Suppression of Cutibacterium acnes-Mediated Inflammatory Reactions by Fibroblast Growth Factor 21 in Skin
Abstract
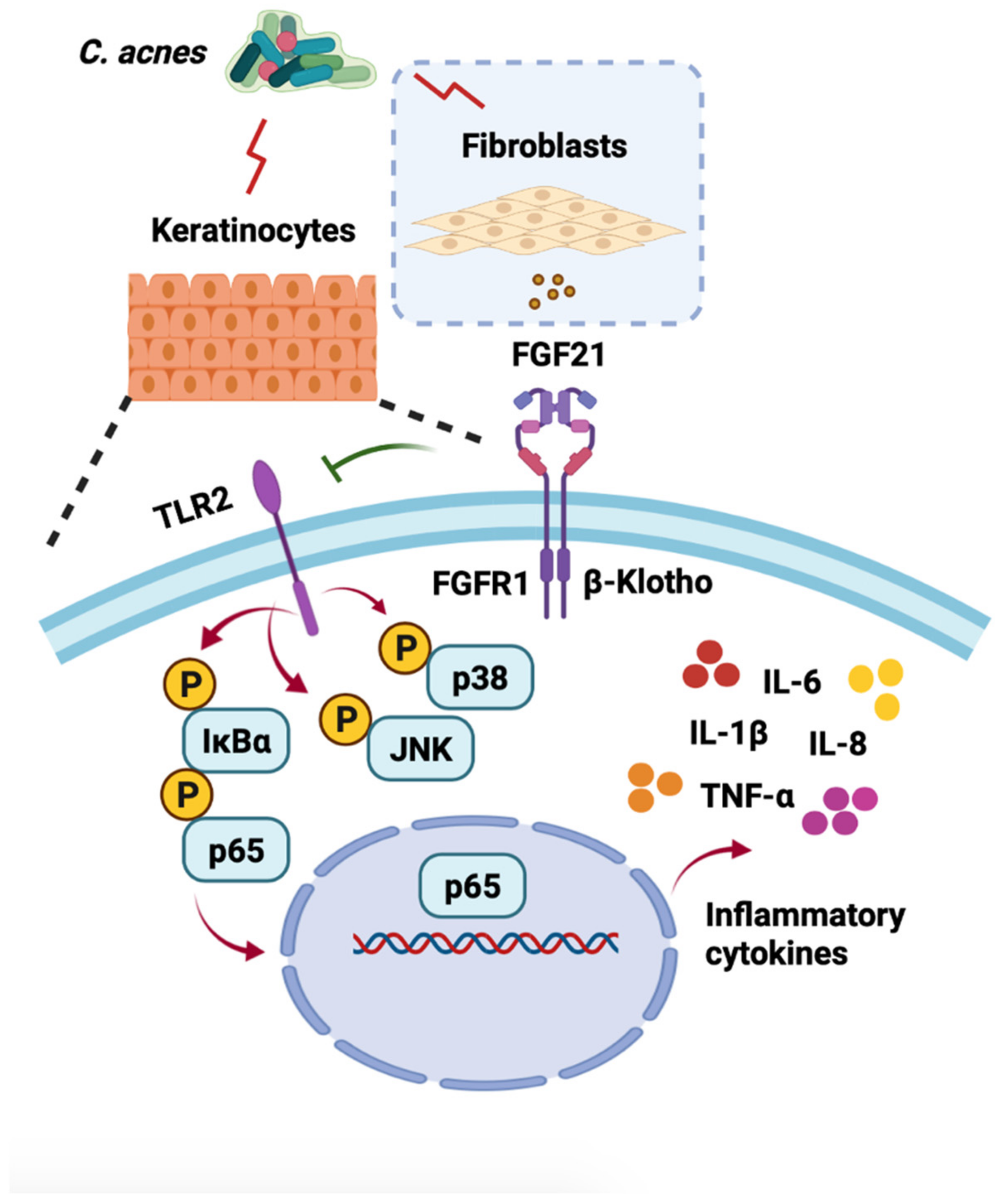
:1. Introduction
2. Results
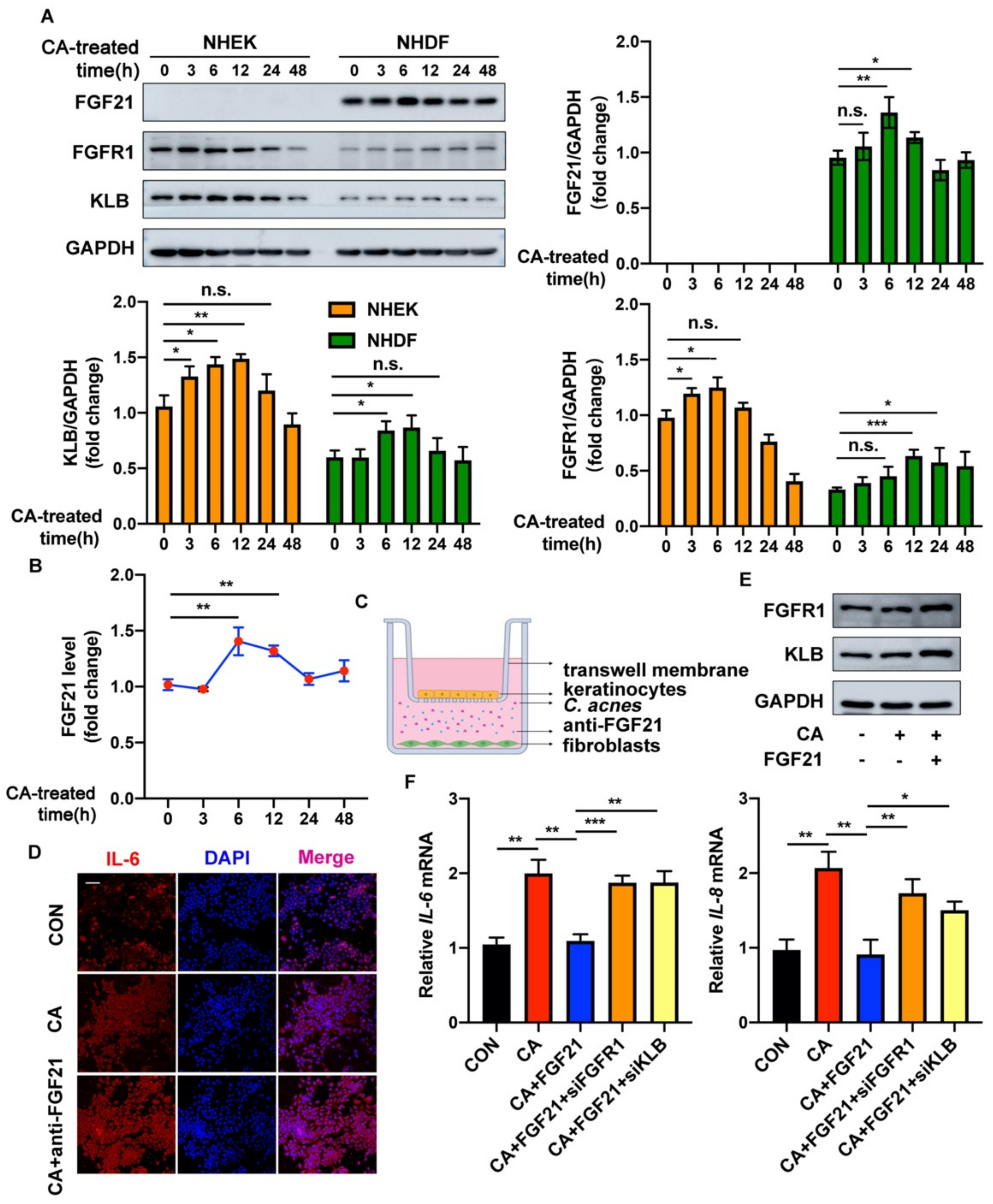
2.1. FGF21 Is Upregulated in C. acnes-Stimulated Skin
2.2. FGF21 Acts in a Paracrine Manner to Exert Anti-Inflammatory Functions in C. acnes-Stimulated Cultured Keratinocytes
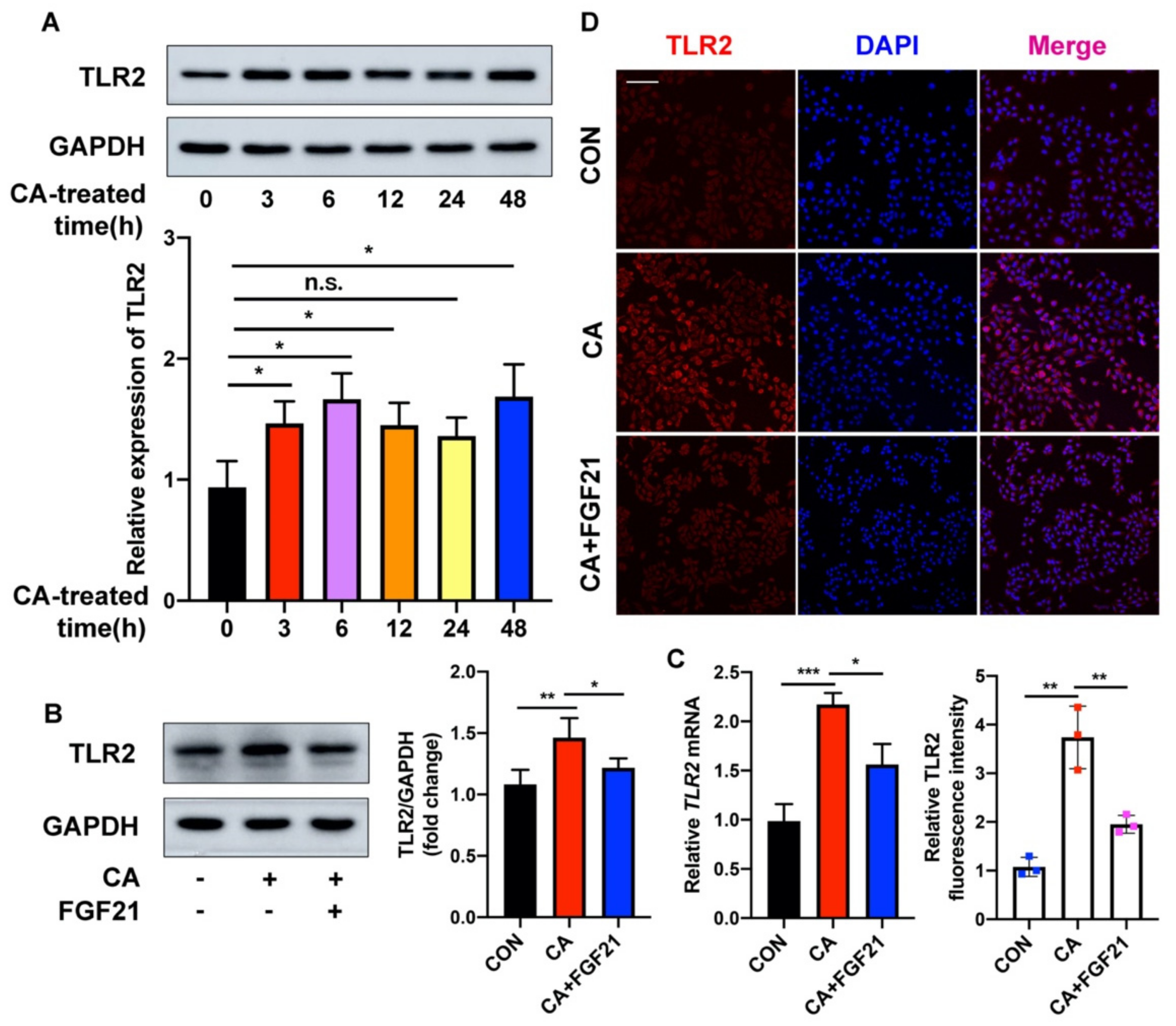
2.3. FGF21 Inhibition of the C. acnes-Induced Proinflammatory Response Is Associated with the TLR2/NF-κB/MAPK Signaling Pathways In Vitro
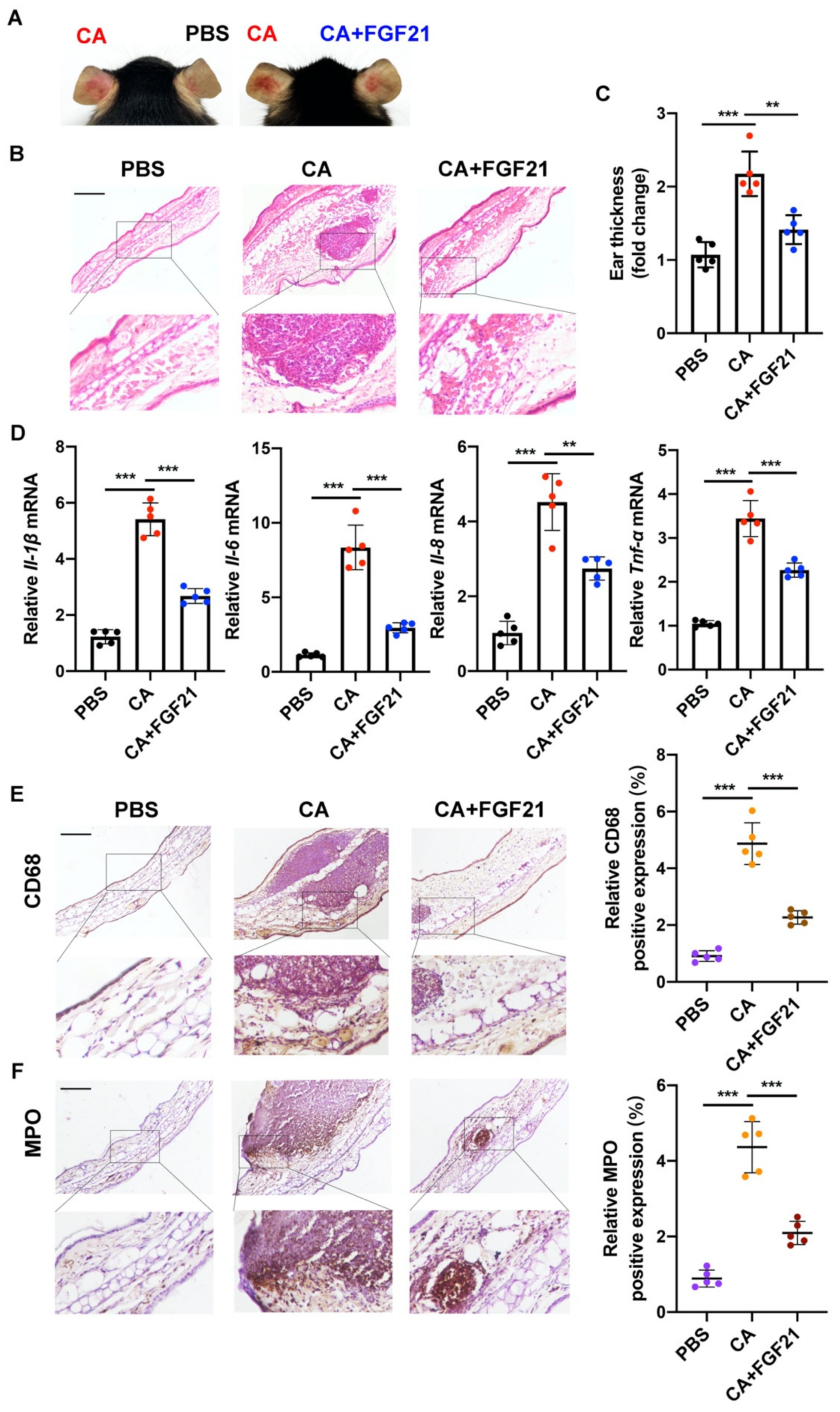
2.4. Anti-Inflammatory Effects of FGF21 on C. acnes Stimulation In Vivo
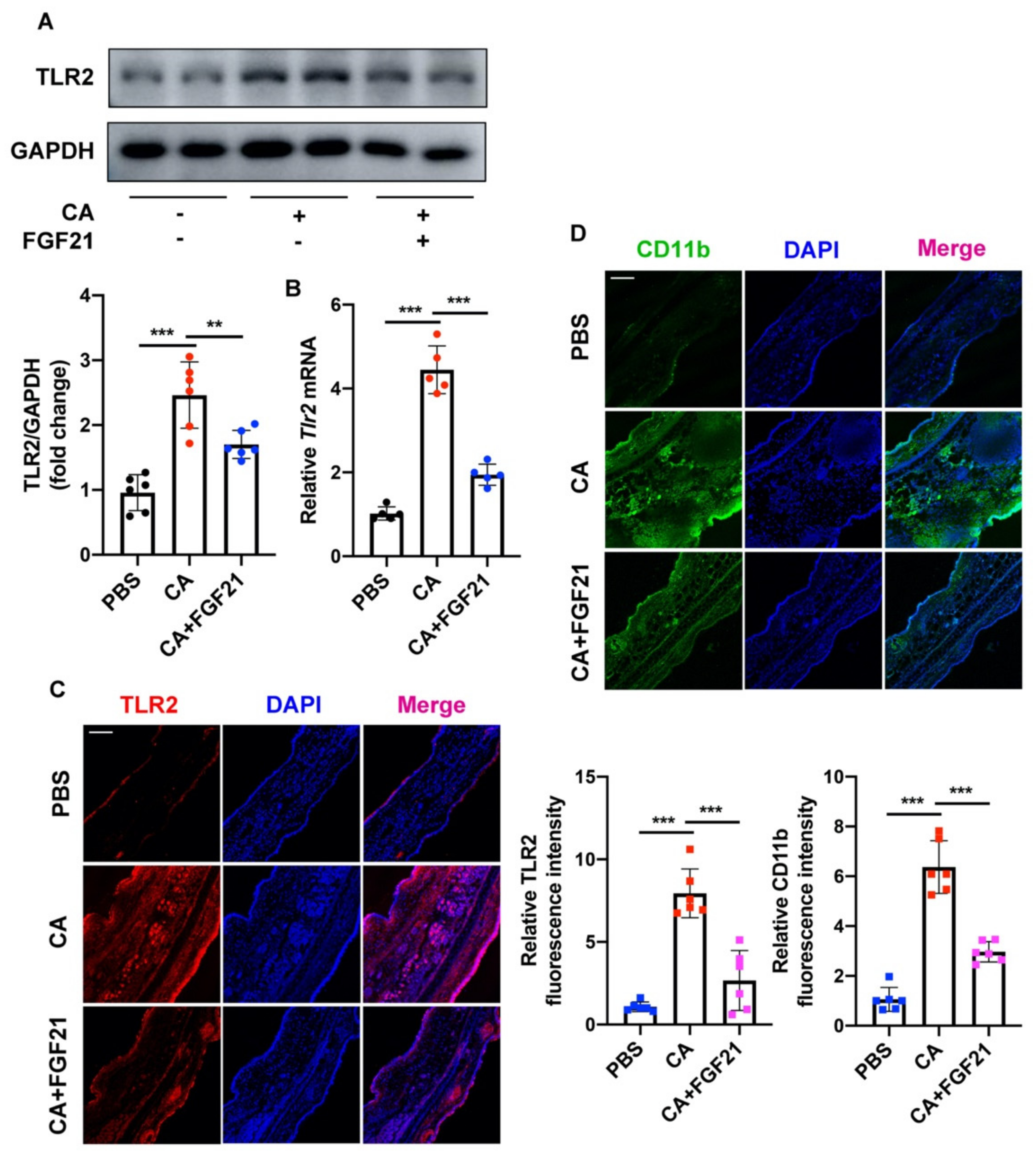
2.5. FGF21 Suppresses C. acnes-Induced Inflammation by Inhibiting the TLR2/NF-κB/MAPK Signaling Pathways In Vivo
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain Culture
4.2. Cell Culture and Stimulation
4.3. Extraction and Culture of Human Primary Epidermal Keratinocytes (NHEKs) and Human Primary Dermal Fibroblasts (NHDFs)
4.4. Co-Culture System
4.5. Animal Model
4.6. Western Blot Analysis
4.7. RNA Extraction and qRT-PCR
4.8. RNA Interference
4.9. Histology, Immunohistochemistry (IHC), and Immunofluorescence Staining
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Moradi Tuchayi, S.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Primers 2015, 1, 15029. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, H.P. From new findings in acne pathogenesis to new approaches in treatment. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 8–12. [Google Scholar] [CrossRef]
- Isard, O.; Knol, A.C.; Ariès, M.F.; Nguyen, J.M.; Khammari, A.; Castex-Rizzi, N.; Dréno, B. Propionibacterium acnes activates the IGF-1/IGF-1R system in the epidermis and induces keratinocyte proliferation. J. Investig. Dermatol. 2011, 131, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mitra, R.; Maitra, A.; Gupta, S.; Kumaran, S.; Chakrabortty, A.; Majumder, P.P. Sebum and Hydration Levels in Specific Regions of Human Face Significantly Predict the Nature and Diversity of Facial Skin Microbiome. Sci. Rep. 2016, 6, 36062. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Achermann, Y.; Goldstein, E.J.; Coenye, T.; Shirtliff, M.E. Propionibacterium acnes: From commensal to opportunistic biofilm-associated implant pathogen. Clin. Microbiol. Rev. 2014, 27, 419–440. [Google Scholar] [CrossRef] [Green Version]
- Jahns, A.C.; Lundskog, B.; Ganceviciene, R.; Palmer, R.H.; Golovleva, I.; Zouboulis, C.C.; McDowell, A.; Patrick, S.; Alexeyev, O.A. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: A case-control study. Br. J. Dermatol. 2012, 167, 50–58. [Google Scholar] [CrossRef]
- O’Neill, A.M.; Gallo, R.L. Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome 2018, 6, 177. [Google Scholar] [CrossRef]
- Kuzina, E.S.; Ung, P.M.; Mohanty, J.; Tome, F.; Choi, J.; Pardon, E.; Steyaert, J.; Lax, I.; Schlessinger, A.; Schlessinger, J.; et al. Structures of ligand-occupied β-Klotho complexes reveal a molecular mechanism underlying endocrine FGF specificity and activity. Proc. Natl. Acad. Sci. USA 2019, 116, 7819–7824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhu, L.; Hu, J.; Guo, R.; Ye, S.; Liu, F.; Wang, D.; Zhao, Y.; Hu, A.; Wang, X.; et al. FGF21 Attenuated LPS-Induced Depressive-Like Behavior via Inhibiting the Inflammatory Pathway. Front. Pharmacol. 2020, 11, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaich, G.; Chien, J.Y.; Fu, H.; Glass, L.C.; Deeg, M.A.; Holland, W.L.; Kharitonenkov, A.; Bumol, T.; Schilske, H.K.; Moller, D.E. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 2013, 18, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Shao, Y.; Wu, F.; Wang, Y.; Xiong, R.; Zheng, J.; Tian, H.; Wang, B.; Wang, Y.; Zhang, Y.; et al. FGF21 Prevents Angiotensin II-Induced Hypertension and Vascular Dysfunction by Activation of ACE2/Angiotensin-(1-7) Axis in Mice. Cell Metab. 2018, 27, 1323–1337.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X. The FGF metabolic axis. Front. Med. 2019, 13, 511–530. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Li, J.; Ma, J.; Yang, X.; Ni, M.; Zhang, Y.; Li, X.; Lin, Z.; Gong, F. Fibroblast growth factor 21 alleviates acute pancreatitis via activation of the Sirt1-autophagy signalling pathway. J. Cell Mol. Med. 2020, 24, 5341–5351. [Google Scholar] [CrossRef] [Green Version]
- Zarei, M.; Pizarro-Delgado, J.; Barroso, E.; Palomer, X.; Vázquez-Carrera, M. Targeting FGF21 for the Treatment of Nonalcoholic Steatohepatitis. Trends Pharmacol. Sci. 2020, 41, 199–208. [Google Scholar] [CrossRef]
- Kendall, A.C.; Nicolaou, A. Bioactive lipid mediators in skin inflammation and immunity. Prog. Lipid Res. 2013, 52, 141–164. [Google Scholar] [CrossRef]
- Rebane, A.; Runnel, T.; Aab, A.; Maslovskaja, J.; Rückert, B.; Zimmermann, M.; Plaas, M.; Kärner, J.; Treis, A.; Pihlap, M.; et al. MicroRNA-146a alleviates chronic skin inflammation in atopic dermatitis through suppression of innate immune responses in keratinocytes. J. Allergy Clin. Immunol. 2014, 134, 836–847.e11. [Google Scholar] [CrossRef]
- Beylot, C.; Auffret, N.; Poli, F.; Claudel, J.P.; Leccia, M.T.; Del Giudice, P.; Dreno, B. Propionibacterium acnes: An update on its role in the pathogenesis of acne. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 271–278. [Google Scholar] [CrossRef]
- Curtis, B.J.; Radek, K.A. Cholinergic regulation of keratinocyte innate immunity and permeability barrier integrity: New perspectives in epidermal immunity and disease. J. Investig. Dermatol. 2012, 132, 28–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, Z.; Liu, K.; Wu, Y.; Jiang, D.; Lai, Y. Staphylococcal LTA-Induced miR-143 Inhibits Propionibacterium acnes-Mediated Inflammatory Response in Skin. J. Investig. Dermatol. 2016, 136, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, C.A.; Stephen, S.; Ashchyan, H.J.; James, W.D.; Micheletti, R.G.; Rosenbach, M. Neutrophilic dermatoses: Pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J. Am. Acad. Dermatol. 2018, 79, 987–1006. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A. Idiopathic pulmonary fibrosis: An epithelial/fibroblastic cross-talk disorder. Respir. Res. 2002, 3, 3. [Google Scholar] [CrossRef]
- Xu, H.; Ling, M.; Xue, J.; Dai, X.; Sun, Q.; Chen, C.; Liu, Y.; Zhou, L.; Liu, J.; Luo, F.; et al. Exosomal microRNA-21 derived from bronchial epithelial cells is involved in aberrant epithelium-fibroblast cross-talk in COPD induced by cigarette smoking. Theranostics 2018, 8, 5419–5433. [Google Scholar] [CrossRef]
- Aden, N.; Nuttall, A.; Shiwen, X.; de Winter, P.; Leask, A.; Black, C.M.; Denton, C.P.; Abraham, D.J.; Stratton, R.J. Epithelial cells promote fibroblast activation via IL-1alpha in systemic sclerosis. J. Investig. Dermatol. 2010, 130, 2191–2200. [Google Scholar] [CrossRef] [Green Version]
- Muir, A.B.; Lim, D.M.; Benitez, A.J.; Modayur Chandramouleeswaran, P.; Lee, A.J.; Ruchelli, E.D.; Spergel, J.M.; Wang, M.L. Esophageal epithelial and mesenchymal cross-talk leads to features of epithelial to mesenchymal transition in vitro. Exp. Cell Res. 2013, 319, 850–859. [Google Scholar] [CrossRef] [Green Version]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [Green Version]
- Werling, D.; Jungi, T.W. TOLL-like receptors linking innate and adaptive immune response. Vet. Immunol. Immunopathol. 2003, 91, 1–12. [Google Scholar] [CrossRef]
- Nakao, M.; Sugaya, M.; Fujita, H.; Miyagaki, T.; Morimura, S.; Shibata, S.; Asano, Y.; Sato, S. Deficiency Exacerbates Imiquimod-Induced Psoriasis-Like Skin Inflammation through Decrease in Regulatory T Cells and Impaired IL-10 Production. Int. J. Mol. Sci. 2020, 21, 8560. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kanada, K.; Macleod, D.T.; Borkowski, A.W.; Morizane, S.; Nakatsuji, T.; Cogen, A.L.; Gallo, R.L. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J. Investig. Dermatol. 2011, 131, 688–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Choteau, L.; Vancraeyneste, H.; Le Roy, D.; Dubuquoy, L.; Romani, L.; Jouault, T.; Poulain, D.; Sendid, B.; Calandra, T.; Roger, T.; et al. Role of TLR1, TLR2 and TLR6 in the modulation of intestinal inflammation and Candida albicans elimination. Gut Pathog. 2017, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, R.; Xu, H.; Liu, Y.; Du, L.; Duan, Z.; Tong, J.; He, Y.; Chen, Q.; Chen, X.; Li, M. miR-146a Inhibits Biofilm-Derived Cutibacterium acnes-Induced Inflammatory Reactions in Human Keratinocytes. J. Investig. Dermatol. 2019, 139, 2488–2496.e4. [Google Scholar] [CrossRef]
- Lee, W.R.; Kim, K.H.; An, H.J.; Kim, J.Y.; Chang, Y.C.; Chung, H.; Park, Y.Y.; Lee, M.L.; Park, K.K. The protective effects of melittin on Propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 1922–1930. [Google Scholar] [CrossRef] [Green Version]
- Kiezel-Tsugunova, M.; Kendall, A.C.; Nicolaou, A. Fatty acids and related lipid mediators in the regulation of cutaneous inflammation. Biochem. Soc. Trans. 2018, 46, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.W.; Choi, J.W.; Park, K.C.; Youn, S.W. Facial sebum affects the development of acne, especially the distribution of inflammatory acne. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 301–306. [Google Scholar] [CrossRef]
- Zaenglein, A.L. Acne Vulgaris. N. Engl. J. Med. 2018, 379, 1343–1352. [Google Scholar] [CrossRef]
- Lim, H.W.; Collins, S.A.B.; Resneck, J.S., Jr.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.e2. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cheng, Y.; Gu, J.; Wang, S.; Zhou, S.; Wang, Y.; Tan, Y.; Feng, W.; Fu, Y.; Mellen, N.; et al. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of Type 1 diabetic mice. Clin. Sci. 2016, 130, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Roberts, D.; Kharitonenkov, A.; Zhang, B.; Hanson, S.M.; Li, Y.C.; Zhang, L.Q.; Wu, Y.H. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci. Rep. 2013, 3, 2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wang, Y.; Zhang, Z.; Liu, Q.; Gu, J. Cardioprotective effects of fibroblast growth factor 21 against doxorubicin-induced toxicity via the SIRT1/LKB1/AMPK pathway. Cell Death Dis. 2017, 8, e3018. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, D.M.; Rocheleau, J.V. The FGF21 Receptor Signaling Complex: Klothoβ, FGFR1c, and Other Regulatory Interactions. Vitam. Horm. 2016, 101, 17–58. [Google Scholar] [PubMed]
- Canady, J.; Arndt, S.; Karrer, S.; Bosserhoff, A.K. Increased KGF expression promotes fibroblast activation in a double paracrine manner resulting in cutaneous fibrosis. J. Investig. Dermatol. 2013, 133, 647–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Tong, G.; Fan, J.; Shen, Y.; Wang, N.; Gong, W.; Hu, Z.; Zhu, K.; Li, X.; Jin, L.; et al. FGF21 promotes migration and differentiation of epidermal cells during wound healing via SIRT1 dependent autophagy. Br. J. Pharmacol. 2021, 179, 1102–1121. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.M.; Mangelsdorf, D.J.; Kliewer, S.A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 2015, 26, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Wang, J.; Lu, S.; Chen, Z.; Fan, S.; Chen, D.; Xue, H.; Shi, W.; He, J. Short lipopeptides specifically inhibit the growth of Propionibacterium acnes with a dual antibacterial and anti-inflammatory action. Br. J. Pharmacol. 2019, 176, 2321–2335. [Google Scholar] [CrossRef]
- Xu, N.; Deng, W.; He, G.; Gan, X.; Gao, S.; Chen, Y.; Gao, Y.; Xu, K.; Qi, J.; Lin, H.; et al. Alpha- and gamma-mangostins exhibit anti-acne activities via multiple mechanisms. Immunopharmacol. Immunotoxicol. 2018, 40, 415–422. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, R.; Chen, Q.; Du, L.; Tong, J.; He, Y.; Xu, H.; Li, M. Curcumin suppresses interleukin-6 production in THP-1 monocytes induced by Propionibacterium acnes extracts via downregulation of Toll-like receptor 2 expression and the nuclear factor kappa B pathway. Br. J. Dermatol. 2019, 181, 1320–1322. [Google Scholar] [CrossRef]
- Lee, E.H.; Shin, J.H.; Kim, S.S.; Joo, J.H.; Choi, E.; Seo, S.R. Suppression of Propionibacterium acnes-Induced Skin Inflammation by Laurus nobilis Extract and Its Major Constituent Eucalyptol. Int. J. Mol. Sci. 2019, 20, 3510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.; Lee, M.Y. Kaempferia parviflora Extract as a Potential Anti-Acne Agent with Anti-Inflammatory, Sebostatic and Anti-Propionibacterium acnes Activity. Int. J. Mol. Sci. 2018, 19, 3457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.M.; Choo, J.E.; Lee, H.J.; Kim, K.N.; Chang, S.E. Epidermal Growth Factor Attenuated the Expression of Inflammatory Cytokines in Human Epidermal Keratinocyte Exposed to Propionibacterium acnes. Ann. Dermatol. 2018, 30, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronet, C.; Passelli, K.; Charmoy, M.; Scarpellino, L.; Myburgh, E.; Hauyon La Torre, Y.; Turco, S.; Mottram, J.C.; Fasel, N.; Luther, S.A.; et al. TLR2 Signaling in Skin Nonhematopoietic Cells Induces Early Neutrophil Recruitment in Response to Leishmania major Infection. J. Investig. Dermatol. 2019, 139, 1318–1328. [Google Scholar] [CrossRef] [Green Version]
- Baud, V.; Collares, D. Post-Translational Modifications of RelB NF-κB Subunit and Associated Functions. Cells 2016, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-López, A.; Bautista-de Lucio, V.M.; Serafín-López, J.; Robles-Sánchez, E.; Garfias, Y. Amniotic membrane modulates innate immune response inhibiting PRRs expression and NF-κB nuclear translocation on limbal myofibroblasts. Exp. Eye Res. 2014, 127, 215–223. [Google Scholar] [CrossRef]
- Barygina, V.; Becatti, M.; Prignano, F.; Lotti, T.; Taddei, N.; Fiorillo, C. Fibroblasts to Keratinocytes Redox Signaling: The Possible Role of ROS in Psoriatic Plaque Formation. Antioxidants 2019, 8, 566. [Google Scholar] [CrossRef] [Green Version]
- Henrot, P.; Laurent, P.; Levionnois, E.; Leleu, D.; Pain, C.; Truchetet, M.E.; Cario, M. A Method for Isolating and Culturing Skin Cells: Application to Endothelial Cells, Fibroblasts, Keratinocytes, and Melanocytes From Punch Biopsies in Systemic Sclerosis Skin. Front. Immunol. 2020, 11, 566607. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Shen, Y.; Zhang, S.; Wang, N.; Luo, L.; Zhu, X.; Xu, X.; Cong, W.; Jin, L.; Zhu, Z. Suppression of Cutibacterium acnes-Mediated Inflammatory Reactions by Fibroblast Growth Factor 21 in Skin. Int. J. Mol. Sci. 2022, 23, 3589. https://doi.org/10.3390/ijms23073589
Yu Y, Shen Y, Zhang S, Wang N, Luo L, Zhu X, Xu X, Cong W, Jin L, Zhu Z. Suppression of Cutibacterium acnes-Mediated Inflammatory Reactions by Fibroblast Growth Factor 21 in Skin. International Journal of Molecular Sciences. 2022; 23(7):3589. https://doi.org/10.3390/ijms23073589
Chicago/Turabian StyleYu, Ying, Yingjie Shen, Siyi Zhang, Nan Wang, Lan Luo, Xinyi Zhu, Xiejun Xu, Weitao Cong, Litai Jin, and Zhongxin Zhu. 2022. "Suppression of Cutibacterium acnes-Mediated Inflammatory Reactions by Fibroblast Growth Factor 21 in Skin" International Journal of Molecular Sciences 23, no. 7: 3589. https://doi.org/10.3390/ijms23073589
APA StyleYu, Y., Shen, Y., Zhang, S., Wang, N., Luo, L., Zhu, X., Xu, X., Cong, W., Jin, L., & Zhu, Z. (2022). Suppression of Cutibacterium acnes-Mediated Inflammatory Reactions by Fibroblast Growth Factor 21 in Skin. International Journal of Molecular Sciences, 23(7), 3589. https://doi.org/10.3390/ijms23073589




