Macrophage-like Cells Are Responsive to Titania Nanotube Intertube Spacing—An In Vitro Study
Abstract
:1. Introduction
2. Results and Discussions
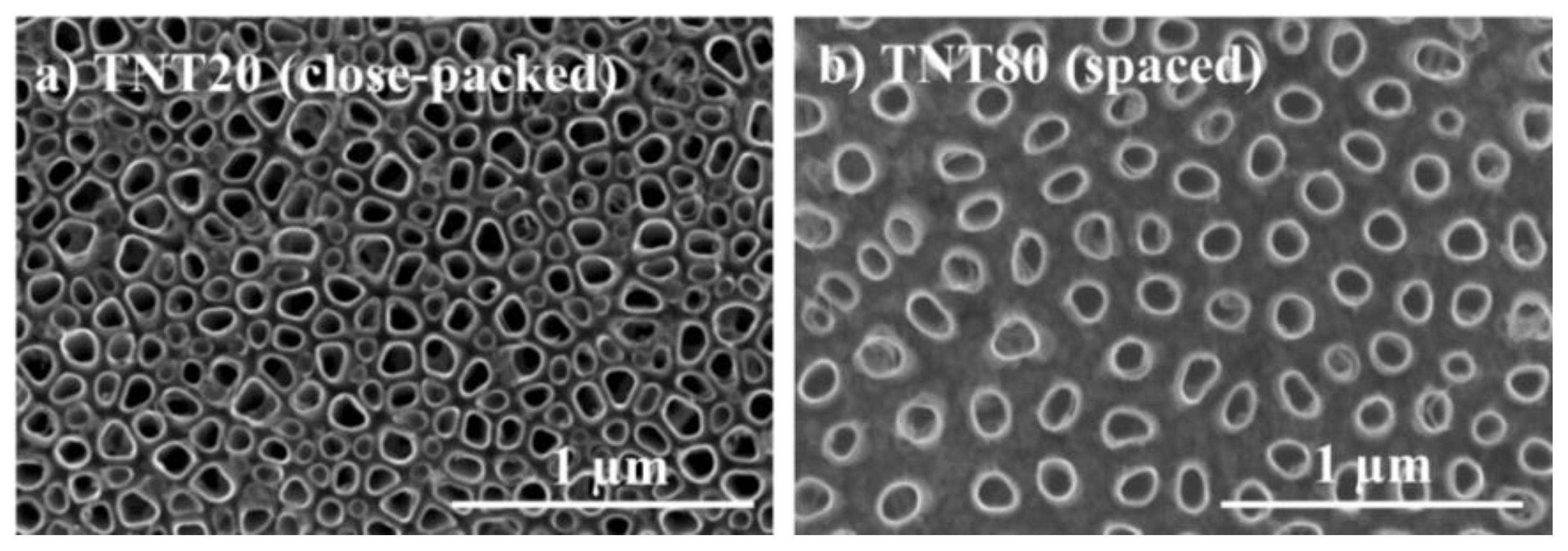
2.1. Nanotube Morphology and Characterization
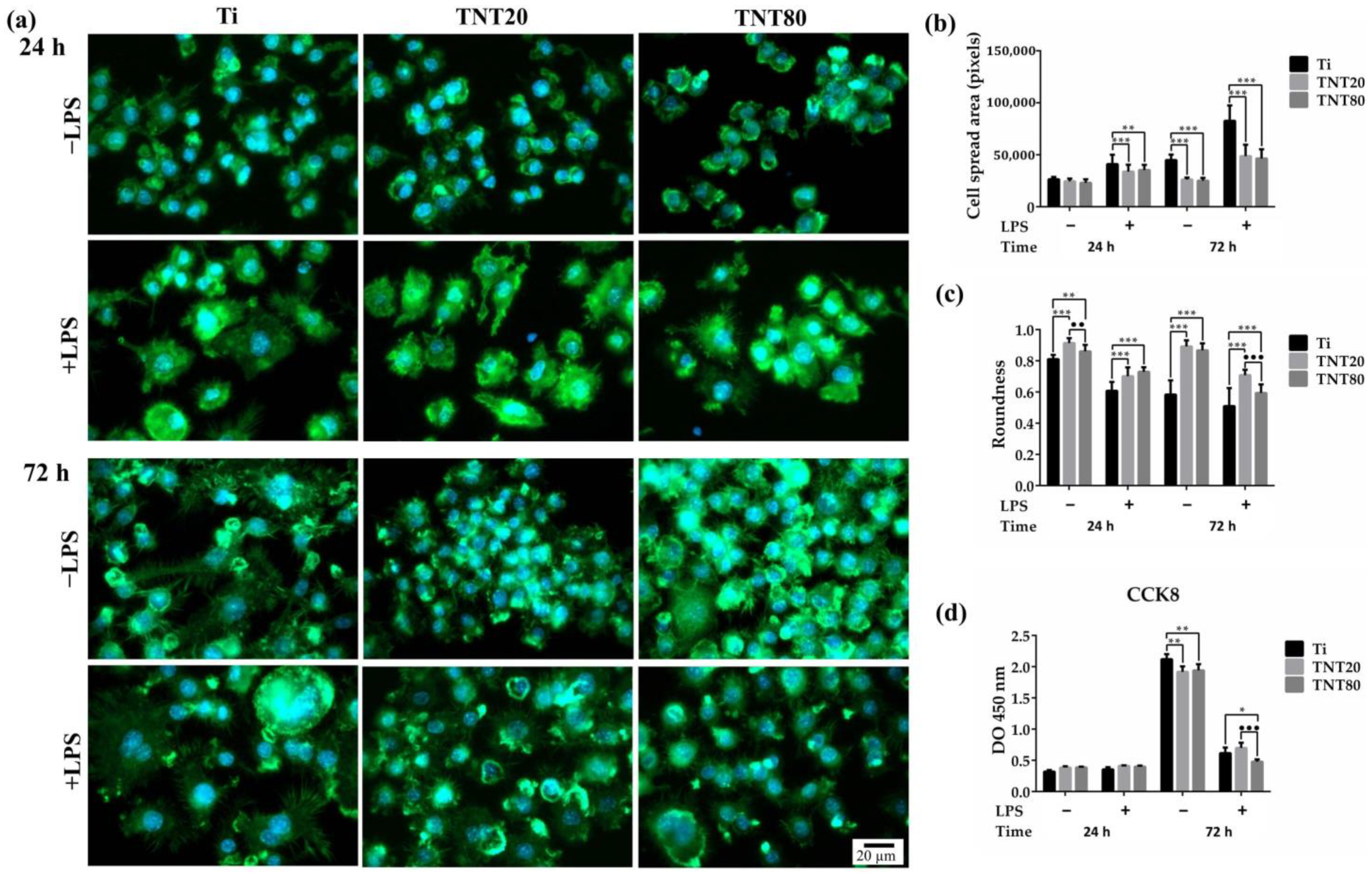
2.2. Macrophage Adhesion, Morphology, and Cell Proliferation
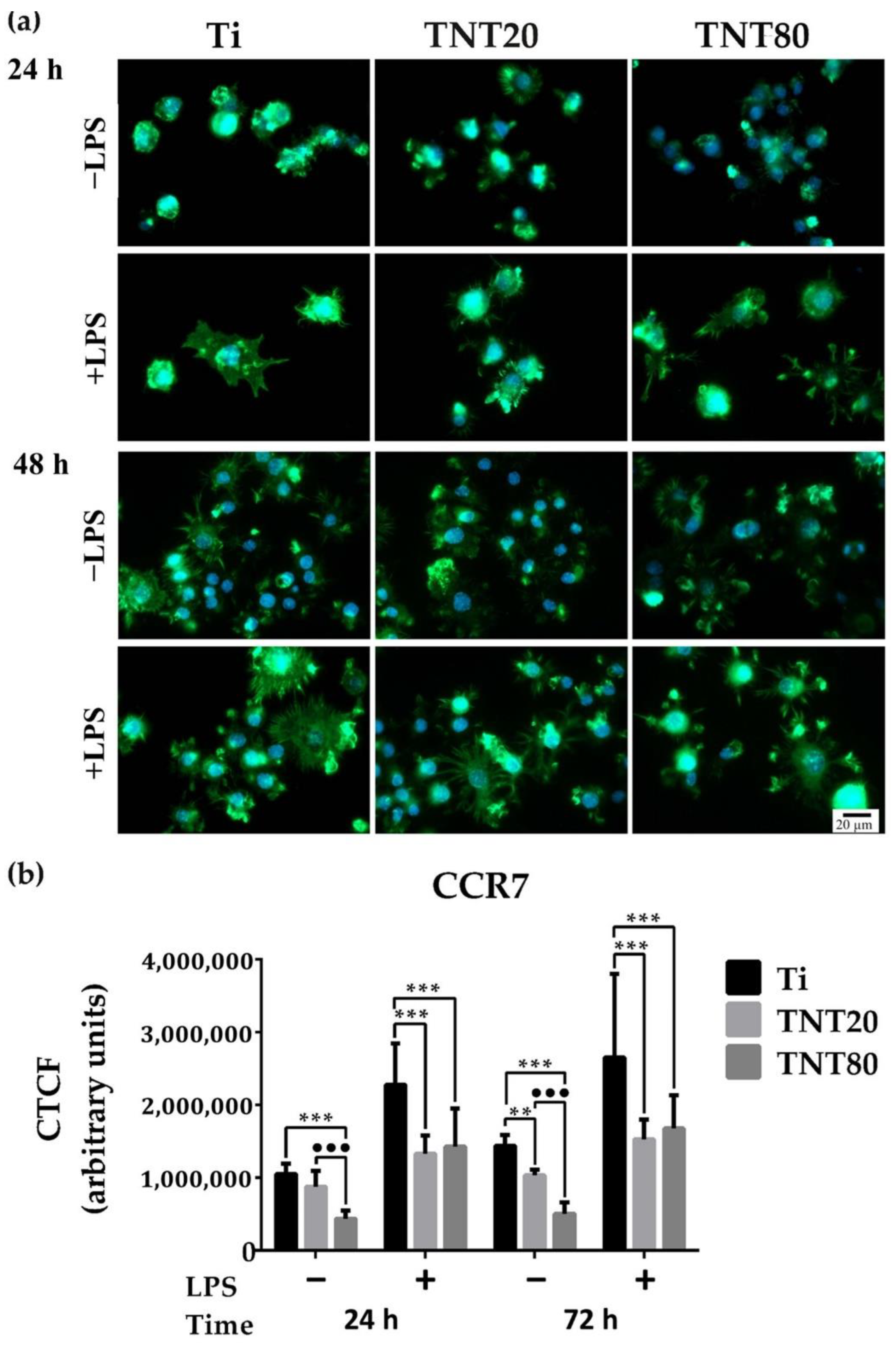
2.3. Macrophage Polarization
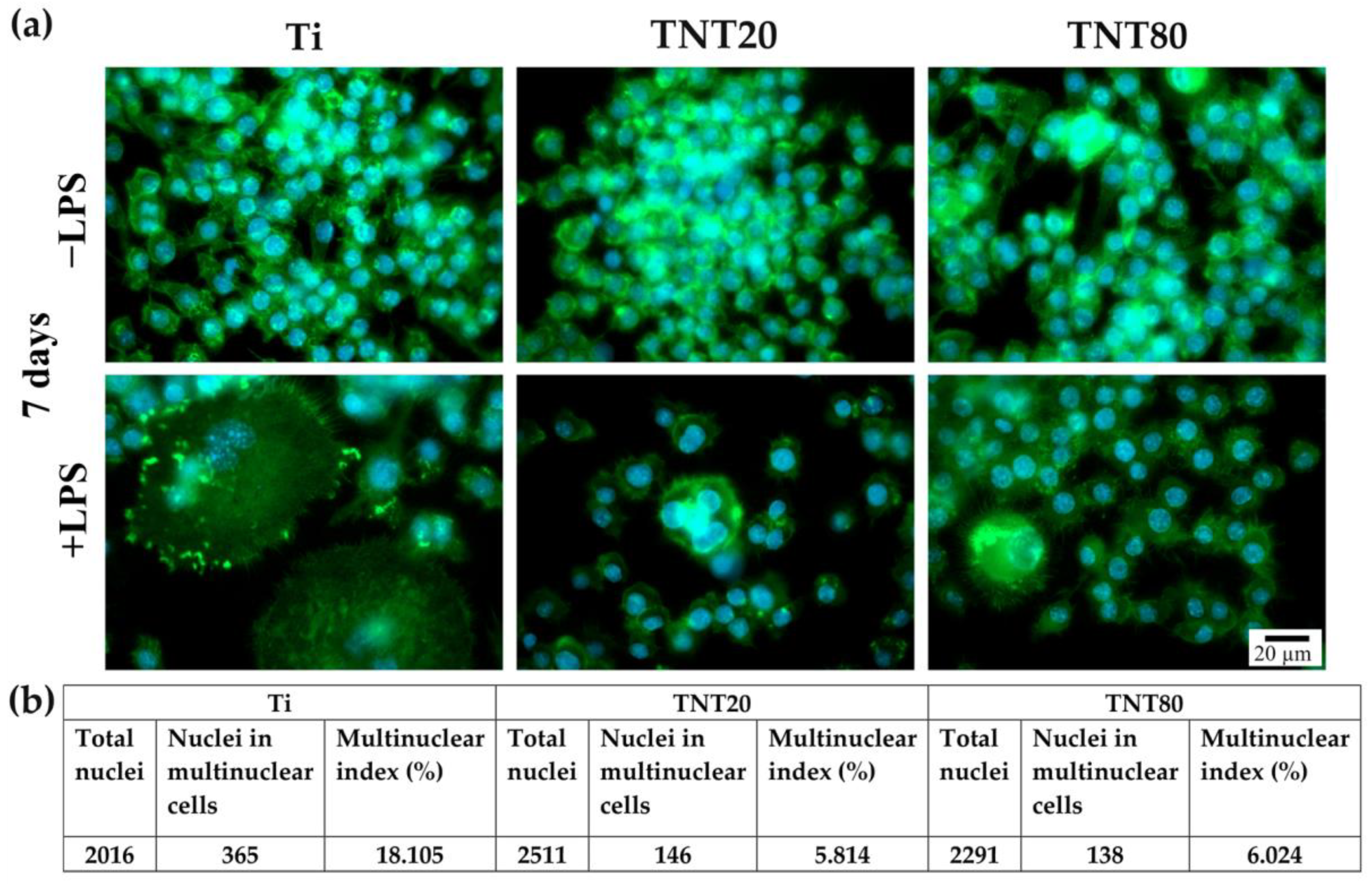
2.4. Foreign Body Giant Cell Formation
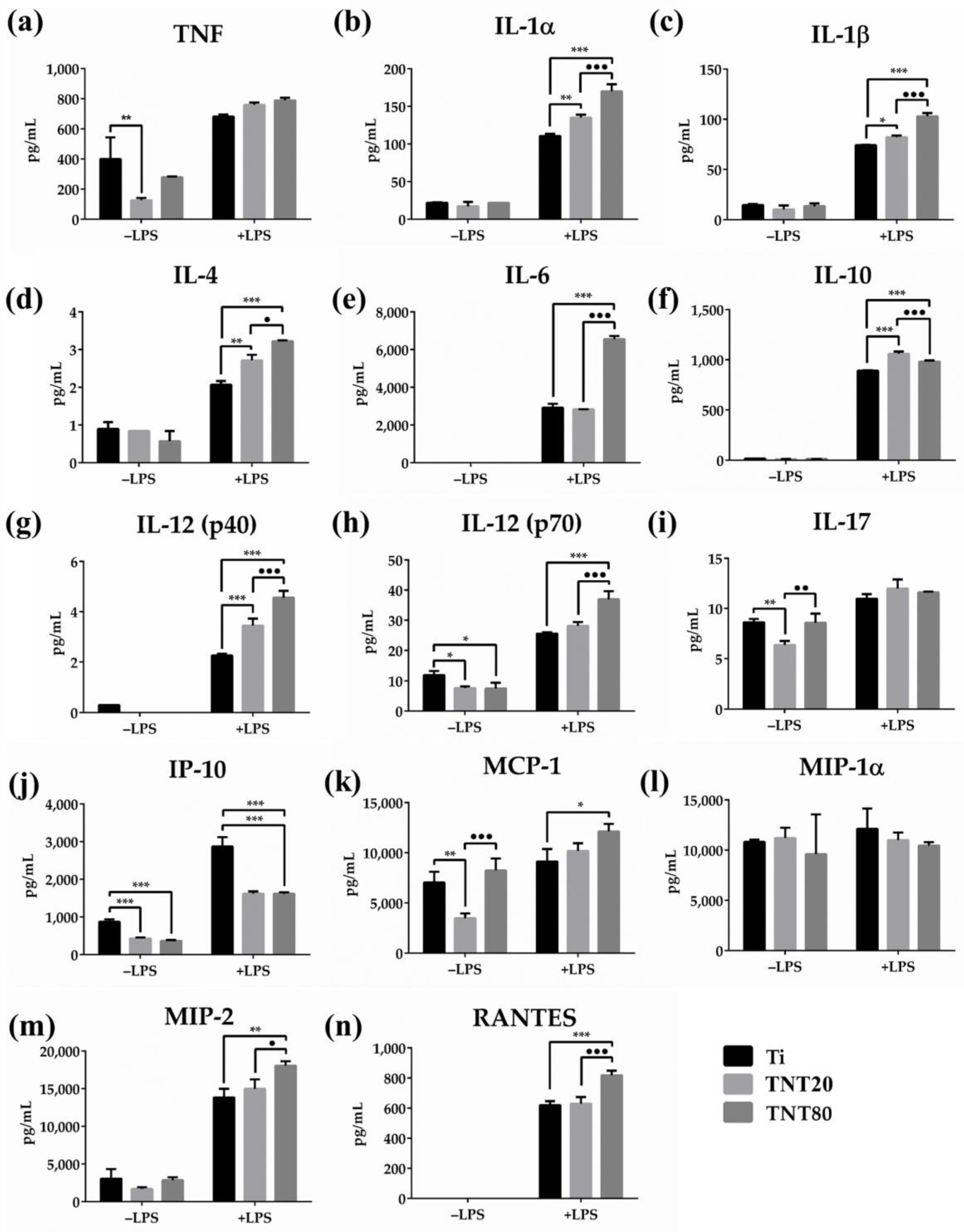
2.5. Cytokine Expression
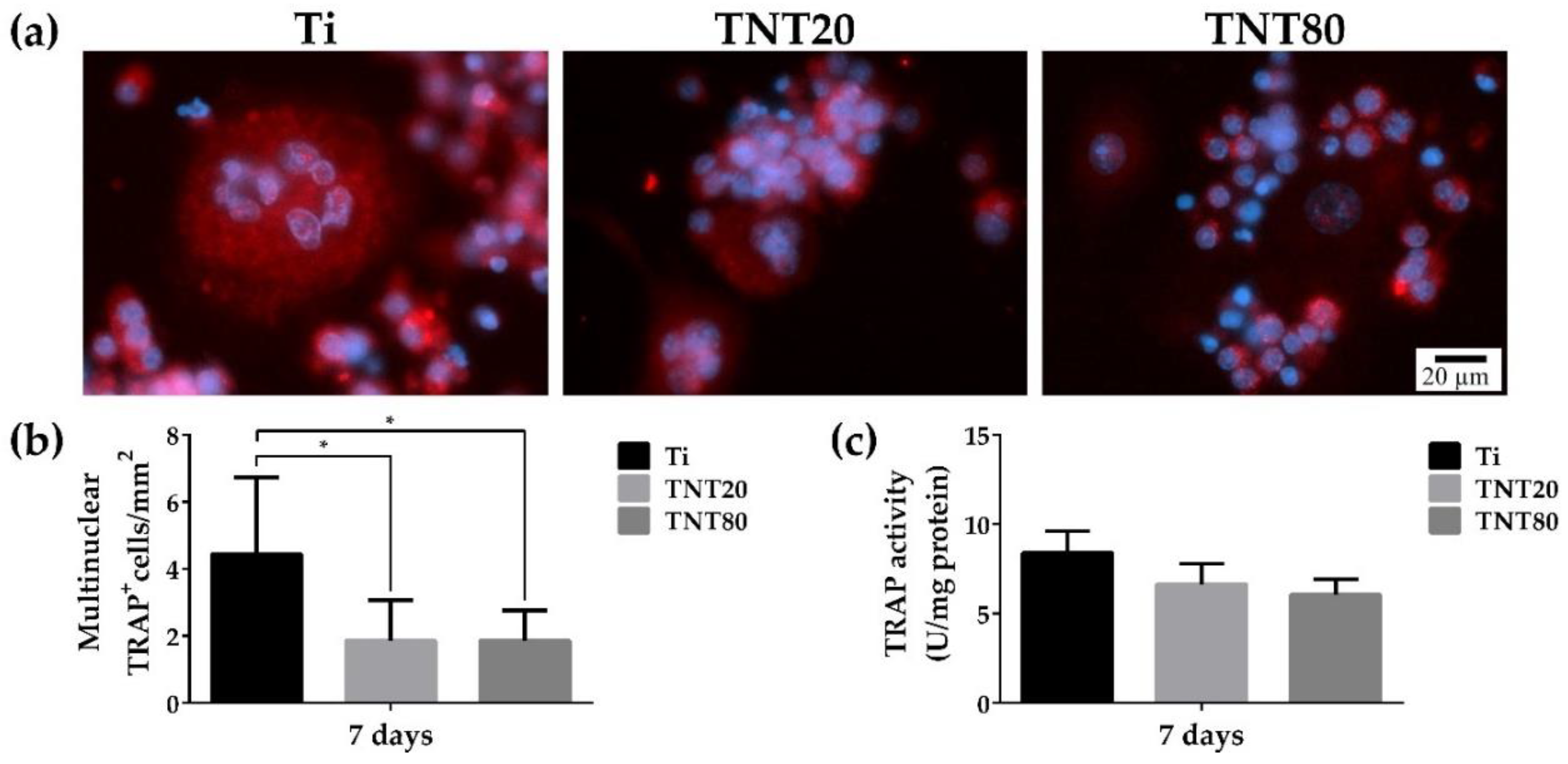
2.6. Osteoclast Differentiation
3. Materials and Methods
3.1. Nanotube Synthesis and Characterization
3.2. Cell Culture
3.3. Cell Survival and Proliferation Assays
3.4. Cell Adhesion and Morphology Assesment
3.5. Expression of Markers Characteristic of M1/M2 Phenotypes
3.6. In Vitro Macrophage Fusion Assay
3.7. Luminex-Based Cytokine/Chemokine Detection and Quantification
3.8. Osteoclast Differentiation Assay
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, A.W.; Pingguan-Murphy, B.; Ahmad, R.; Akbar, S.A. Review of titania nanotubes: Fabrication and cellular response. Ceram. Int. 2012, 38, 4421–4435. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Khoon, C. Titanium Alloys in Orthopaedics. In Titanium Alloys—Advances in Properties Control; Sieniawski, J., Ziaja, W., Eds.; InTech: London, UK, 2013. [Google Scholar]
- Verissimo, N.C.; Chung, S.; Webster, T.J. New nanoscale surface modifications of metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 249–273. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, S.; Spriano, S.; Cazzola, M. Fast and effective osseointegration of dental, spinal, and orthopedic implants through tailored chemistry of inorganic surfaces. In Nanostructured Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 337–377. [Google Scholar]
- Wang, J.; Meng, F.; Song, W.; Jin, J.; Ma, Q.; Fei, D.; Fang, L.; Chen, L.; Wang, Q.; Zhang, Y. Nanostructured titanium regulates osseointegration via influencing macrophage polarization in the osteogenic environment. Int. J. Nanomed. 2018, 13, 4029–4043. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Cimpean, A.; Tesler, A.B.; Mazare, A. Anodic TiO2 Nanotubes: Tailoring Osteoinduction via Drug Delivery. Nanomaterials 2021, 11, 2359. [Google Scholar] [CrossRef]
- Guo, T.; Oztug, N.A.K.; Han, P.; Ivanovski, S.; Gulati, K. Untwining the topography-chemistry interdependence to optimize the bioactivity of nano-engineered titanium implants. Appl. Surf. Sci. 2021, 570, 151083. [Google Scholar] [CrossRef]
- Ion, R.; Necula, M.G.; Mazare, A.; Mitran, V.; Neacsu, P.; Schmuki, P.; Cimpean, A. Drug Delivery Systems Based on Titania Nanotubes and Active Agents for Enhanced Osseointegration of Bone Implants. Curr. Med. Chem. 2020, 27, 854–902. [Google Scholar] [CrossRef]
- Özkan, S.; Nguyen, N.T.; Mazare, A.; Schmuki, P. Controlled spacing of self-organized anodic TiO2 nanotubes. Electrochem. Commun. 2016, 69, 76–79. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Berger, S.; Hauser, C.; Meyer, K.; Yang, M.; Schmuki, P. Transition of TiO2 nanotubes to nanopores for electrolytes with very low water contents. Electrochem. Commun. 2010, 12, 1184–1186. [Google Scholar] [CrossRef]
- Liu, N.; Lee, K.; Schmuki, P. Small diameter TiO2 nanotubes vs. nanopores in dye sensitized solar cells. Electrochem. Commun. 2012, 15, 1–4. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglic, A. Influence of anodization parameters on morphology of TiO2 nanostructured surfaces. Adv. Mater. Lett. 2016, 7, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Lee, K.; Roy, P.; Birajdar, B.I.; Spiecker, E.; Schmuki, P. Formation of a non-thickness-limited titanium dioxide mesosponge and its use in dye-sensitized solar cells. Angew. Chem. Int. Ed. 2009, 48, 9326–9329. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, D.; Schmuki, P. Highly self-ordered nanochannel TiO2 structures by anodization in a hot glycerol electrolyte. Chem. Commun. 2011, 47, 5789–5791. [Google Scholar] [CrossRef]
- Ion, R.; Stoian, A.B.; Dumitriu, C.; Grigorescu, S.; Mazare, A.; Cimpean, A.; Demetrescu, I.; Schmuki, P. Nanochannels formed on TiZr alloy improve biological response. Acta Biomater. 2015, 24, 370–377. [Google Scholar] [CrossRef]
- Ion, R.; Mazare, A.; Dumitriu, C.; Pirvu, C.; Schmuki, P.; Cimpean, A. Nanochannelar Topography Positively Modulates Osteoblast Differentiation and Inhibits Osteoclastogenesis. Coatings 2018, 8, 294. [Google Scholar] [CrossRef] [Green Version]
- Hasanzadeh Kafshgari, M.; Kah, D.; Mazare, A.; Nguyen, N.T.; Distaso, M.; Peukert, W.; Goldmann, W.H.; Schmuki, P.; Fabry, B. Anodic Titanium Dioxide Nanotubes for Magnetically Guided Therapeutic Delivery. Sci. Rep. 2019, 9, 13439. [Google Scholar] [CrossRef] [Green Version]
- Kafshgari, M.H.; Mazare, A.; Distaso, M.; Goldmann, W.H.; Peukert, W.; Fabry, B.; Schmuki, P. Intracellular Drug Delivery with Anodic Titanium Dioxide Nanotubes and Nanocylinders. ACS Appl. Mater. Interfaces 2019, 11, 14980–14985. [Google Scholar] [CrossRef]
- Silverwood, R.K.; Fairhurst, P.G.; Sjöström, T.; Welsh, F.; Sun, Y.; Li, G.; Yu, B.; Young, P.S.; Su, B.; Meek, R.M.D.; et al. Analysis of Osteoclastogenesis/Osteoblastogenesis on Nanotopographical Titania Surfaces. Adv. Healthc. Mater. 2016, 5, 947–955. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Yu, K.; Feng, Y.; Zhang, L.; Yang, G. An effective light activated TiO2 nanodot platform for gene delivery within cell sheets to enhance osseointegration. Chem. Eng. J. 2020, 402, 126170. [Google Scholar] [CrossRef]
- Chen, X.; Fan, H.; Deng, X.; Wu, L.; Yi, T.; Gu, L.; Zhou, C.; Fan, Y.; Zhang, X. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Bauer, S.; Pittrof, A.; Killian, M.S.; Schmuki, P.; von der Mark, K. Synergistic control of mesenchymal stem cell differentiation by nanoscale surface geometry and immobilized growth factors on TiO2 nanotubes. Small 2012, 8, 98–107. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces. Part I: Materials and surfaces. Prog. Mater. Sci. 2012, 58, 261–326. [Google Scholar] [CrossRef]
- Chen, Z.; Bachhuka, A.; Wei, F.; Wang, X.; Liu, G.; Vasilev, K.; Xiao, Y. Nanotopography-based strategy for the precise manipulation of osteoimmunomodulation in bone regeneration. Nanoscale 2017, 9, 18129–18152. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.; Gretzer, C. Macrophage interactions with modified material surfaces. Curr. Opin. Solid State Mater. Sci. 2001, 5, 163–176. [Google Scholar] [CrossRef]
- Refai, A.K.; Textor, M.; Brunette, D.M.; Waterfield, J.D. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J. Biomed. Mater. Res. 2004, 70A, 194–205. [Google Scholar] [CrossRef]
- Tan, K.S.; Qian, L.; Rosado, R.; Flood, P.M.; Cooper, L.F. The role of titanium surface topography on J774A.1 macrophage inflammatory cytokines and nitric oxide production. Biomaterials 2006, 27, 5170–5177. [Google Scholar] [CrossRef]
- Bota, P.C.S.; Collie, A.M.B.; Puolakkainen, P.; Vernon, R.B.; Sage, E.H.; Ratner, B.D.; Stayton, P.S. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J. Biomed. Mater. Res. Part A 2010, 95A, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Barth, K.A.; Waterfield, J.D.; Brunette, D.M. The effect of surface roughness on RAW 264.7 macrophage phenotype. J. Biomed. Mater. Res. Part A 2013, 101A, 2679–2688. [Google Scholar] [CrossRef]
- Christo, S.N.; Bachhuka, A.; Diener, K.R.; Mierczynska, A.; Hayball, J.D.; Vasilev, K. The Role of Surface Nanotopography and Chemistry on Primary Neutrophil and Macrophage Cellular Responses. Adv. Healthc. Mater. 2016, 5, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, S.; Liu, X.; Xu, L.; Miao, X.; Xu, Z.; Cao, L.; Wang, H.; Jiang, X. M2 macrophages contribute to osteogenesis and angiogenesis on nanotubular TiO2 surfaces. J. Mater. Chem. B 2017, 5, 3364–3376. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yu, Y.; Ma, P.; Luo, Z.; Hu, Y.; Li, M.; He, Y.; Zhang, Y.; Peng, Z.; Song, G.; et al. Titania nanotubes promote osteogenesis via mediating crosstalk between macrophages and MSCs under oxidative stress. Colloids Surf. B Biointerfaces 2019, 180, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Feng, X.; Li, W.; Wang, L.N.; Wang, X. Regulation of RAW 264.7 macrophages behavior on anodic TiO2 nanotubular arrays. Front. Mater. Sci. 2017, 11, 318–327. [Google Scholar] [CrossRef]
- Rajyalakashmi, A.; Ercan, B.; Balasubramanian, K.; Webster, T.J. Reduced adhesion of macrophages on anodized titanium with select nanotube surface features. Int. J. Nanomed. 2011, 6, 1765–1771. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, L.M.; Brammer, K.S.; Johnston, G.W.; Chien, S.; Jin, S. Macrophage Inflammatory Response to TiO2 Nanotube Surfaces. J. Biomater. Nanobiotechnol. 2011, 2, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.J.; Yu, W.Q.; Zhang, Y.L.; Jiang, X.Q.; Zhang, F.Q. Effects of TiO2 nanotube layers on RAW 264.7 macrophage behaviour and bone morphogenetic protein-2 expression. Cell Prolif. 2013, 46, 685–694. [Google Scholar] [CrossRef]
- Gao, S.; Lu, R.; Wang, X.; Chou, J.; Wang, N.; Huai, X.; Wang, C.; Zhao, Y.; Chen, S. Immune response of macrophages on super-hydrophilic TiO2 nanotube arrays. J. Biomater. Appl. 2020, 34, 1239–1253. [Google Scholar] [CrossRef]
- Ma, Q.-L.; Zhao, L.-Z.; Liu, R.-R.; Jin, B.-Q.; Song, W.; Wang, Y.; Zhang, Y.-S.; Chen, L.-H.; Zhang, Y.-M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014, 35, 9853–9867. [Google Scholar] [CrossRef]
- Neacsu, P.; Mazare, A.; Cimpean, A.; Park, J.; Costache, M.; Schmuki, P.; Demetrescu, I. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int. J. Biochem. Cell Biol. 2014, 55, 187–195. [Google Scholar] [CrossRef]
- Steeves, A.J.; Ho, W.; Munisso, M.C.; Lomboni, D.J.; Larrañaga, E.; Omelon, S.; Martínez, E.; Spinello, D.; Variola, F. The implication of spatial statistics in human mesenchymal stem cell response to nanotubular architectures. Int. J. Nanomed. 2020, 15, 2151–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neacsu, P.; Mazare, A.; Schmuki, P.; Cimpean, A. Attenuation of the macrophage inflammatory activity by TiO2 nanotubes via inhibition of MAPK and NF-κB pathways. Int. J. Nanomed. 2015, 10, 6455–6467. [Google Scholar] [CrossRef] [Green Version]
- Necula, M.G.; Mazare, A.; Ion, R.N.; Ozkan, S.; Park, J.; Schmuki, P.; Cimpean, A. Lateral spacing of TiO2 nanotubes modulates osteoblast behavior. Materials 2019, 12, 2956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkan, S.; Nguyen, N.T.; Mazare, A.; Hahn, R.; Cerri, I.; Schmuki, P. Fast growth of TiO2 nanotube arrays with controlled tube spacing based on a self-ordering process at two different scales. Electrochem. Commun. 2017, 77, 98–102. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Ozkan, S.; Hwang, I.; Mazare, A.; Schmuki, P. TiO2 nanotubes with laterally spaced ordering enable optimized hierarchical structures with significantly enhanced photocatalytic H2 generation. Nanoscale 2016, 8, 16868–16873. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, S.; Cha, G.; Mazare, A.; Schmuki, P. TiO2 nanotubes with different spacing, Fe2O3 decoration and their evaluation for Li-ion battery application. Nanotechnology 2018, 29, 195402. [Google Scholar] [CrossRef]
- Ozkan, S.; Valle, F.; Mazare, A.; Hwang, I.; Taccardi, N.; Zazpe, R.; Macak, J.M.; Cerri, I.; Schmuki, P. Optimized Polymer Electrolyte Membrane Fuel Cell Electrode Using TiO2 Nanotube Arrays with Well-Defined Spacing. ACS Appl. Nano Mater. 2020, 3, 4157–4170. [Google Scholar] [CrossRef]
- Ozkan, S.; Mazare, A.; Schmuki, P. Critical parameters and factors in the formation of spaced TiO2 nanotubes by self-organizing anodization. Electrochim. Acta 2018, 268, 435–447. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; Schmuki, P.; Von Der Mark, K. Narrow window in nanoscale dependent activation of endothelial cell growth and differentiation on TiO2 nanotube surfaces. Nano Lett. 2009, 9, 3157–3164. [Google Scholar] [CrossRef]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef] [Green Version]
- Kamath, S.; Bhattacharyya, D.; Padukudru, C.; Timmons, R.B.; Tang, L. Surface chemistry influences implant-mediated host tissue responses. J. Biomed. Mater. Res. Part A 2008, 86A, 617–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesure, L.; de Visscher, G.; Vranken, I.; Lebacq, A.; Flameng, W. Gene expression study of monocytes/macrophages during early foreign body reaction and identification of potential precursors of myofibroblasts. PLoS ONE 2010, 5, e12949. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Hulander, M.; Lundgren, A.; Faxälv, L.; Lindahl, T.L.; Palmquist, A.; Berglin, M.; Elwing, H. Gradients in surface nanotopography used to study platelet adhesion and activation. Colloids Surf. B Biointerfaces 2013, 110, 261–269. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Jones, J.A.; Xu, Y.; Low, H.-Y.; Anderson, J.M.; Leong, K.W. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials 2010, 31, 3479–3491. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [Green Version]
- Honma, M.; Ikebuchi, Y.; Kariya, Y.; Suzuki, H. Regulatory Mechanisms of RANKL Presentation to Osteoclast Precursors. Curr. Osteoporos. Rep. 2014, 12, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.U.; Gott, S.C.; Woo, B.W.K.; Rao, M.P.; Liu, W.F. Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype. ACS Appl. Mater. Interfaces 2015, 7, 28665–28672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainslie, K.M.; Thakar, R.G.; Bernards, D.A.; Desai, T.A. Inflammatory Response to Implanted Nanostructured Materials. In Biological Interactions on Materials Surfaces; Puleo, D., Bizios, R., Eds.; Springer US: New York, NY, USA, 2009; pp. 355–371. [Google Scholar]
- Vadiveloo, P.; Keramidaris, E.; Morrison, W.; Stewart, A. Lipopolysaccharide-induced cell cycle arrest in macrophages occurs independently of nitric oxide synthase II induction. Biochim. Biophys. Acta Mol. Cell Res. 2001, 1539, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Zhao, C.; Wang, Y.; Li, Y.; Duan, K.; Wang, J.; Weng, J.; Feng, B. Nano-topographic titanium modulates macrophage response in vitro and in an implant-associated rat infection model. RSC Adv. 2016, 6, 111919–111927. [Google Scholar] [CrossRef]
- Smith, B.S.; Capellato, P.; Kelley, S.; Gonzalez-Juarrero, M.; Popat, K.C. Reduced in vitro immune response on titania nanotube arrays compared to titanium surface. Biomater. Sci. 2013, 1, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zheng, T.; Wen, L.M.; Li, R.; Zhang, Y.B.; Bi, W.J.; Feng, X.J.; Qi, M.C. Osteogenic capability of strontium and icariin-loaded TiO2 nanotube coatings in vitro and in osteoporotic rats. J. Biomater. Appl. 2021, 35, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Guo, S.; Yang, D.; Li, B.; Guo, Z.; Han, Y. Interrod spacing dependent angiogenesis and osseointegration of Na2TiO3 nanorods-patterned arrays via immunoregulation. Chem. Eng. J. 2021, 426, 131187. [Google Scholar] [CrossRef]
- Yu, D.; Guo, S.; Yu, M.; Liu, W.; Li, X.; Chen, D.; Li, B.; Guo, Z.; Han, Y. Immunomodulation and osseointegration activities of Na2TiO3 nanorods-arrayed coatings doped with different Sr content. Bioact. Mater. 2022, 10, 323–334. [Google Scholar] [CrossRef]
- Kwiecień, I.; Polubiec-Kownacka, M.; Dziedzic, D.; Wołosz, D.; Rzepecki, P.; Domagała-Kulawik, J. CD163 and CCR7 as markers for macrophage polarisation in lung cancer microenvironment. Cent. Eur. J. Immunol. 2019, 44, 395–402. [Google Scholar] [CrossRef]
- Xia, Z.; Triffitt, J.T. A review on macrophage responses to biomaterials. Biomed. Mater. 2006, 1, R1–R9. [Google Scholar] [CrossRef]
- Sheikh, Z.; Brooks, P.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padmanabhan, J.; Kyriakides, T.R. Nanomaterials, Inflammation, and Tissue Engineering. WIREs Nanomed. Nanobiotechnol. 2015, 7, 355–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klopfleisch, R. Macrophage reaction against biomaterials in the mouse model—Phenotypes, functions and markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D. OsteoMacs: Key players around bone biomaterials. Biomaterials 2016, 82, 1–19. [Google Scholar] [CrossRef]
- Hallab, N.J.; Jacobs, J.J. Chemokines Associated with Pathologic Responses to Orthopedic Implant Debris. Front. Endocrinol. 2017, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Gibon, E.; Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Lu, L.; Nabeshima, A.; Yao, Z.; Goodman, S.B. Aging Affects Bone Marrow Macrophage Polarization: Relevance to Bone Healing. Regen. Eng. Transl. Med. 2016, 2, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.A.; Chang, D.T.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. Part A 2007, 83A, 585–596. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G. Biology of implant osseointegration. Univ. Connect. Health Cent. 2011, 103, e22–e25. [Google Scholar]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.P.; Borish, L.; Steinke, J.W. Immunologic messenger molecules: Cytokines, interferons, and chemokines. J. Allergy Clin. Immunol. 2010, 125, S53–S72. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453. [Google Scholar] [CrossRef] [Green Version]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- AI-Aql, Z.S.; Alagl, A.S.; Graves, D.T.; Gerstenfeld, L.C.; Einhorn, T.A. Molecular Mechanisms Controlling Bone Formation during Fracture Healing and Distraction Osteogenesis. J. Dent. Res. 2008, 87, 107–118. [Google Scholar] [CrossRef]
- Prystaz, K.; Kaiser, K.; Kovtun, A.; Haffner-Luntzer, M.; Fischer, V.; Rapp, A.E.; Liedert, A.; Strauss, G.; Waetzig, G.H.; Rose-John, S.; et al. Distinct Effects of IL-6 Classic and Trans -Signaling in Bone Fracture Healing. Am. J. Pathol. 2018, 188, 474–490. [Google Scholar] [CrossRef] [Green Version]
- Fernando, M.R.; Reyes, J.L.; Iannuzzi, J.; Leung, G.; McKay, D.M. The Pro-Inflammatory Cytokine, Interleukin-6, Enhances the Polarization of Alternatively Activated Macrophages. PLoS ONE 2014, 9, e94188. [Google Scholar] [CrossRef]
- Wallace, A.; Cooney, T.E.; Englund, R.; Lubahn, J.D. Effects of Interleukin-6 Ablation on Fracture Healing in Mice. J. Orthop. Res. 2011, 29, 1437–1442. [Google Scholar] [CrossRef]
- Glass, G.E.; Chan, J.K.; Freidin, A.; Feldmann, M.; Horwood, N.J.; Nanchahal, J. TNF- promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 1585–1590. [Google Scholar] [CrossRef] [Green Version]
- Eming, S.A.; Hammerschmidt, M.; Krieg, T.; Roers, A. Interrelation of immunity and tissue repair or regeneration. Semin. Cell Dev. Biol. 2009, 20, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef] [PubMed]
- Hamlet, S.; Alfarsi, M.; George, R.; Ivanovski, S. The effect of hydrophilic titanium surface modification on macrophage inflammatory cytokine gene expression. Clin. Oral Implant. Res. 2012, 23, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Naito, A.; Inoue, J.-I.; Satoh, M.; Santee-Cooper, S.M.; Ware, C.F.; Togawa, A.; Nishikawa, S.; Nishikawa, S.-I. Different Cytokines Induce Surface Lymphotoxin-αβ on IL-7 Receptor-α Cells that Differentially Engender Lymph Nodes and Peyer’s Patches. Immunity 2002, 17, 823–833. [Google Scholar] [CrossRef] [Green Version]
- Abdi, K. IL-12: The Role of p40 Versus p75. Scand. J. Immunol. 2002, 56, 1–11. [Google Scholar] [CrossRef]
- Ono, T.; Okamoto, K.; Nakashima, T.; Nitta, T.; Hori, S.; Iwakura, Y.; Takayanagi, H. IL-17-producing γδ T cells enhance bone regeneration. Nat. Commun. 2016, 7, 10928. [Google Scholar] [CrossRef] [Green Version]
- Croes, M.; Kruyt, M.C.; Groen, W.M.; van Dorenmalen, K.M.A.; Dhert, W.J.A.; Öner, F.C.; Alblas, J. Interleukin 17 enhances bone morphogenetic protein-2-induced ectopic bone formation. Sci. Rep. 2018, 8, 7269. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, C.; Jin, L.; Yang, Y. IL-17 alters the mesenchymal stem cell niche towards osteogenesis in cooperation with osteocytes. J. Cell. Physiol. 2020, 235, 4466–4480. [Google Scholar] [CrossRef] [Green Version]
- Grütz, G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J. Leukoc. Biol. 2005, 77, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar]
- Takakura, R.; Kiyohara, T.; Murayama, Y.; Miyazaki, Y.; Miyoshi, Y.; Shinomura, Y.; Matsuzawa, Y. Enhanced macrophage responsiveness to lipopolysaccharide and CD40 stimulation in a murine model of inflammatory bowel disease: IL-10-deficient mice. Inflamm. Res. 2002, 51, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.-Y.; Zheng, G.-H.; Zhao, L.; Wu, J.-G.; Zhang, X.-Y.; Zhang, S.-L.; Huang, Z.-J.; Xiong, F.-L.; Li, C.-M. Anti-inflammatory effects of ethyl acetate fraction from Melilotus suaveolens Ledeb on LPS-stimulated RAW 264.7 cells. J. Ethnopharmacol. 2009, 123, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Takito, J.; Inoue, S.; Nakamura, M. The Sealing Zone in Osteoclasts: A Self-Organized Structure on the Bone. Int. J. Mol. Sci. 2018, 19, 984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, M.; Jin, Z.; Yang, X.; Wang, H.; Xu, K. The controlled release of simvastatin from TiO2 nanotubes to promote osteoblast differentiation and inhibit osteoclast resorption. Appl. Surf. Sci. 2017, 396, 1741–1751. [Google Scholar] [CrossRef]
- Tesler, A.B.; Prado, L.H.; Khusniyarov, M.M.; Thievessen, I.; Mazare, A.; Fischer, L.; Virtanen, S.; Goldmann, W.H.; Schmuki, P. A One-Pot Universal Approach to Fabricate Lubricant-Infused Slippery Surfaces on Solid Substrates. Adv. Funct. Mater. 2021, 31, 2101090. [Google Scholar] [CrossRef]
- Dascălu, C.A.; Maidaniuc, A.; Pandele, A.M.; Voicu, S.I.; Machedon-Pisu, T.; Stan, G.E.; Cîmpean, A.; Mitran, V.; Antoniac, I.V.; Miculescu, F. Synthesis and characterization of biocompatible polymer-ceramic film structures as favorable interface in guided bone regeneration. Appl. Surf. Sci. 2019, 494, 335–352. [Google Scholar] [CrossRef]
- Negrescu, A.-M.; Necula, M.-G.; Gebaur, A.; Golgovici, F.; Nica, C.; Curti, F.; Iovu, H.; Costache, M.; Cimpean, A. In Vitro Macrophage Immunomodulation by Poly(ε-caprolactone) Based-Coated AZ31 Mg Alloy. Int. J. Mol. Sci. 2021, 22, 909. [Google Scholar] [CrossRef]
 ; white arrow—filopodia
; white arrow—filopodia  ; dashed yellow arrow—membrane ruffling
; dashed yellow arrow—membrane ruffling  ). Insets in (a), marked red squares, show the morphology of the corresponding substrate. In the higher magnification images of the area marked with green squares for 24 h, +LPS condition (b), the cell membrane is delineated (orange dashed line) and the filopodia (white arrows) are indicated.
). Insets in (a), marked red squares, show the morphology of the corresponding substrate. In the higher magnification images of the area marked with green squares for 24 h, +LPS condition (b), the cell membrane is delineated (orange dashed line) and the filopodia (white arrows) are indicated.
 ; white arrow—filopodia
; white arrow—filopodia  ; dashed yellow arrow—membrane ruffling
; dashed yellow arrow—membrane ruffling  ). Insets in (a), marked red squares, show the morphology of the corresponding substrate. In the higher magnification images of the area marked with green squares for 24 h, +LPS condition (b), the cell membrane is delineated (orange dashed line) and the filopodia (white arrows) are indicated.
). Insets in (a), marked red squares, show the morphology of the corresponding substrate. In the higher magnification images of the area marked with green squares for 24 h, +LPS condition (b), the cell membrane is delineated (orange dashed line) and the filopodia (white arrows) are indicated.

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Necula, M.G.; Mazare, A.; Negrescu, A.M.; Mitran, V.; Ozkan, S.; Trusca, R.; Park, J.; Schmuki, P.; Cimpean, A. Macrophage-like Cells Are Responsive to Titania Nanotube Intertube Spacing—An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 3558. https://doi.org/10.3390/ijms23073558
Necula MG, Mazare A, Negrescu AM, Mitran V, Ozkan S, Trusca R, Park J, Schmuki P, Cimpean A. Macrophage-like Cells Are Responsive to Titania Nanotube Intertube Spacing—An In Vitro Study. International Journal of Molecular Sciences. 2022; 23(7):3558. https://doi.org/10.3390/ijms23073558
Chicago/Turabian StyleNecula, Madalina Georgiana, Anca Mazare, Andreea Mariana Negrescu, Valentina Mitran, Selda Ozkan, Roxana Trusca, Jung Park, Patrik Schmuki, and Anisoara Cimpean. 2022. "Macrophage-like Cells Are Responsive to Titania Nanotube Intertube Spacing—An In Vitro Study" International Journal of Molecular Sciences 23, no. 7: 3558. https://doi.org/10.3390/ijms23073558
APA StyleNecula, M. G., Mazare, A., Negrescu, A. M., Mitran, V., Ozkan, S., Trusca, R., Park, J., Schmuki, P., & Cimpean, A. (2022). Macrophage-like Cells Are Responsive to Titania Nanotube Intertube Spacing—An In Vitro Study. International Journal of Molecular Sciences, 23(7), 3558. https://doi.org/10.3390/ijms23073558








