The ATF3–OPG Axis Contributes to Bone Formation by Regulating the Differentiation of Osteoclasts, Osteoblasts, and Adipocytes
Abstract
:1. Introduction
2. Results
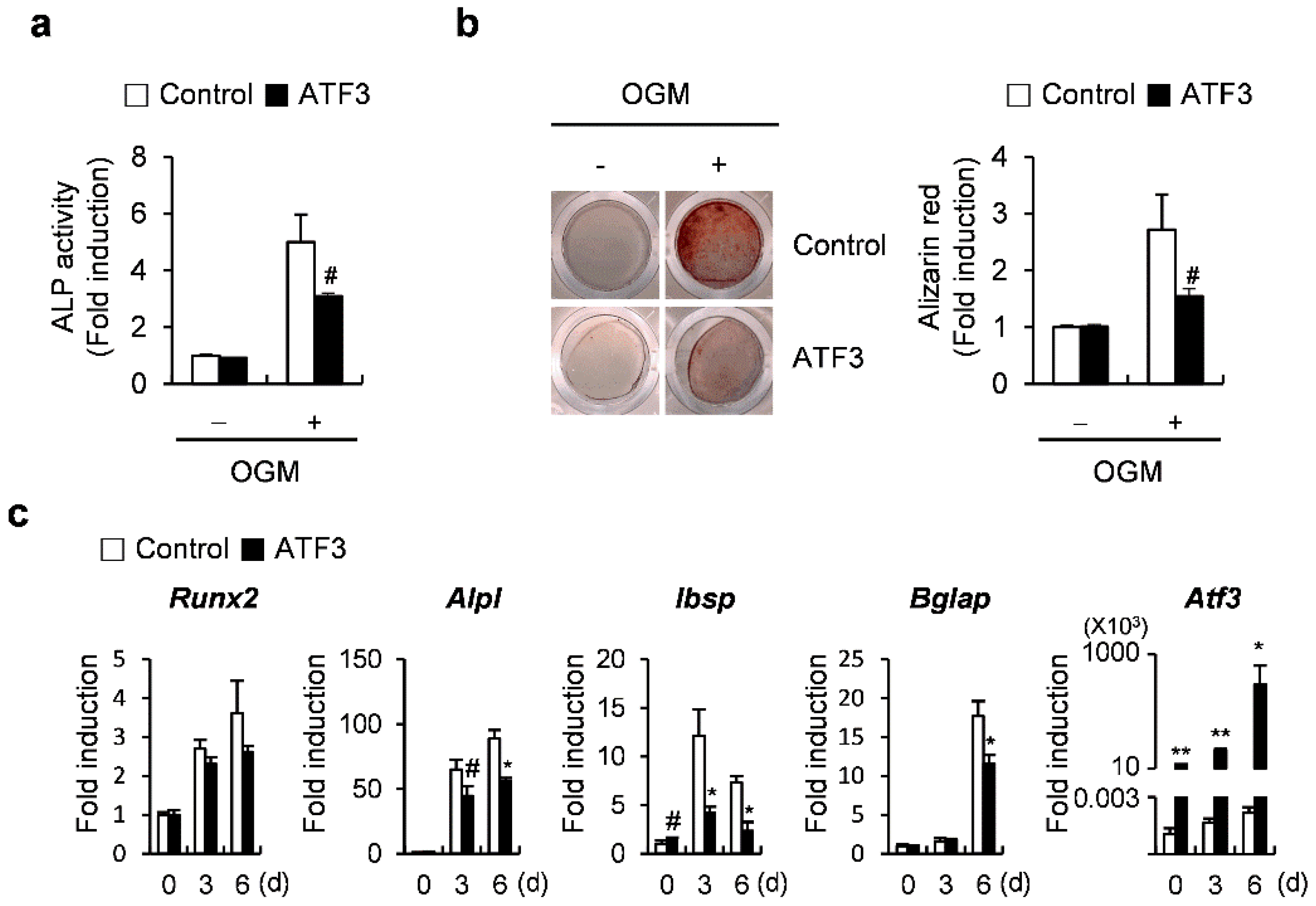
2.1. ATF3 Inhibits Osteoblast Differentiation and Bone Formation in Primary Osteoblasts
2.2. ATF3 Induces the Expression of Tnfrsf11b (Opg)
2.3. The Net Effect of ATF3 May Be Biased towards Inhibition of Osteoclastogenesis
2.4. OPG Promotes Osteoblast Differentiation and Attenuates Transdifferentiation of Osteoblasts to Adipocytes
2.5. ATF3 Acts like OPG in the Differentiation of Osteoblasts and the Transdifferentiation of Osteoblasts to Adipocytes
2.6. ATF3 Is Involved in Controlling the Differentiation of Osteoclasts, Osteoblasts, and Adipocytes through OPG Induction
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Osteoblast Differentiation
4.3. Osteoclast Differentiation
4.4. Adipocyte Differentiation
4.5. Retroviral Gene Transduction
4.6. siRNA Transfection
4.7. OPG Neutralization
4.8. ChIP
4.9. Real-Time PCR
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, T.; Li, L. The stem cell niches in bone. J. Clin. Investig. 2006, 116, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.C.; Oh, S.H.; Lee, M.N.; Koh, J.T. Macrophage-Stimulating Protein Enhances Osteoblastic Differentiation via the Recepteur d’Origine Nantais Receptor and Extracellular Signal-Regulated Kinase Signaling Pathway. J. Bone Metab. 2020, 27, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metab. 2021, 39, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Bodine, P.V.; Zhao, W.; Kharode, Y.P.; Bex, F.J.; Lambert, A.J.; Goad, M.B.; Gaur, T.; Stein, G.S.; Lian, J.B.; Komm, B.S. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol. Endocrinol. 2004, 18, 1222–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, R.; Rawadi, G.; Roman-Roman, S. Wnt signaling: A key regulator of bone mass. Curr. Top. Dev. Biol. 2006, 76, 103–127. [Google Scholar] [CrossRef]
- Hu, H.; Hilton, M.J.; Tu, X.; Yu, K.; Ornitz, D.M.; Long, F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 2005, 132, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Harris, M.A.; Rossini, G.; Dunstan, C.R.; Dallas, S.L.; Feng, J.Q.; Mundy, G.R.; Harris, S.E. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif. Tissue Int. 1997, 60, 283–290. [Google Scholar] [CrossRef]
- Nishimura, R.; Hata, K.; Harris, S.E.; Ikeda, F.; Yoneda, T. Core-binding factor alpha 1 (Cbfa1) induces osteoblastic differentiation of C2C12 cells without interactions with Smad1 and Smad5. Bone 2002, 31, 303–312. [Google Scholar] [CrossRef]
- Hanai, J.; Chen, L.F.; Kanno, T.; Ohtani-Fujita, N.; Kim, W.Y.; Guo, W.H.; Imamura, T.; Ishidou, Y.; Fukuchi, M.; Shi, M.J.; et al. Interaction and functional cooperation of PEBP2/CBF with Smads. Synergistic induction of the immunoglobulin germline Calpha promoter. J. Biol. Chem. 1999, 274, 31577–31582. [Google Scholar] [CrossRef] [Green Version]
- Javed, A.; Barnes, G.L.; Jasanya, B.O.; Stein, J.L.; Gerstenfeld, L.; Lian, J.B.; Stein, G.S. Runt homology domain transcription factors (Runx, Cbfa, and AML) mediate repression of the bone sialoprotein promoter: Evidence for promoter context-dependent activity of Cbfa proteins. Mol. Cell. Biol. 2001, 21, 2891–2905. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, N. Bone Cell Communication Factors Provide a New Therapeutic Strategy for Osteoporosis. Chonnam Med. J. 2020, 56, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yang, Y.R.; Kwon, K.S.; Kim, N. Anti-Müllerian Hormone Negatively Regulates Osteoclast Differentiation by Suppressing the Receptor Activator of Nuclear Factor-κB Ligand Pathway. J. Bone Metab. 2021, 28, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Alrowaili, M.G.; Hussein, A.M.; Eid, E.A.; Serria, M.S.; Abdellatif, H.; Sakr, H.F. Effect of Intermittent Fasting on Glucose Homeostasis and Bone Remodeling in Glucocorticoid-Induced Osteoporosis Rat Model. J. Bone Metab. 2021, 28, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Arai, F.; Miyamoto, T.; Ohneda, O.; Inada, T.; Sudo, T.; Brasel, K.; Miyata, T.; Anderson, D.M.; Suda, T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J. Exp. Med. 1999, 190, 1741–1754. [Google Scholar] [CrossRef]
- Dougall, W.C.; Glaccum, M.; Charrier, K.; Rohrbach, K.; Brasel, K.; De Smedt, T.; Daro, E.; Smith, J.; Tometsko, M.E.; Maliszewski, C.R.; et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999, 13, 2412–2424. [Google Scholar] [CrossRef]
- Gimble, J.M. The function of adipocytes in the bone marrow stroma. New Biol. 1990, 2, 304–312. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Hardouin, P.; Pansini, V.; Cortet, B. Bone marrow fat. Jt. Bone Spine 2014, 81, 313–319. [Google Scholar] [CrossRef]
- Justesen, J.; Stenderup, K.; Ebbesen, E.N.; Mosekilde, L.; Steiniche, T.; Kassem, M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001, 2, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Meunier, P.; Aaron, J.; Edouard, C.; Vignon, G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin. Orthop. Relat. Res. 1971, 80, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Clabaut, A.; Grare, C.; Rolland-Valognes, G.; Letarouilly, J.-G.; Bourrier, C.; Andersen, T.L.; Sikjær, T.; Rejnmark, L.; Ejersted, C.; Pastoureau, P.; et al. Adipocyte-induced transdifferentiation of osteoblasts and its potential role in age-related bone loss. PLoS ONE 2021, 16, e0245014. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, K.; Park, G.; Iezaki, T.; Horie, T.; Kanayama, T.; Ozaki, K.; Onishi, Y.; Takahata, Y.; Yoneda, Y.; Takarada, T.; et al. ATF3 controls proliferation of osteoclast precursor and bone remodeling. Sci. Rep. 2016, 6, 30918. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.P.; Liang, G.; Whelan, J.; Hai, T. ATF3 and ATF3 delta Zip. Transcriptional repression versus activation by alternatively spliced isoforms. J. Biol. Chem. 1994, 269, 15819–15826. [Google Scholar] [CrossRef]
- Liang, G.; Wolfgang, C.D.; Chen, B.P.; Chen, T.H.; Hai, T. ATF3 gene. Genomic organization, promoter, and regulation. J. Biol. Chem. 1996, 271, 1695–1701. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.-C.; Kim, J.H.; Kim, K.; Kim, I.; Seong, S.; Kim, N. ATF3 modulates calcium signaling in osteoclast differentiation and activity by associating with c-Fos and NFATc1 proteins. Bone 2017, 95, 33–40. [Google Scholar] [CrossRef]
- Jeong, B.-C. ATF3 mediates the inhibitory action of TNF-α on osteoblast differentiation through the JNK signaling pathway. Biochem. Biophys. Res. Commun. 2018, 499, 696–701. [Google Scholar] [CrossRef]
- Kajimura, D.; Hinoi, E.; Ferron, M.; Kode, A.; Riley, K.J.; Zhou, B.; Guo, X.E.; Karsenty, G. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J. Exp. Med. 2011, 208, 841–851. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Patel, M.S.; Bradley, A.; Wagner, E.F.; Karsenty, G. The molecular clock mediates leptin-regulated bone formation. Cell 2005, 122, 803–815. [Google Scholar] [CrossRef] [Green Version]
- Cong, Q.; Jia, H.; Biswas, S.; Li, P.; Qiu, S.; Deng, Q.; Guo, X.; Ma, G.; Ling Chau, J.F.; Wang, Y.; et al. p38α MAPK Regulates Lineage Commitment and OPG Synthesis of Bone Marrow Stromal Cells to Prevent Bone Loss under Physiological and Pathological Conditions. Stem Cell Rep. 2016, 6, 566–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.T.; Kim, E.H.; Luong, T.T.; Pyo, S.; Rhee, D.K. TLR4 mediates pneumolysin-induced ATF3 expression through the JNK/p38 pathway in Streptococcus pneumoniae-infected RAW 264.7 cells. Mol. Cells 2015, 38, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenblatt, M.B.; Shim, J.H.; Glimcher, L.H. Mitogen-activated protein kinase pathways in osteoblasts. Annu. Rev. Cell Dev. Biol. 2013, 29, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Thouverey, C.; Caverzasio, J. The p38α MAPK positively regulates osteoblast function and postnatal bone acquisition. Cell. Mol. Life Sci. 2012, 69, 3115–3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, H.C.; Cheng, C.F. Master Regulator Activating Transcription Factor 3 (ATF3) in Metabolic Homeostasis and Cancer. Front. Endocrinol. 2020, 11, 556. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Guo, M.; Yu, J.; Yan, C. The common stress responsive transcription factor ATF3 binds genomic sites enriched with p300 and H3K27ac for transcriptional regulation. BMC Genom. 2016, 17, 335. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, M.; Zhou, X.; Liu, Y.; Jing, B.; Wang, X.; Zhang, Q.; Sun, Y. Role of Osteoprotegerin (OPG) in Bone Marrow Adipogenesis. Cell. Physiol. Biochem. 2016, 40, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Choe, N.; Kwon, D.-H.; Ryu, J.; Shin, S.; Cho, H.J.; Joung, H.; Eom, G.H.; Ahn, Y.; Park, W.J.; Nam, K.-I.; et al. miR-27a-3p Targets ATF3 to Reduce Calcium Deposition in Vascular Smooth Muscle Cells. Mol. Ther. Nucleic Acids 2020, 22, 627–639. [Google Scholar] [CrossRef]
- Jang, M.K.; Kim, C.H.; Seong, J.K.; Jung, M.H. ATF3 inhibits adipocyte differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2012, 421, 38–43. [Google Scholar] [CrossRef]
- Jang, M.K.; Jung, M.H. ATF3 inhibits PPARgamma-stimulated transactivation in adipocyte cells. Biochem. Biophys. Res. Commun. 2015, 456, 80–85. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Kim, K.; Kim, I.; Seong, S.; Koh, J.-T.; Kim, N. The ATF3–OPG Axis Contributes to Bone Formation by Regulating the Differentiation of Osteoclasts, Osteoblasts, and Adipocytes. Int. J. Mol. Sci. 2022, 23, 3500. https://doi.org/10.3390/ijms23073500
Kim JH, Kim K, Kim I, Seong S, Koh J-T, Kim N. The ATF3–OPG Axis Contributes to Bone Formation by Regulating the Differentiation of Osteoclasts, Osteoblasts, and Adipocytes. International Journal of Molecular Sciences. 2022; 23(7):3500. https://doi.org/10.3390/ijms23073500
Chicago/Turabian StyleKim, Jung Ha, Kabsun Kim, Inyoung Kim, Semun Seong, Jeong-Tae Koh, and Nacksung Kim. 2022. "The ATF3–OPG Axis Contributes to Bone Formation by Regulating the Differentiation of Osteoclasts, Osteoblasts, and Adipocytes" International Journal of Molecular Sciences 23, no. 7: 3500. https://doi.org/10.3390/ijms23073500
APA StyleKim, J. H., Kim, K., Kim, I., Seong, S., Koh, J.-T., & Kim, N. (2022). The ATF3–OPG Axis Contributes to Bone Formation by Regulating the Differentiation of Osteoclasts, Osteoblasts, and Adipocytes. International Journal of Molecular Sciences, 23(7), 3500. https://doi.org/10.3390/ijms23073500





