrossRef] [PubMed]
- Radveikiené, I.; Vidžiúnaité, R.; Meškiené, R.; Meškys, R.; Časaité, V. Characterization of a yellow laccase from Botrytis cinerea 241. J. Fungi 2021, 7, 143. [Google Scholar] [CrossRef]
- Mot, A.C.; Coman, C.; Hadade, N.; Damian, G.; Silaghi-Dumitrescu, R.; Heering, H. “Yellow” laccase from Sclerotinia sclerotiorum is a blue laccase that enhances its substrate affinity by forming a reversible tyrosyl-product adduct. PLoS ONE 2020, 15, e0225530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehra, R.; Kepp, K.P. Contribution of substrate reorganization energies of electron transfer to laccase activity. Phys. Chem. Chem. Phys. 2019, 21, 15805–15814. [Google Scholar] [CrossRef]
- Mehra, R.; Meyer, A.S.; Kepp, K.P. Molecular dynamics derived life times of active substrate binding poses explain KM of laccase mutants. RSC Adv. 2018, 8, 36915–36926. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H. Chemistry of lacquer (urushi). J. Chem. Soc. 1883, 43, 472–486. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.K.S.; Otari, S.V.; Li, J.; Kim, D.R.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J. Hazard. Mater. 2018, 347, 442–450. [Google Scholar] [CrossRef]
- Vipotnik, Z.; Michelin, M.; Tavares, T. Rehabilitation of a historically contaminated soil by different laccases and laccase-mediator system. J. Soils Sediments 2022, 1–9. [Google Scholar] [CrossRef]
- Sorrentino, I.; Carriere, M.; Jamet, H.; Stanzione, I.; Piscitelli, A.; Giardina, P.; Le Goff, A. The laccase mediator system at carbon nanotubes for anthracene oxidation and femtomolar electrochemical biosensing. Analyst 2022, 147, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-catalysed oxidations of naturally occurring phenols: From in vivo biosynthetic pathways to green synthetic applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef] [Green Version]
- Debnath, R.; Saha, T. An insight into the production strategies and applications of the ligninolytic enzyme laccase from bacteria and fungi. Biocatal. Agric. Biotechnol. 2020, 26, 101645. [Google Scholar] [CrossRef]
- Khatami, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various types and applications. Biotechnol. Appl. Biochem. 2022. [Google Scholar] [CrossRef]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C. Recent trends in fungal laccase for various industrial applications: An eco-friendly approach—A review. Biotechnol. Bioproc. Eng. 2016, 21, 19–38. [Google Scholar] [CrossRef]
- Rostami, A.; Abdelrasoul, A.; Shokri, Z.; Shirvandi, Z. Applications and mechanisms of free and immobilized laccase in detoxification of phenolic compounds—A review. Korean J. Chem. Eng. 2022, 1–12. [Google Scholar] [CrossRef]
- Martínková, L.; Kotik, M.; Marková, E.; Homolka, L. Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: A review. Chemosphere 2016, 149, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, H.M.N.; Zhao, Y.P. Hazardous contaminants in the environment and their laccase-assisted degradation—A review. J. Environ. Manag. 2019, 234, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Estrada, C.; Rostro-Alanis, M.D.; Muñoz-Gutiérrez, B.D.; Iqbal, H.M.N.; Kannan, S.; Parra-Saldívar, R. Emergent contaminants: Endocrine disruptors and their laccase-assisted degradation—A review. Sci. Total Environ. 2018, 612, 1516–1531. [Google Scholar] [CrossRef]
- Yashas, S.R.; Shivakumara, B.P.; Udayashankara, T.H.; Krishna, B.M. Laccase biosensor: Green technique for quantification of phenols in wastewater (a review). Orient. J. Chem. 2018, 34, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Li, S.Y.; Si, Y.B.; Huang, Q.G. Advances in laccase-triggered anabolism for biotechnology applications. Crit. Rev. Biotechnol. 2021, 41, 969–993. [Google Scholar] [CrossRef]
- Slagman, S.; Zuilhof, H.; Franssen, M.C.R. Laccase-mediated grafting on biopolymers and synthetic polymers: A critical review. ChemBioChem 2018, 19, 288–311. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Luo, G.; Ngo, H.H.; Guo, W.S.; Zhang, S.C. Advances in thermostable laccase and its current application in lignin-first biorefinery: A review. Bioresour. Technol. 2020, 298, 122511. [Google Scholar] [CrossRef]
- Mayolo-Deloisa, K.; González-González, M.; Rito-Palomares, M. Laccases in food industry: Bioprocessing, potential industrial and biotechnological applications. Front. Bioeng. Biotechnol. 2020, 8, 222. [Google Scholar] [CrossRef] [Green Version]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Nordin, N.A.; Lamaming, J.; Asim, M. Laccase, an emerging tool to fabricate green composites: A review. Bioresources 2015, 10, 6262–6284. [Google Scholar] [CrossRef] [Green Version]
- Adamian, Y.; Lonappan, L.; Alokpa, K.; Agathos, S.N.; Cabana, H. Recent developments in the immobilization of laccase on carbonaceous supports for environmental applications—A critical review. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Vieira, Y.A.; Gurgel, D.; Henriques, R.O.; Machado, R.A.F.; de Oliveira, D.; Oechsler, B.F.; Furigo, A. A perspective review on the application of polyacrylonitrile-based supports for laccase immobilization. Chem. Rec. 2021, e202100215. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.H.; Yuan, L.; Jia, L.N.; Xue, P.; Yao, H.Q. Recent developments of a co-immobilized laccase-mediator system: A review. RSC Adv. 2021, 11, 29498–29506. [Google Scholar] [CrossRef]
- Zhou, W.T.; Zhang, W.X.; Cai, Y.P. Laccase immobilization for water purification: A comprehensive review. Chem. Eng. J. 2021, 403, 126272. [Google Scholar] [CrossRef]
- Daronch, N.A.; Kelbert, M.; Pereira, C.S.; de Araújo, P.H.H.; de Oliveira, D. Elucidating the choice for a precise matrix for laccase immobilization: A review. Chem. Eng. J. 2020, 397, 125506. [Google Scholar] [CrossRef]
- Ren, D.J.; Wang, Z.B.; Jiang, S.; Yu, H.Y.; Zhang, S.Q.; Zhang, X.Q.; Taylor; Francis, L. Recent environmental applications of and development prospects for immobilized laccase: A review. Biotechnol. Genet. Eng. Rev. 2020, 36, 81–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, X.; Sun, J.L.; Chen, S.C. Stimulation of laccase biocatalysis in ionic liquids: A review on recent progress. Curr. Prot. Pept. Sci. 2018, 19, 100–111. [Google Scholar] [CrossRef]
- Lei, L.L.; Yang, X.Y.; Song, Y.D.; Huang, H.; Li, Y.X. Current research progress on laccase-like nanomaterials. New J. Chem. 2022, 46, 3541–3550. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases-occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [Green Version]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg. Med. Chem. Lett. 2014, 22, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.A.; Riley, P.A. Mechanistic aspects of the tyrosinase oxidation of hydroquinone. Bioorg. Med. Chem. Lett. 2014, 24, 2463–2464. [Google Scholar] [CrossRef]
- Halaouli, S.; Asther, M.; Sigoillot, J.C.; Hamdi, M.; Lomascolo, A. Fungal tyrosinases: New prospects in molecular characteristics, bioengineering and biotechnological applications. J. Appl. Microbiol. 2006, 100, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Son, S.; Yun, H.Y.; Kim, D.H.; Chun, P.; Moon, H.R. Tyrosinase inhibitors: A patent review (2011–2015). Expert Opin. Ther. Pat. 2016, 26, 347–362. [Google Scholar] [CrossRef]
- Burlando, B.; Clericuzio, M.; Cornara, L. Moraceae plants with tyrosinase inhibitory activity: A review. Mini-Rev. Med. Chem. 2017, 17, 108–121. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Khan, M.T.H.; Muñoz-Muñoz, J.; García-Molina, F.; García-Cánovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhibit. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Opperman, L.; De Kock, M.; Klaasen, J.; Rahiman, F. Tyrosinase and melanogenesis inhibition by indigenous african plants: A review. Cosmetics 2020, 7, 60. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Wang, G.C.; Zeng, Q.H.; Li, Y.F.; Liu, H.Q.; Wang, J.J.; Zhao, Y. A systematic review of synthetic tyrosinase inhibitors and their structure-activity relationship. Crit. Rev. Food Sci. Nutr. 2021, 1–42. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Kerkar, S. Microorganisms as a source of tyrosinase inhibitors: A review. Ann. Microbiol. 2017, 67, 343–358. [Google Scholar] [CrossRef]
- García-Molina, P.; García-Molina, F.; Teruel-Puche, J.A.; Rodríguez-López, J.N.; García-Cánovas, F.; Muñoz-Muñoz, J.L. Considerations about the kinetic mechanism of tyrosinase in its action on monophenols: A review. Mol. Catal. 2022, 518, 112072. [Google Scholar] [CrossRef]
- Mogharabi, M.; Faramarzi, M.A. Laccase and laccase-mediated systems in the synthesis of organic compounds. Adv. Synth. Catal. 2014, 356, 897–927. [Google Scholar] [CrossRef]
- Kudanga, T.; Nemadziva, B.; Le Roes-Hill, M. Laccase catalysis for the synthesis of bioactive compounds. Appl. Microbiol. Biotechnol. 2017, 101, 13–33. [Google Scholar] [CrossRef] [PubMed]
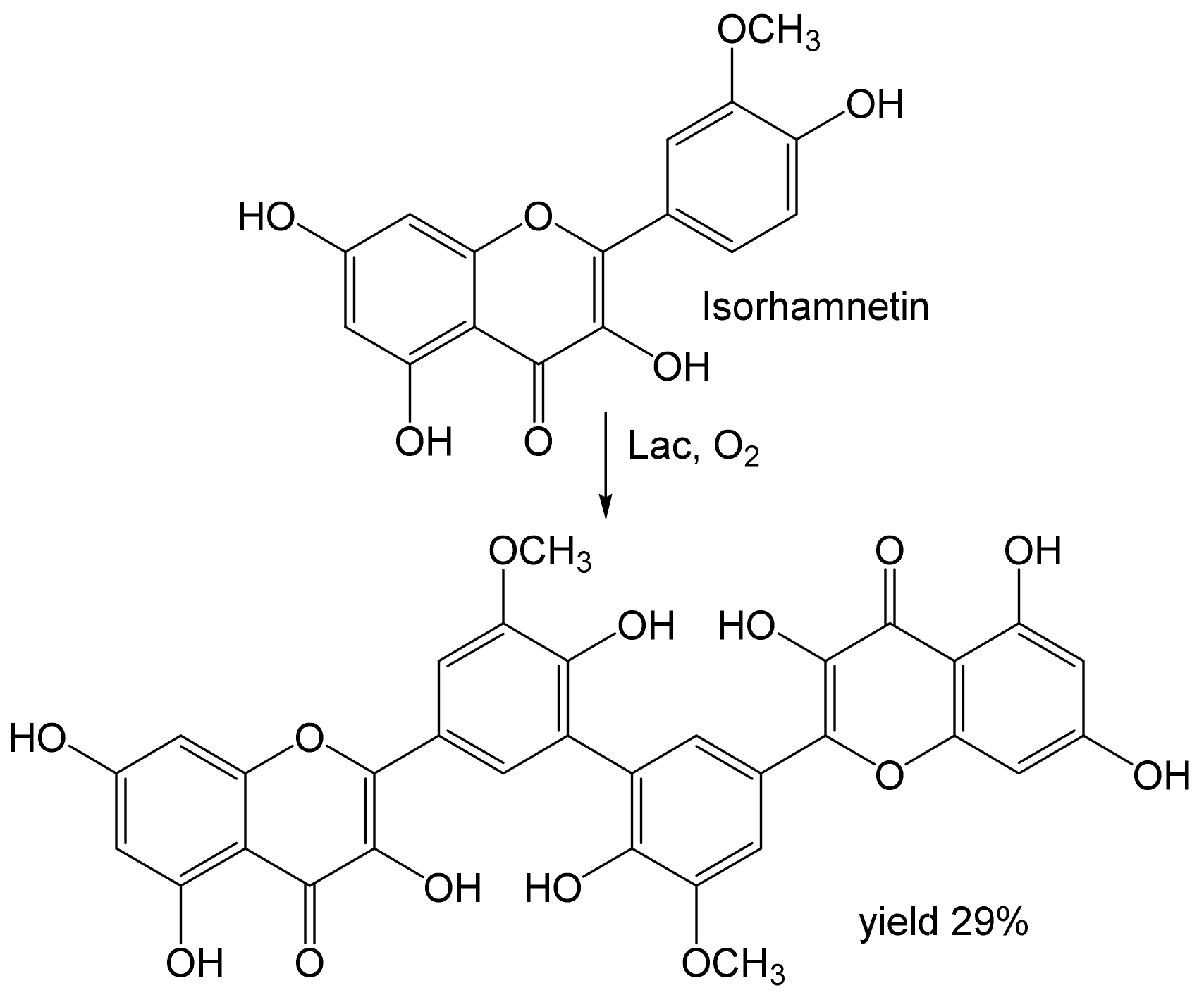
- Aruwa, C.E.; Amoo, S.O.; Koorbanally, N.; Kudanga, T. Laccase-mediated modification of isorhamnetin improves antioxidant and antibacterial activities. Process Biochem. 2022, 112, 53–61. [Google Scholar] [CrossRef]
- Gavezzotti, P.; Bertacchi, F.; Fronza, G.; Křen, V.; Monti, D.; Riva, S. Laccase-catalyzed dimerization of piceid, a resveratrol glucoside, and its further enzymatic elaboration. Adv. Synth. Catal. 2015, 357, 1831–1839. [Google Scholar] [CrossRef]
- Bassanini, I.; Gavezzotti, P.; Monti, D.; Krejzova, J.; Kren, V.; Riva, S. Laccase-catalyzed dimerization of glycosylated lignols. J. Mol. Catal. B-Enzym. 2016, 134, 295–301. [Google Scholar] [CrossRef]
- Muñiz-Mouro, A.; Ferreira, A.M.; Coutinho, J.A.P.; Freire, M.G.; Tavares, A.P.M.; Gullón, P.; González-García, S.; Eibes, G. Integrated biocatalytic platform based on aqueous biphasic systems for the sustainable oligomerization of rutin. ACS Sustain. Chem. Eng. 2021, 9, 9941–9950. [Google Scholar] [CrossRef]
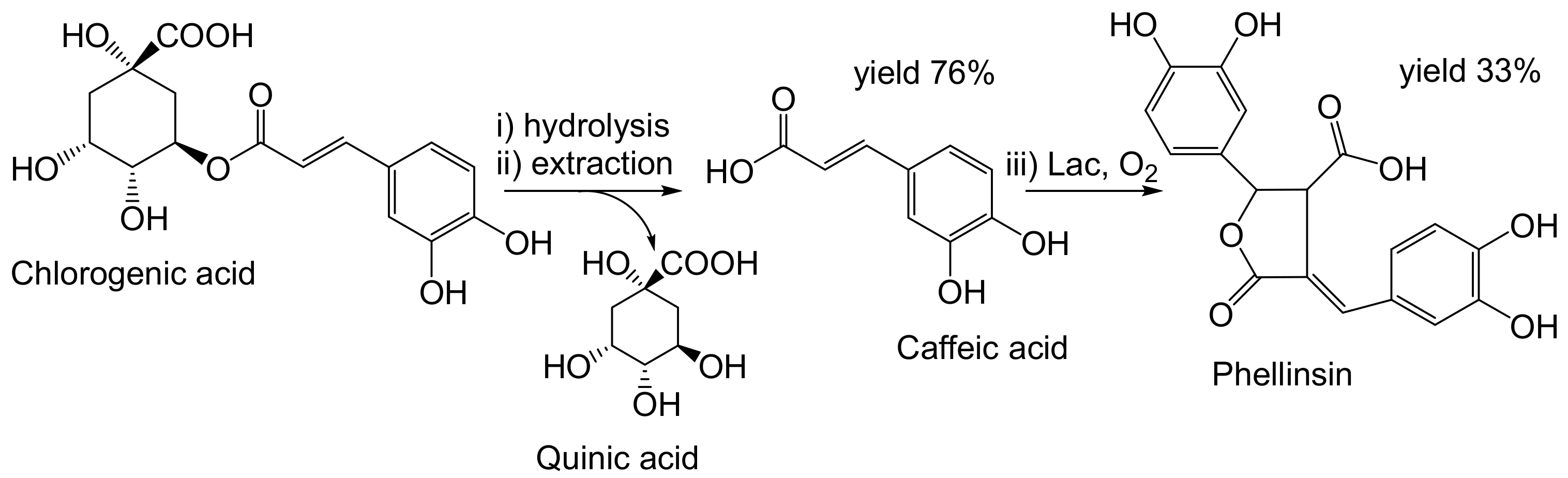
- Nemadziva, B.; Le Roes-Hill, M.; Koorbanally, N.; Kudanga, T. Small laccase-catalyzed synthesis of a caffeic acid dimer with high antioxidant capacity. Process Biochem. 2018, 69, 99–105. [Google Scholar] [CrossRef]
- Nemadziva, B.; Ngubane, S.; Ruzengwe, F.M.; Kasumbwe, K.; Kudanga, T. Potato peels as feedstock for laccase-catalysed synthesis of phellinsin A. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
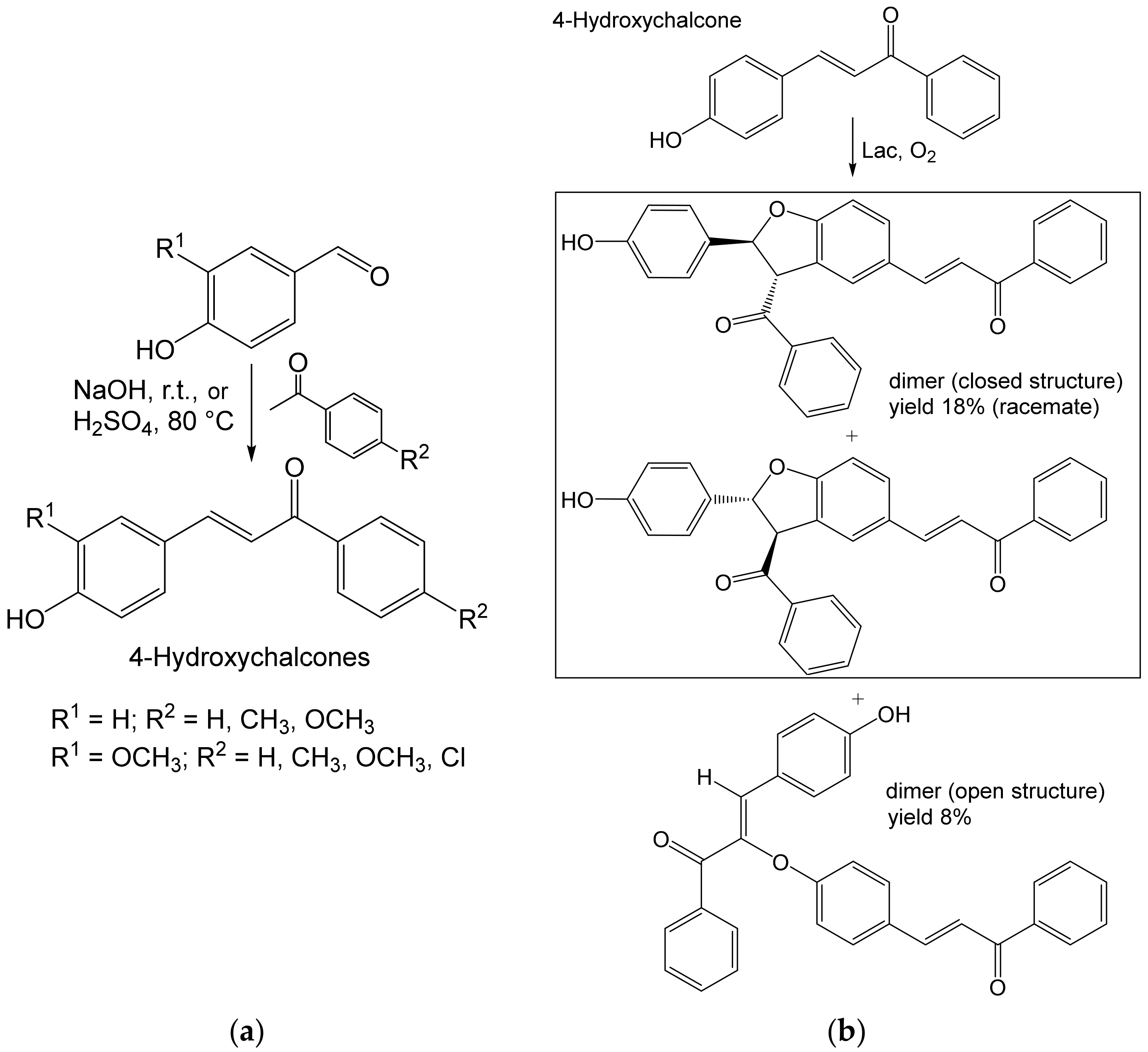
- Grosso, S.; Radaelli, F.; Fronza, G.; Passarella, D.; Monti, D.; Riva, S. Studies on the laccase-catalyzed oxidation of 4-hydroxy-chalcones. Adv. Synth. Catal. 2019, 361, 2696–2705. [Google Scholar] [CrossRef] [Green Version]
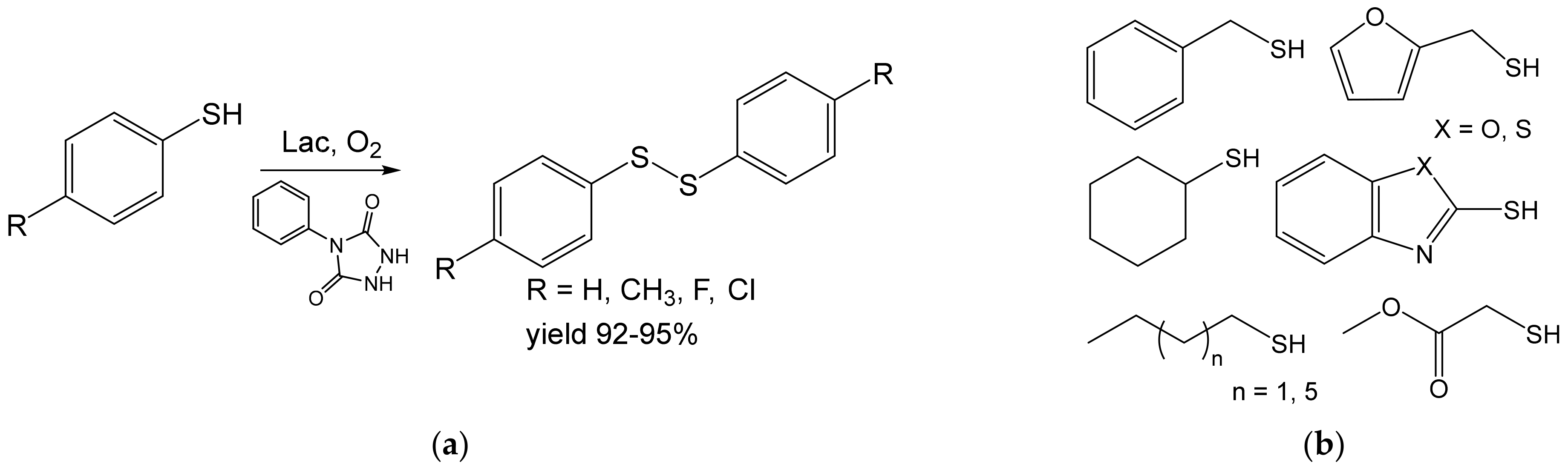
- Khaledian, D.; Rostami, A.; Zarei, S.A. Laccase-catalyzed in situ generation and regeneration of N-phenyltriazolinedione for the aerobic oxidative homo-coupling of thiols to disulfides. Catal. Commun. 2018, 114, 75–78. [Google Scholar] [CrossRef]
- Mikolasch, A.; Hammer, E.; Witt, S.; Lindequist, U. Laccase-catalyzed derivatization of 6-aminopenicillanic, 7-aminocephalosporanic and 7-aminodesacetoxycephalosporanic acid. AMB Express 2020, 10, 177. [Google Scholar] [CrossRef]
- Mikolasch, A.; Hahn, V. Laccase-catalyzed derivatization of antibiotics with sulfonamide or sulfone structures. Microorganisms 2021, 9, 2199. [Google Scholar] [CrossRef]
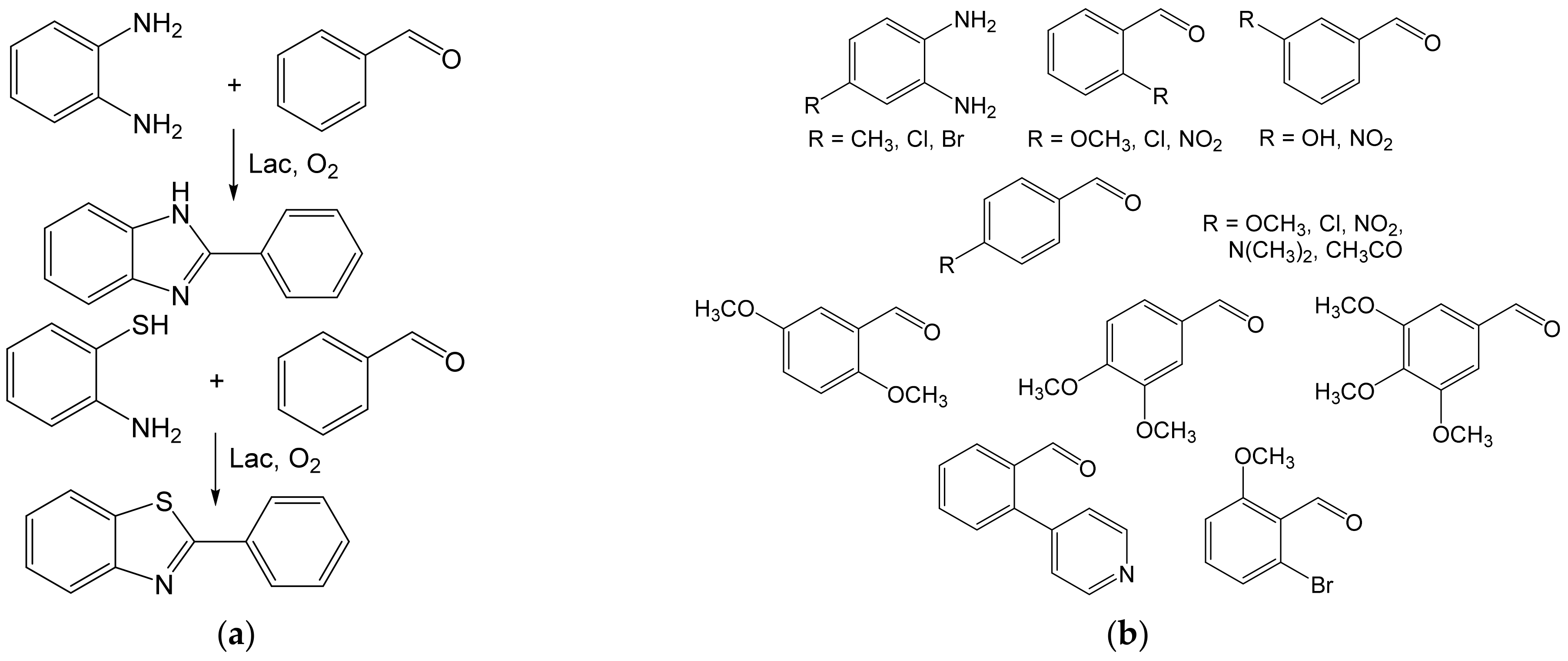
- Maphupha, M.; Juma, W.P.; de Koning, C.B.; Brady, D. A modern and practical laccase-catalysed route suitable for the synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles. RSC Adv. 2018, 8, 39496–39510. [Google Scholar] [CrossRef] [Green Version]
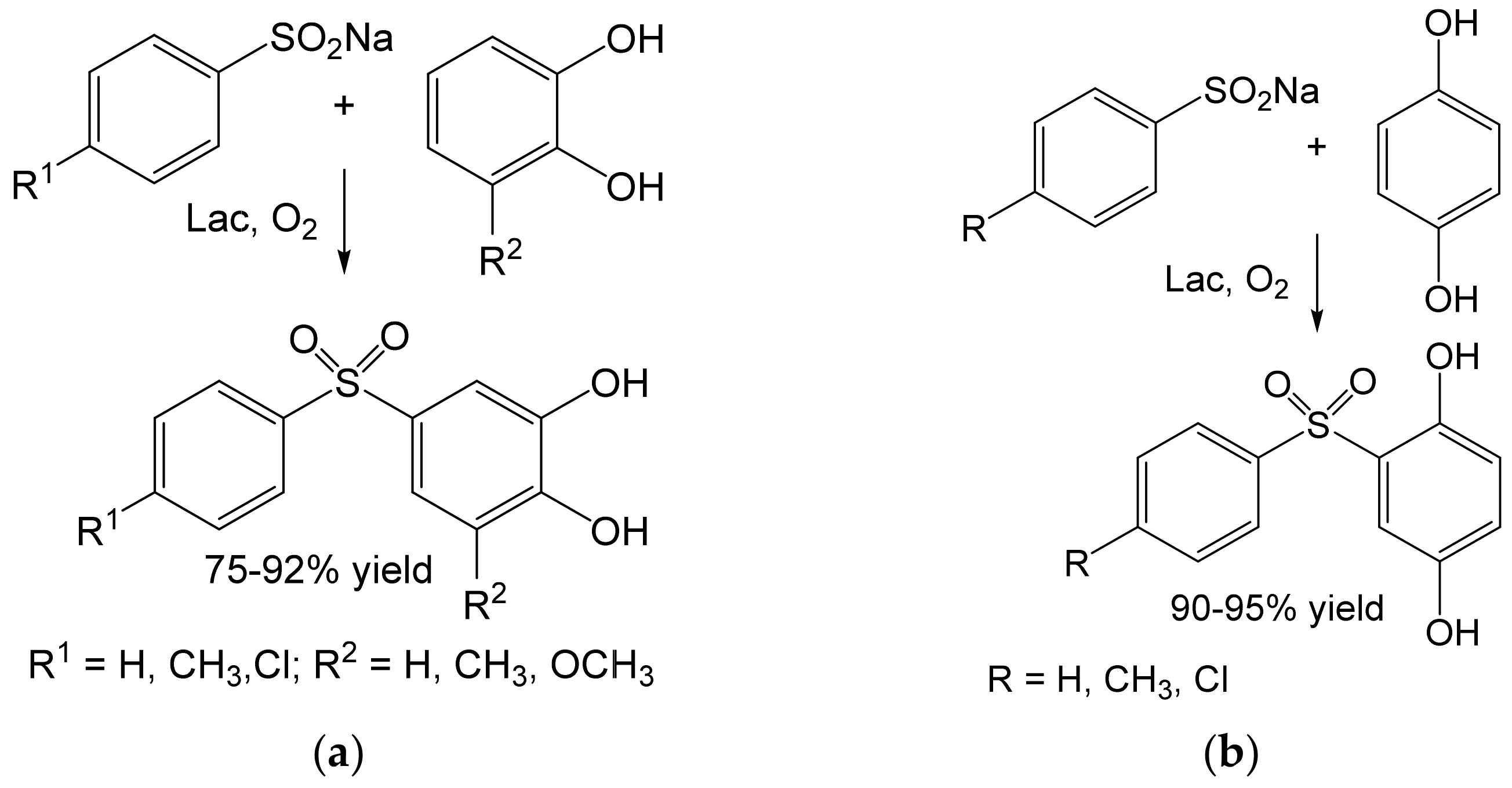
- Habibi, D.; Rahimi, A.; Rostami, A.; Moradi, S. Green and mild laccase-catalyzed aerobic oxidative coupling of benzenediol derivatives with various sodium benzenesulfinates. Tetrahedron Lett. 2017, 58, 289–293. [Google Scholar] [CrossRef]
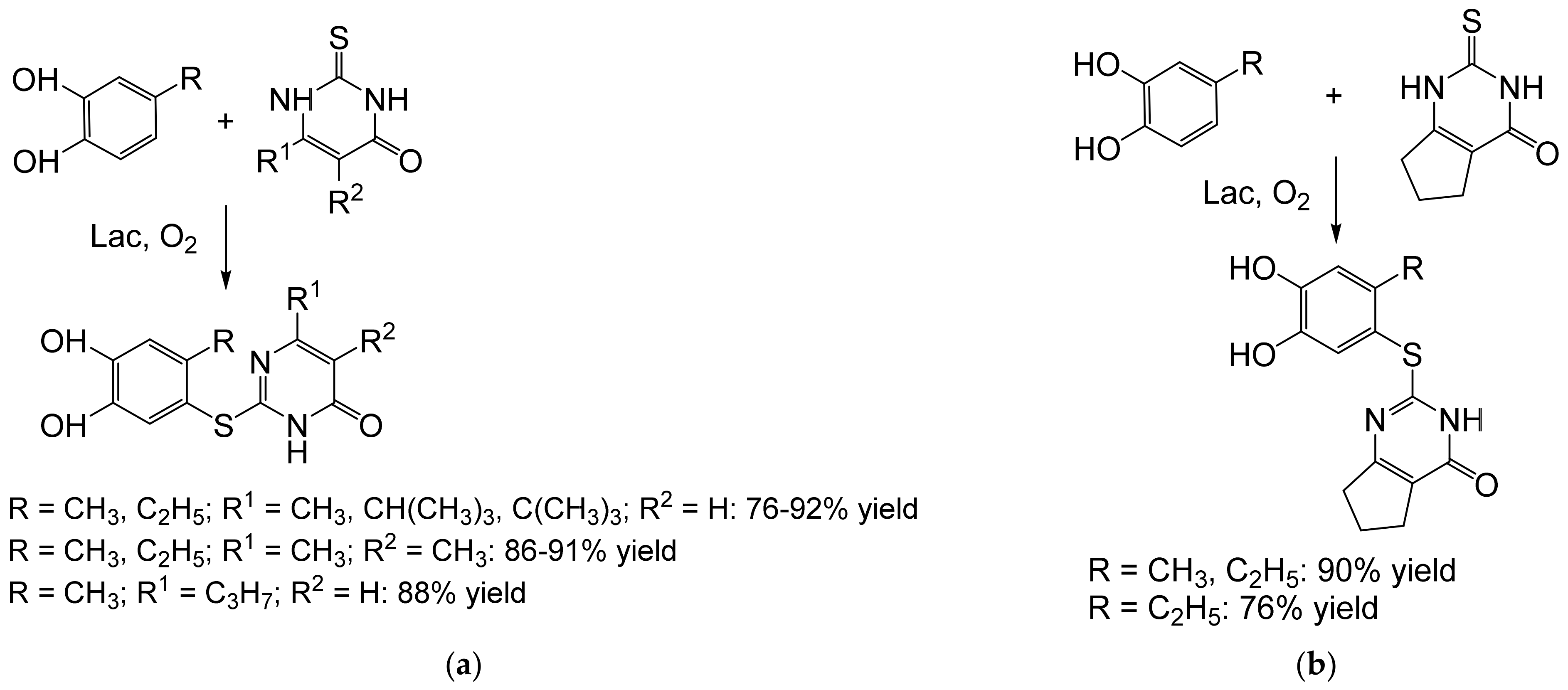
- Abdel-Mohsen, H.T.; Conrad, J.; Harms, K.; Nohr, D.; Beifuss, U. Laccase-catalyzed green synthesis and cytotoxic activity of novel pyrimidobenzothiazoles and catechol thioethers. RSC Adv. 2017, 7, 17427–17441. [Google Scholar] [CrossRef] [Green Version]
- Benny, L.; Cherian, A.R.; Varghese, A.; Sangwan, N.; Avti, P.K.; Hegde, G. A novel laccase-based biocatalyst for selective electro-oxidation of 2-thiophene methanol. Mol. Catal. 2021, 516, 111999. [Google Scholar] [CrossRef]
- González-Granda, S.; Méndez-Sánchez, D.; Lavandera, I.; Gotor-Fernández, V. Laccase-mediated oxidations of propargylic alcohols. application in the deracemization of 1-arylprop-2-yn-1-ols in combination with alcohol dehydrogenases. ChemCatChem 2020, 12, 520–527. [Google Scholar] [CrossRef]
- Ramos-Martín, M.; Lecuna, R.; Cicco, L.; Vitale, P.; Capriati, V.; Rios-Lombardía, N.; González-Sabin, J.; Soto, A.P.; García-Álvarez, J. A one-pot two-step synthesis of tertiary alcohols combining the biocatalytic laccase/TEMPO oxidation system with organolithium reagents in aerobic aqueous media at room temperature. Chem. Commun. 2021, 57, 13534–13537. [Google Scholar] [CrossRef]
- Mayr, S.A.; Subagia, R.; Weiss, R.; Schwaiger, N.; Weber, H.K.; Leitner, J.; Ribitsch, D.; Nyanhongo, G.S.; Guebitz, G.M. Oxidation of various kraft lignins with a bacterial laccase enzyme. Int. J. Mol. Sci. 2021, 22, 13161. [Google Scholar] [CrossRef]
- Zhu, J.; Song, S.Q.; Lian, L.D.; Shi, L.; Ren, A.; Zhao, M.W. Improvement of laccase activity by silencing PacC in Ganoderma lucidum. World J. Microbiol. Biotechnol. 2022, 38, 32. [Google Scholar] [CrossRef]
- Aza, P.; Molpeceres, G.; Ruiz-Dueñas, F.J.; Camarero, S. Heterologous expression, engineering and characterization of a novel laccase of Agrocybe pediades with promising properties as biocatalyst. J. Fungi 2021, 7, 359. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Kalia, V.C.; Kim, S.Y.; Lee, J.K.; Kim, I.W. Immobilization of laccase through inorganic-protein hybrids using various metal ions. Indian J. Microbiol. 2022, 1–5. [Google Scholar] [CrossRef]
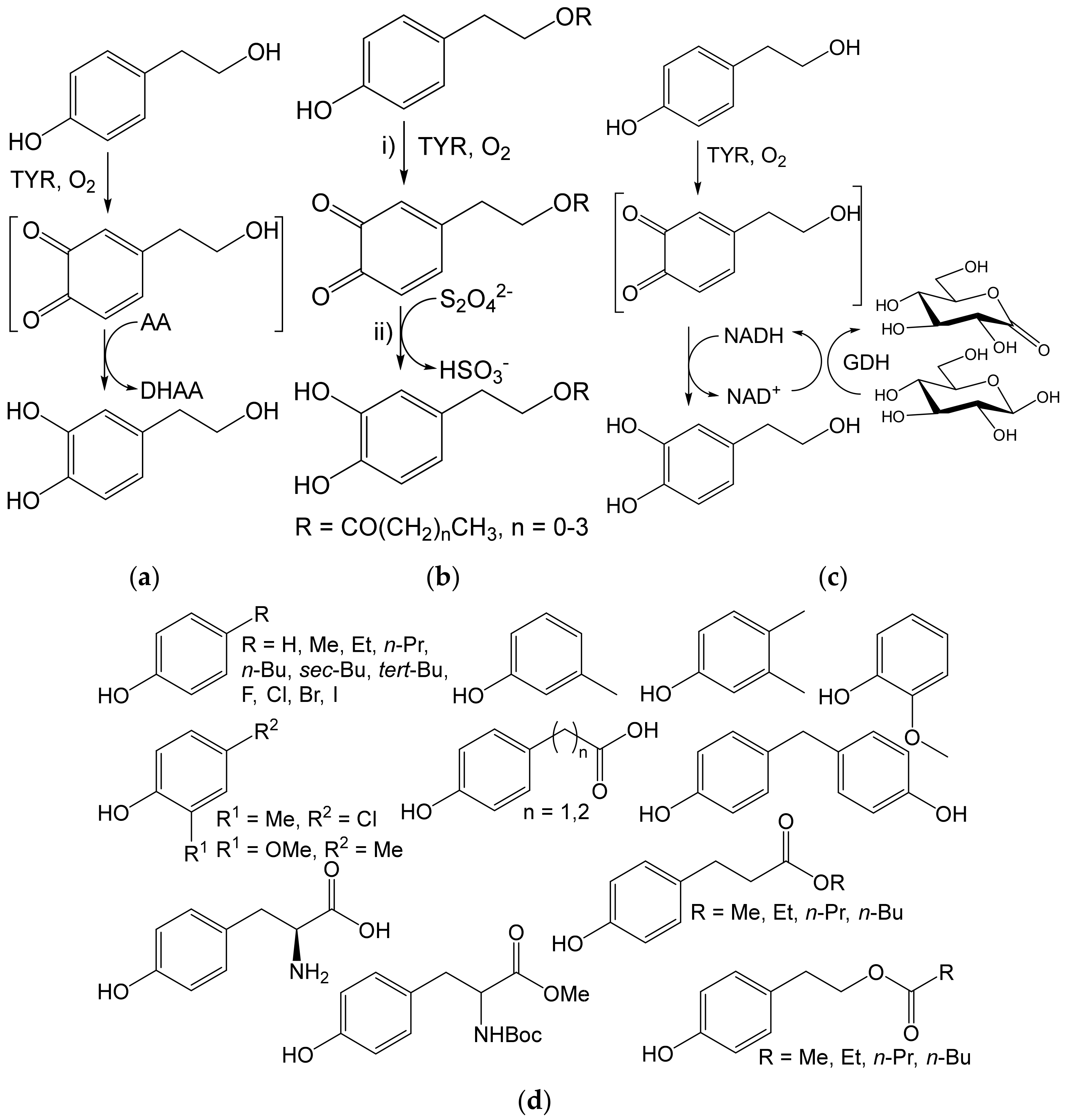
- Espín, J.C.; Soler-Rivas, C.; Cantos, E.; Tomas-Barberán, F.A.; Wichers, H.J. Synthesis of the antioxidant hydroxytyrosol using tyrosinase as biocatalyst. J. Agric. Food Chem. 2001, 49, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Guazzaroni, M.; Crestini, C.; Saladino, R. Layer-by-Layer coated tyrosinase: An efficient and selective synthesis of catechols. Bioorg. Med. Chem. 2012, 20, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botta, G.; Bizzarri, B.M.; Garozzo, A.; Timpanaro, R.; Bisignano, B.; Amatore, D.; Palamara, A.T.; Nencioni, L.; Saladino, R. Carbon nanotubes supported tyrosinase in the synthesis of lipophilic hydroxytyrosol and dihydrocaffeoyl catechols with antiviral activity against DNA and RNA viruses. Bioorg. Med. Chem. 2015, 23, 5345–5351. [Google Scholar] [CrossRef]
- Deri-Zenaty, B.; Bachar, S.; Rebros, M.; Fishman, A. A coupled enzymatic reaction of tyrosinase and glucose dehydrogenase for the production of hydroxytyrosol. Appl. Microbiol. Biotechnol. 2020, 104, 4945–4955. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Pieri, C.; Botta, G.; Arabuli, L.; Mosesso, P.; Cinelli, S.; Schinoppi, A.; Saladino, R. Synthesis and antioxidant activity of DOPA peptidomimetics by a novel IBX mediated aromatic oxidative functionalization. RSC Adv. 2015, 5, 60354–60364. [Google Scholar] [CrossRef]
- Botta, G.; Delfino, M.; Guazzaroni, M.; Crestini, C.; Onofri, S.; Saladino, R. Selective synthesis of DOPA and DOPA peptides by native and immobilized tyrosinase in organic solvent. ChemPlusChem 2013, 78, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Bozzini, T.; Botta, G.; Delfino, M.; Onofri, S.; Saladino, R.; Amatore, D.; Sgarbanti, R.; Nencioni, L.; Palamara, A.T. Tyrosinase and Layer-by-Layer supported tyrosinases in the synthesis of lipophilic catechols with antiinfluenza activity. Bioorg. Med. Chem. 2013, 21, 7699–7708. [Google Scholar] [CrossRef]
- Guazzaroni, M.; Pasqualini, M.; Botta, G.; Saladino, R. A Novel synthesis of bioactive catechols by layer-by-layer immobilized tyrosinase in an organic solvent medium. ChemCatChem 2012, 4, 89–99. [Google Scholar] [CrossRef]
- Martínková, L.; Příhodová, R.; Kulik, N.; Pelantová, H.; Křístková, B.; Petrásková, L.; Biedermann, D. Biocatalyzed reactions towards functional food components 4-alkylcatechols and their analogues. Catalysts 2020, 10, 1077. [Google Scholar] [CrossRef]
- Davis, R.; Molloy, S.; Quigley, B.; Nikodinovic-Runic, J.; Solano, F.; O’Connor, K.E. Biocatalytic versatility of engineered and wild-type tyrosinase from R. solanacearum for the synthesis of 4-halocatechols. Appl. Microbiol. Biotechnol. 2018, 102, 5121–5131. [Google Scholar] [CrossRef] [PubMed]
- Algieri, C.; Donato, L.; Bonacci, P.; Giorno, L. Tyrosinase immobilised on polyamide tubular membrane for the l-DOPA production: Total recycle and continuous reactor study. Biochem. Eng. J. 2012, 66, 14–19. [Google Scholar] [CrossRef]
- Donato, L.; Algieri, C.; Rizzi, A.; Giorno, L. Kinetic study of tyrosinase immobilized on polymeric membrane. J. Membr. Sci. 2014, 454, 346–350. [Google Scholar] [CrossRef]
- Senger, D.R.; Li, D.; Jaminet, S.C.; Cao, S.G. Activation of the Nrf2 cell defense pathway by ancient foods: Disease prevention by important molecules and microbes lost from the modern western diet. PLoS ONE 2016, 11, e0148042. [Google Scholar] [CrossRef]
- Lezzi, C.; Bleve, G.; Spagnolo, S.; Perrotta, C.; Grieco, F. Production of recombinant Agaricus bisporus tyrosinase in Saccharomyces cerevisiae cells. J. Ind. Microbiol. Biotechnol. 2012, 39, 1875–1880. [Google Scholar] [CrossRef]
- Kawamura-Konishi, Y.; Maekawa, S.; Tsuji, M.; Goto, H. C-terminal processing of tyrosinase is responsible for activation of Pholiota microspora proenzyme. Appl. Microbiol. Biotechnol. 2011, 90, 227–234. [Google Scholar] [CrossRef]
- Moe, L.L.; Maekawa, S.; Kawamura-Konishi, Y. The pro-enzyme C-terminal processing domain of Pholiota nameko tyrosinase is responsible for folding of the N-terminal catalytic domain. Appl. Microbiol. Biotechnol. 2015, 99, 5499–5510. [Google Scholar] [CrossRef]
- Haudecoeur, R.; Gouron, A.; Dubois, C.; Jamet, H.; Lightbody, M.; Hardré, R.; Milet, A.; Bergantino, E.; Bubacco, L.; Belle, C.; et al. Investigation of binding-site homology between mushroom and bacterial tyrosinases by using aurones as effectors. ChemBioChem. 2014, 15, 1325–1333. [Google Scholar] [CrossRef]
- Marková, E.; Kotik, M.; Křenková, A.; Man, P.; Haudecoeur, R.; Boumendjel, A.; Hardré, R.; Mekmouche, Y.; Courvoisier-Dezord, E.; Réglier, M.; et al. Recombinant tyrosinase from Polyporus arcularius: Overproduction in escherichia coli, characterization, and use in a study of aurones as tyrosinase effectors. J. Agric. Food Chem. 2016, 64, 2925–2931. [Google Scholar] [CrossRef]
- Halaouli, S.; Record, E.; Casalot, L.; Hamdi, M.; Sigoillot, J.C.; Asther, M.; Lomascolo, A. Cloning and characterization of a tyrosinase gene from the white-rot fungus Pycnoporus sanguineus, and overproduction of the recombinant protein in Aspergillus niger. Appl. Microbiol. Biotechnol. 2006, 70, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Westerholm-Parvinen, A.; Selinheimo, E.; Boer, H.; Kalkkinen, N.; Mattinen, M.; Saloheimo, M. Expression of the Trichoderma reesei tyrosinase 2 in Pichia pastoris: Isotopic labeling and physicochemical characterization. Protein Expr. Purif. 2007, 55, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Uhnáková, B.; Ludwig, R.; Pěknicová, J.; Homolka, L.; Lisá, L.; Šulc, M.; Petříčková, A.; Elzeinová, F.; Pelantová, H.; Monti, D.; et al. Biodegradation of tetrabromobisphenol A by oxidases in basidiomycetous fungi and estrogenic activity of the biotransformation products. Bioresour. Technol. 2011, 102, 9409–9415. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínková, L.; Křístková, B.; Křen, V. Laccases and Tyrosinases in Organic Synthesis. Int. J. Mol. Sci. 2022, 23, 3462. https://doi.org/10.3390/ijms23073462
Martínková L, Křístková B, Křen V. Laccases and Tyrosinases in Organic Synthesis. International Journal of Molecular Sciences. 2022; 23(7):3462. https://doi.org/10.3390/ijms23073462
Chicago/Turabian StyleMartínková, Ludmila, Barbora Křístková, and Vladimír Křen. 2022. "Laccases and Tyrosinases in Organic Synthesis" International Journal of Molecular Sciences 23, no. 7: 3462. https://doi.org/10.3390/ijms23073462
APA StyleMartínková, L., Křístková, B., & Křen, V. (2022). Laccases and Tyrosinases in Organic Synthesis. International Journal of Molecular Sciences, 23(7), 3462. https://doi.org/10.3390/ijms23073462








