IL11 Activates Pancreatic Stellate Cells and Causes Pancreatic Inflammation, Fibrosis and Atrophy in a Mouse Model of Pancreatitis
Abstract
1. Introduction
2. Results
2.1. IL11 Induces Pancreatic Stellate Cell Activation
2.2. IL11-Dependent ERK Signaling Is Required for Pancreatic Stellate Cell Activation
2.3. A Neutralizing IL11RA Antibody Reduces Tissue Damage in Acute Pancreatitis
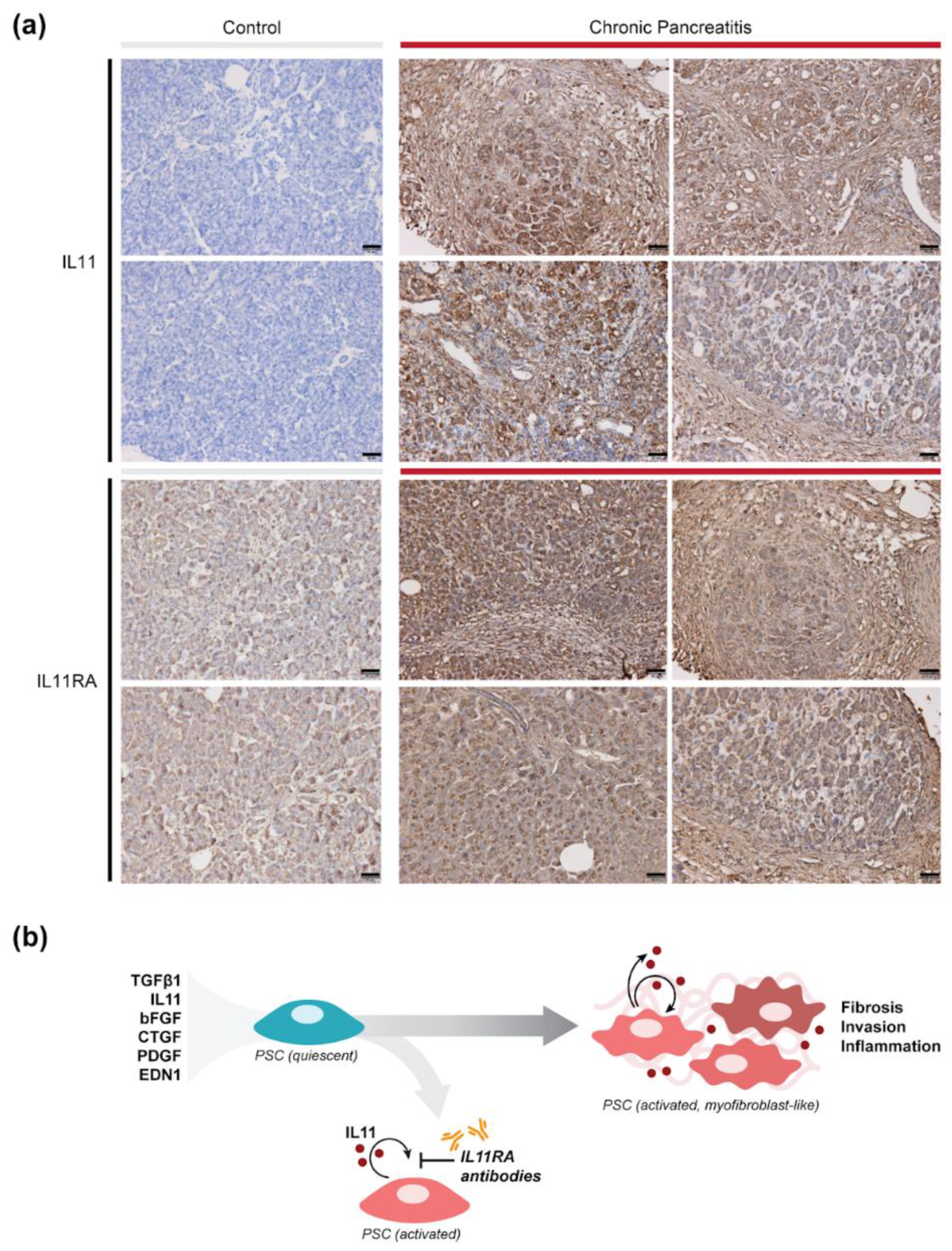
2.4. IL11 and IL11RA Are Elevated Chronic Pancreatitis in Humans
3. Discussion
4. Materials and Methods
4.1. Recombinant Proteins
4.2. Chemicals
4.3. Cell Culture
4.4. Immunofluorescence Staining
4.5. High-Content Imaging Assays
4.6. ELISA and Sirius Red Collagen Assay
4.7. RT-qPCR
4.8. Matrigel Invasion Assay
4.9. Pancreatic Duct Ligation (PDL) Model
4.10. Histology and Immunohistochemistry
4.11. Immunoblotting
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forsmark, C.E.; Vege, S.S.; Wilcox, C.M. Acute Pancreatitis. N. Engl. J. Med. 2017, 376, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Whitcomb, D.C.; Shimosegawa, T.; Esposito, I.; Lerch, M.M.; Gress, T.; Mayerle, J.; Drewes, A.M.; Rebours, V.; Akisik, F.; et al. Chronic Pancreatitis. Nat. Rev. Dis. Primers 2017, 3, 17060. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, G.; Detlefsen, S.; Feyerabend, B. Fibrosis of the Pancreas: The Initial Tissue Damage and the Resulting Pattern. Virchows Arch. 2004, 445, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Steer, M.L.; Waxman, I.; Freedman, S. Chronic Pancreatitis. N. Engl. J. Med. 1995, 332, 1482–1490. [Google Scholar] [CrossRef]
- Witt, H.; Apte, M.V.; Keim, V.; Wilson, J.S. Chronic Pancreatitis: Challenges and Advances in Pathogenesis, Genetics, Diagnosis, and Therapy. Gastroenterology 2007, 132, 1557–1573. [Google Scholar] [CrossRef]
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic Pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef]
- Apte, M.V.; Pirola, R.C.; Wilson, J.S. Pancreatic Stellate Cells: A Starring Role in Normal and Diseased Pancreas. Front. Physiol. 2012, 3, 344. [Google Scholar] [CrossRef]
- Apte, M.; Pirola, R.C.; Wilson, J.S. Pancreatic Stellate Cell: Physiologic Role, Role in Fibrosis and Cancer. Curr. Opin. Gastroenterol. 2015, 31, 416–423. [Google Scholar] [CrossRef]
- Bachem, M.G.; Zhou, Z.; Zhou, S.; Siech, M. Role of Stellate Cells in Pancreatic Fibrogenesis Associated with Acute and Chronic Pancreatitis. J. Gastroenterol. Hepatol. 2006, 21 (Suppl. 3), S92–S96. [Google Scholar] [CrossRef]
- Jin, G.; Hong, W.; Guo, Y.; Bai, Y.; Chen, B. Molecular Mechanism of Pancreatic Stellate Cells Activation in Chronic Pancreatitis and Pancreatic Cancer. J. Cancer 2020, 11, 1505–1515. [Google Scholar] [CrossRef]
- Erkan, M.; Adler, G.; Apte, M.V.; Bachem, M.G.; Buchholz, M.; Detlefsen, S.; Esposito, I.; Friess, H.; Gress, T.M.; Habisch, H.-J.; et al. StellaTUM: Current Consensus and Discussion on Pancreatic Stellate Cell Research. Gut 2012, 61, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, M.; Kestler, H.A.; Holzmann, K.; Ellenrieder, V.; Schneiderhan, W.; Siech, M.; Adler, G.; Bachem, M.G.; Gress, T.M. Transcriptome Analysis of Human Hepatic and Pancreatic Stellate Cells: Organ-Specific Variations of a Common Transcriptional Phenotype. J. Mol. Med. 2005, 83, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.A.; Schafer, S. Hiding in Plain Sight: Interleukin-11 Emerges as a Master Regulator of Fibrosis, Tissue Integrity, and Stromal Inflammation. Annu. Rev. Med. 2020, 71, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Li, H.; Sun, L.; Brigstock, D.R.; Gao, R. Interleukin-6 Participates in Human Pancreatic Stellate Cell Activation and Collagen I Production via TGF-β1/Smad Pathway. Cytokine 2021, 143, 155536. [Google Scholar] [CrossRef]
- Viedma, J.A.; Pérez-Mateo, M.; Domínguez, J.E.; Carballo, F. Role of Interleukin-6 in Acute Pancreatitis. Comparison with C-Reactive Protein and Phospholipase A. Gut 1992, 33, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Wang, S.S.; Lu, R.H.; Chang, F.Y.; Lee, S.D. Serum Interleukin 10 and Interleukin 11 in Patients with Acute Pancreatitis. Gut 1999, 45, 895–899. [Google Scholar] [CrossRef][Green Version]
- Sathyanarayan, G.; Garg, P.K.; Prasad, H.K.; Tandon, R.K. Elevated Level of Interleukin-6 Predicts Organ Failure and Severe Disease in Patients with Acute Pancreatitis. J. Gastroenterol. Hepatol. 2007, 22, 550–554. [Google Scholar] [CrossRef]
- Shimizu, T.; Shiratori, K.; Sawada, T.; Kobayashi, M.; Hayashi, N.; Saotome, H.; Keith, J.C. Recombinant Human Interleukin-11 Decreases Severity of Acute Necrotizing Pancreatitis in Mice. Pancreas 2000, 21, 134–140. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Singh, B.K.; Adami, E.; Viswanathan, S.; Dong, J.; D’Agostino, G.A.; Ng, B.; Lim, W.W.; Tan, J.; Paleja, B.S.; et al. Inhibiting Interleukin 11 Signaling Reduces Hepatocyte Death and Liver Fibrosis, Inflammation, and Steatosis in Mouse Models of Non-Alcoholic Steatohepatitis. Gastroenterology 2019, 157, 777–792.e14. [Google Scholar] [CrossRef]
- Tabula Muris Consortium; Overall Coordination; Logistical Coordination; Organ Collection and Processing; Library Preparation and Sequencing; Computational Data Analysis; Cell Type Annotation; Writing Group; Supplemental Text Writing Group. Principal investigators Single-Cell Transcriptomics of 20 Mouse Organs Creates a Tabula Muris. Nature 2018, 562, 367–372. [Google Scholar] [CrossRef]
- Schafer, S.; Viswanathan, S.; Widjaja, A.A.; Lim, W.-W.; Moreno-Moral, A.; DeLaughter, D.M.; Ng, B.; Patone, G.; Chow, K.; Khin, E.; et al. IL-11 Is a Crucial Determinant of Cardiovascular Fibrosis. Nature 2017, 552, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, A.A.; Viswanathan, S.; Jinrui, D.; Singh, B.K.; Tan, J.; Wei Ting, J.G.; Lamb, D.; Shekeran, S.G.; George, B.L.; Schafer, S.; et al. Molecular Dissection of Pro-Fibrotic IL11 Signaling in Cardiac and Pulmonary Fibroblasts. Front. Mol. Biosci. 2021, 8, 926. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Abe, K.; Anbo, Y.; Katoh, H. Changes in the Mouse Exocrine Pancreas after Pancreatic Duct Ligation: A Qualitative and Quantitative Histological Study. Arch. Histol. Cytol. 1995, 58, 365–374. [Google Scholar] [CrossRef]
- Sendler, M.; Beyer, G.; Mahajan, U.M.; Kauschke, V.; Maertin, S.; Schurmann, C.; Homuth, G.; Völker, U.; Völzke, H.; Halangk, W.; et al. Complement Component 5 Mediates Development of Fibrosis, via Activation of Stellate Cells, in 2 Mouse Models of Chronic Pancreatitis. Gastroenterology 2015, 149, 765–776.e10. [Google Scholar] [CrossRef]
- Ng, B.; Dong, J.; Viswanathan, S.; Widjaja, A.A.; Paleja, B.S.; Adami, E.; Ko, N.S.J.; Wang, M.; Lim, S.; Tan, J.; et al. Fibroblast-specific IL11 Signaling Drives Chronic Inflammation in Murine Fibrotic Lung Disease. FASEB J. 2020, 34, 11802–11815. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, H.; Miyata, T.; Yasuda, H.; Satoh, Y.; Hanatsuka, K.; Kita, H.; Ohashi, A.; Tamada, K.; Makita, N.; Iiri, T.; et al. Distinct Roles of Smad2-, Smad3-, and ERK-Dependent Pathways in Transforming Growth Factor-β1 Regulation of Pancreatic Stellate Cellular Functions. J. Biol. Chem. 2004, 279, 8873–8878. [Google Scholar] [CrossRef]
- Sun, L.; Qu, L.; Brigstock, D.R.; Li, H.; Li, Y.; Gao, R. Biological and Proteomic Characteristics of an Immortalized Human Pancreatic Stellate Cell Line. Int. J. Med. Sci. 2020, 17, 137–144. [Google Scholar] [CrossRef]
- Viswanathan, S.; Ng, B.; Widjaja, A.A.; Pua, C.J.; Tham, N.; Tan, J.; Cook, S.A.; Schafer, S. Critical Conditions for Studying Interleukin-11 Signaling In Vitro and Avoiding Experimental Artefacts. Curr. Protoc. 2021, 1, e251. [Google Scholar] [CrossRef]
- Clemons, A.P.; Holstein, D.M.; Galli, A.; Saunders, C. Cerulein-Induced Acute Pancreatitis in the Rat Is Significantly Ameliorated by Treatment with MEK1/2 Inhibitors U0126 and PD98059. Pancreas 2002, 25, 251–259. [Google Scholar] [CrossRef]
- Mazzon, E.; Impellizzeri, D.; Di Paola, R.; Paterniti, I.; Esposito, E.; Cappellani, A.; Bramanti, P.; Cuzzocrea, S. Effects of Mitogen-Activated Protein Kinase Signaling Pathway Inhibition on the Development of Cerulein-Induced Acute Pancreatitis in Mice. Pancreas 2012, 41, 560–570. [Google Scholar] [CrossRef]
- Shigekawa, M.; Hikita, H.; Kodama, T.; Shimizu, S.; Li, W.; Uemura, A.; Miyagi, T.; Hosui, A.; Kanto, T.; Hiramatsu, N.; et al. Pancreatic STAT3 Protects Mice against Caerulein-Induced Pancreatitis via PAP1 Induction. Am. J. Pathol. 2012, 181, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.-W.; Ng, B.; Widjaja, A.; Xie, C.; Su, L.; Ko, N.; Lim, S.-Y.; Kwek, X.-Y.; Lim, S.; Cook, S.A.; et al. Transgenic Interleukin 11 Expression Causes Cross-Tissue Fibro-Inflammation and an Inflammatory Bowel Phenotype in Mice. PLoS ONE 2020, 15, e0227505. [Google Scholar] [CrossRef] [PubMed]
- Jasso, G.J.; Jaiswal, A.; Varma, M.; Laszewski, T.; Grauel, A.; Omar, A.; Silva, N.; Dranoff, G.; Porter, J.A.; Mansfield, K.; et al. Colon Stroma Mediates an Inflammation-Driven Fibroblastic Response Controlling Matrix Remodeling and Healing. PLoS Biol. 2022, 20, e3001532. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, A.A.; Dong, J.; Adami, E.; Viswanathan, S.; Ng, B.; Pakkiri, L.S.; Chothani, S.P.; Singh, B.K.; Lim, W.W.; Zhou, J.; et al. Redefining IL11 as a Regeneration-Limiting Hepatotoxin and Therapeutic Target in Acetaminophen-Induced Liver Injury. Sci. Transl. Med. 2021, 13, eaba8146. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Viswanathan, S.; Adami, E.; Singh, B.K.; Chothani, S.P.; Ng, B.; Lim, W.W.; Zhou, J.; Tripathi, M.; Ko, N.S.J.; et al. Hepatocyte-Specific IL11 Cis-Signaling Drives Lipotoxicity and Underlies the Transition from NAFLD to NASH. Nat. Commun. 2021, 12, 66. [Google Scholar] [CrossRef]
- Ng, B.; Cook, S.A.; Schafer, S. Interleukin-11 Signaling Underlies Fibrosis, Parenchymal Dysfunction, and Chronic Inflammation of the Airway. Exp. Mol. Med. 2020, 52, 1871–1878. [Google Scholar] [CrossRef]
- Strikoudis, A.; Cieślak, A.; Loffredo, L.; Chen, Y.-W.; Patel, N.; Saqi, A.; Lederer, D.J.; Snoeck, H.-W. Modeling of Fibrotic Lung Disease Using 3D Organoids Derived from Human Pluripotent Stem Cells. Cell Rep. 2019, 27, 3709–3723.e5. [Google Scholar] [CrossRef]
- Widjaja, A.; Shekeran, S.; Adami, E.; Goh, J.; Tan, J.; Viswanathan, S.; Lim, S.Y.; Tan, P.H.; Hubner, N.; Coffman, T.; et al. A Neutralizing IL-11 Antibody Improves Renal Function and Increases Lifespan in a Mouse Model of Alport Syndrome. J. Am. Soc. Nephrol. 2022. [Google Scholar] [CrossRef]
- Ng, B.; Dong, J.; D’Agostino, G.; Viswanathan, S.; Widjaja, A.A.; Lim, W.-W.; Ko, N.S.J.; Tan, J.; Chothani, S.P.; Huang, B.; et al. Interleukin-11 Is a Therapeutic Target in Idiopathic Pulmonary Fibrosis. Sci. Transl. Med. 2019, 11, eaaw1237. [Google Scholar] [CrossRef]





| Antibodies | Source | Catalogue | Usage (Dilution) |
|---|---|---|---|
| Anti-α-Smooth Muscle Actin | Abcam | ab7817 | Immunofluorescence (1:500) |
| Anti-Collagen I | Abcam | ab34710 | Immunofluorescence (1:500) |
| Anti-Collagen I | Abcam | ab21286 | Immunohistochemistry (1:100) |
| Anti-gp130 | Thermo Fisher | PA5-99526 | Immunofluorescence (1:100) |
| Anti-IL6RA | Thermo Fisher | MA1-80456 | Immunofluorescence (1:100) |
| Anti-IL11RA | Abcam | ab125015 | Immunofluorescence (1:100) |
| Anti-IL11RA | Aldevron | X209 | Immunohistochemistry (1:100) |
| Anti-rabbit Alexa Fluor 488 secondary antibody | Abcam | ab15077 | Immunofluorescence (1:1000) |
| Anti-mouse Alexa Fluor 488 secondary antibody | Abcam | ab1510113 | Immunofluorescence (1:1000) |
| Anti-IL11 | Aldevron [39] | X203 | Western blot (1:5000); Immunohistochemistry (1:500) |
| Anti-IL6 | Cell Signaling Technology | 12912 | Western blot (1:1000) |
| Anti-IL1β | Cell Signaling Technology | 12242 | Western blot (1:1000) |
| Anti-TNFα | Cell Signaling Technology | 11948 | Western blot (1:1000) |
| Anti-pERK1/2 | Cell Signaling Technology | 4370 | Western blot (1:1000) |
| Anti-ERK1/2 | Cell Signaling Technology | 4695 | Western blot (1:1000) |
| Anti-pSTAT3 | Cell Signaling Technology | 4113 | Western blot (1:1000) |
| Anti-STAT3 | Cell Signaling Technology | 4904 | Western blot (1:1000) |
| Anti-pNF-κB | Cell Signaling Technology | 3033 | Western blot (1:1000) |
| Anti-NF-κB | Cell Signaling Technology | 8242 | Western blot (1:1000) |
| Anti-Cleaved Caspase-3 | Cell Signaling Technology | 9664 | Western blot (1:1000) |
| Anti-Caspase-3 | Cell Signaling Technology | 9662 | Western blot (1:1000) |
| Anti-α-Smooth Muscle Actin | Cell Signaling Technology | 19245 | Western blot (1:1000) |
| Anti-GAPDH | Cell Signaling Technology | 2118 | Western blot (1:1000) |
| Anti-Fibronectin | Abcam | ab2413 | Western blot (1:1000) |
| Anti-rabbit IgG, HRP-linked antibody | Cell Signaling Technology | 7074 | Western blot (1:2000) |
| Anti-mouse IgG, HRP-linked antibody | Cell Signaling Technology | 7076 | Western blot (1:2000) |
| Host | Gene | Forward Primer | Reverse Primer |
|---|---|---|---|
| Human | ACTA2 | 5′-CTGTTGTAGGTGGTTTCATGGA-3′ | 5′-AGAGTTACGAGTTGCCTGATG-3′ |
| Human | COL1A1 | 5′-GAGGGCCAAGACGAAGACATC-3′ | 5′-CAGATCACGTCATCGCACAAC-3′ |
| Human | IL11 | 5′-GCAGCGGACAGGGAAGGGTT-3′ | 5′-CCACAGGCTCAGCACGACCA-3′ |
| Human | TIMP1 | 5′-GTGGCACTCATTGCTTGTGG-3′ | 5′-CAAGGTGACGGGACTGGAAG-3′ |
| Human | GAPDH | 5′-ACAACTTTGGTATCGTGGAAGG-3′ | 5′-GCCATCACGCCACAGTTTC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, B.; Viswanathan, S.; Widjaja, A.A.; Lim, W.-W.; Shekeran, S.G.; Goh, J.W.T.; Tan, J.; Kuthubudeen, F.; Lim, S.Y.; Xie, C.; et al. IL11 Activates Pancreatic Stellate Cells and Causes Pancreatic Inflammation, Fibrosis and Atrophy in a Mouse Model of Pancreatitis. Int. J. Mol. Sci. 2022, 23, 3549. https://doi.org/10.3390/ijms23073549
Ng B, Viswanathan S, Widjaja AA, Lim W-W, Shekeran SG, Goh JWT, Tan J, Kuthubudeen F, Lim SY, Xie C, et al. IL11 Activates Pancreatic Stellate Cells and Causes Pancreatic Inflammation, Fibrosis and Atrophy in a Mouse Model of Pancreatitis. International Journal of Molecular Sciences. 2022; 23(7):3549. https://doi.org/10.3390/ijms23073549
Chicago/Turabian StyleNg, Benjamin, Sivakumar Viswanathan, Anissa A. Widjaja, Wei-Wen Lim, Shamini G. Shekeran, Joyce Wei Ting Goh, Jessie Tan, Fathima Kuthubudeen, Sze Yun Lim, Chen Xie, and et al. 2022. "IL11 Activates Pancreatic Stellate Cells and Causes Pancreatic Inflammation, Fibrosis and Atrophy in a Mouse Model of Pancreatitis" International Journal of Molecular Sciences 23, no. 7: 3549. https://doi.org/10.3390/ijms23073549
APA StyleNg, B., Viswanathan, S., Widjaja, A. A., Lim, W.-W., Shekeran, S. G., Goh, J. W. T., Tan, J., Kuthubudeen, F., Lim, S. Y., Xie, C., Schafer, S., Adami, E., & Cook, S. A. (2022). IL11 Activates Pancreatic Stellate Cells and Causes Pancreatic Inflammation, Fibrosis and Atrophy in a Mouse Model of Pancreatitis. International Journal of Molecular Sciences, 23(7), 3549. https://doi.org/10.3390/ijms23073549







