How Light Reactions of Photosynthesis in C4 Plants Are Optimized and Protected under High Light Conditions
Abstract
:1. Diversity of C4 Photosynthesis
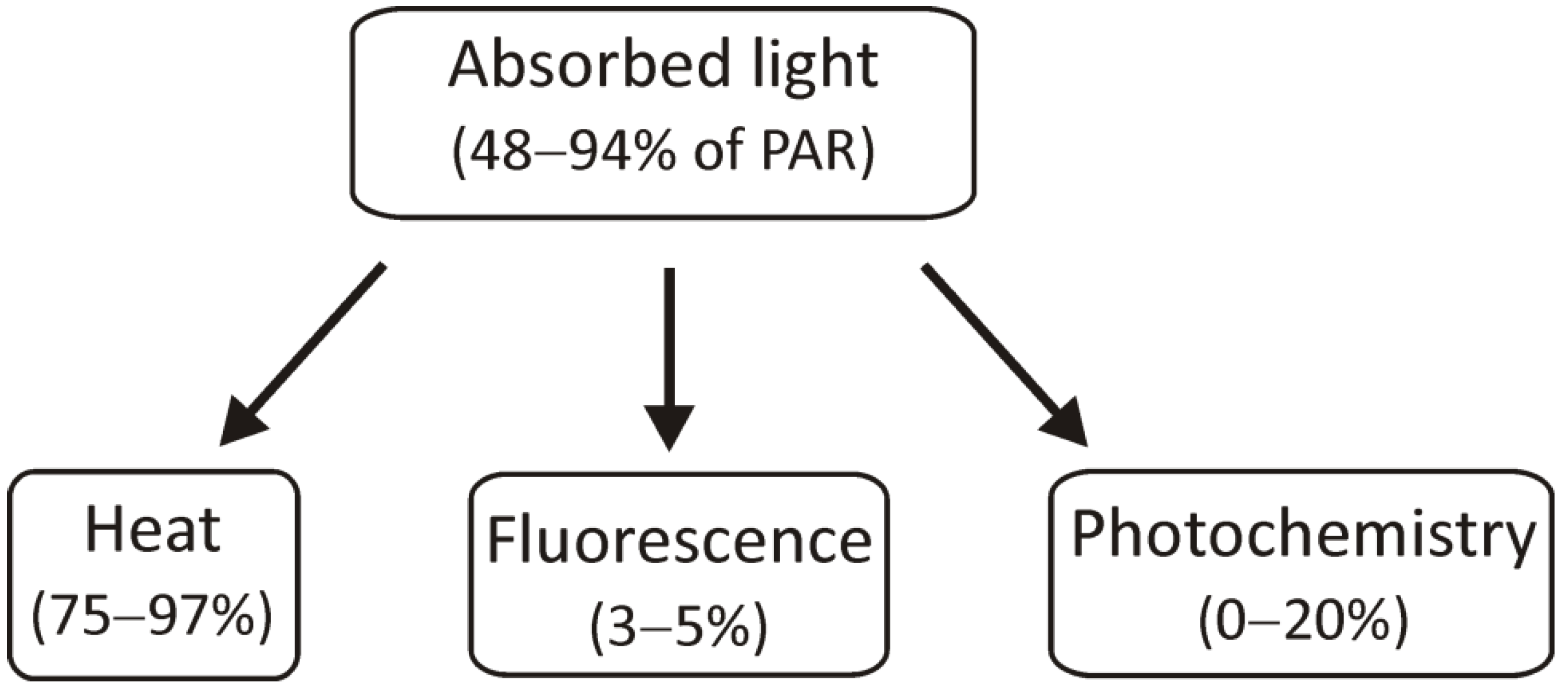
2. Ways of Light Energy Utilization: Balanced Distribution between Photosystems and Emission of Excess Energy as a Heat
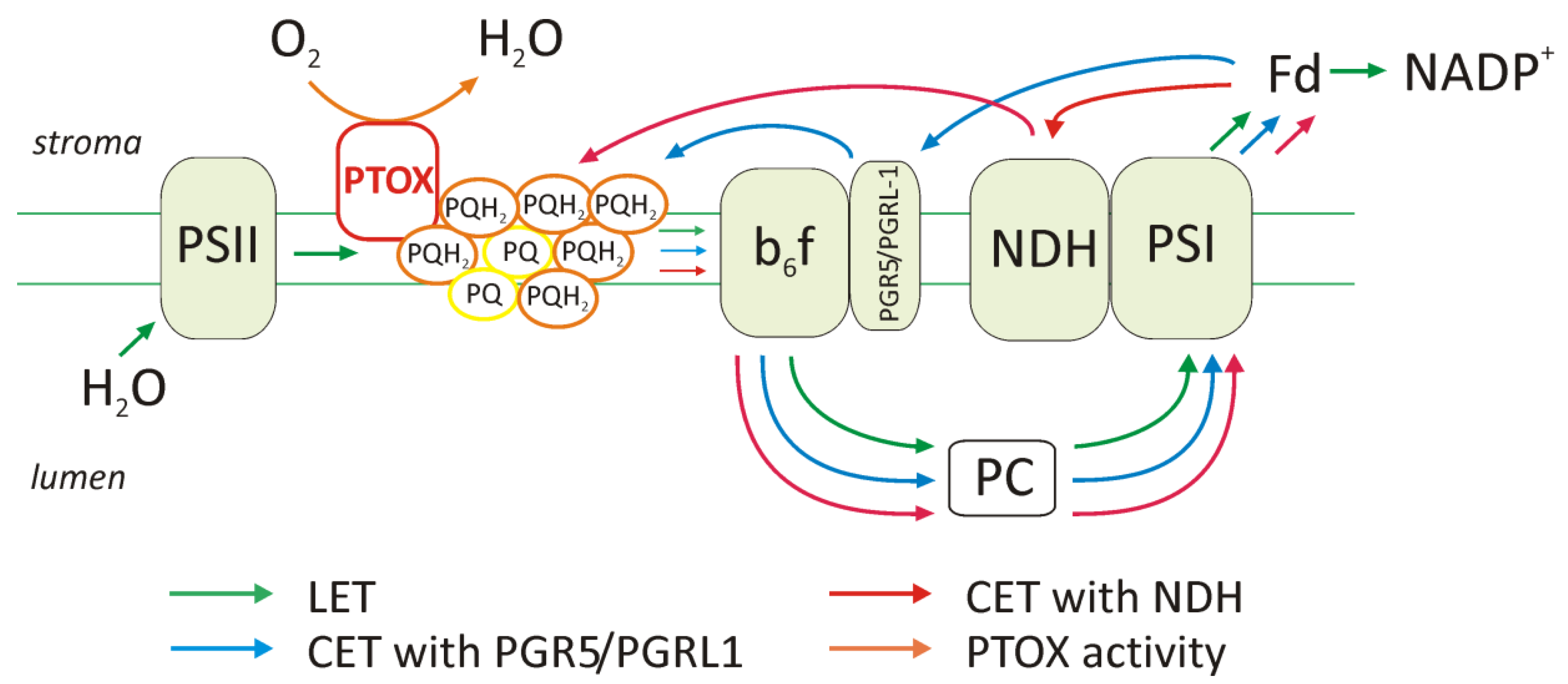
2.1. Elevation of Cyclic Electron Transport Components
2.2. Function of PTOX Protein and Chlororespiration
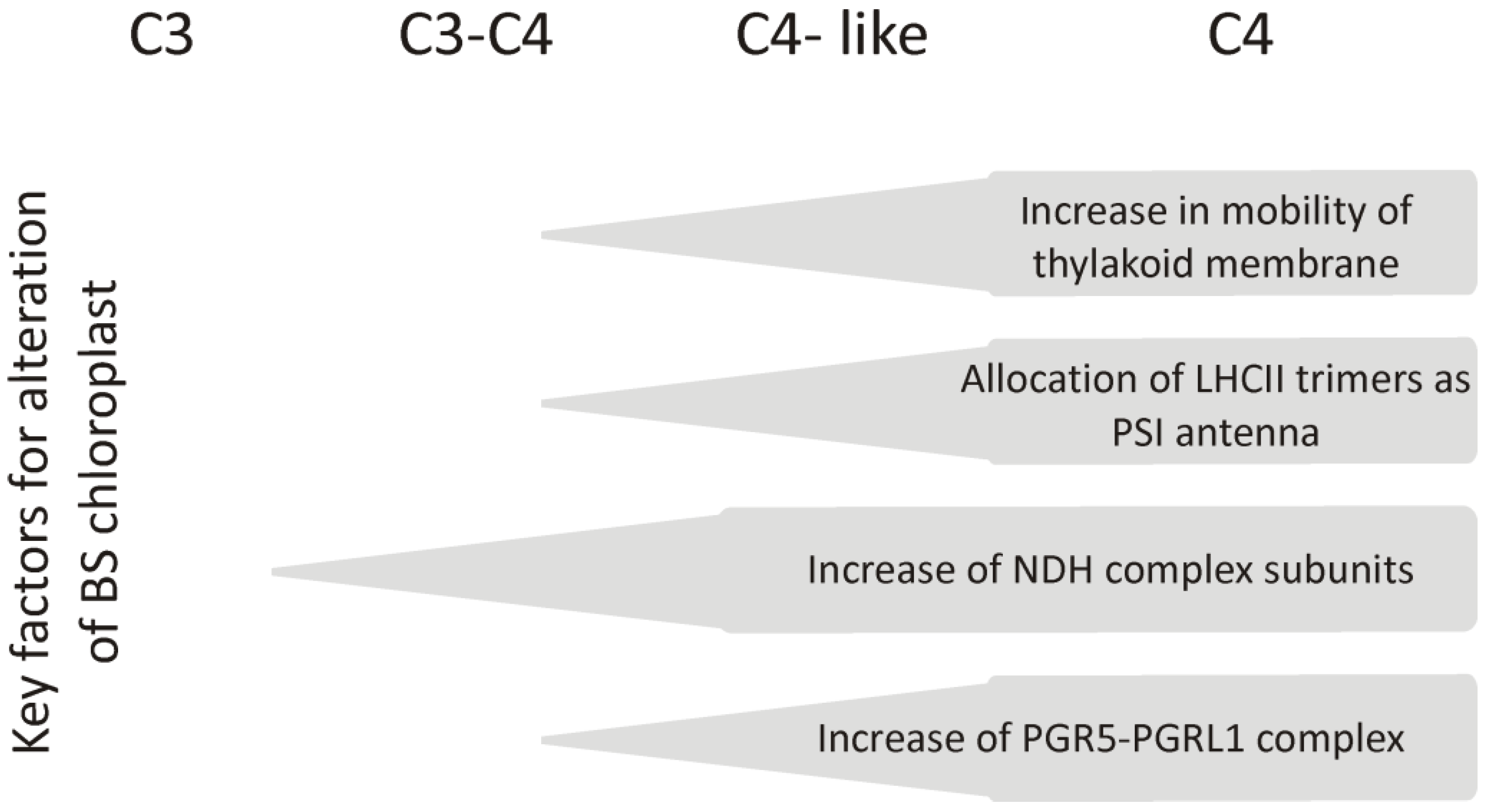
2.3. Changes in the Amount of Thylakoid Complexes and Rearrangement of Super- and Megacomplexes
2.4. Photoinhibition and Role of D1 Protein Phosphorylation
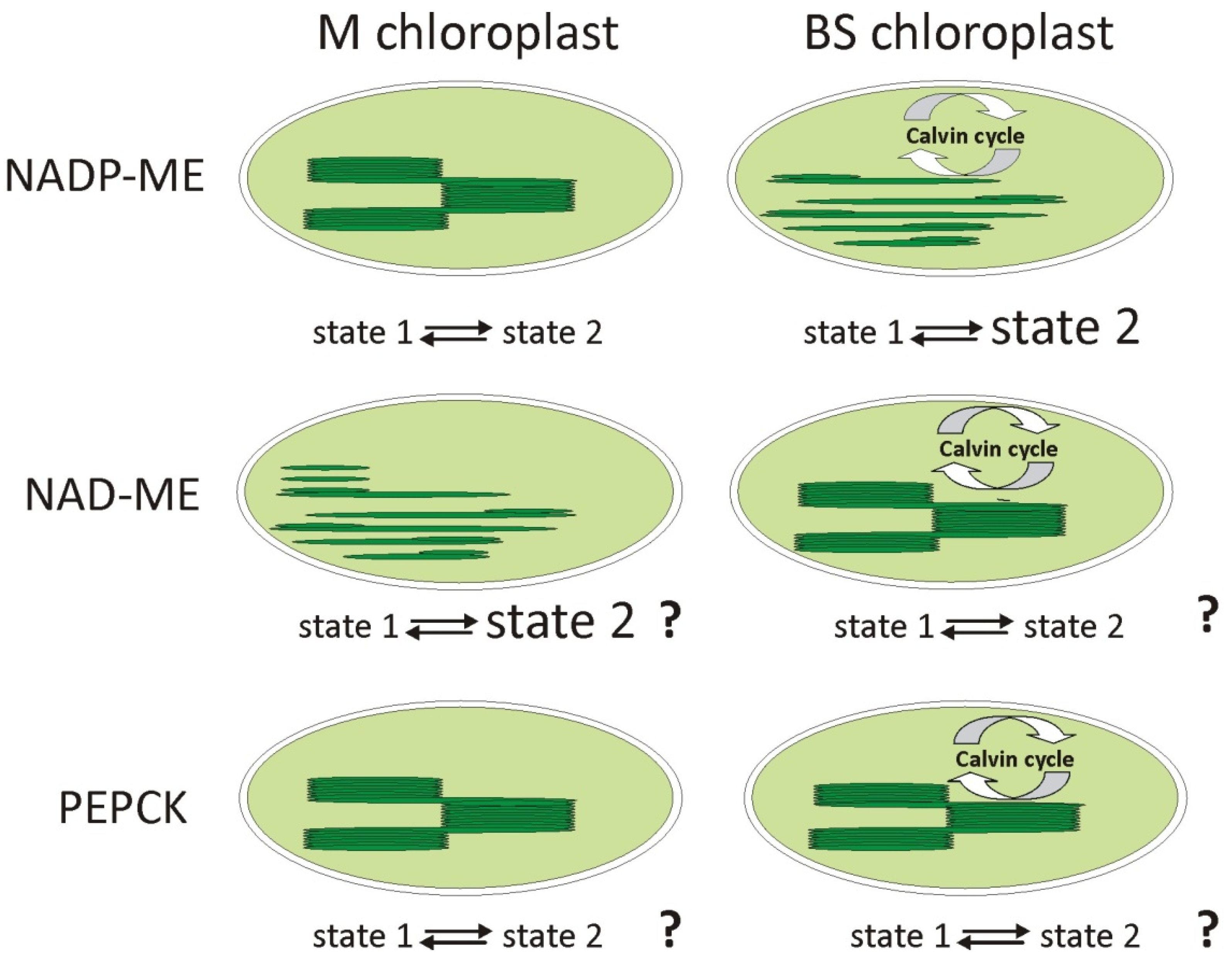
2.5. State Transitions and Phosphorylation of LHCII
2.6. Xanthophyll Cycle and Heat Dissipation
3. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Hatch, M.D.; Slack, C.R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem. J. 1966, 101, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Slack, C.R.; Hatch, M.D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem. J. 1967, 103, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osmond, C.B. Beta-carboxylation during photosynthesis in Atriplex. Biochim. Biophys. Acta 1967, 141, 197–199. [Google Scholar] [CrossRef]
- Hatch, M.D. C4 photosynthesis: A unique elend of modified biochemistry, anatomy and ultrastructure. Biochim. Biophys. Acta Rev. Bioenerg. 1987, 895, 81–106. [Google Scholar] [CrossRef]
- Gutierrez, M.; Gracen, V.E.; Edwards, G.E. Biochemical and cytological relationships in C4 plants. Planta 1974, 119, 279–300. [Google Scholar] [CrossRef]
- Hatch, M.; Kagawa, T.; Craig, S. Subdivision of C4-Pathway Species Based on Differing C4 Acid Decarboxylating Systems and Ultrastructural Features. Funct. Plant Biol. 1975, 2, 111–128. [Google Scholar] [CrossRef]
- Furbank, R.T. Evolution of the C4 photosynthetic mechanism: Are there really three C4 acid decarboxylation types? J. Exp. Bot. 2011, 62, 3103–3108. [Google Scholar] [CrossRef] [Green Version]
- Edwards, G.E.; Voznesenskaya, E.V. Chapter 4 C4 Photosynthesis: Kranz Forms and Single-Cell C4 in Terrestrial Plants. In C4 Photosynthesis and Related CO2 Concentrating Mechanisms; Springer: Dordrecht, The Netherlands, 2011; pp. 29–61. [Google Scholar] [CrossRef]
- Walker, B.J.; Kramer, D.M.; Fisher, N.; Fu, X. Flexibility in the Energy Balancing Network of Photosynthesis Enables Safe Operation under Changing Environmental Conditions. Plants 2020, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Hatch, M.D. C4 Photosynthesis: An Unlikely Process Full of Surprises. Plant Cell Physiol. 1992, 33, 333–342. [Google Scholar] [CrossRef]
- Hatch, M.D.; Osmond, C.B. 4. Compartmentation and Transport in C4 Photosynthesis. In Encyclopedia of Plant Physiology, New Series Vol 3, Transport in Plants III; Stocking, C.R., Heber, U., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1976; pp. 144–184. [Google Scholar]
- Yoshimura, Y.; Kubota, F.; Ueno, O. Structural and biochemical bases of photorespiration in C4 plants: Quantification of organelles and glycine decarboxylase. Planta 2004, 220, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.D.; Schenk, H.J.; Thorsch, J.A.; Ferren, W.R. Leaf anatomy and subgeneric affiliations of C3 and C4 species of Suaeda (Chenopodiaceae) in North America. Am. J. Bot. 1997, 84, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Voznesenskaya, E.V.; Franceschi, V.R.; Pyankov, V.I.; Edwards, G.E. Anatomy, chloroplast structure and compartmentation of enzymes relative to photosynthetic mechanisms in leaves and cotyledons of species in the tribe Salsoleae (Chenopodiaceae). J. Exp. Bot. 1999, 50, 1779–1795. [Google Scholar] [CrossRef]
- Voznesenskaya, E.V.; Franceschi, V.R.; Chuong, S.D.X.; Edwards, G.E. Functional characterization of phosphoenolpyruvate carboxykinase-type C4 leaf anatomy: Immuno-, cytochemical and ultrastructural analyses. Ann. Bot. 2006, 98, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Furbank, R.; Hatch, M. Mechanism of C 4 Photosynthesis: The Size and Composition of the Inorganic Carbon Pool in Bundle Sheath Cells. Plant Physiol. 1987, 85, 958–95864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnell, J.N.; Hatch, M.D. Low Bundle Sheath Carbonic Anhydrase Is Apparently Essential for Effective C4 Pathway Operation. Plant Physiol. 1988, 86, 1252–1256. [Google Scholar] [CrossRef] [Green Version]
- Sage, R.F.; Christin, P.-A.; Edwards, E.J. The C4 plant lineages of planet Earth. J. Exp. Bot. 2011, 62, 3155–3169. [Google Scholar] [CrossRef]
- Sage, R.F.; Stata, M. Photosynthetic diversity meets biodiversity: The C4 plant example. J. Plant Physiol. 2015, 172, 104–119. [Google Scholar] [CrossRef]
- Brown, R.H. A Difference in N Use Efficiency in C3 and C4 Plants and its Implications in Adaptation and Evolution. Crop Sci. 1978, 18, 93–98. [Google Scholar] [CrossRef]
- Sage, R.F.; Pearcy, R.W.; Seemann, J.R. The Nitrogen Use Efficiency of C3 and C4 Plants: III. Leaf Nitrogen Effects on the Activity of Carboxylating Enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol. 1987, 85, 355. [Google Scholar] [CrossRef] [Green Version]
- Jordan, D.B.; Ogren, W.L. The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase. Planta 1984, 161, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F. A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: Species number, evolutionary lineages, and Hall of Fame. J. Exp. Bot. 2017, 68, e11–e28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianconi, M.E.; Dunning, L.T.; Moreno-Villena, J.J.; Osborne, C.P.; Christin, P.A. Gene duplication and dosage effects during the early emergence of C4 photosynthesis in the grass genus Alloteropsis. J. Exp. Bot. 2018, 69, 1967–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, B.P.; Aubry, S.; Hibberd, J.M. Molecular evolution of genes recruited into C4 photosynthesis. Trends Plant Sci. 2012, 17, 213–220. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Furbank, R.T. Strategies for improving C4 photosynthesis. Curr. Opin. Plant Biol. 2016, 31, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, Y.; Murata, N. Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Appl. Microbiol. Biotechnol. 2014, 98, 8777–8796. [Google Scholar] [CrossRef]
- Ghannoum, O. C4 photosynthesis and water stress. Ann. Bot. 2009, 103, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Osborne, C.P.; Freckleton, R.P. Ecological selection pressures for C4 photosynthesis in the grasses. Proc. R. Soc. B Biol. Sci. 2009, 276, 1753–1760. [Google Scholar] [CrossRef] [Green Version]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Ishikawa, N.; Takabayashi, A.; Noguchi, K.; Tazoe, Y.; Yamamoto, H.; Von Caemmerer, S.; Sato, F.; Endo, T. NDH-Mediated Cyclic Electron Flow Around Photosystem I is Crucial for C4 Photosynthesis. Plant Cell Physiol. 2016, 57, 2020–2028. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, N.; Iwano, M.; Havaux, M.; Yokota, A.; Munekage, Y.N. Promotion of cyclic electron transport around photosystem I during the evolution of NADP-malic enzyme-type C4 photosynthesis in the genus Flaveria. New Phytol. 2013, 199, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Hertle, A.P.; Blunder, T.; Wunder, T.; Pesaresi, P.; Pribil, M.; Armbruster, U.; Leister, D. PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol. Cell 2013, 49, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darie, C.C.; Biniossek, M.L.; Winter, V.; Mutschler, B.; Haehnel, W. Isolation and structural characterization of the Ndh complex from mesophyll and bundle sheath chloroplasts of Zea mays. FEBS J. 2005, 272, 2705–2716. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, A.; Funk, E.; Westhoff, P.; Steinmüller, K. Differential expression of plastome-encoded ndh genes in mesophyll and bundle-sheath chloroplasts of the C4 plant Sorghum bicolor indicates that the complex I-homologous NAD(P)H-plastoquinone oxidoreductase is involved in cyclic electron transport. Planta 1996, 199, 276–281. [Google Scholar] [CrossRef]
- Majeran, W.; Zybailov, B.; Ytterberg, A.J.; Dunsmore, J.; Sun, Q.; van Wijk, K.J. Consequences of C4 differentiation for chloroplast membrane proteomes in maize mesophyll and bundle sheath cells. Mol. Cell. Proteom. 2008, 7, 1609–1638. [Google Scholar] [CrossRef] [Green Version]
- Munekage, Y.N.; Taniguchi, Y.Y. Promotion of Cyclic Electron Transport around Photosystem I with the Development of C4 Photosynthesis. Plant Cell Physiol. 2016, 57, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Takabayashi, A.; Kishine, M.; Asada, K.; Endo, T.; Sato, F. Differential use of two cyclic electron flows around photosystem I for driving CO2-concentration mechanism in C4 photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 16898–16903. [Google Scholar] [CrossRef] [Green Version]
- Sazanov, L.A.; Burrows, P.A.; Nixon, P.J. The chloroplast Ndh complex mediates the dark reduction of the plastoquinone pool in response to heat stress in tobacco leaves. FEBS Lett. 1998, 429, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Rogowski, P.; Wasilewska-Dębowska, W.; Urban, A.; Romanowska, E. Maize bundle sheath chloroplasts—A unique model of permanent State 2. Environ. Exp. Bot. 2018, 155, 321–331. [Google Scholar] [CrossRef]
- Romanowska, E.; Powikrowska, M.; Zienkiewicz, M.; Drozak, A.; Pokorska, B. High light induced accumulation of two isoforms of the CF1 α-subunit in mesophyll and bundle sheath chloroplasts of C4 plants. Acta Biochim. Pol. 2008, 55, 175–182. [Google Scholar] [CrossRef]
- Shikanai, T. Chloroplast NDH: A different enzyme with a structure similar to that of respiratory NADH dehydrogenase. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Liszkay, A.; Feilke, K. The Dual Role of the Plastid Terminal Oxidase PTOX: Between a Protective and a Pro-oxidant Function. Front. Plant Sci. 2016, 6, 1147:1–1147:3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennon, A.M.; Prommeenate, P.; Nixon, P.J. Location, expression and orientation of the putative chlororespiratory enzymes, Ndh and IMMUTANS, in higher-plant plastids. Planta 2003, 218, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Streb, P.; Josse, E.M.; Gallouët, E.; Baptist, F.; Kuntz, M.; Cornic, G. Evidence for alternative electron sinks to photosynthetic carbon assimilation in the high mountain plant species Ranunculus glacialis. Plant. Cell Environ. 2005, 28, 1123–1135. [Google Scholar] [CrossRef]
- Majeran, W.; van Wijk, K.J. Cell-type-specific differentiation of chloroplasts in C4 plants. Trends Plant Sci. 2009, 14, 100–109. [Google Scholar] [CrossRef]
- Essemine, J.; Lyu, M.J.A.; Qu, M.; Perveen, S.; Khan, N.; Song, Q.; Chen, G.; Zhu, X.G. Contrasting Responses of Plastid Terminal Oxidase Activity Under Salt Stress in Two C4 Species With Different Salt Tolerance. Front. Plant Sci. 2020, 11, 1009. [Google Scholar] [CrossRef]
- Rumeau, D.; Peltier, G.; Cournac, L. Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant. Cell Environ. 2007, 30, 1041–1051. [Google Scholar] [CrossRef]
- Leong, T.Y.; Anderson, J.M. Adaptation of the thylakoid membranes of pea chloroplasts to light intensities. I. Study on the distribution of chlorophyll-protein complexes. Photosynth. Res. 1984, 5, 105–115. [Google Scholar] [CrossRef]
- Drozak, A.; Romanowska, E. Acclimation of mesophyll and bundle sheath chloroplasts of maize to different irradiances during growth. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 1539–1546. [Google Scholar] [CrossRef] [Green Version]
- Pfannschmidt, T.; Nilsson, A.; Allen, J.F. Photosynthetic control of chloroplast gene expression. Nature 1999, 397, 625–628. [Google Scholar] [CrossRef]
- Urban, A.; Rogowski, P.; Wasilewska-Dębowska, W.; Romanowska, E. Effect of light on the rearrangements of PSI super-and megacomplexes in the non-appressed thylakoid domains of maize mesophyll chloroplasts. Plant Sci. 2020, 301, 110655. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demmig-Adams, B.; Cohu, C.M.; Muller, O.; Adams, W.W. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynth. Res. 2012, 113, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Kangasjärvi, S.; Neukermans, J.; Li, S.; Aro, E.M.; Noctor, G. Photosynthesis, photorespiration, and light signalling in defence responses. J. Exp. Bot. 2012, 63, 1619–1636. [Google Scholar] [CrossRef] [Green Version]
- Tikkanen, M.; Grebe, S. Switching off photoprotection of photosystem I—A novel tool for gradual PSI photoinhibition. Physiol. Plant. 2018, 162, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Mekala, N.R.; Aro, E.M. Photosystem II photoinhibition-repair cycle protects Photosystem I from irreversible damage. Biochim. Biophys. Acta 2014, 1837, 210–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballottari, M.; Alcocer, M.J.P.; D’Andrea, C.; Viola, D.; Ahn, T.K.; Petrozza, A.; Polli, D.; Fleming, G.R.; Cerullo, G.; Bassi, R. Regulation of photosystem I light harvesting by zeaxanthin. Proc. Natl. Acad. Sci. USA 2014, 111, E2431–E2438. [Google Scholar] [CrossRef] [Green Version]
- Lima-Melo, Y.; Alencar, V.T.C.B.; Lobo, A.K.M.; Sousa, R.H.V.; Tikkanen, M.; Aro, E.M.; Silveira, J.A.G.; Gollan, P.J. Photoinhibition of Photosystem I Provides Oxidative Protection During Imbalanced Photosynthetic Electron Transport in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 916:1–916:13. [Google Scholar] [CrossRef] [Green Version]
- Pokorska, B.; Zienkiewicz, M.; Powikrowska, M.; Drozak, A.; Romanowska, E. Differential turnover of the photosystem II reaction centre D1 protein in mesophyll and bundle sheath chloroplasts of maize. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 1161–1169. [Google Scholar] [CrossRef] [Green Version]
- Rintamäki, E.; Kettunen, R.; Aro, E.M. Differential D1 Dephosphorylation in Functional and Photodamaged Photosystem II Centers: Dephosphorylation is a prerequisite for degradation of damaged D1. J. Biol. Chem. 1996, 271, 14870–14875. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Paakkarinen, V.; Van Wijk, K.J.; Aro, E.M. Biogenesis of the chloroplast-encoded D1 protein: Regulation of translation elongation, insertion, and assembly into photosystem II. Plant Cell 2000, 12, 1769–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikkanen, M.; Grieco, M.; Kangasjärvi, S.; Aro, E.M. Thylakoid Protein Phosphorylation in Higher Plant Chloroplasts Optimizes Electron Transfer under Fluctuating Light. Plant Physiol. 2010, 152, 723–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zienkiewicz, M.; Krupnik, T.; Drozak, A.; Golke, A.; Romanowska, E. Chloramphenicol acetyltransferase- a new selectable marker in stable nuclear transformation of the red alga Cyanidioschyzon merolae. Protoplasma 2017, 254, 587–596. [Google Scholar] [CrossRef]
- Sharma, P.K.; Hall, D.O. Changes in Carotenoid Composition and Photosynthesis in Sorghum under High Light and Salt Stresses. J. Plant Physiol. 1992, 140, 661–666. [Google Scholar] [CrossRef]
- Bergantino, E.; Segalla, A.; Brunetta, A.; Teardo, E.; Rigoni, F.; Giacometti, G.M.; Szabò, I. Light- and pH-dependent structural changes in the PsbS subunit of photosystem II. Proc. Natl. Acad. Sci. USA 2003, 100, 15265–15270. [Google Scholar] [CrossRef] [Green Version]

| Process Taking Place in Chloroplasts | C3 Plants | C4 Plants |
|---|---|---|
| Xanthophyll cycle and heat dissipation | Typical, occurring with the zeaxanthin and PsbS protein [65]. | |
| State transitions and LHCII phosphorylation | Function of state 1 and state 2, depending on phosphorylation of the LHCII antenna [64]. | Permanent state 2 in agranal BS maize (NADP-ME) chloroplasts. LHCII in phosphorylated form, regardless of the condition [41]. |
| Photoinhibition and phosphorylation of D1 protein | Damaged D1 is directed to the thylakoid stroma, dephosphorylated, and then degraded. | D1 degradation is faster in the BS chloroplast of maize [61]. Photodamage of some PSII pools for protection against PSI excess [57]. |
| Cyclic electron transport components | Lower ATP demand resulting from metabolism. | Elevation of the CET ad alternative CET pathway with NDH complex for higher efficiency of ATP production [33,36,37]. |
| PTOX functioning and chlororespiration | Minor importance, activity mainly under stressful conditions. | High amount and activity in maize BS chloroplasts for better protection against ROS formation during elevated cyclic electron transport [47]. |
| Changes in antenna and reaction centers amount | Higher content of LHCII antenna in low light intensities. Higher content of reaction centers at high light intensities [51]. | |
| Additional mechanism(s) | No data available. | Formation of megacomplexes in maize mesophyll chloroplasts [53]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewska-Dębowska, W.; Zienkiewicz, M.; Drozak, A. How Light Reactions of Photosynthesis in C4 Plants Are Optimized and Protected under High Light Conditions. Int. J. Mol. Sci. 2022, 23, 3626. https://doi.org/10.3390/ijms23073626
Wasilewska-Dębowska W, Zienkiewicz M, Drozak A. How Light Reactions of Photosynthesis in C4 Plants Are Optimized and Protected under High Light Conditions. International Journal of Molecular Sciences. 2022; 23(7):3626. https://doi.org/10.3390/ijms23073626
Chicago/Turabian StyleWasilewska-Dębowska, Wioleta, Maksymilian Zienkiewicz, and Anna Drozak. 2022. "How Light Reactions of Photosynthesis in C4 Plants Are Optimized and Protected under High Light Conditions" International Journal of Molecular Sciences 23, no. 7: 3626. https://doi.org/10.3390/ijms23073626






