The Beneficial Effects of Triterpenic Acid and Acteoside in an In Vitro Model of Nonalcoholic Steatohepatitis (NASH)
Abstract
:1. Introduction
2. Results
2.1. Determination of Experimental Concentration
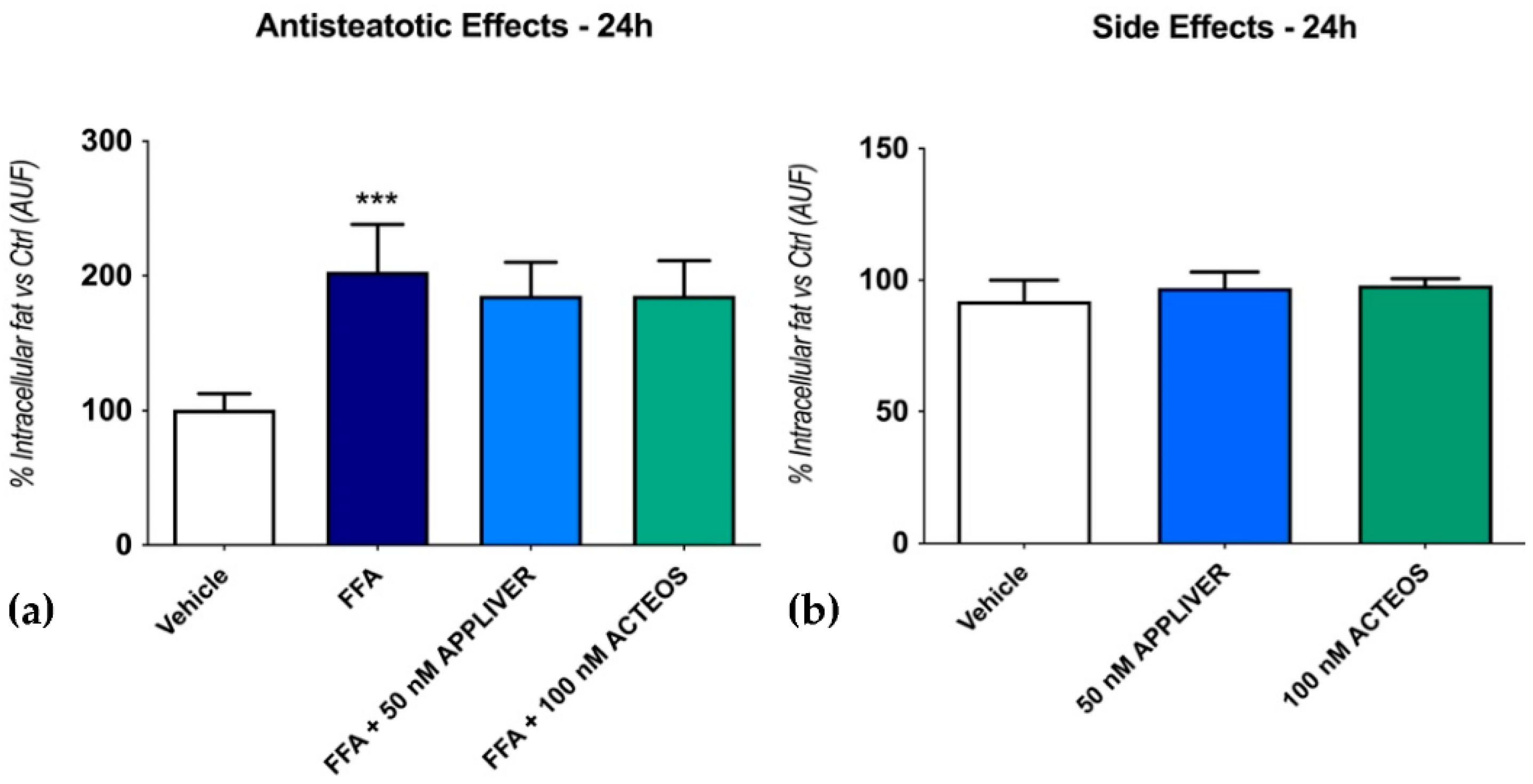
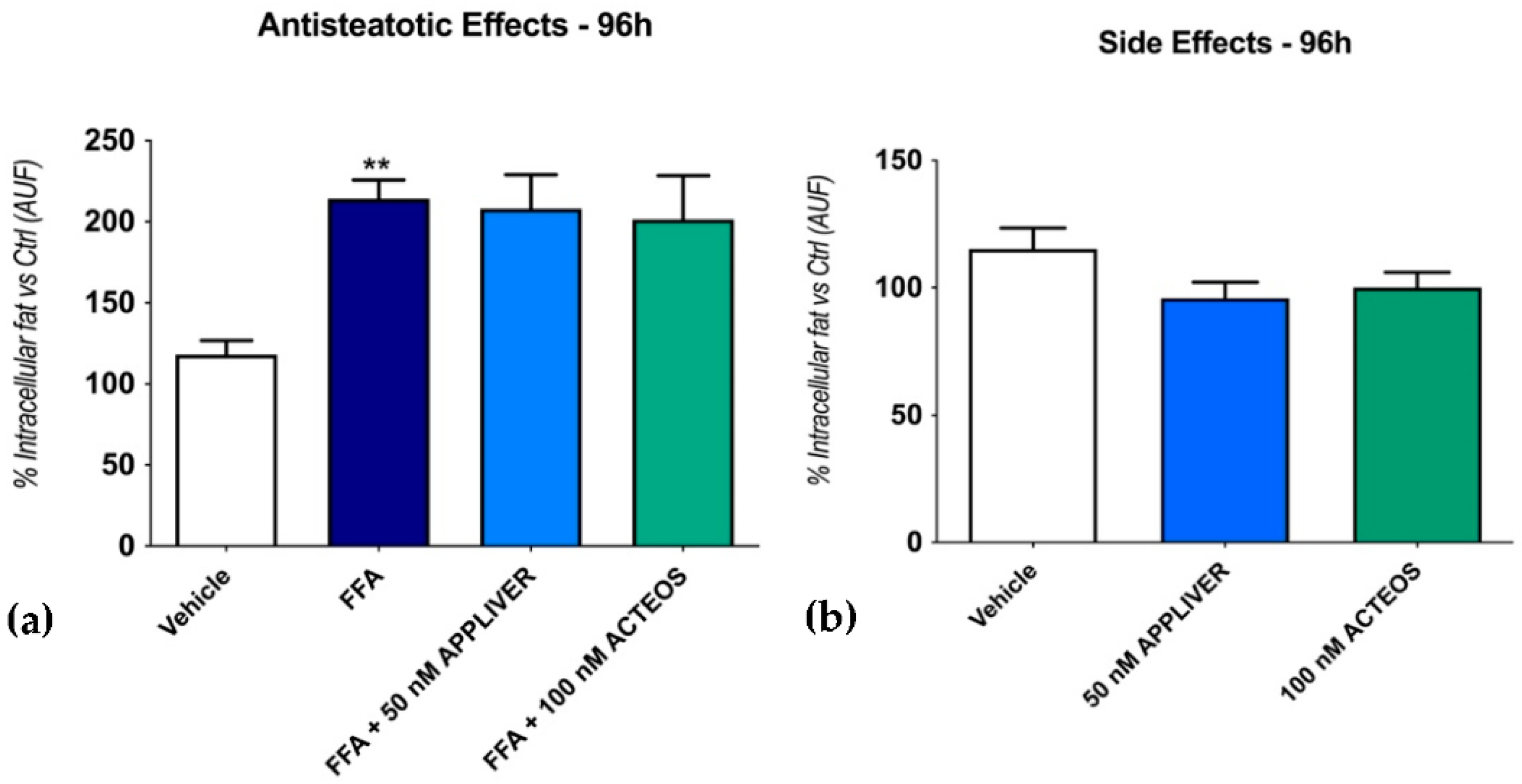
2.2. Effects of APPLIVER and ACTEOS on Fat Accumulation
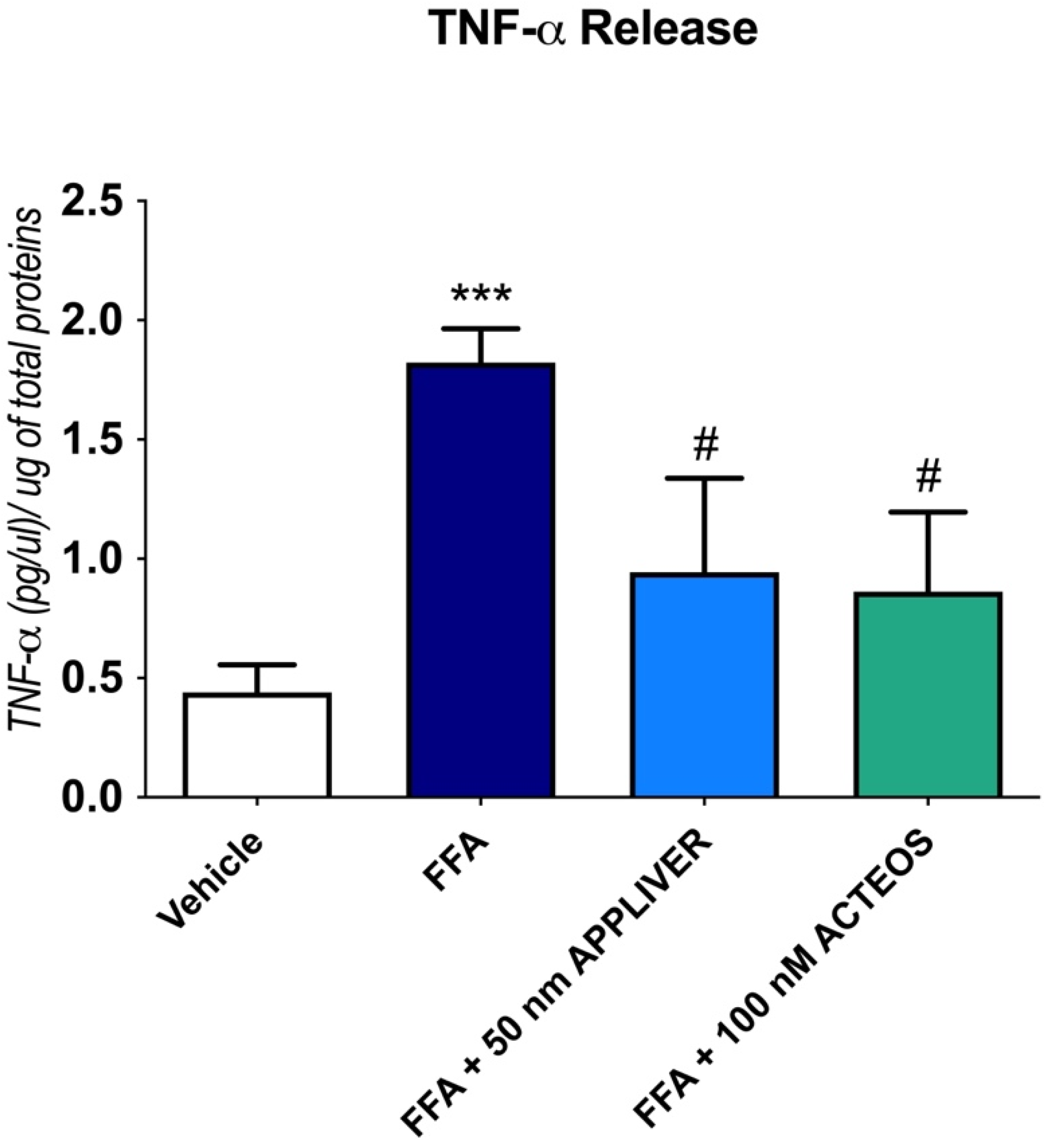
2.3. Effects of APPLIVER and ACTEOS on the Inflammatory Response
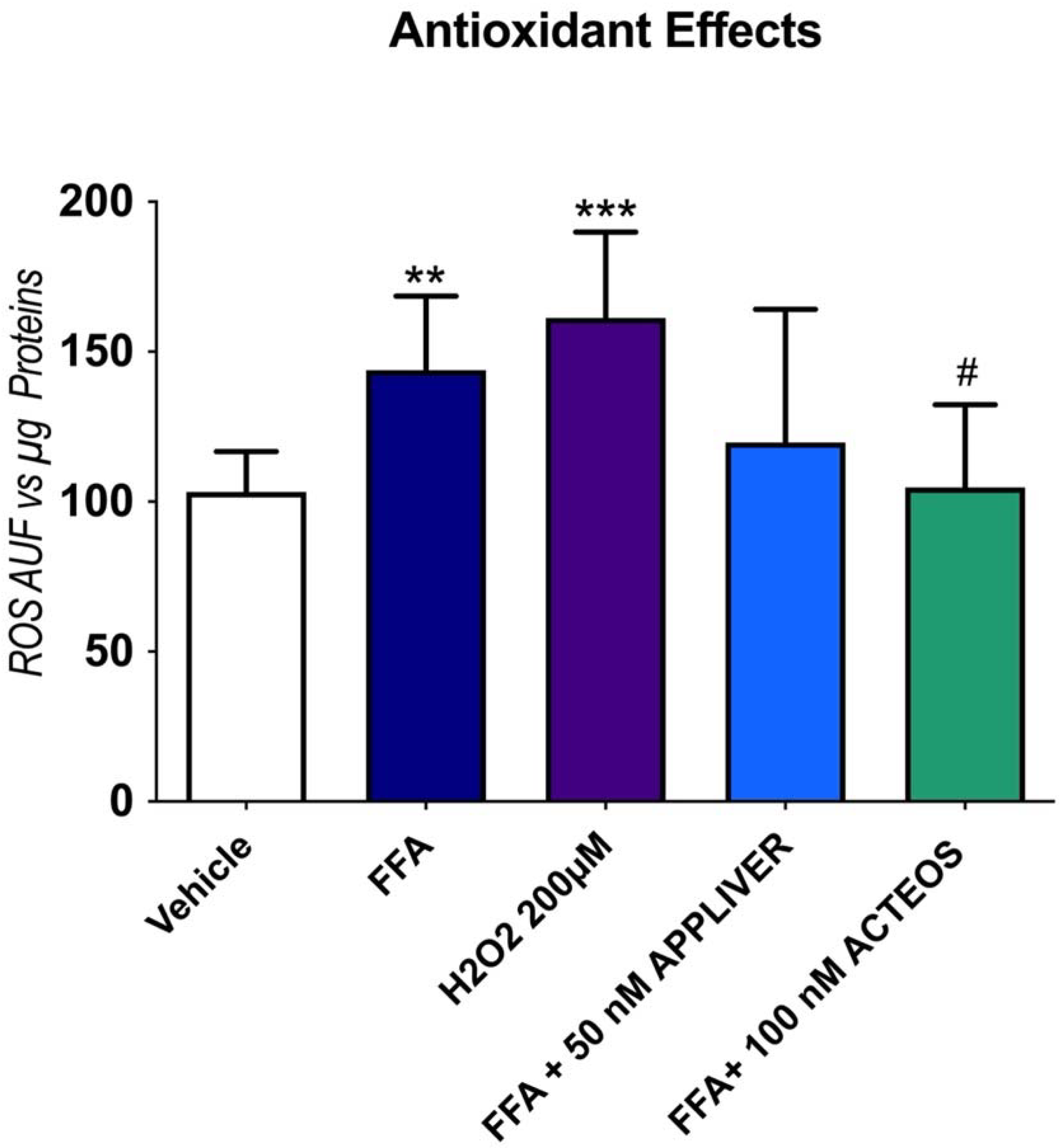
2.4. Effects of APPLIVER and ACTEOS on the Production Reactive Oxygen Species
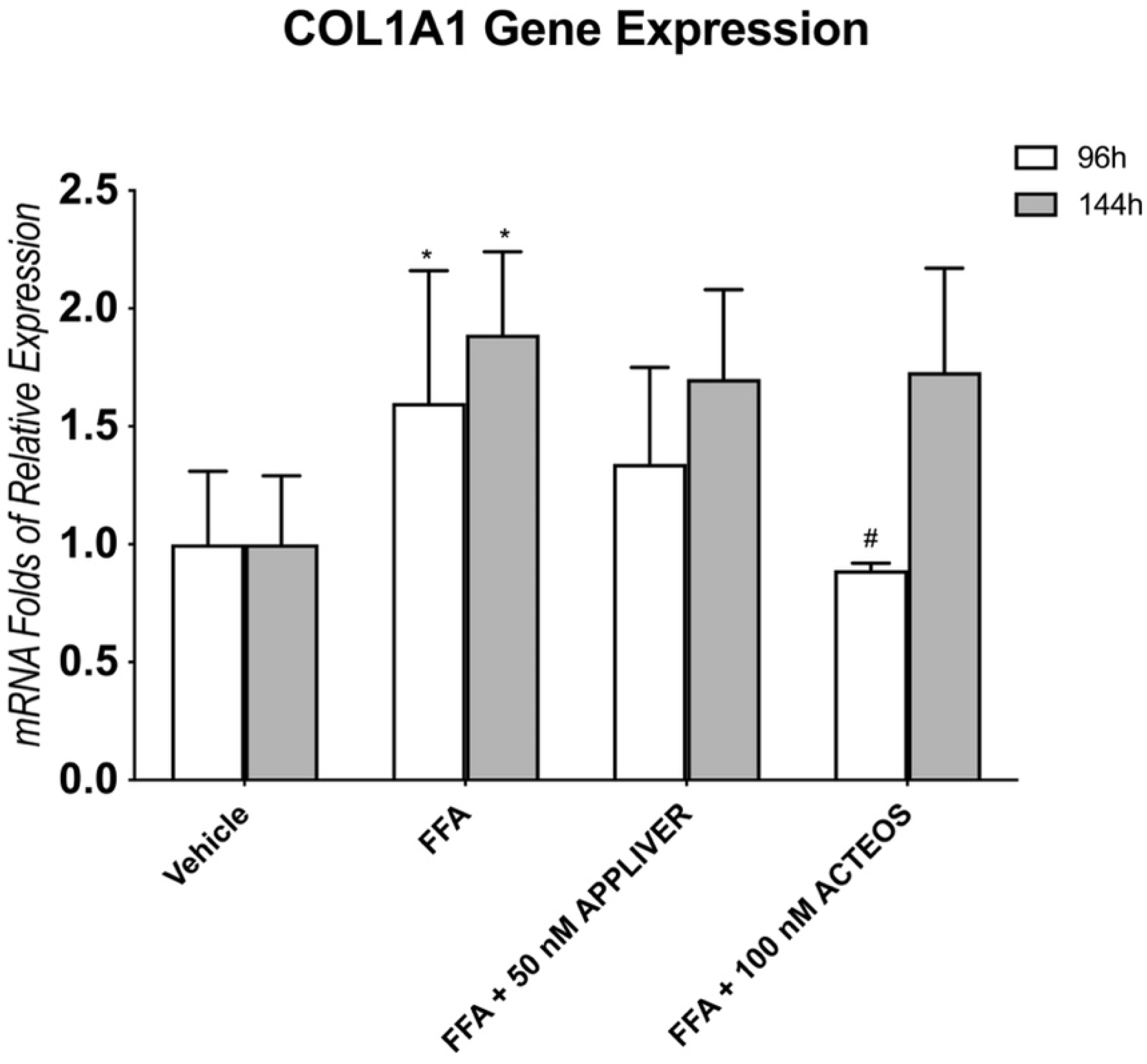
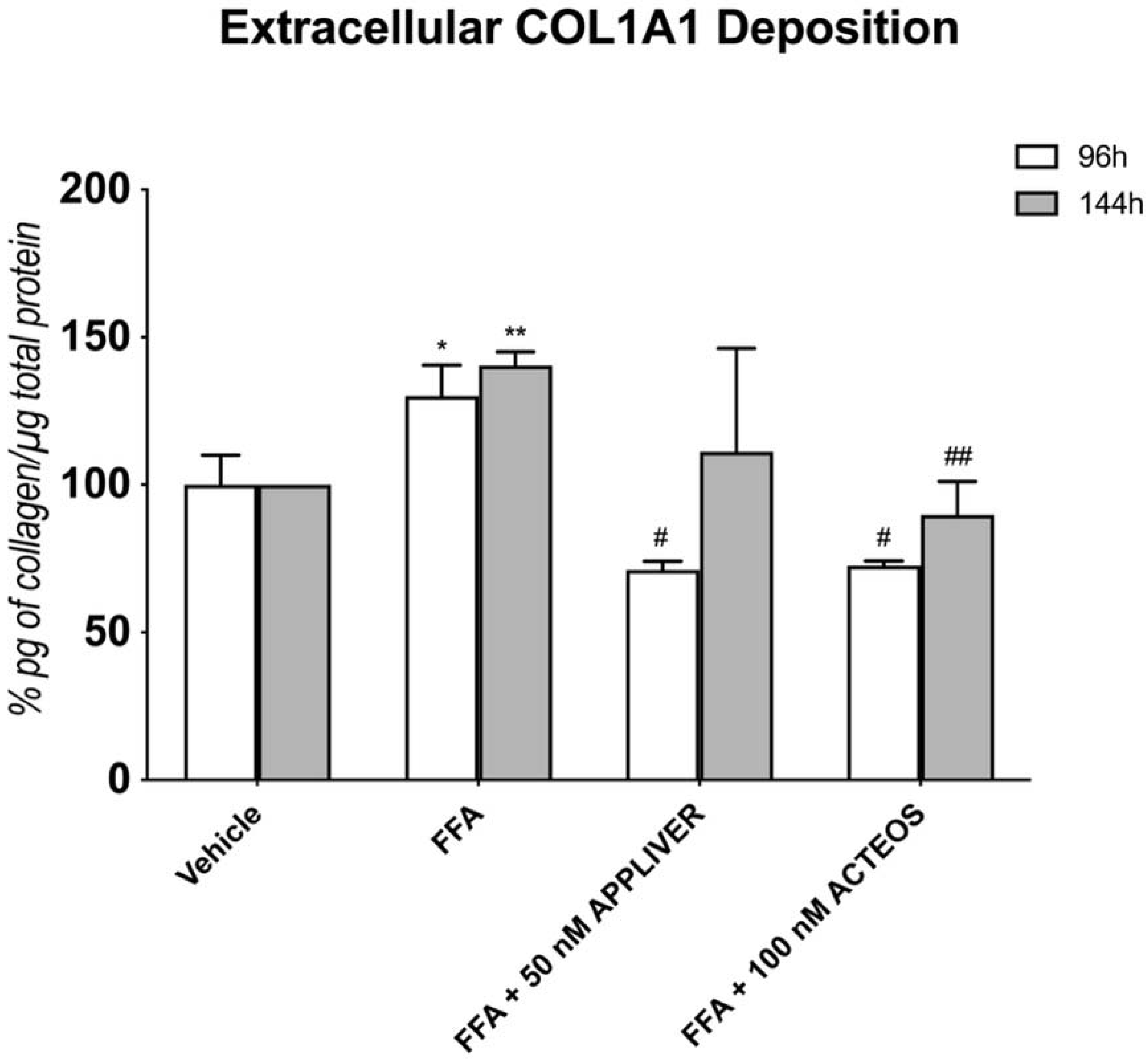
2.5. Effects of APPLIVER and ACTEOS on Collagen (COL1A1) Production of SCC
3. Discussion
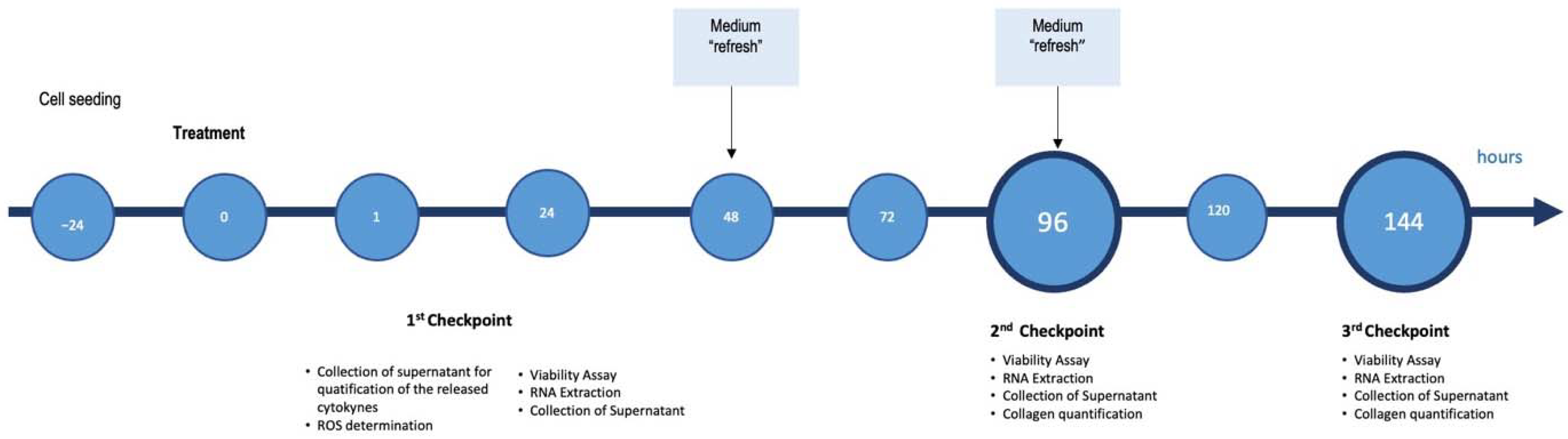
4. Materials and Methods
4.1. Compounds and Chemicals
4.2. Cell Culture
4.3. Experimental Dose Determination and Cell Viability Assay
4.4. Fluorimetric Determination of Intracellular Fat Content—Nile Red Staining
4.5. RNA Extraction, cDNA Synthesis, and Gene Expression Analysis by qRT-PCR
4.6. Intracellular ROS Generation by H2DCFDA
4.7. Quantification of COL1A1 Deposition (AlphaLISA Quantification)
4.8. Normalization vs. µg of Total Proteins
4.9. TNF-α ELISA and Bicinchoninic Acid (BCA) Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Araújo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global Epidemiology of Non-Alcoholic Fatty Liver Disease/Non-Alcoholic Steatohepatitis: What We Need in the Future. Liver Int. Off. J. Int. Assoc. Study Liver 2018, 38 (Suppl. 1), 47–51. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S.; HCC-NAFLD Italian Study Group. Clinical Patterns of Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease: A Multicenter Prospective Study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Sarin, S.K.; Wong, V.W.-S.; Fan, J.-G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.-H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver Clinical Practice Guidelines for the Diagnosis and Management of Metabolic Associated Fatty Liver Disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Rosso, N.; Chavez-Tapia, N.C.; Tiribelli, C.; Bellentani, S. Translational Approaches: From Fatty Liver to Non-Alcoholic Steatohepatitis. World J. Gastroenterol. WJG 2014, 20, 9038–9049. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis Stage Is the Strongest Predictor for Disease-Specific Mortality in NAFLD after up to 33 Years of Follow-Up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukada, S.; Parsons, C.J.; Rippe, R.A. Mechanisms of Liver Fibrosis. Clin. Chim. Acta 2006, 364, 33–60. [Google Scholar] [CrossRef]
- Giraudi, P.J.; Barbero Becerra, V.J.; Marin, V.; Chavez-Tapia, N.C.; Tiribelli, C.; Rosso, N. The Importance of the Interaction between Hepatocyte and Hepatic Stellate Cells in Fibrogenesis Induced by Fatty Accumulation. Exp. Mol. Pathol. 2015, 98, 85–92. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Olech, M.; Ziemichód, W.; Nowacka-Jechalke, N. The Occurrence and Biological Activity of Tormentic Acid—A Review. Molecules 2021, 26, 3797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-T.; Yang, L.; Jiang, J.-G. Tormentic Acid in Foods Exerts Anti-Proliferation Efficacy through Inducing Apoptosis and Cell Cycle Arrest. J. Funct. Foods 2015, 19, 575–583. [Google Scholar] [CrossRef]
- Villar, A.; Payá, M.; Hortigüela, M.D.; Cortes, D. Tormentic Acid, a New Hypoglycemic Agent from Poterium Ancistroides. Planta Med. 1986, 52, 43–45. [Google Scholar] [CrossRef]
- Wang, A.; Luo, J.; Moore, W.; Alkhalidy, H.; Wu, L.; Zhang, J.; Zhen, W.; Wang, Y.; Clegg, D.J.; Xu, B.; et al. GPR30 Regulates Diet-Induced Adiposity in Female Mice and Adipogenesis in Vitro. Sci. Rep. 2016, 6, 34302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Liu, T.; Ma, L.; Yan, M.; Zhao, Y.; Gu, Z.; Huang, Y. Protective Effect of Acteoside on Immunological Liver Injury Induced by Bacillus Calmette-Guerin plus Lipopolysaccharide. Planta Med. 2009, 75, 1463–1469. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Cho, I.J.; Kim, J.W.; Lee, M.; Ku, S.K.; Choi, J.; Lee, H. Hepatoprotective Effects of Blue Honeysuckle on CCl4-induced Acute Liver Damaged Mice. Food Sci. Nutr. 2018, 7, 322–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soret, P.-A.; Magusto, J.; Housset, C.; Gautheron, J. In Vitro and In Vivo Models of Non-Alcoholic Fatty Liver Disease: A Critical Appraisal. J. Clin. Med. 2020, 10, 36. [Google Scholar] [CrossRef]
- Anfuso, B.; Tiribelli, C.; Adorini, L.; Rosso, N. Obeticholic Acid and INT-767 Modulate Collagen Deposition in a NASH in Vitro Model. Sci. Rep. 2020, 10, 1699. [Google Scholar] [CrossRef]
- Barbero-Becerra, V.J.; Giraudi, P.J.; Chávez-Tapia, N.C.; Uribe, M.; Tiribelli, C.; Rosso, N. The Interplay between Hepatic Stellate Cells and Hepatocytes in an in Vitro Model of NASH. Toxicol. In Vitro 2015, 29, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- 14:00–17:00 ISO 10993-5:2009. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/03/64/36406.html (accessed on 7 February 2022).
- Chavez-Tapia, N.C.; Rosso, N.; Tiribelli, C. Effect of Intracellular Lipid Accumulation in a New Model of Non-Alcoholic Fatty Liver Disease. BMC Gastroenterol. 2012, 12, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, J.C.; Bland, L.A.; Oettinger, C.W.; Arduino, M.J.; McAllister, S.K.; Aguero, S.M.; Favero, M.S. Cytokine Kinetics in an in Vitro Whole Blood Model Following an Endotoxin Challenge. Lymphokine Cytokine Res. 1993, 12, 115–120. [Google Scholar] [PubMed]
- Held, F.; Hoppe, E.; Cvijovic, M.; Jirstrand, M.; Gabrielsson, J. Challenge Model of TNFα Turnover at Varying LPS and Drug Provocations. J. Pharmacokinet. Pharmacodyn. 2019, 46, 223–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Novel Recombinant Slow-Release TNF α-Derived Peptide Effectively Inhibits Tumor Growth and Angiogensis | Scientific Reports. Available online: https://www.nature.com/articles/srep13595 (accessed on 21 January 2022).
- Available online: https://abres.it/en/estratti-naturali/ (accessed on 21 January 2021).
- Domitrović, R.; Potočnjak, I. A Comprehensive Overview of Hepatoprotective Natural Compounds: Mechanism of Action and Clinical Perspectives. Arch. Toxicol. 2016, 90, 39–79. [Google Scholar] [CrossRef]
- Jiang, W.-P.; Huang, S.-S.; Matsuda, Y.; Saito, H.; Uramaru, N.; Ho, H.-Y.; Wu, J.-B.; Huang, G.-J. Protective Effects of Tormentic Acid, a Major Component of Suspension Cultures of Eriobotrya Japonica Cells, on Acetaminophen-Induced Hepatotoxicity in Mice. Molecules 2017, 22, 830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monica, R.L.; Marco, B.; Nicodemo, G.P.; Antoine, S.; Francesco, M.; Rosa, T. Antiproliferative Activities on Renal, Prostate and Melanoma Cancer Cell Lines of Sarcopoterium Spinosum Aerial Parts and Its Major Constituent Tormentic Acid. Anticancer Agents Med. Chem. 2013, 13, 768–776. [Google Scholar]
- Ma, A.; Wang, Y.; Zhang, Q. Tormentic Acid Reduces Inflammation in BV-2 Microglia by Activating the Liver X Receptor Alpha. Neuroscience 2015, 287, 9–14. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, S.; Huang, R.; Tan, S.; Liang, S.; Wu, X.; Zhuo, L.; Huang, Q. Protective Effect of Tormentic Acid from Potentilla Chinensis against Lipopolysaccharide/d-Galactosamine Induced Fulminant Hepatic Failure in Mice. Int. Immunopharmacol. 2014, 19, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A Review of Its Occurrence, (Bio)Synthesis and Pharmacological Significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Attia, Y.M.; El-Kersh, D.M.; Wagdy, H.A.; Elmazar, M.M. Verbascoside: Identification, Quantification, and Potential Sensitization of Colorectal Cancer Cells to 5-FU by Targeting PI3K/AKT Pathway. Sci. Rep. 2018, 8, 16939. [Google Scholar] [CrossRef] [Green Version]
- Burgos, C.; Muñoz-Mingarro, D.; Navarro, I.; Martín-Cordero, C.; Acero, N. Neuroprotective Potential of Verbascoside Isolated from Acanthus mollis L. Leaves through Its Enzymatic Inhibition and Free Radical Scavenging Ability. Antioxidants 2020, 9, 1207. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Adams, L.A.; de Lédinghen, V.; Wong, G.L.-H.; Sookoian, S. Noninvasive Biomarkers in NAFLD and NASH—Current Progress and Future Promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression From NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Anfuso, B.; Giraudi, P.J.; Tiribelli, C.; Rosso, N. Silybin Modulates Collagen Turnover in an In Vitro Model of NASH. Molecules 2019, 24, 1280. [Google Scholar] [CrossRef] [Green Version]
- Kakino, S.; Ohki, T.; Nakayama, H.; Yuan, X.; Otabe, S.; Hashinaga, T.; Wada, N.; Kurita, Y.; Tanaka, K.; Hara, K.; et al. Pivotal Role of TNF-α in the Development and Progression of Nonalcoholic Fatty Liver Disease in a Murine Model. Horm. Metab. Res. 2018, 50, 80–87. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Delli Bovi, A.P.; Marciano, F.; Mandato, C.; Siano, M.A.; Savoia, M.; Vajro, P. Oxidative Stress in Non-Alcoholic Fatty Liver Disease. An Updated Mini Review. Front. Med. 2021, 8, 165. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Sun, G.-Y.; Zhang, Y.; He, J.-J.; Zheng, S.; Lin, J.-N. Tormentic Acid Inhibits H2O2-Induced Oxidative Stress and Inflammation in Rat Vascular Smooth Muscle Cells via Inhibition of the NF-ΚB Signaling Pathway. Mol. Med. Rep. 2016, 14, 3559–3564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-H.; Chen, C.-H.; Hsieh, P.-F.; Lee, Y.-H.; Kuo, W.W.-T.; Wu, R.C.-Y.; Hung, C.-H.; Yang, Y.-L.; Lin, V.C. Verbascoside Inhibits the Epithelial-Mesenchymal Transition of Prostate Cancer Cells through High-Mobility Group Box 1/Receptor for Advanced Glycation End-Products/TGF-β Pathway. Environ. Toxicol. 2021, 36, 1080–1089. [Google Scholar] [CrossRef]
- Stefanovic, B.; Hellerbrand, C.; Holcik, M.; Briendl, M.; Aliebhaber, S.; Brenner, D.A. Posttranscriptional Regulation of Collagen Alpha1(I) MRNA in Hepatic Stellate Cells. Mol. Cell. Biol. 1997, 17, 5201–5209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Li, Y.; Zhang, X.; Wei, Y.; Wen, S.; Lu, Z.; Huang, Q.; Wei, J. Tormentic Acid Inhibits Hepatic Stellate Cells Activation via Blocking PI3K/Akt/MTOR and NF-ΚB Signalling Pathways. Cell Biochem. Funct. 2021, 39, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hui, A.Y.; Albanis, E.; Arthur, M.J.; O’Byrne, S.M.; Blaner, W.S.; Mukherjee, P.; Friedman, S.L.; Eng, F.J. Human Hepatic Stellate Cell Lines, LX-1 and LX-2: New Tools for Analysis of Hepatic Fibrosis. Gut 2005, 54, 142–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taimr, P.; Higuchi, H.; Kocova, E.; Rippe, R.A.; Friedman, S.; Gores, G.J. Activated Stellate Cells Express the TRAIL Receptor-2/Death Receptor-5 and Undergo TRAIL-Mediated Apoptosis. Hepatology 2003, 37, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Balis, U.J.; Yarmush, M.L.; Toner, M. Effect of Cell–Cell Interactions in Preservation of Cellular Phenotype: Cocultivation of Hepatocytes and Nonparenchymal Cells. FASEB J. 1999, 13, 1883–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Treatment | Huh7 Monoculture | Huh7—LX2 Simultaneous Co-Culture | ||
|---|---|---|---|---|
| 24 h | 96 h | 144 h | ||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| +FFA | Vehicle | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| FFA | 99 ± 11 | 86 ± 7 | 91 ± 5 | |
| APPLIVER 10 nM | 89 ± 18 | 83 ± 2 | 97 ± 5 | |
| APPLIVER 50 nM | 94 ± 22 | 69 ± 28 * | 111 ± 8 | |
| ACTEOS 100 nM | 98 ± 6 | 81 ± 9 | 90 ± 3 | |
| ACTEOS 10,000 nM | 96 ± 11 | 75 ± 7 * | 88 ± 1 | |
| −FFA | Vehicle | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| APPLIVER 10 nM | 99 ± 17 | 99 ± 3 | 118 ± 37 | |
| APPLIVER 50 nM | 90 ± 16 | 82 ± 1 | 105 ± 21 | |
| ACTEOS 100 nM | 93 ± 12 | 82 ± 3 | 96 ± 20 | |
| ACTEOS 10,000 nM | 82 ± 7 | 69 ± 7 * | 71 ± 6 * | |
| Gene Name | Accession Number | Forward | Reverse |
|---|---|---|---|
| IL-8 | NM_000584 | GACATACTCCAAACCTTTCCAC | CTTCTCCACAACCCTCTGC |
| IL-6 | NM_000600 | ACAGATTTGAGAGTAGTGAGGAAC | GGCTGGCATTTGTGGTTGG |
| TNF-α | NM_000594 | GTGAGGAGGACGAACATC | GAGCCAGAAGAGGTTGAG |
| COL1A1 | NM_000088 | CGGAGGAGAGTCAGGAAG | ACACAAGGAACAGAACAGTC |
| 18S | NR_003286.2 | TAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
| HPRT | NM_000194. | ACATCTGGAGTCCTATTGACATCG | CCGCCCAAAGGGAACTGATAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvoza, N.; Bedin, C.; Saccani, A.; Tiribelli, C.; Rosso, N. The Beneficial Effects of Triterpenic Acid and Acteoside in an In Vitro Model of Nonalcoholic Steatohepatitis (NASH). Int. J. Mol. Sci. 2022, 23, 3562. https://doi.org/10.3390/ijms23073562
Salvoza N, Bedin C, Saccani A, Tiribelli C, Rosso N. The Beneficial Effects of Triterpenic Acid and Acteoside in an In Vitro Model of Nonalcoholic Steatohepatitis (NASH). International Journal of Molecular Sciences. 2022; 23(7):3562. https://doi.org/10.3390/ijms23073562
Chicago/Turabian StyleSalvoza, Noel, Chiara Bedin, Andrea Saccani, Claudio Tiribelli, and Natalia Rosso. 2022. "The Beneficial Effects of Triterpenic Acid and Acteoside in an In Vitro Model of Nonalcoholic Steatohepatitis (NASH)" International Journal of Molecular Sciences 23, no. 7: 3562. https://doi.org/10.3390/ijms23073562
APA StyleSalvoza, N., Bedin, C., Saccani, A., Tiribelli, C., & Rosso, N. (2022). The Beneficial Effects of Triterpenic Acid and Acteoside in an In Vitro Model of Nonalcoholic Steatohepatitis (NASH). International Journal of Molecular Sciences, 23(7), 3562. https://doi.org/10.3390/ijms23073562







