Roles of Oxidative Stress in Acute Tendon Injury and Degenerative Tendinopathy—A Target for Intervention
Abstract
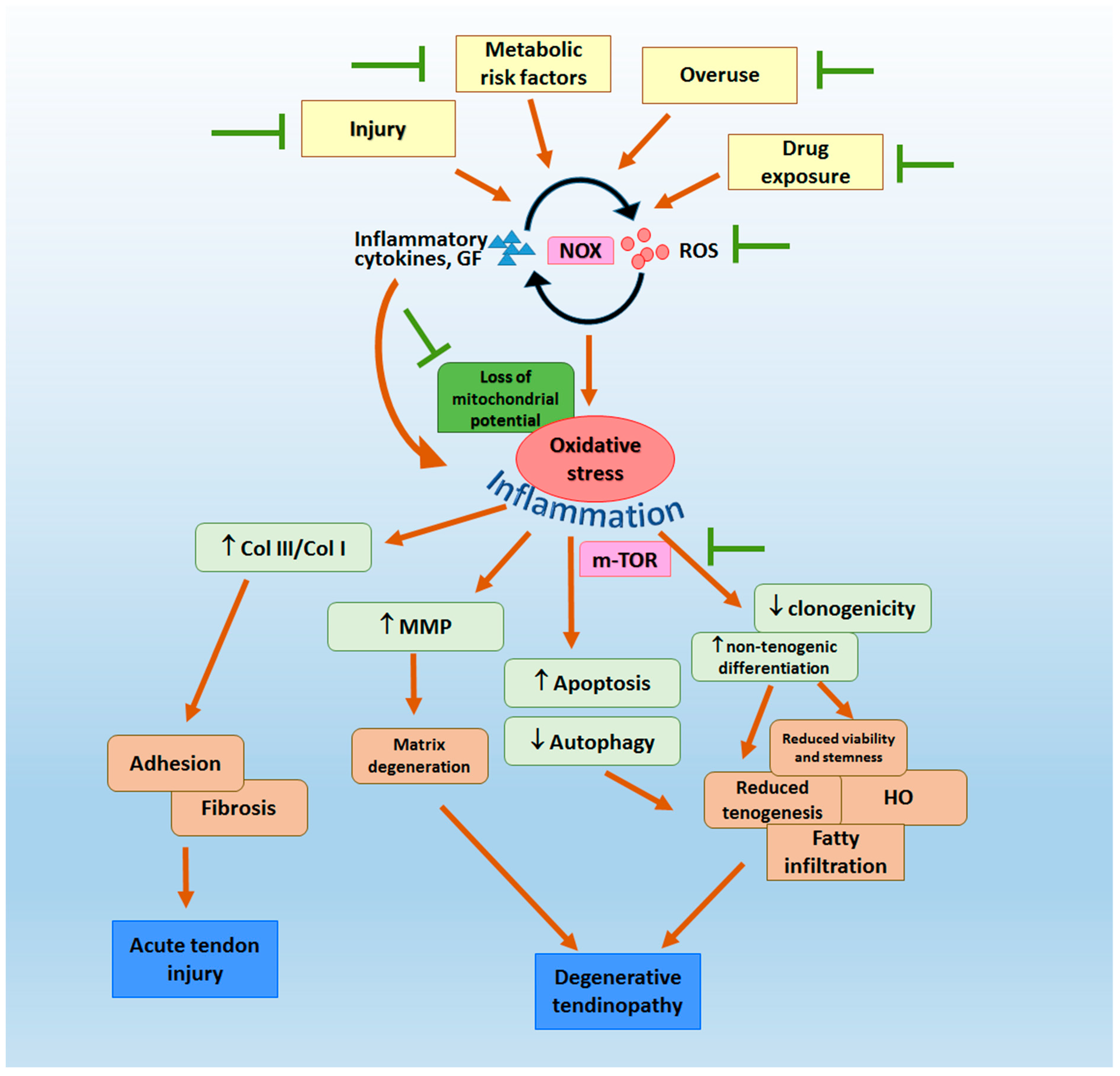
:1. Tendon Fibrosis, Adhesion and Degenerative Tendinopathy—Unresolved Sports Medicine Problems
2. Oxidative Stress in the Pathogenesis of Tendon Fibrosis, Adhesion and Degenerative Tendinopathy
3. Clinical and Pre-Clinical Evidence of Potential Sources and Mechanisms of Oxidative Stress in Tendon
3.1. Clinical Evidence
3.2. Pre-Clinical Evidence
3.2.1. Tendon Injury
3.2.2. Overuse
3.2.3. mTOR Pathway
3.2.4. Metabolic Risk Factors
3.2.5. Drug Exposure
4. Anti-Oxidative Therapies for the Promotion of Tendon Repair
4.1. Clinical Trials
4.2. Pre-Clinical Studies
4.2.1. mTOR Pathway Inhibitors
4.2.2. Hyaluronic Acid
4.2.3. Natural Antioxidants from Plants
4.2.4. Ascorbic Acid/Vitamin C
4.2.5. Vitamin D
4.2.6. Vitamin E
4.2.7. Protection of Mitochondria
4.2.8. Others
5. Future Research Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaux, J.F.; Forthomme, B.; Le Goff, C.; Crielaard, J.M.; Croisier, J.L. Current opinions on tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253. [Google Scholar] [PubMed]
- Engebretson, B.; Mussett, Z.; Williams, C.; Simmons, A.; Sikavitsas, V. Chapter 12—Tendon tissue engineering: Combined tissue engineering approach for the regeneration of tendons. In Tendon Regeneration; Gomes, M.E., Reis, R.L., Rodrigues, M.T., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 321–347. [Google Scholar]
- Davies, M.R.; Lee, L.; Feeley, B.T.; Kim, H.T.; Liu, X. Lysophosphatidic acid-induced RhoA signaling and prolonged macrophage infiltration worsens fibrosis and fatty infiltration following rotator cuff tears. J. Orthop. Res. 2017, 35, 1539–1547. [Google Scholar] [PubMed] [Green Version]
- de la Durantaye, M.; Piette, A.B.; van Rooijen, N.; Frenette, J. Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons. J. Orthop. Res. 2014, 32, 279–285. [Google Scholar] [PubMed]
- Cui, H.; He, Y.; Chen, S.; Zhang, D.; Yu, Y.; Fan, C. Macrophage-derived miRNA-containing exosomes induce peritendinous fibrosis after tendon injury through the miR-21-5p/Smad7 pathway. Mol. Ther. Nucleic. Acids. 2019, 14, 114–130. [Google Scholar] [PubMed] [Green Version]
- Zhang, J.; Wang, L.; Chu, J.; Ao, X.; Jiang, T.; Yan, B.; Huang, M.; Zhang, Z. Macrophage-derived neurotrophin-3 promotes heterotopic ossification in rats. Lab. Investig. 2020, 100, 762–776. [Google Scholar] [PubMed]
- Hu, J.-J.; Yin, Z.; Shen, W.-L.; Xie, Y.-B.; Zhu, T.; Lu, P.; Cai, Y.-Z.; Kong, M.-J.; Heng, B.C.; Zhou, Y.-T.; et al. Pharmacological regulation of in situ tissue stem cells differentiation for soft tissue calcification treatment. Stem Cells 2016, 34, 1083–1096. [Google Scholar]
- Brandt, L.; Schubert, S.; Scheibe, P.; Brehm, W.; Franzen, J.; Gross, C.; Burk, J. Tenogenic properties of mesenchymal progenitor cells are compromised in an inflammatory environment. Int. J. Mol. Sci. 2018, 19, 2549. [Google Scholar]
- Asai, S.; Otsuru, S.; Candela, M.E.; Cantley, L.; Uchibe, K.; Hofmann, T.J.; Zhang, K.; Wapner, K.L.; Soslowsky, L.J.; Horwitz, E.M.; et al. Tendon progenitor cells in injured tendons have strong chondrogenic potential: The CD105-negative subpopulation induces chondrogenic degeneration. Stem Cells 2014, 32, 3266–3277. [Google Scholar]
- Longo, U.G.; Olivia, F.; Denaro, V.; Maffulli, N. Oxygen species and overuse tendinopathy in athletes. Disabil. Rehabil. 2008, 30, 1563–1571. [Google Scholar] [PubMed]
- Wang, M.X.; Wei, A.; Yuan, J.; Clippe, A.; Bernard, A.; Knoops, B.; Murrell, G.A. Antioxidant enzyme peroxiredoxin 5 is upregulated in degenerative human tendon. Biochem. Biophys. Res. Commun. 2001, 284, 667–673. [Google Scholar]
- Yuan, J.; Murrell, G.A.; Trickett, A.; Landtmeters, M.; Knoops, B.; Wang, M.X. Overexpression of antioxidant enzyme peroxiredoxin 5 protects human tendon cells against apoptosis and loss of cellular function during oxidative stress. Biochim. Biophys. Acta 2004, 1693, 37–45. [Google Scholar] [PubMed] [Green Version]
- Itoigawa, Y.; Yoshida, K.; Nojiri, H.; Morikawa, D.; Kawasaki, T.; Wada, T.; Koga, A.; Maruyama, Y.; Ishijima, M. Association of recurrent tear after arthroscopic rotator cuff repair and superoxide-induced oxidative stress. Am. J. Sports Med. 2021, 49, 2048–2055. [Google Scholar] [PubMed]
- Yoshida, K.; Itoigawa, Y.; Wada, T.; Maruyama, Y.; Nojiri, H.; Kawasaki, T.; Kaneko, K. Association of superoxide-induced oxidative stress with rotator cuff tears in human patients. J. Orthop. Res. 2020, 38, 212–218. [Google Scholar] [PubMed]
- Noh, K.C.; Park, S.H.; Yang, C.J.; Lee, G.W.; Kim, M.K.; Kang, Y.H. Involvement of synovial matrix degradation and angiogenesis in oxidative stress-exposed degenerative rotator cuff tears with osteoarthritis. J. Shoulder Elb. Surg. 2018, 27, 141–150. [Google Scholar]
- Yuan, T.; Qian, H.; Yu, X.; Meng, J.; Lai, C.T.; Jiang, H.; Zhao, J.N.; Bao, N.R. Proteomic analysis reveals rotator cuff injury caused by oxidative stress. Ther. Adv. Chronic Dis. 2021, 12, 2040622320987057. [Google Scholar] [PubMed]
- Abate, M.; Di Carlo, L.; Cocco, G.; Cocco, A.; Sabatini, E.; Salini, V. Oxidative stress and abnormal tendon sonographic features in elite soccer players (a pilot study). Rev. Bras. Ortop. 2021, 56, 432–437. [Google Scholar]
- Meier, B.; Radeke, H.H.; Selle, S.; Younes, M.; Sies, H.; Resch, K.; Habermehl, G.G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-α. Biochem. J. 1989, 263, 539–545. [Google Scholar] [PubMed]
- Ribitsch, I.; Bileck, A.; Aldoshin, A.; Kańduła, M.; Mayer, R.; Egerbacher, M.; Gabner, S.; Auer, U.; Gültekin, S.; Huber, J.; et al. Molecular mechanisms of fetal tendon regeneration versus adult fibrous repair. Int. J. Mol. Sci. 2021, 22, 5619. [Google Scholar]
- Li, X.; Su, Z.; Shen, K.; Wang, Q.; Xu, C.; Wang, F.; Zhang, Y.; Jiang, D. Eugenol-preconditioned mesenchymal stem cell-derived extracellular vesicles promote antioxidant capacity of tendon stem cells in vitro and in vivo. Oxidative Med. Cell. Longev. 2022, 2022, 3945195. [Google Scholar]
- Morikawa, D.; Itoigawa, Y.; Nojiri, H.; Sano, H.; Itoi, E.; Saijo, Y.; Kaneko, K.; Shimizu, T. Contribution of oxidative stress to the degeneration of rotator cuff entheses. J. Shoulder Elb. Surg. 2014, 23, 628–635. [Google Scholar]
- Semis, H.S.; Gur, C.; Ileriturk, M.; Kandemir, F.M.; Kaynar, O. Evaluation of therapeutic effects of quercetin against Achilles tendinopathy in rats via oxidative stress, inflammation, apoptosis, autophagy, and metalloproteinases. Am. J. Sports Med. 2022, 50, 486–498. [Google Scholar]
- Liu, Y.-C.; Wang, H.-L.; Huang, Y.-Z.; Weng, Y.-H.; Chen, R.-S.; Tsai, W.-C.; Yeh, T.-H.; Lu, C.-S.; Chen, Y.-L.; Lin, Y.-W.; et al. Alda-1, an activator of ALDH2, ameliorates Achilles tendinopathy in cellular and mouse models. Biochem. Pharmacol. 2020, 175, 113919. [Google Scholar]
- Yuan, J.; Murrell, G.A.C.; Trickett, A.; Wang, M.-X. Involvement of cytochrome c release and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochim. Biophys. Acta-Mol. Cell Res. 2003, 1641, 35–41. [Google Scholar]
- Kim, R.J.; An, S.H.; Gwark, J.Y.; Park, H.B. Antioxidant effects on hypoxia-induced oxidative stress and apoptosis in rat rotator cuff fibroblasts. Eur. Cells Mater. 2021, 41, 680–693. [Google Scholar]
- Cheung, T.S.; Lau, P.M.; Lu, H.; Ho, H.P.; Lui, P.P.Y.; Kong, S.K. Cytotoxic and sublethal effects of silver nanoparticles on tendon-derived stem cells—implications for tendon engineering. Toxicol. Res. 2016, 5, 318–330. [Google Scholar]
- Wang, F.; Murrell, G.A.; Wang, M.X. Oxidative stress-induced c-Jun N-terminal kinase (JNK) activation in tendon cells upregulates MMP1 mRNA and protein expression. J. Orthop. Res. 2007, 25, 378–389. [Google Scholar]
- Sun, Y.; Chen, H.; Ye, H.; Liang, W.; Lam, K.K.; Cheng, B.; Lu, Y.; Jiang, C. Nudt21-mediated alternative polyadenylation of HMGA2 3′-UTR impairs stemness of human tendon stem cell. Aging 2020, 12, 18436–18452. [Google Scholar] [PubMed]
- Lee, Y.W.; Fu, S.C.; Yeung, M.Y.; Lau, C.M.L.; Chan, K.M.; Hung, L.K. Effects of redox modulation on cell proliferation, viability, and migration in cultured rat and human tendon progenitor cells. Oxidative Med. Cell Longev. 2017, 2017, 8785042. [Google Scholar]
- Chen, H.; Ge, H.A.; Wu, G.B.; Cheng, B.; Lu, Y.; Jiang, C. Autophagy prevents oxidative stress-induced loss of self-renewal capacity and stemness in human tendon stem cells by reducing ROS accumulation. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 39, 2227–2238. [Google Scholar]
- Fu, S.C.; Yeung, M.Y.; Rolf, C.G.; Yung, P.S.; Chan, K.M.; Hung, L.K. Hydrogen peroxide induced tendinopathic changes in a rat model of patellar tendon injury. J. Orthop. Res. 2018, 36, 3268–3274. [Google Scholar] [PubMed] [Green Version]
- Meng, J.; Yu, P.; Tong, J.; Sun, W.; Jiang, H.; Wang, Y.; Xue, K.; Xie, F.; Qian, H.; Liu, N.; et al. Hydrogen treatment reduces tendon adhesion and inflammatory response. J. Cell. Biochem. 2018, 120, 1610–1619. [Google Scholar]
- Li, P.; Zhou, H.; Tu, T.; Lu, H. Dynamic exacerbation in inflammation and oxidative stress during the formation of peritendinous adhesion resulted from acute tendon injury. J. Orthop. Surg. Res. 2021, 16, 293. [Google Scholar]
- Hung, L.K.; Fu, S.C.; Lee, Y.W.; Mok, T.Y.; Chan, K.M. Local vitamin-C injection reduced tendon adhesion in a chicken model of flexor digitorum profundus tendon injury. J. Bone Jt. Surg. 2013, 95, e41. [Google Scholar]
- Eliasson, P.; Andersson, T.; Aspenberg, P. Influence of a single loading episode on gene expression in healing rat Achilles tendons. J. Appl. Physiol. 2012, 112, 279–288. [Google Scholar] [PubMed]
- Kocadal, O.; Pepe, M.; Akyurek, N.; Gunes, Z.; Surer, H.; Aksahin, E.; Ogut, B.; Aktekin, C.N. The evaluation of exogenous melatonin administration in supraspinatus overuse tendinopathy in an experimental rat model. Clin. Shoulder Elb. 2019, 22, 79–86. [Google Scholar] [PubMed] [Green Version]
- Wunderli, S.L.; Blache, U.; Piccoli, A.B.; Niederöst, B.; Holenstein, C.N.; Passini, F.S.; Silván, U.; Bundgaard, L.; Keller, U.A.D.; Snedeker, J.G. Tendon response to matrix unloading is determined by the patho-physiological niche. Matrix Biol. 2020, 89, 11–26. [Google Scholar]
- Zapp, C.; Obarska-Kosinska, A.; Rennekamp, B.; Kurth, M.; Hudson, D.M.; Mercadante, D.; Barayeu, U.; Dick, T.P.; Denysenkov, V.; Prisner, T.; et al. Mechanoradicals in tensed tendon collagen as a source of oxidative stress. Nat. Commun. 2020, 11, 2315. [Google Scholar]
- Banerjee, P.; Mehta, A.; Shanthi, C. Investigation into the cyto-protective and wound healing properties of cryptic peptides from bovine Achilles tendon collagen. Chem.-Biol. Interact. 2014, 211, 1–10. [Google Scholar] [PubMed]
- Cong, X.X.; Rao, X.S.; Lin, J.X.; Liu, X.C.; Zhang, G.A.; Gao, X.K.; He, M.Y.; Shen, W.L.; Fan, W.; Pioletti, D.; et al. Activation of AKT-mTOR signaling directs tenogenesis of mesenchymal stem cells. Stem Cells 2018, 36, 527–539. [Google Scholar]
- Rui, Y.F.; Lui, P.P.; Rolf, C.G.; Wong, Y.M.; Lee, Y.W.; Chan, K.M. Expression of chondro-osteogenic BMPs in clinical samples of patellar tendinopathy. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2012, 20, 1409–1417. [Google Scholar]
- Lui, P.P.; Lee, Y.W.; Wong, Y.M.; Zhang, X.; Dai, K.; Rolf, C.G. Expression of Wnt pathway mediators in metaplasic tissue in animal model and clinical samples of tendinopathy. Rheumatology 2013, 52, 1609–1618. [Google Scholar] [PubMed] [Green Version]
- Lui, P.P.; Chan, L.S.; Fu, S.C.; Chan, K.M. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am. J. Sports Med. 2010, 38, 757–764. [Google Scholar] [PubMed]
- Hashimoto, T.; Nobuhara, K.; Hamada, T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin. Orthop. Relat. Res. 2003, 415, 111–120. [Google Scholar]
- Lui, P.P.; Chan, L.S.; Cheuk, Y.C.; Lee, Y.W.; Chan, K.M. Expression of bone morphogenetic protein-2 in the chondrogenic and ossifying sites of calcific tendinopathy and traumatic tendon injury rat models. J. Orthop. Surg. Res. 2009, 4, 27. [Google Scholar] [PubMed] [Green Version]
- Rui, Y.F.; Lui, P.P.; Wong, Y.M.; Tan, Q.; Chan, K.M. Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem Cells Dev. 2013, 22, 1076–1085. [Google Scholar] [PubMed] [Green Version]
- Ichinose, T.; Yamamoto, A.; Kobayashi, T.; Shitara, H.; Shimoyama, D.; Iizuka, H.; Koibuchi, N.; Takagishi, K. Compensatory hypertrophy of the teres minor muscle after large rotator cuff tear model in adult male rat. J. Shoulder Elb. Surg. 2016, 25, 316–321. [Google Scholar]
- Joshi, S.K.; Liu, X.; Samagh, S.P.; Lovett, D.H.; Bodine, S.C.; Kim, H.T.; Feeley, B.T. mTOR regulates fatty infiltration through SREBP-1 and PPARgamma after a combined massive rotator cuff tear and suprascapular nerve injury in rats. J. Orthop. Res. 2013, 31, 724–730. [Google Scholar]
- Liu, X.; Joshi, S.K.; Samagh, S.P.; Dang, Y.X.; Laron, D.; Lovett, D.H.; Bodine, S.C.; Kim, H.T.; Feeley, B.T. Evaluation of Akt/mTOR activity in muscle atrophy after rotator cuff tears in a rat model. J. Orthop. Res. 2012, 30, 1440–1446. [Google Scholar] [PubMed]
- Nie, D.; Zhou, Y.; Wang, W.; Zhang, J.; Wang, J.H. Mechanical overloading induced-activation of mTOR signaling in tendon stem/progenitor cells contributes to tendinopathy development. Front. Cell Dev. Biol. 2021, 9, 687856. [Google Scholar] [PubMed]
- Chen, G.; Jiang, H.; Tian, X.; Tang, J.; Bai, X.; Zhang, Z.; Wang, L. Mechanical loading modulates heterotopic ossification in calcific tendinopathy through the mTORC1 signaling pathway. Mol. Med. Rep. 2017, 16, 5901–5907. [Google Scholar]
- Xu, K.; Zhang, Z.; Chen, M.; Moqbel, S.A.A.; He, Y.; Ma, C.; Jiang, L.; Xiong, Y.; Wu, L. Nesfatin-1 promotes the osteogenic differentiation of tendon-derived stem cells and the pathogenesis of heterotopic ossification inrat tendons via the mTOR Pathway. Front. Cell Dev. Biol. 2020, 8, 547342. [Google Scholar] [PubMed]
- Chen, Y.; Shen, W.; Tang, C.; Huang, J.; Fan, C.; Yin, Z.; Hu, Y.; Chen, W.; Ouyang, H.; Zhou, Y.; et al. Targeted pathological collagen delivery of sustained-release rapamycin to prevent heterotopic ossification. Sci. Adv. 2020, 6, 9526. [Google Scholar]
- Hu, Y.; Wang, Z. Rapamycin prevents heterotopic ossification by inhibiting the mTOR pathway and oxidative stress. Biochem. Biophys. Res. Commun. 2021, 573, 171–178. [Google Scholar] [PubMed]
- Skovgaard, D.; Siersma, V.D.; Klausen, S.B.; Visnes, H.; Haukenes, I.; Bang, C.W.; Bager, P.; Silbernagel, K.G.; Gaida, J.; Magnusson, S.P.; et al. Chronic hyperglycemia, hypercholesterolemia, and metabolic syndrome are associated with risk of tendon injury. Scand. J. Med. Sci. Sports 2021, 31, 1822–1831. [Google Scholar]
- Ahn, H.S.; Kim, H.J.; Kang, T.U.; Kazmi, S.Z.; Suh, J.S.; Choi, J.Y. Dyslipidemia Is associated with increased risk of Achilles tendon disorders in underweight individuals to a greater extent than obese individuals: A nationwide, population-based, longitudinal cohort study. Orthop. J. Sports Med. 2021, 9, 23259671211042599. [Google Scholar]
- Macchi, M.; Spezia, M.; Elli, S.; Schiaffini, G.; Chisari, E. Obesity increases the risk of tendinopathy, tendon tear and rupture, and postoperative complications: A systematic review of clinical studies. Clin. Orthop. Relat. Res. 2020, 478, 1839–1847. [Google Scholar]
- Lui, P.P.Y. Tendinopathy in diabetes mellitus patients-Epidemiology, pathogenesis, and management. Scand. J. Med. Sci. Sports 2017, 27, 776–787. [Google Scholar]
- Lui, P.P.Y.; Yung, P.S.H. Inflammatory mechanisms linking obesity and tendinopathy. J. Orthop. Transl. 2021, 31, 80–90. [Google Scholar]
- Dodson, M.; Redmann, M.; Rajasekaran, N.S.; Darley-Usmar, V.; Zhang, J. KEAP1-NRF2 signalling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem. J. 2015, 469, 347–355. [Google Scholar]
- Jiang, H.; Chen, Y.; Chen, G.; Tian, X.; Tang, J.; Luo, L.; Huang, M.; Yan, B.; Ao, X.; Zhou, W.; et al. Leptin accelerates the pathogenesis of heterotopic ossification in rat tendon tissues via mTORC1 signaling. J. Cell. Physiol. 2018, 233, 1017–1028. [Google Scholar]
- Poulsen, R.C.; Knowles, H.J.; Carr, A.J.; Hulley, P.A. Cell differentiation versus cell death: Extracellular glucose is a key determinant of cell fate following oxidative stress exposure. Cell Death Dis. 2014, 5, e1074. [Google Scholar] [PubMed] [Green Version]
- Tsai, W.C.; Liang, F.C.; Cheng, J.W.; Lin, L.P.; Chang, S.C.; Chen, H.H.; Pang, J.H. High glucose concentration up-regulates the expression of matrix metalloproteinase-9 and -13 in tendon cells. BMC Musculoskelet. Disord. 2013, 14, 255. [Google Scholar]
- Ueda, Y.; Inui, A.; Mifune, Y.; Sakata, R.; Muto, T.; Harada, Y.; Takase, F.; Kataoka, T.; Kokubu, T.; Kuroda, R. The effects of high glucose condition on rat tenocytes in vitro and rat Achilles tendon in vivo. Bone Jt. Res. 2018, 7, 362–372. [Google Scholar]
- Mukohara, S.; Mifune, Y.; Inui, A.; Nishimoto, H.; Kurosawa, T.; Yamaura, K.; Yoshikawa, T.; Kuroda, R. In vitro and in vivo tenocyte-protective effectiveness of dehydroepiandrosterone against high glucose-induced oxidative stress. BMC Musculoskelet. Disord. 2021, 22, 519. [Google Scholar]
- Babior, B.M. The activity of leukocyte NADPH oxidase: Regulation by p47PHOX cysteine and serine residues. Antioxid. Redox Signal. 2002, 4, 35–38. [Google Scholar]
- Elgawish, A.; Glomb, M.; Friedlander, M.; Monnier, V.M. Involvement of hydrogen peroxide in collagen cross-linking by high glucose in vitro and in vivo. J. Biol. Chem. 1996, 271, 12964–12971. [Google Scholar]
- Sajithlal, G.B.; Chithra, P.; Chandrakasan, G. The role of metal-catalyzed oxidation in the formation of advanced glycation end products: An in vitro study on collagen. Free Radic. Biol. Med. 1998, 25, 265–269. [Google Scholar]
- Sajithlal, G.B.; Chithra, P.; Chandrakasan, G. An in vitro study on the role of metal catalyzed oxidation in glycation and crosslinking of collagen. Mol. Cell. Biochem. 1999, 194, 257–263. [Google Scholar]
- Li, K.; Deng, Y.; Deng, G.; Chen, P.; Wang, Y.; Wu, H.; Ji, Z.; Yao, Z.; Zhang, X.; Yu, B.; et al. High cholesterol induces apoptosis and autophagy through the ROS-activated AKT/FOXO1 pathway in tendon-derived stem cells. Stem Cell Res. Ther. 2020, 11, 131. [Google Scholar]
- Li, K.; Deng, G.; Deng, Y.; Chen, S.; Wu, H.; Cheng, C.; Zhang, X.; Yu, B.; Zhang, K. High cholesterol inhibits tendon-related gene expressions in tendon-derived stem cells through reactive oxygen species-activated nuclear factor-kappaB signaling. J. Cell. Physiol. 2019, 234, 18017–18028. [Google Scholar]
- Alfredson, H.; Thorsen, K.; Lorentzon, R. In situ microdialysis in tendon tissue: High levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 1999, 7, 378–381. [Google Scholar]
- Alfredson, H.; Ljung, B.O.; Thorsen, K.; Lorentzon, R. In vivo investigation of ECRB tendons with microdialysis technique--no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop. Scand. 2000, 71, 475–479. [Google Scholar] [PubMed] [Green Version]
- Alfredson, H.; Forsgren, S.; Thorsen, K.; Lorentzon, R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. J. Orthop. Res. 2001, 19, 881–886. [Google Scholar] [PubMed]
- Kim, R.J.; Hah, Y.S.; Gwark, J.Y.; Park, H.B. N-acetylcysteine reduces glutamate-induced cytotoxicity to fibroblasts of rat supraspinatus tendons. Connect. Tissue Res. 2019, 60, 431–443. [Google Scholar]
- Lowes, D.A.; Wallace, C.; Murphy, M.P.; Webster, N.R.; Galley, H.F. The mitochondria targeted antioxidant MitoQ protects against fluoroquinolone-induced oxidative stress and mitochondrial membrane damage in human Achilles tendon cells. Free Radic. Res. 2009, 43, 323–328. [Google Scholar]
- Pouzaud, F.; Bernard-Beaubois, K.; Thevenin, M.; Warnet, J.M.; Hayem, G.; Rat, P. In vitro discrimination of fluoroquinolones toxicity on tendon cells: Involvement of oxidative stress. J. Pharmacol. Exp. Ther. 2004, 308, 394–402. [Google Scholar]
- Pouzaud, F.; Dutot, M.; Martin, C.; Debray, M.; Warnet, J.M.; Rat, P. Age-dependent effects on redox status, oxidative stress, mitochondrial activity and toxicity induced by fluoroquinolones on primary cultures of rabbit tendon cells. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 232–241. [Google Scholar]
- Simonin, M.A.; Gegout-Pottie, P.; Minn, A.; Gillet, P.; Netter, P.; Terlain, B. Pefloxacin-induced achilles tendon toxicity in rodents: Biochemical changes in proteoglycan synthesis and oxidative damage to collagen. Antimicrob. Agents Chemother. 2000, 44, 867–872. [Google Scholar]
- Lehner, C.; Gehwolf, R.; Hirzinger, C.; Stephan, D.; Augat, P.; Tauber, M.; Resch, H.; Bauer, H.C.; Bauer, H.; Tempfer, H. Bupivacaine induces short-term alterations and impairment in rat tendons. Am. J. Sports Med. 2013, 41, 1411–1418. [Google Scholar]
- Fu, S.; He, Z.; Tang, Y.; Lan, B. Effects and Mechanism of Berberine on the Dexamethasone-induced Injury of human tendon cells. Evid.-Based Complement. Altern. Med. 2020, 2020, 8832218. [Google Scholar]
- Min, K.; Lee, J.M.; Kim, M.J.; Jung, S.Y.; Kim, K.S.; Lee, S.; Choi, Y.S. Restoration of cellular proliferation and characteristics of human tenocytes by vitamin D. J. Orthop. Res. 2019, 37, 2241–2248. [Google Scholar] [PubMed]
- Gumina, S.; Passaretti, D.; Gurzì, M.; Candela, V. Arginine L-alpha-ketoglutarate, methylsulfonylmethane, hydrolyzed type I collagen and bromelain in rotator cuff tear repair: A prospective randomized study. Curr. Med. Res. Opin. 2012, 28, 1767–1774. [Google Scholar] [PubMed]
- Notarnicola, A.; Pesce, V.; Vicenti, G.; Tafuri, S.; Forcignanò, M.; Moretti, B. SWAAT study: Extracorporeal shock wave therapy and arginine supplementation and other nutraceuticals for insertional Achilles tendinopathy. Adv. Ther. 2012, 29, 799–814. [Google Scholar] [PubMed]
- Mavrogenis, S.; Johannessen, E.; Jensen, P.; Sindberg, C. The effect of essential fatty acids and antioxidants combined with physiotherapy treatment in recreational athletes with chronic tendon disorders. Phys. Ther. Sport 2004, 5, 194–199. [Google Scholar]
- Martel, M.; Laumonerie, P.; Girard, M.; Dauzere, F.; Mansat, P.; Bonnevialle, N. Does vitamin C supplementation improve rotator cuff healing? A preliminary study. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 63–70. [Google Scholar]
- Fillipin, L.I.; Mauriz, J.L.; Vedovelli, K.; Moreira, A.J.; Zettler, C.G.; Lech, O.; Marroni, N.P.; González-Gallego, J. Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg. Med. 2005, 37, 293–300. [Google Scholar] [PubMed]
- Sielski, Ł.; Sutkowy, P.; Katarzyna, P.O.; Woźniak, A.; Skopkowska, A.; Woźniak, B.; Czuczejko, J. The impact of high-intensity laser therapy on oxidative stress, lysosomal enzymes, and protease inhibitor in athletes. Chin. J. Physiol. 2019, 62, 273–278. [Google Scholar]
- Tripodi, N.; Feehan, J.; Husaric, M.; Sidiroglou, F.; Apostolopoulos, V. The effect of low-level red and near-infrared photobiomodulation on pain and function in tendinopathy: A systematic review and meta-analysis of randomized control trials. BMC Sports Sci. Med. Rehabil. 2021, 13, 91. [Google Scholar]
- Yao, Z.; Li, J.; Xiong, H.; Cui, H.; Ning, J.; Wang, S.; Ouyang, X.; Qian, Y.; Fan, C. MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J. Nanobiotechnol. 2021, 19, 169. [Google Scholar]
- Vassallo, V.; Stellavato, A.; Cimini, D.; Pirozzi, A.V.A.; Alfano, A.; Cammarota, M.; Balato, G.; D’Addona, A.; Ruosi, C.; Schiraldi, C. Unsulfated biotechnological chondroitin by itself as well as in combination with high molecular weight hyaluronan improves the inflammation profile in osteoarthritis in vitro model. J. Cell. Biochem. 2021, 122, 1021–1036. [Google Scholar]
- Yoshida, M.; Funasaki, H.; Kubota, M.; Marumo, K. Therapeutic effects of high molecular weight hyaluronan injections for tendinopathy in a rat model. J. Orthop. Sci. 2015, 20, 186–195. [Google Scholar] [PubMed]
- Frizziero, A.; Salamanna, F.; Giavaresi, G.; Ferrari, A.; Martini, L.; Marini, M.; Veicsteinas, A.; Maffulli, N.; Masiero, S.; Fini, M.; et al. Hyaluronic acid injections protect patellar tendon from detraining-associated damage. Histol. Histopathol. 2015, 30, 1079–1088. [Google Scholar] [PubMed]
- Honda, H.; Gotoh, M.; Kanazawa, T.; Ohzono, H.; Nakamura, H.; Ohta, K.; Nakamura, K.-I.; Fukuda, K.; Teramura, T.; Hashimoto, T.; et al. Hyaluronic acid accelerates tendon-to-bone healing after rotator cuff repair. Am. J. Sports Med. 2017, 45, 3322–3330. [Google Scholar]
- Li, H.; Chen, Y.; Chen, S. Enhancement of rotator cuff tendon-bone healing using bone marrow-stimulating technique along with hyaluronic acid. J. Orthop. Transl. 2019, 17, 96–102. [Google Scholar]
- Miki, Y.; Teramura, T.; Tomiyama, T.; Onodera, Y.; Matsuoka, T.; Fukuda, K.; Hamanishi, C. Hyaluronan reversed proteoglycan synthesis inhibited by mechanical stress: Possible involvement of antioxidant effect. Inflamm. Res. 2010, 59, 471–477. [Google Scholar] [PubMed]
- Gallorini, M.; Berardi, A.C.; Gissi, C.; Cataldi, A.; Osti, L. Nrf2-mediated cytoprotective effect of four different hyaluronic acids by molecular weight in human tenocytes. J. Drug Target. 2020, 28, 212–224. [Google Scholar] [PubMed]
- Oliva, F.; Gallorini, M.; Antonetti Lamorgese Passeri, C.; Gissi, C.; Ricci, A.; Cataldi, A.; Colosimo, A.; Berardi, A.C. Conjugation with methylsulfonylmethane improves hyaluronic acid anti-inflammatory activity in a hydrogen peroxide-exposed tenocyte culture in vitro model. Int. J. Mol. Sci. 2020, 21, 7956. [Google Scholar]
- Liang, Y.; Xu, K.; Zhang, P.; Zhang, J.; Chen, P.; He, J.; Fang, Y.; Zhou, Y.; Wang, J.; Bai, J. Quercetin reduces tendon adhesion in rat through suppression of oxidative stress. BMC Musculoskelet. Disord. 2020, 21, 608. [Google Scholar]
- Sajithlal, G.B.; Chithra, P.; Chandrakasan, G. Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochem. Pharmacol. 1998, 56, 1607–1614. [Google Scholar]
- Zhang, J.; Xiao, C.; Zhang, X.; Lin, Y.; Yang, H.; Zhang, Y.S.; Ding, J. An oxidative stress-responsive electrospun polyester membrane capable of releasing anti-bacterial and anti-inflammatory agents for postoperative anti-adhesion. J. Control. Release 2021, 335, 359–368. [Google Scholar]
- Sun, W.; Meng, J.; Wang, Z.; Yuan, T.; Qian, H.; Chen, W.; Tong, J.; Xie, Y.; Zhang, Y.; Zhao, J.; et al. Proanthocyanidins Attenuation of H2O2-induced oxidative damage in tendon-derived stem cells via upregulating Nrf-2 signaling pathway. BioMed Res. Int. 2017, 2017, 7529104. [Google Scholar] [PubMed] [Green Version]
- Nam, D.C.; Hah, Y.S.; Nam, J.B.; Kim, R.J.; Park, H.B. Cytoprotective mechanism of cyanidin and delphinidin against oxidative stress-induced tenofibroblast death. Biomol. Ther. 2016, 24, 426–432. [Google Scholar]
- Park, H.B.; Hah, Y.S.; Yang, J.W.; Nam, J.B.; Cho, S.H.; Jeong, S.T. Antiapoptotic effects of anthocyanins on rotator cuff tenofibroblasts. J. Orthop. Res. 2010, 28, 1162–1169. [Google Scholar] [PubMed]
- Kim, R.J.; Hah, Y.S.; Sung, C.M.; Kang, J.R.; Park, H.B. Do antioxidants inhibit oxidative-stress-induced autophagy of tenofibroblasts? J. Orthop. Res. 2014, 32, 937–943. [Google Scholar]
- Kurosawa, T.; Mifune, Y.; Inui, A.; Nishimoto, H.; Ueda, Y.; Kataoka, T.; Yamaura, K.; Mukohara, S.; Kuroda, R. Evaluation of apocynin in vitro on high glucose-induced oxidative stress on tenocytes. Bone Jt. Res. 2020, 9, 23–28. [Google Scholar]
- Hsiao, M.Y.; Lin, A.C.; Liao, W.H.; Wang, T.G.; Hsu, C.H.; Chen, W.S.; Lin, F.H. Drug-loaded hyaluronic acid hydrogel as a sustained-release regimen with dual effects in early intervention of tendinopathy. Sci. Rep. 2019, 9, 4784. [Google Scholar]
- Hsiao, M.-Y.; Lin, P.-C.; Liao, W.-H.; Chen, W.-S.; Hsu, C.-H.; He, C.-K.; Wu, Y.-W.; Gefen, A.; Iafisco, M.; Liu, L.; et al. The Effect of the Repression of Oxidative Stress on Tenocyte Differentiation: A preliminary study of a rat cell model using a novel differential tensile strain bioreactor. Int. J. Mol. Sci. 2019, 20, 3437. [Google Scholar]
- Perez Gutierrez, R.M.; de Jesus Martinez Ortiz, M. Beneficial effect of Azadirachta indica on advanced glycation end-product in streptozotocin-diabetic rat. Pharm. Biol. 2014, 52, 1435–1444. [Google Scholar]
- Jagtap, A.G.; Patil, P.B. Antihyperglycemic activity and inhibition of advanced glycation end product formation by Cuminum cyminum in streptozotocin induced diabetic rats. Food Chem. Toxicol. 2010, 48, 2030–2036. [Google Scholar]
- Morikawa, D.; Nojiri, H.; Itoigawa, Y.; Ozawa, Y.; Kaneko, K.; Shimizu, T. Antioxidant treatment with vitamin C attenuated rotator cuff degeneration caused by oxidative stress in Sod1-deficient mice. JSES Open Access 2018, 2, 91–96. [Google Scholar]
- Chiu, C.H.; Chen, P.; Chen, A.C.; Chan, Y.S.; Hsu, K.Y.; Rei, H.; Lei, K.F. Real-Time Monitoring of ascorbic acid-mediated reduction of cytotoxic effects of analgesics and NSAIDs on tenocytes proliferation. Dose-Response 2019, 17, 1559325819832143. [Google Scholar] [PubMed]
- Lee, Y.W.; Fu, S.C.; Mok, T.Y.; Chan, K.M.; Hung, L.K. Local administration of Trolox, a vitamin E analog, reduced tendon adhesion in a chicken model of flexor digitorum profundus tendon injury. J. Orthop. Transl. 2017, 10, 102–107. [Google Scholar]
- Zhang, X.; Wada, S.; Zhang, Y.; Chen, D.; Deng, X.H.; Rodeo, S.A. Assessment of mitochondrial dysfunction in a murine model of supraspinatus tendinopathy. J. Bone Jt. Surg. 2021, 103, 174–183. [Google Scholar]
- Lee, J.M.; Hwang, J.W.; Kim, M.J.; Jung, S.Y.; Kim, K.S.; Ahn, E.H.; Min, K.; Choi, Y.S. Mitochondrial transplantation modulates inflammation and apoptosis, alleviating tendinopathy both in vivo and in vitro. Antioxidants 2021, 10, 696. [Google Scholar] [PubMed]
- Yao, Z.; Qian, Y.; Jin, Y.; Wang, S.; Li, J.; Yuan, W.E.; Fan, C. Biomimetic multilayer polycaprolactone/sodium alginate hydrogel scaffolds loaded with melatonin facilitate tendon regeneration. Carbohydr. Polym. 2022, 277, 118865. [Google Scholar]
- Japjec, M.; Pavlov, K.H.; Petrovic, A.; Staresinic, M.; Sebecic, B.; Buljan, M.; Vranes, H.; Giljanovic, A.; Drmic, D.; Japjec, M.; et al. Stable gastric pentadecapeptide BPC 157 as a therapy for the disable myotendinous junctions in rats. Biomedicines 2021, 9, 1547. [Google Scholar]
- Appetecchia, F.; Consalvi, S.; Berrino, E.; Gallorini, M.; Granese, A.; Campestre, C.; Carradori, S.; Biava, M.; Poce, G. A novel class of dual-acting DCH-CORMs counteracts oxidative stress-induced inflammation in human primary tenocytes. Antioxidants 2021, 10, 1828. [Google Scholar]
- Gallorini, M.; Berardi, A.C.; Ricci, A.; Antonetti Lamorgese Passeri, C.; Zara, S.; Oliva, F.; Cataldi, A.; Carta, F.; Carradori, S. Dual acting carbon monoxide releasing molecules and carbonic anhydrase inhibitors differentially modulate inflammation in human tenocytes. Biomedicines 2021, 9, 141. [Google Scholar]
- Pouzaud, F.; Christen, M.O.; Warnet, J.M.; Rat, P. Anethole dithiolethione: An antioxidant agent against tenotoxicity induced by fluoroquinolones. Pathol. Biol. 2004, 52, 308–313. [Google Scholar]
- Tohidnezhad, M.; Varoga, D.; Wruck, C.J.; Brandenburg, L.O.; Seekamp, A.; Shakibaei, M.; Sönmez, T.T.; Pufe, T.; Lippross, S. Platelet-released growth factors can accelerate tenocyte proliferation and activate the anti-oxidant response element. Histochem. Cell Biol. 2011, 135, 453–460. [Google Scholar]
- Muto, T.; Kokubu, T.; Mifune, Y.; Inui, A.; Sakata, R.; Harada, Y.; Takase, F.; Kurosaka, M. Effects of platelet-rich plasma and triamcinolone acetonide on interleukin-1ß-stimulated human rotator cuff-derived cells. Bone Jt. Res. 2016, 5, 602–609. [Google Scholar]
- Hudgens, J.L.; Sugg, K.B.; Grekin, J.A.; Gumucio, J.P.; Bedi, A.; Mendias, C.L. Platelet-Rich Plasma Activates proinflammatory signaling pathways and induces oxidative stress in tendon fibroblasts. Am. J. Sports Med. 2016, 44, 1931–1940. [Google Scholar] [PubMed] [Green Version]
- Zhu, Y.; Xie, A.; Li, M.; Zhang, C.; Ni, T. Noninvasive photochemical sealing for Achilles tendon rupture by combination of upconversion nanoparticles and photochemical tissue bonding technology. BioMed Res. Int. 2020, 2020, 1753152. [Google Scholar] [PubMed]
- Yao, Z.; Wang, X.; Zhang, W.; Liu, Y.; Ni, T. Photochemical tissue bonding promotes the proliferation and migration of injured tenocytes through ROS/RhoA/NF-kappaB/Dynamin 2 signaling pathway. J. Cell Physiol. 2018, 233, 7047–7056. [Google Scholar] [PubMed]
- Sayampanathan, A.A.; Basha, M.; Mitra, A.K. Risk factors of lateral epicondylitis: A meta-analysis. Surgeon 2020, 18, 122–128. [Google Scholar]
- Zabrzyński, J.; Szukalski, J.; Paczesny, L.; Szwedowski, D.; Grzanka, D. Cigarette smoking intensifies tendinopathy of the LHBT. A microscopic study after arthroscopic treatment. Pol. J. Pathol. 2019, 70, 134–138. [Google Scholar]
- Golbidi, S.; Edvinsson, L.; Laher, I. Smoking and endothelial dysfunction. Curr. Vasc. Pharmacol. 2020, 18, 1–11. [Google Scholar]
- Malińska, D.; Wieckowski, M.; Michalska, B.; Drabik, K.; Prill, M.; Patalas-Krawczyk, P.; Walczak, J.; Szymański, J.; Mathis, C.; van der Toorn, M.; et al. Mitochondria as a possible target for nicotine action. J. Bioenerg. Biomembr. 2019, 51, 259–276. [Google Scholar]
- Hatta, T.; Sano, H.; Sakamoto, N.; Kishimoto, K.N.; Sato, M.; Itoi, E. Nicotine reduced MMP-9 expression in the primary porcine tenocytes exposed to cyclic stretch. J. Orthop. Res. 2013, 31, 645–650. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lui, P.P.Y.; Zhang, X.; Yao, S.; Sun, H.; Huang, C. Roles of Oxidative Stress in Acute Tendon Injury and Degenerative Tendinopathy—A Target for Intervention. Int. J. Mol. Sci. 2022, 23, 3571. https://doi.org/10.3390/ijms23073571
Lui PPY, Zhang X, Yao S, Sun H, Huang C. Roles of Oxidative Stress in Acute Tendon Injury and Degenerative Tendinopathy—A Target for Intervention. International Journal of Molecular Sciences. 2022; 23(7):3571. https://doi.org/10.3390/ijms23073571
Chicago/Turabian StyleLui, Pauline Po Yee, Xing Zhang, Shiyi Yao, Haonan Sun, and Caihao Huang. 2022. "Roles of Oxidative Stress in Acute Tendon Injury and Degenerative Tendinopathy—A Target for Intervention" International Journal of Molecular Sciences 23, no. 7: 3571. https://doi.org/10.3390/ijms23073571
APA StyleLui, P. P. Y., Zhang, X., Yao, S., Sun, H., & Huang, C. (2022). Roles of Oxidative Stress in Acute Tendon Injury and Degenerative Tendinopathy—A Target for Intervention. International Journal of Molecular Sciences, 23(7), 3571. https://doi.org/10.3390/ijms23073571






