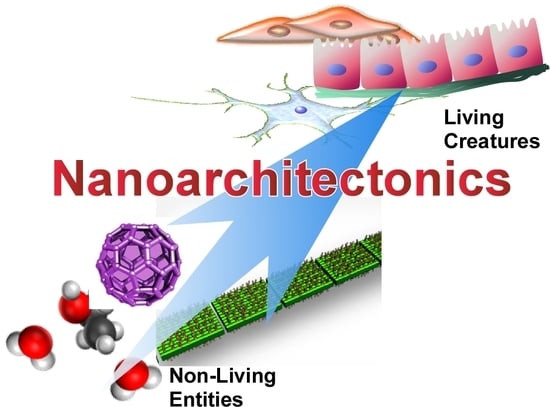
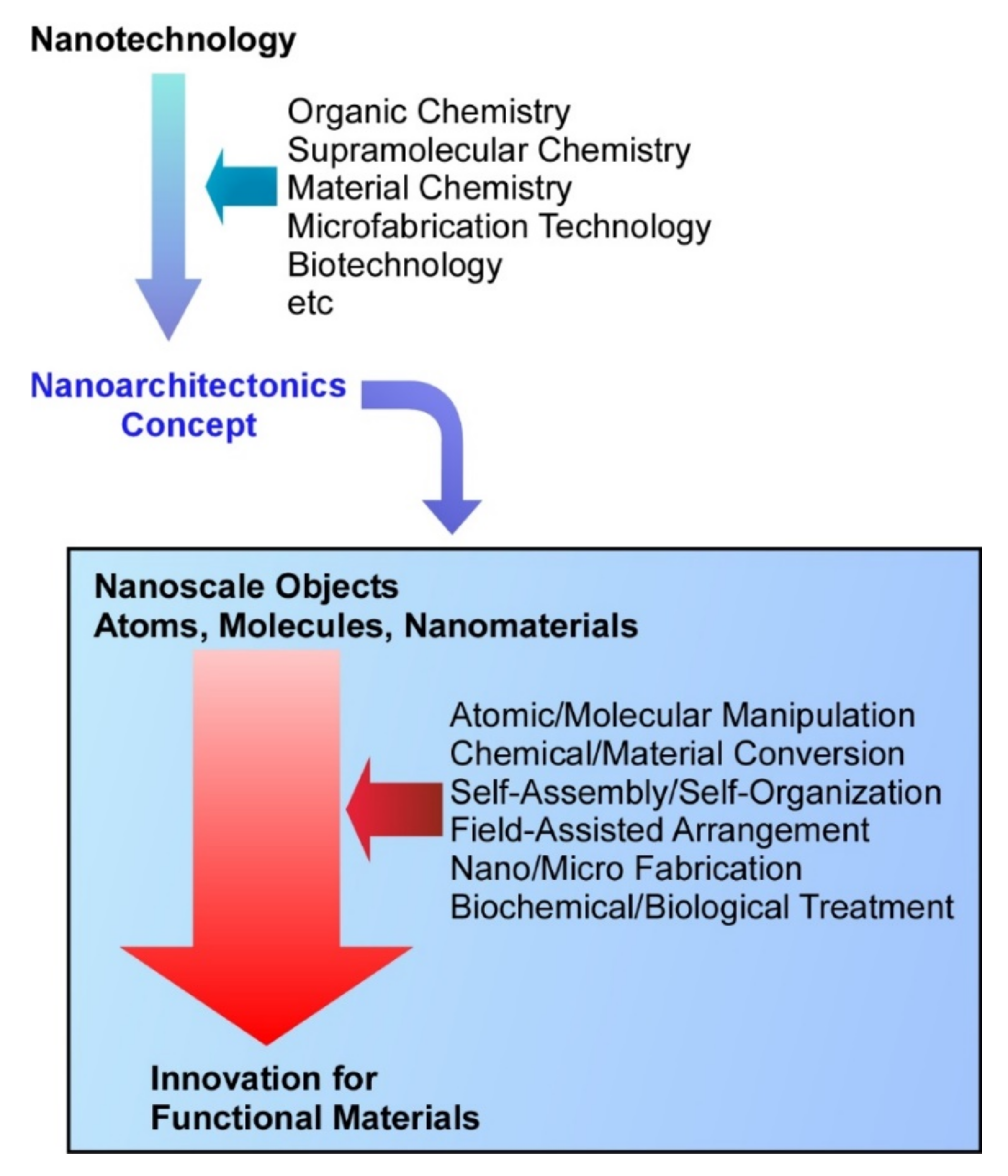
Biomimetic and Biological Nanoarchitectonics
Abstract
:1. Introduction
2. Synthetic Nanoarchitectonics for Bio-Related Units
3. Self-Assembly Nanoarchitectonics with Bio-Related Units, General
4. Nanoarchitectonics with Nucleic Acids
5. Nanoarchitectonics with Peptides
6. Nanoarchitectonics with Proteins
7. Bio-Related Nanoarchitectonics in Conjugation with Materials
8. Summary and Future Perspectives
Funding
Conflicts of Interest
References
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, D.A.; Canniffe, D.P. How nature designs light-harvesting antenna systems: Design principles and functional realization in chlorophototrophic prokaryotes. J. Phys. B Atomic Mol. Opt. Phys. 2018, 51, 033001. [Google Scholar] [CrossRef]
- Kamimura, Y.R.; Kanai, M. Chemical insights into liquid-liquid phase separation in molecular biology. Bull. Chem. Soc. Jpn. 2021, 94, 1045–1058. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef] [Green Version]
- Uzieliene, I.; Bironaite, D.; Bernotas, P.; Sobolev, A.; Bernotiene, E. Mechanotransducive biomimetic systems for chondrogenic differentiation in vitro. Int. J. Mol. Sci. 2021, 22, 9690. [Google Scholar] [CrossRef]
- Williams, M.S.; Turos, E. The chemistry of the ketogenic diet: Updates and opportunities in organic synthesis. Int. J. Mol. Sci. 2021, 22, 5230. [Google Scholar] [CrossRef]
- Au, Y.K.; Xi, Z. Recent advances in transition metal-catalyzed selective B-H functionalization of o-carboranes. Bull. Chem. Soc. Jpn. 2021, 94, 879–899. [Google Scholar] [CrossRef]
- Oda, S.; Hatakeyama, T. Development of one-shot/one-pot borylation reactions toward organoboron-based materials. Bull. Chem. Soc. Jpn. 2021, 94, 950–960. [Google Scholar] [CrossRef]
- Li, Y.; Henzie, J.; Park, T.; Wang, J.; Young, C.; Xie, H.; Yi, J.W.; Li, J.; Kim, M.; Kim, J.; et al. Fabrication of flexible microsupercapacitors with binder-free ZIF-8 derived carbon films via electrophoretic deposition. Bull. Chem. Soc. Jpn. 2020, 93, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Lee, J.M.; Kothandam, G.; Palanisami, T.; Al-Muhtaseb, A.H.; Karakoti, A.; Yi, J.; Bolan, N.; Vinu, A. A review on the synthesis and applications of nanoporous carbons for the removal of complex chemical contaminants. Bull. Chem. Soc. Jpn. 2021, 94, 1232–1257. [Google Scholar] [CrossRef]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional supramolecular polymers. Science 2012, 335, 813–817. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Miao, T.; Qian, Y.; Zhang, Z.; Zhang, W.; Zhu, X. Supramolecular chirality in azobenzene-containing polymer system: Traditional postpolymerization self-assembly versus in situ supramolecular self-assembly strategy. Int. J. Mol. Sci. 2020, 21, 6186. [Google Scholar] [CrossRef]
- Yamasumi, K.; Maeda, H. Charged porphyrins: π-Electronic systems that form ion-pairing assembled structures. Bull. Chem. Soc. Jpn. 2021, 94, 2252–2262. [Google Scholar] [CrossRef]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper coordination compounds as biologically active agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef]
- Hosono, N. Design of porous coordination materials with dynamic properties. Bull. Chem. Soc. Jpn. 2021, 94, 60–69. [Google Scholar] [CrossRef]
- Ariga, K.; Shionoya, K. Nanoarchitectonics for coordination asymmetry and related chemistry. Bull. Chem. Soc. Jpn. 2021, 94, 839–859. [Google Scholar] [CrossRef]
- Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Yunus, R.M.; Lee, T.K.; Ahmad, A.; Chong, S.T. Review of chitosan-based polymers as proton exchange membranes and roles of chitosan-supported ionic liquids. Int. J. Mol. Sci. 2020, 21, 632. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Gupta, M.; Yamaguchi, D.; Gan, K.P.; Nakayama, M. Supramolecular association and nanostructure formation of liquid crystals and polymers for new functional materials. Bull. Chem. Soc. Jpn. 2021, 94, 357–376. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K. Helical polymers with dynamic and static macromolecular helicity memory: The power of helicity memory for helical polymer synthesis and applications. Bull. Chem. Soc. Jpn. 2021, 94, 2637–2661. [Google Scholar] [CrossRef]
- Higashi, S.L.; Rozi, N.; Hanifah, S.A.; Ikeda, M. Supramolecular architectures of nucleic acid/peptide hybrids. Int. J. Mol. Sci. 2020, 21, 9458. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M. Next generation multifunctional nano-science of advanced metal complexes with quantum effect and nonlinearity. Bull. Chem. Soc. Jpn. 2021, 94, 209–264. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Hill, J.P. Mechanical control of nanomaterials and nanosystems. Adv. Mater. 2012, 24, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Miwa, K.; Imada, H.; Imai-Imada, M.; Kawahara, S.; Takeya, J.; Kawai, M.; Galperin, M.; Kim, Y. Selective triplet exciton formation in a single molecule. Nature 2019, 570, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Kazuma, K. Real-space studies of plasmon-induced dissociation reactions with an STM. Bull. Chem. Soc. Jpn. 2020, 93, 1552–1557. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, M.; Yang, D.; Li, Z.; Shi, M.; Sun, K.; Yang, J.; Wang, J. Strain-relief patterns and Kagome lattice in self-assembled C60 thin films grown on Cd(0001). Int. J. Mol. Sci. 2021, 22, 6880. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and applications of microfluidic devices: A review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Yamamoto, H.M. Phase-transition devices based on organic Mott insulators. Bull. Chem. Soc. Jpn. 2021, 94, 2505–2539. [Google Scholar] [CrossRef]
- Kim, J.J.; Wang, Y.; Wang, H.; Lee, S.; Yokota, T.; Someya, T. Skin electronics: Next-generation device platform for virtual and augmented reality. Adv. Funct. Mater. 2021, 31, 2009602. [Google Scholar] [CrossRef]
- Ikeda, A.; Shinkai, S. Novel cavity design using calix[n]arene skeletons: Toward molecular recognition and metal binding. Chem. Rev. 1997, 97, 1713–1734. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Kunitake, T. Molecular Recognition at air−water and related interfaces: Complementary hydrogenbonding and multisite iInteraction. Acc. Chem. Res. 1998, 31, 371–378. [Google Scholar] [CrossRef]
- Sasaki, Y.; Lyu, X.; Tang, W.; Wu, H.; Minami, T. Polythiophene-based chemical sensors: Toward on-site supramolecular analytical devices. Bull. Chem. Soc. Jpn. 2021, 94, 2613–2622. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akutagawa, T. Chemical design and physical properties of dynamic molecular assemblies. Bull. Chem. Soc. Jpn. 2021, 94, 1400–1420. [Google Scholar] [CrossRef]
- Neal, E.A.; Nakanishi, T. Alkyl-fullerene materials of tunable morphology and function. Bull. Chem. Soc. Jpn. 2021, 94, 1769–1788. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Pou, P.; Abe, M.; Jelinek, P.; Pérez, R.; Morita, S.; Custance, Ó. Chemical identification of individual surface atoms by atomic force microscopy. Nature 2007, 446, 64–67. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Shioya, N. MAIRS: Innovation of molecular orientation analysis in a thin film. Bull. Chem. Soc. Jpn. 2020, 93, 1127–1138. [Google Scholar] [CrossRef]
- Rabiee, N.; Bagherzadeh, M.; Ghasemi, A.; Zare, H.; Ahmadi, S.; Fatahi, Y.; Dinarvand, R.; Rabiee, M.; Ramakrishna, S.; Shokouhimehr, M.; et al. Point-of-use rapid detection of SARS-CoV-2: Nanotechnology-enabled solutions for the COVID-19 pandemic. Int. J. Mol. Sci. 2020, 21, 5126. [Google Scholar] [CrossRef]
- Shimizu, T.; Lungerich, D.; Stuckner, J.; Murayama, M.; Harano, K.; Nakamura, E. Real-time video imaging of mechanical motions of a single molecular shuttle with sub-millisecond sub-angstrom precision. Bull. Chem. Soc. Jpn. 2020, 93, 1079–1085. [Google Scholar] [CrossRef]
- Ariga, K. Nanoarchitectonics: What’s coming next after nanotechnology? Nanoscale Horiz. 2021, 6, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P. There’s plenty of room at the bottom. Calif. Inst. Technol. J. Eng. Sci. 1960, 4, 23–36. [Google Scholar]
- Roukes, M. Plenty of room, indeed. Sci. Am. 2001, 285, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Ji, Q.; Hill, J.P.; Bando, Y.; Aono, M. Forming nanomaterials as layered functional structures toward materials nanoarchitectonics. NPG Asia Mater. 2012, 4, e17. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K. Nanoarchitectonics revolution and evolution: From small science to big technology. Small Sci. 2021, 1, 2000032. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Aono, M. Nanoarchitectonics: A new materials horizon for nanotechnology. Mater. Horiz. 2015, 2, 406–413. [Google Scholar] [CrossRef]
- Ariga, K.; Minami, K.; Ebara, M.; Nakanishi, J. What are the emerging concepts and challenges in NANO? Nanoarchitectonics, hand-operating nanotechnology and mechanobiology. Polym. J. 2016, 48, 371–389. [Google Scholar] [CrossRef]
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J.P. Nanoarchitectonics for dynamic functional materials from atomic-/molecular-level manipulation to macroscopic action. Adv. Mater. 2016, 28, 1251–1286. [Google Scholar] [CrossRef]
- Tirayaphanitchkul, C.; Imwiset, K.; Ogawa, M. Nanoarchitectonics through organic modification of oxide based layered materials; concepts, methods and functions. Bull. Chem. Soc. Jpn. 2021, 94, 678–693. [Google Scholar] [CrossRef]
- Karami-Darehnaranji, M.; Taghizadeh, S.-M.; Mirzaei, E.; Berenjian, A.; Ebrahiminezhad, A. Size tuned synthesis of FeOOH nanorods toward self-assembled nanoarchitectonics. Langmuir 2021, 37, 115–123. [Google Scholar] [CrossRef]
- Siddiqui, A.; Thawarkar, S.; Singh, S.P. A novel perylenediimide molecule: Synthesis, structural property relationship and nanoarchitectonic. J. Solid State Chem. 2022, 306, 122687. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, M. Nanoarchitectonics through supramolecular gelation: Formation and switching of diverse nanostructures. Mol. Syst. Des. Eng. 2019, 4, 11–28. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Li, J. Langmuir nanoarchitectonics from basic to frontier. Langmuir 2019, 35, 3585–3599. [Google Scholar] [CrossRef]
- Ariga, K.; Mori, T.; Kitao, T.; Uemura, T. Supramolecular chiral nanoarchitectonics. Adv. Mater. 2020, 32, 1905657. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Ghosh, S.; Pradhan, B.; Dalui, A.; Shrestha, L.K.; Acharya, S.; Ariga, K. Two-dimensional (2D) nanomaterials towards electrochemical nanoarchitectonics in energy-related applications. Bull. Chem. Soc. Jpn. 2017, 90, 627–648. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Zhang, W.; Lee, C.-S. Interlayer nanoarchitectonics of two-dimensional transition-metal dichalcogenides nanosheets for energy storage and conversion applications. Adv. Energy Mater. 2017, 7, 1700571. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-active polymers for energy storage nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Zhou, L.; Yu, H.; Dai, Y.; Ouyang, J.; Liu, Z.; Wang, Y.; Le, Z.; Adesina, A.A. Nanoarchitectonics of poly(vinyl alcohol)/graphene oxide composite electrodes for highly efficient electrosorptive removal of U(VI) from aqueous solution. Sep. Purif. Technol. 2021, 278, 119604. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Darder, M.; Boutahala, M.; Aranda, P.; Ruiz-Hitzky, E. Composite nanoarchitectonics: Alginate beads encapsulating sepiolite/magnetite/Prussian blue for removal of cesium ions from water. Bull. Chem. Soc. Jpn. 2021, 94, 122–132. [Google Scholar] [CrossRef]
- Deng, G.; Xie, L.; Xu, S.; Kang, X.; Ma, J. Fiber nanoarchitectonics for pre-treatments in facile detection of short-chain fatty acids in waste water and faecal samples. Polymers 2021, 13, 3906. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, A.; Krishnan, V. Ultrathin Au–Ag heterojunctions on nanoarchitectonics based biomimetic substrates for dip catalysis. J. Inorg. Organomet. Polym. 2021, 31, 1954–1966. [Google Scholar] [CrossRef]
- Chen, G.; Sciortino, F.; Ariga, K. Atomic nanoarchitectonics for catalysis. Adv. Mater. Interfaces 2021, 8, 2001395. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Na, J.; Yao, Y.; Azhar, A.; Yan, X.; Qi, J.; Yamauchi, Y.; Li, J. 0D–1D hybrid nanoarchitectonics: Tailored design of FeCo@N–C yolk–shell nanoreactors with dual sites for excellent Fenton-like catalysis. Chem. Sci. 2021, 12, 15418–15422. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.P.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746. [Google Scholar] [CrossRef]
- Komiyama, M.; Mori, T.; Ariga, K. Molecular imprinting: Materials nanoarchitectonics with molecular information. Bull. Chem. Soc. Jpn. 2018, 91, 1075–1111. [Google Scholar] [CrossRef]
- Zhai, B.; Huang, R.; Tang, J.; Li, M.; Yang, J.; Wang, G.; Liu, K.; Fang, Y. Film nanoarchitectonics of pillar[5]arene for high-performance fluorescent sensing: A proof-of-concept study. ACS Appl. Mater. Interfaces 2021, 13, 54561–54569. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, M.; Mori, T.; Watanabe, S.; Takeya, J. Atom/molecular nanoarchitectonics for devices and related applications. Nano Today 2019, 28, 100762. [Google Scholar] [CrossRef]
- Ariga, K.; Makita, T.; Ito, M.; Mori, T.; Watanabe, S.; Takeya, J. Review of advanced sensor devices employing nanoarchitectonics concepts. Beilstein J. Nanotechnol. 2019, 10, 2014–2030. [Google Scholar] [CrossRef]
- Terabe, K.; Tsuchiya, T.; Tsuruoka, T. A variety of functional devices realized by ionic nanoarchitectonics, complementing electronics components. Adv. Electron. Mater. 2021, 2021, 2100645. [Google Scholar] [CrossRef]
- Kandori, H. Structure/function study of photoreceptive proteins by FTIR spectroscopy. Bull. Chem. Soc. Jpn. 2020, 93, 904–926. [Google Scholar] [CrossRef]
- Avinash, M.B.; Govindaraju, T. Nanoarchitectonics of biomolecular assemblies for functional applications. Nanoscale 2014, 6, 13348–13369. [Google Scholar] [CrossRef] [PubMed]
- Pivato, R.V.; Rossi, F.; Ferro, M.; Castiglione, F.; Trotta, F.; Mele, A. β-Cyclodextrin nanosponge hydrogels as drug delivery nanoarchitectonics for multistep drug release kinetics. ACS Appl. Polym. Mater. 2021, 3, 6562–6571. [Google Scholar] [CrossRef]
- Momekova, D.B.; Gugleva, V.E.; Petrov, P.D. Nanoarchitectonics of multifunctional niosomes for advanced drug delivery. ACS Omega 2021, 6, 33265–33273. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Liu, K.; Abbas, M.; Yan, X. Peptide-modulated self-assembly of chromophores toward biomimetic light-harvesting nanoarchitectonics. Adv. Mater. 2016, 28, 1031–1043. [Google Scholar] [CrossRef]
- Dutta, S.; Kim, J.; Hsieh, P.-H.; Hsu, Y.-S.; Kaneti, Y.V.; Shieh, F.-K.; Yamauchi, Y.; Wu, K.C.-W. Nanoarchitectonics of biofunctionalized metal–organic frameworks with biological macromolecules and living cells. Small Methods 2019, 3, 1900213. [Google Scholar] [CrossRef]
- Komiyama, M.; Ariga, K. Nanoarchitectonics to prepare practically useful artificial enzymes. Mol. Catal. 2019, 475, 110492. [Google Scholar] [CrossRef]
- Eom, S.; Choi, G.; Hiroyuki Nakamura, H.; Choy, J.-H. 2-Dimensional nanomaterials with imaging and diagnostic functions for nanomedicine; A review. Bull. Chem. Soc. Jpn. 2020, 93, 1–12. [Google Scholar] [CrossRef]
- Paris, J.L.; Vallet-Regí, N. Ultrasound-activated nanomaterials for therapeutics. Bull. Chem. Soc. Jpn. 2020, 93, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Karthik, V.; Poornima, S.; Vigneshwaran, A.; Raj, D.P.R.D.D.; Subbaiya, R.; Manikandan, S.; Saravanan, M. Nanoarchitectonics is an emerging drug/gene delivery and targeting strategy—A critical review. J. Mol. Struct. 2021, 1243, 130844. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Asthagiri, A.R.; Lauffenburger, D.A. Bioengineering models of cell signaling. Annu. Rev. Biomed. Eng. 2000, 2, 31–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariga, K.; Jia, X.; Song, J.; Hill, J.P.; Leong, D.T.; Jia, Y.; Li, J. Nanoarchitectonics beyond self-assembly: Challenges to create bio-like hierarchic organization. Angew. Chem. Int. Ed. 2020, 59, 15424–15446. [Google Scholar] [CrossRef] [PubMed]
- Aono, M.; Ariga, K. The way to nanoarchitectonics and the way of nanoarchitectonics. Adv. Mater. 2016, 28, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K. Nanoarchitectonics: A navigator from materials to life. Mater. Chem. Front. 2017, 1, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Ishijima, A.; Doi, T.; Sakurada, K.; Yanagida, T. Sub-piconewton force fluctuations of actomyosin in vitro. Nature 1991, 352, 301–306. [Google Scholar] [CrossRef]
- Yanadida, T.; Ishii, Y. Single molecule detection, thermal fluctuation and life. Proc. Jpn. Acad. Ser. B 2017, 93, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Ariga, K.; Yamauchi, Y. Nanoarchitectonics from atom to life. Chem. Asian J. 2020, 15, 718–728. [Google Scholar] [CrossRef]
- Sarikaya, M.; Tamerler, C.; Jen, A.K.-Y.; Schulten, K.; Baneyx, F. Molecular biomimetics: Nanotechnology through biology. Nat. Mater. 2003, 2, 577–585. [Google Scholar] [CrossRef]
- Higuchi, A.; Ling, Q.-D.; Hsu, S.-T.; Umezawa, A. Biomimetic cell culture proteins as extracellular matrices for stem cell differentiation. Chem. Rev. 2012, 112, 4507–4540. [Google Scholar] [CrossRef]
- Jian, Y.; Handschuh-Wang, S.; Zhang, J.; Lu, W.; Zhou, X.; Chen, T. Biomimetic anti-freezing polymeric hydrogels: Keeping soft-wet materials active in cold environments. Mater. Horiz. 2021, 8, 351–369. [Google Scholar] [CrossRef]
- Guo, C.; Sedgwick, A.C.; Hirao, T.; Sessler, J.L. Supramolecular fluorescent sensors: An historical overview and update. Coord. Chem. Rev. 2021, 427, 213560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yamada, T.; Kimizuka, N. Supramolecular thermocells based on thermo-responsiveness of host guest chemistry. Bull. Chem. Soc. Jpn. 2021, 94, 1525–1546. [Google Scholar] [CrossRef]
- Kakuta, T.; Yamagishi, T.; Ogoshi, T. Stimuli-responsive supramolecular assemblies constructed from pillar[n]arenes. Acc. Chem. Res. 2018, 51, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Stockdale, T.P.; Lam, N.Y.S.; Anketell, M.J.; Paterson, I. The Stereocontrolled total synthesis of polyketide natural products: A thirty-year journey. Bull. Chem. Soc. Jpn. 2021, 94, 713–731. [Google Scholar] [CrossRef]
- Hirama, M.; Oishi, T.; Uehara, H.; Inoue, M.; Maruyama, M.; Oguri, H.; Satake, M. Total synthesis of ciguatoxin CTX3C. Science 2001, 294, 1904–1907. [Google Scholar] [CrossRef]
- Muramatsu, W.; Hattori, T.; Yamamoto, H. Game change from reagent- to substrate-controlled peptide synthesis. Bull. Chem. Soc. Jpn. 2020, 93, 759–767. [Google Scholar] [CrossRef]
- Yang, B.; Gao, S. Recent advances in the application of Diels–Alder reactions involving o-quinodimethanes, aza-o-quinone methides and o-quinone methides in natural product total synthesis. Chem. Soc. Rev. 2018, 47, 7926–7953. [Google Scholar] [CrossRef]
- Kitamura, K.; Ando, Y.; Matsumoto, T.; Suzuki, K. Total synthesis of aryl C-glycoside natural products: Strategies and tactics. Chem. Rev. 2018, 118, 1495–1598. [Google Scholar] [CrossRef]
- Inaba, T.; Ishizaki, Y.; Igarashi, M.; Yoshida, M.; Kigoshi, H. Total synthesis of Hytramycin V, an antibiotic cyclopeptide. Bull. Chem. Soc. Jpn. 2021, 94, 1922–1930. [Google Scholar] [CrossRef]
- Oishi, T. Structure determination, chemical synthesis, and evaluation of biological activity of super carbon chain natural products. Bull. Chem. Soc. Jpn. 2020, 93, 1350–1360. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Yamaji, F.; Miyanishi, W.; Ojika, M.; Igarashi, Y.; Ito, Y. Binding evaluation of pradimicins for oligomannose motifs from fungal mannans. Bull. Chem. Soc. Jpn. 2021, 94, 732–754. [Google Scholar] [CrossRef]
- Min, I.; Tamaki, Y.; Ishitani, O.; Serizawa, T.; Ito, Y.; Uzawa, T. Effective suppression of O2 quenching of photo-excited ruthenium complex using RNA aptamer. Bull. Chem. Soc. Jpn. 2020, 93, 1386–1392. [Google Scholar] [CrossRef]
- Kuriki, R.; Yamamoto, M.; Higuchi, K.; Yamamoto, Y.; Akatsuka, M.; Lu, D.; Yagi, S.; Yoshida, T.; Ishitani, O.; Maeda, K. Robust binding between carbon nitride nanosheets and a binuclear ruthenium(II) complex enabling durable, selective CO2 reduction under visible light in aqueous solution. Angew. Chem. Int. Ed. 2017, 56, 4867–4871. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Ishitani, O. Supramolecular photocatalysts for the reduction of CO2. ACS Catal. 2017, 7, 3394–3409. [Google Scholar] [CrossRef]
- Ogasawara, S.; Takahashi, T.; Kitagawa, Y.; Tamiaki, H. Synthesis of highly fluorescent cationic chlorophyll-a derivatives possessing a p-aminopyridinio group at the 31-position. Bull. Chem. Soc. Jpn. 2021, 94, 1201–1203. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Yano, Y.; Ono, T.; Hisaeda, Y.; Shimakoshi, H. Aerobic electrochemical transformations of DDT to oxygen-incorporated products catalyzed by a B12 derivative. Bull. Chem. Soc. Jpn. 2021, 94, 2784–2791. [Google Scholar] [CrossRef]
- Kawai, S.; Sang, H.; Kantorovich, L.; Takahashi, K.; Nozaki, K.; Ito, S. An endergonic synthesis of single Sondheimer–Wong diyne by local probe chemistry. Angew. Chem. Int. Ed. 2020, 59, 10842–10847. [Google Scholar] [CrossRef]
- Kawai, S.; Krejčí, O.; Nishiuchi, T.; Sahara, K.; Kodama, T.; Pawlak, R.; Meyer, E.; Kubo, T.; Foster, A.S. Three-dimensional graphene nanoribbons as a framework for molecular assembly and local probe chemistry. Sci. Adv. 2020, 6, eaay8913. [Google Scholar] [CrossRef] [Green Version]
- Kawai, S.; Nakatsuka, S.; Hatakeyama, T.; Pawlak, R.; Meier, T.; Tracey, J.; Meyer, E.; Foster, A.S. Multiple heteroatom substitution to graphene nanoribbon. Sci. Adv. 2018, 4, eaar7181. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.; Gao, H.-Y.; Fuchs, H. Frontiers of on-surface synthesis: From principles to applications. Nano Today 2017, 13, 77–96. [Google Scholar] [CrossRef]
- Clair, S.; de Oteyza, D.G. Controlling a chemical coupling reaction on a surface: Tools and strategies for on-surface synthesis. Chem. Rev. 2019, 119, 4717–4776. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Müllen, K.; Narita, A. Syntheses and characterizations of functional polycyclic aromatic hydrocarbons and graphene nanoribbons. Bull. Chem. Soc. Jpn. 2020, 93, 490–506. [Google Scholar] [CrossRef]
- Xu, X.; Kinikar, A.; Giovannantonio, M.D.; Ruffieux, P.; Müllen, K.; Fasel, R.; Narita, A. On-surface synthesis of dibenzohexacenohexacene and dibenzopentaphenoheptaphene. Bull. Chem. Soc. Jpn. 2021, 94, 997–999. [Google Scholar] [CrossRef]
- Kitamoto, D.; Morita, T.; Fukuoka, T.; Konishi, M.; Imura, T. Self-assembling properties of glycolipid biosurfactants and their potential applications. Curr. Opin. Colloid Interface Sci. 2009, 14, 315–328. [Google Scholar] [CrossRef]
- Matsuurua, K. Rational design of self-assembled proteins and peptides for nano- and micro-sized architectures. RSC Adv. 2014, 4, 2942–2953. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Kikuchi, K.; Maity, B.; Ueno, T. The versatile manipulations of self-assembled proteins in vaccine design. Int. J. Mol. Sci. 2021, 22, 1934. [Google Scholar] [CrossRef]
- Nakanishi, W.; Minami, K.; Shrestha, L.K.; Ji, Q.; Hill, J.P.; Ariga, K. Bioactive nanocarbon assemblies: Nanoarchitectonics and applications. Nano Today 2014, 9, 378–394. [Google Scholar] [CrossRef] [Green Version]
- Komiyama, M.; Yoshimoto, K.; Sisido, M.; Ariga, K. Chemistry can make strict and fuzzy controls for bio-systems: DNA nanoarchitectonics and cell-macromolecular nanoarchitectonics. Bull. Chem. Soc. Jpn. 2017, 90, 967–1004. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, H.; Akiyama, K.; Fujie, T. Biopotential measurement of plant leaves with ultra-light and flexible conductive polymer nanosheets. Bull. Chem. Soc. Jpn. 2020, 93, 1007–1013. [Google Scholar] [CrossRef]
- Percec, V.; Xiao, Q. Helical self-organizations and emerging functions in architectures, biological and synthetic macromolecules. Bull. Chem. Soc. Jpn. 2021, 94, 900–928. [Google Scholar] [CrossRef]
- Kunitake, T.; Okahata, Y. A totally synthetic bilayer membrane. J. Am. Chem. Soc. 1977, 99, 3860–3861. [Google Scholar] [CrossRef]
- Antonietti, M.; Förster, S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Kunitake, T. Synthetic bilayer membranes: Molecular design, self-organization, and application. Angew. Chem. Int. Ed. Engl. 1992, 31, 709–726. [Google Scholar] [CrossRef]
- Ramanathan, M.; Shrestha, L.K.; Mori, T.; Ji, Q.; Hill, J.P.; Ariga, K. Amphiphile nanoarchitectonics: From basic physical chemistry to advanced applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ding, W.; Kameta, N. Soft-matter nanotubes: A platform for diverse functions and applications. Chem. Rev. 2020, 120, 2347–2407. [Google Scholar] [CrossRef]
- Kameta, N.; Ding, W.; Masuda, M. Effect of glycine position on the inner diameter of supramolecular nanotubes consisting of Glycolipid monolayer membranes. Bull. Chem. Soc. Jpn. 2021, 94, 1172–1178. [Google Scholar] [CrossRef]
- Hata, Y.; Serizawa, T. Robust gels composed of self-assembled cello-oligosaccharide networks. Bull. Chem. Soc. Jpn. 2021, 94, 2279–2289. [Google Scholar] [CrossRef]
- Vergaro, V.; Scarlino, F.; Bellomo, C.; Rinaldi, R.; Vergara, D.; Michele Maffia, M.; Baldassarre, F.; Giannelli, G.; Zhang, X.; Lvov, Y.M.; et al. Drug-loaded polyelectrolyte microcapsules for sustained targeting of cancer cells. Adv. Drug Deliv. Rev. 2011, 63, 847–864. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Hatami, J.; Mano, F. Coating strategies using layer-by-layer deposition for cell encapsulation. Chem. Asian J. 2016, 11, 1753–1764. [Google Scholar] [CrossRef]
- Ariga, K.; Lvov, Y.; Decher, G. There is still plenty of room for layer-by-layer assembly for constructing nanoarchitectonicsbased materials and devices. Phys. Chem. Chem. Phys. 2022, 24, 4097–4115. [Google Scholar] [CrossRef]
- Ariga, K.; Fakhrullin, R. Nanoarchitectonics on living cells. RSC Adv. 2021, 11, 18898–18914. [Google Scholar] [CrossRef]
- Akashi, M.; Akagi, T. Composite materials by building block chemistry using weak interaction. Bull. Chem. Soc. Jpn. 2021, 94, 1903–1921. [Google Scholar] [CrossRef]
- Han, D.; Pal, S.; Nangreave, J.; Deng, Z.; Liu, Y.; Yan, H. DNA origami with complex curvatures in three-dimensional space. Science 2011, 332, 342–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komiyama, M.; Shigi, N.; Ariga, K. DNA-based nanoarchitectures as eminent vehicles for smart drug delivery systems. Adv. Funct. Mater. 2022; in press. [Google Scholar] [CrossRef]
- Yonamine, Y.; Cervantes-Salguero, K.; Minami, K.; Kawamat, I.; Nakanishi, W.; Hill, J.P.; Murata, S.; Ariga, K. Supramolecular 1-D polymerization of DNA origami through a dynamic process at the 2-dimensionally confined air–water interface. Phys. Chem. Chem. Phys. 2016, 18, 12576–12581. [Google Scholar] [CrossRef]
- Sugimoto, N.; Endoh, T.; Takahashi, S.; Tateishi-Karimata, H. Chemical biology of double helical and non-double helical nucleic acids: “To B or Not To B, that is the question”. Bull. Chem. Soc. Jpn. 2021, 94, 1970–1998. [Google Scholar] [CrossRef]
- Bochman, M.; Paeschke, K.; Zakian, V. DNA secondary structures: Stability and function of G-quadruplex structures. Nat. Rev. Genet. 2012, 13, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Asamitsu, S.; Obata, S.; Yu, Z.; Bando, T.; Sugiyama, H. Recent progress of targeted G-quadruplex-preferred ligands toward cancer therapy. Molecules 2019, 24, 429. [Google Scholar] [CrossRef] [Green Version]
- Hori, D.; Yum, J.H.; Sugiyama, H.; Park, S. Tropylium derivatives as new entrants that sense quadruplex structures. Bull. Chem. Soc. Jpn. 2021, 94, 1948–1953. [Google Scholar] [CrossRef]
- Hayasaka, K.; Shibata, T.; Sugahara, A.; Momotake, A.; Matsui, T.; Neya, S.; Ishizuka, T.; Xu, Y.; Yamamoto, Y. Characterization of structure and catalytic Activity of a complex between heme and an all parallel-stranded tetrameric G-quadruplex formed from DNA/RNA chimera sequence d(TTA)r(GGG)dT. Bull. Chem. Soc. Jpn. 2020, 93, 621–629. [Google Scholar] [CrossRef]
- Uchiyama, M.; Momotake, A.; Ikeue, T.; Yamamoto, Y. Photogeneration of reactive oxygen species from water-soluble phthalocyanine derivatives bound to a G-quadruplex DNA. Bull. Chem. Soc. Jpn. 2020, 93, 1504–1508. [Google Scholar] [CrossRef]
- Liang, X.; Li, L.; Tang, J.; Komiyama, M.; Ariga, K. Dynamism of supramolecular DNA/RNA nanoarchitectonics: From interlocked structures to molecular machines. Bull. Chem. Soc. Jpn. 2020, 93, 581–603. [Google Scholar] [CrossRef]
- Liang, X.; Chen, H.; Li, L.; An, R.; Komiyama, M. Ring-structured DNA and RNA as key players in vivo and in vitro. Bull. Chem. Soc. Jpn. 2021, 94, 141–157. [Google Scholar] [CrossRef]
- Egholm, M.; Buchardt, O.; Christensen, L.; Behrens, C.; Freier, S.M.; Driver, D.A.; Berg, R.H.; Kim, S.K.; Norden, B.; Nielsen, P.E. PNA hybridizes to complementary oligonucleotides obeying the Watson–Crick hydrogen-bonding rules. Nature 1993, 365, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, M.; Komiyama, M. Recognition of target site in various forms of DNA and RNA by peptide nucleic acid (PNA): From fundamentals to practical applications. Bull. Chem. Soc. Jpn. 2021, 94, 1737–1756. [Google Scholar] [CrossRef]
- Sato, Y. Design of fluorescent peptide nucleic acid probes carrying cyanine dyes for targeting double-stranded RNAs for analytical applications. Bull. Chem. Soc. Jpn. 2020, 93, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Podder, A.; Lee, H.J.; Kim, B.H. Fluorescent nucleic acid systems for biosensors. Bull. Chem. Soc. Jpn. 2021, 94, 1010–1035. [Google Scholar] [CrossRef]
- Bando, T.; Sugiyama, H. Sequence-specific PI polyamides make it possible to regulate DNA structure and function. Bull. Chem. Soc. Jpn. 2020, 93, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Pandeeswar, M.; Khare, H.; Ramakumar, S.; Govindaraju, T. Crystallographic insight-guided nanoarchitectonics and conductivity modulation of an n-type organic semiconductor through peptide conjugation. Chem. Commun. 2015, 51, 8315–8318. [Google Scholar] [CrossRef]
- Balachandra, C.; Padhi, D.; Govindaraju, T. Cyclic dipeptide: A privileged molecular scaffold to derive structural diversity and functional utility. ChemMedChem 2021, 16, 2558–2587. [Google Scholar] [CrossRef]
- Cha, X.; Ariga, K.; Kunitake, T. Molecular recognition of aqueous dipeptides at multiple hydrogen-bonding sites of mixed peptide monolayers. J. Am. Chem. Soc. 1996, 118, 9545–9551. [Google Scholar] [CrossRef]
- Ariga, K.; Kamino, A.; Cha, X.; Kunitake, T. Multisite recognition of aqueous dipeptides by oligoglycine arrays mixed with guanidinium and other receptor units at the air−water interface. Langmuir 1999, 15, 3875–3885. [Google Scholar] [CrossRef]
- Ariga, K.; Ito, H.; Hill, J.P.; Tsukube, H. Molecular recognition: From solution science to nano/materials technology. Chem. Soc. Rev. 2012, 41, 5800–5835. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Kikuchi, J.; Naito, M.; Koyama, E.; Yamada, N. Modulated supramolecular assemblies composed of tripeptide derivatives: formation of micrometer-scale rods, nanometer-size needles, and regular patterns with molecular-level flatness from the same compound. Langmuir 2000, 16, 4929–4939. [Google Scholar] [CrossRef]
- Li, Q.; Jia, Y.; Yang, X.; Dai, L.; Das, B.; Acharya, S.; Sun, B.; Yang, Y.; Liu, X.; Ariga, K.; et al. Unidirectional branching growth of dipeptide single crystals for remote light multiplication and collection. ACS Appl. Mater. Interfaces 2019, 11, 31–36. [Google Scholar] [CrossRef]
- Song, J.; Xing, R.; Jiao, T.; Peng, Q.; Yuan, C.; Möhwald, H.; Yan, X. Crystalline dipeptide nanobelts based on solid–solid phase transformation self-assembly and their polarization imaging of cells. ACS Appl. Mater. Interfaces 2018, 10, 2368–2376. [Google Scholar] [CrossRef]
- Eimura, H.; Niwa, A.; Uchida, J.; Kato, T. Self-assembly of peptide-containing mesogens: Thermotropic liquid-crystalline properties and macroscopic alignment of amphiphilic bioconjugates. Bull. Chem. Soc. Jpn. 2021, 94, 1588–1593. [Google Scholar] [CrossRef]
- Mijiddorj, B.; Shirakata, H.; Nakagawa, T.; Ueda, K.; Yokoyama, Y.; Kawamura, I. Stereochemical effects on the self-assembly of pyrenylalanine-phenylalanine dipeptide. Bull. Chem. Soc. Jpn. 2020, 93, 969–977. [Google Scholar] [CrossRef]
- Arora, H.; Ramesh, M.; Rajasekhar, K.; Govindaraju, T. Molecular tools to detect alloforms of Aβ and Tau: Implications for multiplexing and multimodal diagnosis of Alzheimer’s disease. Bull. Chem. Soc. Jpn. 2020, 93, 507–546. [Google Scholar] [CrossRef] [Green Version]
- Katoh, T.; Suga, H. Development of bioactive foldamers using ribosomally synthesized nonstandard peptide libraries. Bull. Chem. Soc. Jpn. 2021, 94, 549–557. [Google Scholar] [CrossRef]
- Sawada, T.; Fujita, M. Orderly entangled nanostructures of metal peptide strands. Bull. Chem. Soc. Jpn. 2021, 94, 2342–2350. [Google Scholar] [CrossRef]
- Inaba, H.; Matsuura, K. Modulation of microtubule properties and functions by encapsulation of nanomaterials using a Tau-derived peptide. Bull. Chem. Soc. Jpn. 2021, 94, 2100–2112. [Google Scholar] [CrossRef]
- Gao, L.; Meiring, J.C.M.; Kraus, Y.; Wranik, M.; Weinert, T.; Pritzl, S.D.; Bingham, R.; Ntouliou, E.; Jansen, K.I.; Olieric, N.; et al. GFP-Orthogonal photoswitchable inhibitor scaffold extends optical control over the microtubule cytoskeleton. Cell Chem. Biol. 2021, 28, 228–241. [Google Scholar] [CrossRef]
- Mafy, N.N.; Matsuo, K.; Hiruma, S.; Uehara, R.; Tamaoki, N. Photoswitchable CENP-E inhibitor enabling the dynamic control of chromosome movement and mitotic progression. J. Am. Chem. Soc. 2020, 142, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Van Geel, O.; Cheung, S.; Gadella, T.W.J. Combining optogenetics with sensitive FRET imaging to monitor local microtubule manipulations. Sci. Rep. 2020, 10, 6034. [Google Scholar] [CrossRef]
- Muraoka, T. Biofunctional molecules inspired by protein mimicry and manipulation. Bull. Chem. Soc. Jpn. 2020, 93, 138–153. [Google Scholar] [CrossRef]
- Ravishankar, S.; Suzuki, S.; Sawada, T.; Lim, S.; Serizawa, T. Preparation and dynamic behavior of protein-polymer complexes formed with polymer-binding peptides. Bull. Chem. Soc. Jpn. 2020, 93, 790–793. [Google Scholar] [CrossRef]
- Watanabe, Y.; Aiba, Y.; Ariyasu, S.; Satoshi Abe, S. Molecular design and regulation of metalloenzyme activities through two novel approaches: Ferritin and P450s. Bull. Chem. Soc. Jpn. 2020, 93, 379–392. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Vong, K. The journey to in vivo synthetic chemistry: From azaelectrocyclization to artificial metalloenzymes. Bull. Chem. Soc. Jpn. 2020, 93, 1275–1286. [Google Scholar] [CrossRef]
- Vong, K.; INasibullin, I.; Tanaka, K. Exploring and adapting the molecular selectivity of artificial metalloenzymes. Bull. Chem. Soc. Jpn. 2021, 94, 382–396. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Mori, T.; Naito, M.; Yamauchi, Y.; Abe, H.; Hill, J.P. Enzyme nanoarchitectonics: Organization and device application. Chem. Soc. Rev. 2013, 42, 6322–6345. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, H.; Morishima, N.; Obuse, S.; Isoshima, T.; Akimoto, J.; Ito, Y. SARS-CoV-2 Proteins microarray by photoimmobilization for Serodiagnosis of the Antibodies. Bull. Chem. Soc. Jpn. 2021, 94, 2435–2443. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y.; Mori, T.; Hill, J.P. 25th Anniversary article: What can be done with the Langmuir-Blodgett method? Recent developments and its critical role in materials science. Adv. Mater. 2013, 25, 6477–6512. [Google Scholar] [CrossRef]
- Ariga, K. Don’t forget Langmuir–Blodgett films 2020: Interfacial nanoarchitectonics with molecules, materials, and living objects. Langmuir 2020, 36, 7158–7180. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, O.N., Jr.; Caseli, L.; Ariga, K. The past and the future of Langmuir and Langmuir–Blodgett films. Chem. Rev. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Okahata, Y.; Tsuruta, T.; Ijiro, K.; Ariga, K. Langmuir-Blodgett films of an enzyme-lipid complex for sensor membranes. Langmuir 1988, 4, 1373–1375. [Google Scholar] [CrossRef]
- Okahata, Y.; Tsuruta, T.; Ijiro, K.; Ariga, K. Preparations of Langmuir-Blodgett films of enzyme-lipid complexes: A glucose sensor membrane. Thin Solid Films 1989, 180, 65–72. [Google Scholar] [CrossRef]
- Rydzek, G.; Ji, Q.; Li, M.; Schaaf, P.; Hill, J.P.; Boulmedais, F.; Ariga, K. Electrochemical nanoarchitectonics and layer-by-layer assembly: From basics to future. Nano Today 2015, 10, 138–167. [Google Scholar] [CrossRef] [Green Version]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Li, J. Molecular assembly of rotary and linear motor proteins. Acc. Chem. Res. 2019, 52, 1623–1631. [Google Scholar] [CrossRef]
- Lvov, Y.; Ariga, K.; Ichinose, I.; Kunitake, T. Assembly of multicomponent protein films by means of electrostatic Layer-by-layer adsorption. J. Am. Chem. Soc. 1995, 117, 6117–6123. [Google Scholar] [CrossRef]
- Ai, H.; Jones, S.A.; Lvov, Y.M. Biomedical applications of electrostatic layer-by-layer nano-assembly of polymers, enzymes, and nanoparticles. Cell Biochem. Biophys. 2003, 39, 23. [Google Scholar] [CrossRef]
- Lvov, Y.; Ariga, K.; Ichinose, I.; Kunitake, T. Layer-by-layer architectures of concanavalin A by means of electrostatic and biospecific interactions. J. Chem. Soc. Chem. Commun. 1995, 22, 2313–2314. [Google Scholar] [CrossRef]
- Anzai, J.; Kobayashi, Y. Construction of multilayer thin films of enzymes by means of sugar−lectin interactions. Langmuir 2000, 16, 2851–2856. [Google Scholar]
- Onda, M.; Lvov, Y.; Ariga, K.; Kunitake, T. Sequential actions of glucose oxidase and peroxidase in molecular films assembled by layer-by-layer alternate adsorption. Biotechnol. Bioeng. 1996, 51, 163–167. [Google Scholar] [CrossRef]
- Onda, M.; Lvov, Y.; Ariga, K.; Kunitake, T. Sequential reaction and product separation on molecular films of glucoamylase and glucose oxidase assembled on an ultrafilter. J. Ferment. Bioeng. 1996, 82, 502–506. [Google Scholar]
- Onda, M.; Ariga, K.; Kunitake, T. Activity and stability of glucose oxidase in molecular films assembled alternately with polyions. J. Biosci. Bioeng. 1999, 87, 69–75. [Google Scholar]
- Matsuda, N.; Okabe, H.; Nagamura, T.; Nakano, K. Direct electron transfer reaction of cytochrome c immobilized on a bare ITO electrode. Bull. Chem. Soc. Jpn. 2021, 94, 433–439. [Google Scholar]
- Hartmann, M. Ordered mesoporous materials for bioadsorption and biocatalysis. Chem. Mater. 2005, 17, 4577–4593. [Google Scholar]
- Wang, Y.; Caruso, F. Mesoporous silica spheres as supports for enzyme immobilization and encapsulation. Chem. Mater. 2005, 17, 953–961. [Google Scholar]
- Fujiwara, M.; Shoji, S.; Murakami, Y.; Ishikawa, K. Macroporous silica microcapsules immobilizing esterase with high hydrolysis reactivity. Bull. Chem. Soc. Jpn. 2020, 93, 1043–1045. [Google Scholar] [CrossRef]
- Vinu, A.; Miyahara, M.; Ariga, K. Biomaterial immobilization in nanoporous carbon molecular sieves: Influence of solution pH, pore volume, and pore diameter. J. Phys. Chem. B 2005, 109, 6436–6441. [Google Scholar] [CrossRef] [PubMed]
- Lakhi, K.S.; Park, D.-H.; Al-Bahily, K.; Cha, W.; Viswanathan, B.; Choy, J.-H.; Vinu, A. Mesoporous carbon nitrides: Synthesis, functionalization, and applications. Chem. Soc. Rev. 2017, 46, 72–101. [Google Scholar] [CrossRef] [PubMed]
- Shitanda, I.; Kato, T.; Suzuki, R.; Aikawa, T.; Hoshi, Y.; Itagaki, M.; Tsujimura, S. Stable immobilization of enzyme on pendant glycidyl group-modified mesoporous carbon by graft polymerization of poly(glycidyl methacrylate). Bull. Chem. Soc. Jpn. 2020, 93, 32–36. [Google Scholar] [CrossRef]
- Yan, D.; Liu, L.; Wang, X.; Xu, K.; Zhong, J. Biomass-derived activated carbon nanoarchitectonics with hibiscus flowers for high-performance supercapacitor electrode applications. Chem. Eng. Technol. 2022, 45, 649–657. [Google Scholar] [CrossRef]
- Sheng, X.; Xu, X.; Wu, Y.; Zhang, X.; Lin, P.; Eid, K.; Abdullah, A.M.; Li, Z.; Yang, T.; Nanjundan, A.K.; et al. Nitrogenization of biomass-derived porous carbon microtubes promotes capacitive deionization performance. Bull. Chem. Soc. Jpn. 2021, 94, 1645–1650. [Google Scholar] [CrossRef]
- Lu, T.; Xu, X.; Zhang, S.; Pan, L.; Wang, Y.; Alshehri, S.M.; Ahamad, T.; Kim, M.; Na, J.; Hossain, M.S.A.; et al. High-performance capacitive deionization by lignocellulose-derived eco-friendly porous carbon materials. Bull. Chem. Soc. Jpn. 2020, 93, 1014–1019. [Google Scholar] [CrossRef]
- Messner, M.; Kurkov, S.V.; Jansook, P.; Loftsson, T. Self-assembled cyclodextrin aggregates and nanoparticles. Int. J. Pharm. 2010, 387, 199–208. [Google Scholar] [CrossRef]
- Au-Duong, A.N.; Kuo, C.C.; Chiu, Y.C. Self-assembled oligosaccharide-based block copolymers as charge-storage materials for memory devices. Polym. J. 2018, 50, 649–658. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Hashidzume, A.; Yamaguchi, H. Supramolecular polymers and materials formed by host-guest interactions. Bull. Chem. Soc. Jpn. 2021, 94, 2381–2389. [Google Scholar] [CrossRef]
- Matsuda, N.; Shimizu, T.; Yamato, M.; Okano, T. Tissue engineering based on cell sheet technology. Adv. Mater. 2007, 19, 3089–3099. [Google Scholar] [CrossRef]
- Thorp, H.; Kim, K.; Kondo, M.; Maak, T.; Grainger, D.W.; Okano, T. Trends in articular cartilage tissue engineering: 3D mesenchymal stem cell sheets as candidates for engineered hyaline-like cartilage. Cells 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, A.; Yoshida, H.; Matsusaki, M.; Akashi, M. Rapid construction of three-dimensional multilayered tissues with endothelial tube networks by the cell-accumulation technique. Adv. Mater. 2011, 23, 3506–3510. [Google Scholar] [CrossRef] [PubMed]
- Fakhrullin, R.F.; Zamaleeva, A.I.; Minullina, R.T.; Konnova, S.A.; Paunov, V.N. Cyborg cells: Functionalisation of living cells with polymers and nanomaterials. Chem. Soc. Rev. 2012, 41, 4189–4206. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Mori, T.; Nakanishi, W.; Shigi, N.; Nakanishi, J.; Hill, J.P.; Komiyama, M.; Ariga, K. Suppression of myogenic differentiation of mammalian cells caused by fluidity of a liquid–liquid interface. ACS Appl. Mater. Interfaces 2017, 9, 30553–30560. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Minami, K.; Uto, K.; Chang, A.C.; Hill, J.P.; Ueki, T.; Nakanishi, J.; Ariga, K. Modulation of mesenchymal stem cells mechanosensing at fluid interfaces by tailored self-assembled protein monolayers. Small 2019, 15, 1804640. [Google Scholar] [CrossRef]
- Jia, X.; Minami, K.; Uto, K.; Chang, A.C.; Hill, J.P.; Nakanishi, J.; Ariga, K. Adaptive liquid interfacially assembled protein nanosheets for guiding mesenchymal stem cell fate. Adv. Mater. 2020, 32, 1905942. [Google Scholar] [CrossRef]
- Minami, K.; Kasuya, Y.; Yamazaki, T.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Sakai, H.; Ariga, K. Highly ordered 1D fullerene crystals for concurrent control of macroscopic cellular orientation and differentiation toward large-scale tissue engineering. Adv. Mater. 2015, 27, 4020–4026. [Google Scholar] [CrossRef]
- Krishnan, V.; Kasuya, Y.; Ji, Q.; Sathish, M.; Shrestha, L.K.; Ishihara, S.; Minami, K.; Morita, H.; Yamazaki, T.; Hanagata, N.; et al. Vortex-aligned fullerene nanowhiskers as a scaffold for orienting cell growth. ACS Appl. Mater. Interfaces 2015, 7, 15667–15673. [Google Scholar] [CrossRef]
- Song, J.; Jia, X.; Minami, K.; Hill, J.P.; Nakanishi, J.; Shrestha, L.K.; Ariga, K. Large-area aligned fullerene nanocrystal scaffolds as culture substrates for enhancing mesenchymal stem cell self-renewal and multipotency. ACS Appl. Nano Mater. 2020, 3, 6497–6506. [Google Scholar] [CrossRef]
- Neto, M.P.; Soares, A.C.; Oliveira, O.N., Jr.; Paulovich, F.V. Machine learning used to create a multidimensional calibration space for sensing and biosensing data. Bull. Chem. Soc. Jpn. 2021, 94, 1553–1562. [Google Scholar] [CrossRef]
- Westermayr, J.; Marquetand, P. Machine learning for electronically excited states of molecules. Chem. Rev. 2021, 121, 9873–9926. [Google Scholar] [CrossRef] [PubMed]
- Oaki, Y.; Igarashi, Y. Materials informatics for 2D materials combined with sparse modeling and chemical perspective: Toward small-data-driven chemistry and materials science. Bull. Chem. Soc. Jpn. 2021, 94, 2410–2422. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Yamauchi, Y.; Ariga, K. Material evolution with nanotechnology, nanoarchitectonics, and materials informatics: What will be the next paradigm shift in nanoporous materials? Adv. Mater. 2022, 34, 2107212. [Google Scholar] [CrossRef] [PubMed]


























Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariga, K. Biomimetic and Biological Nanoarchitectonics. Int. J. Mol. Sci. 2022, 23, 3577. https://doi.org/10.3390/ijms23073577
Ariga K. Biomimetic and Biological Nanoarchitectonics. International Journal of Molecular Sciences. 2022; 23(7):3577. https://doi.org/10.3390/ijms23073577
Chicago/Turabian StyleAriga, Katsuhiko. 2022. "Biomimetic and Biological Nanoarchitectonics" International Journal of Molecular Sciences 23, no. 7: 3577. https://doi.org/10.3390/ijms23073577
APA StyleAriga, K. (2022). Biomimetic and Biological Nanoarchitectonics. International Journal of Molecular Sciences, 23(7), 3577. https://doi.org/10.3390/ijms23073577