Histamine Causes Pyroptosis of Liver by Regulating Gut-Liver Axis in Mice
Abstract
:1. Introduction
2. Results
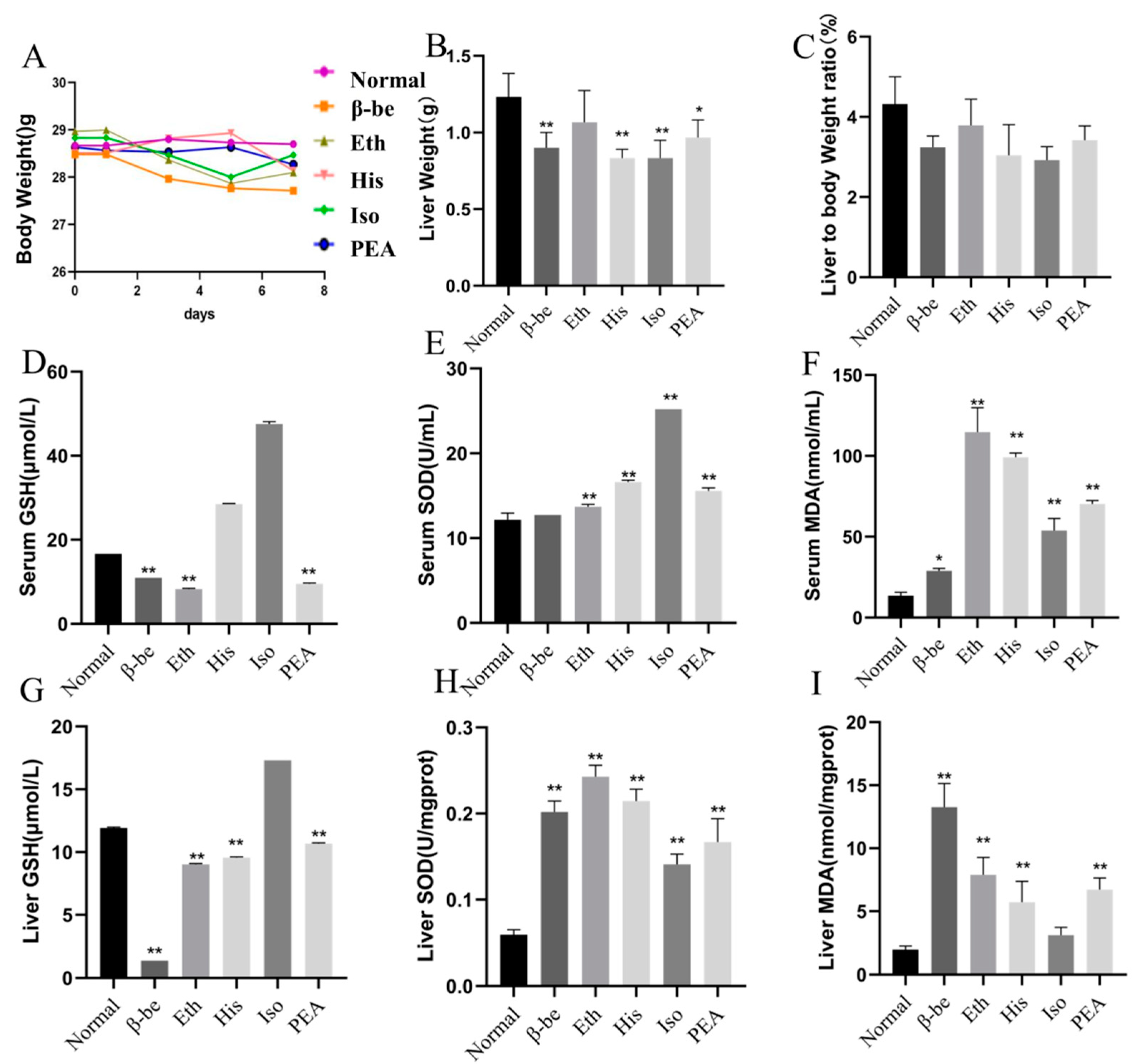
2.1. Effects of His, PEA, β-Be, and Iso on Body Weight, Liver Weight, and Level of Oxidative Stress in Serum and Liver of Mice
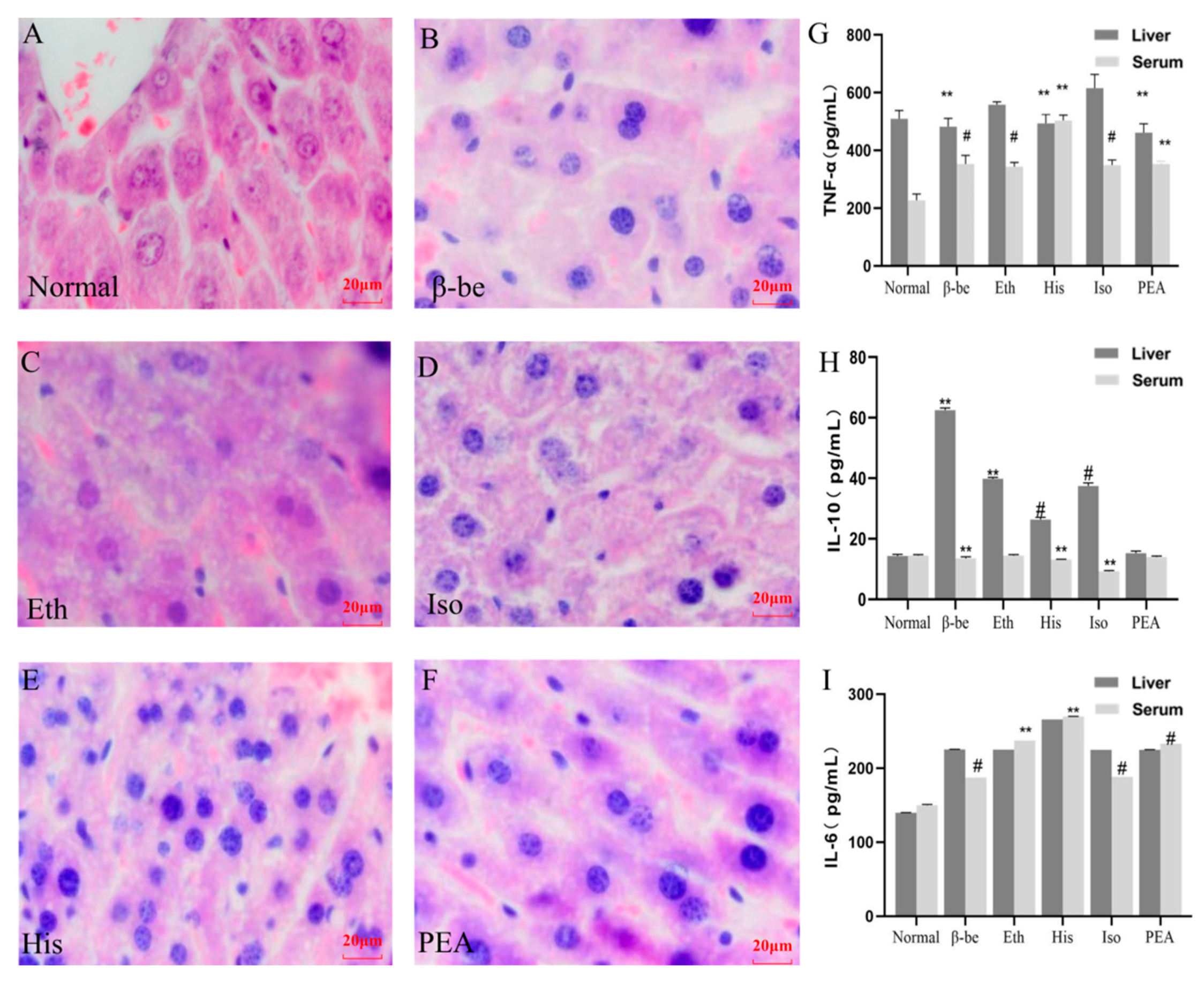
2.2. Effects of His, PEA, β-Be, and Iso on Inflammatory Cytokines in the Liver and Serum
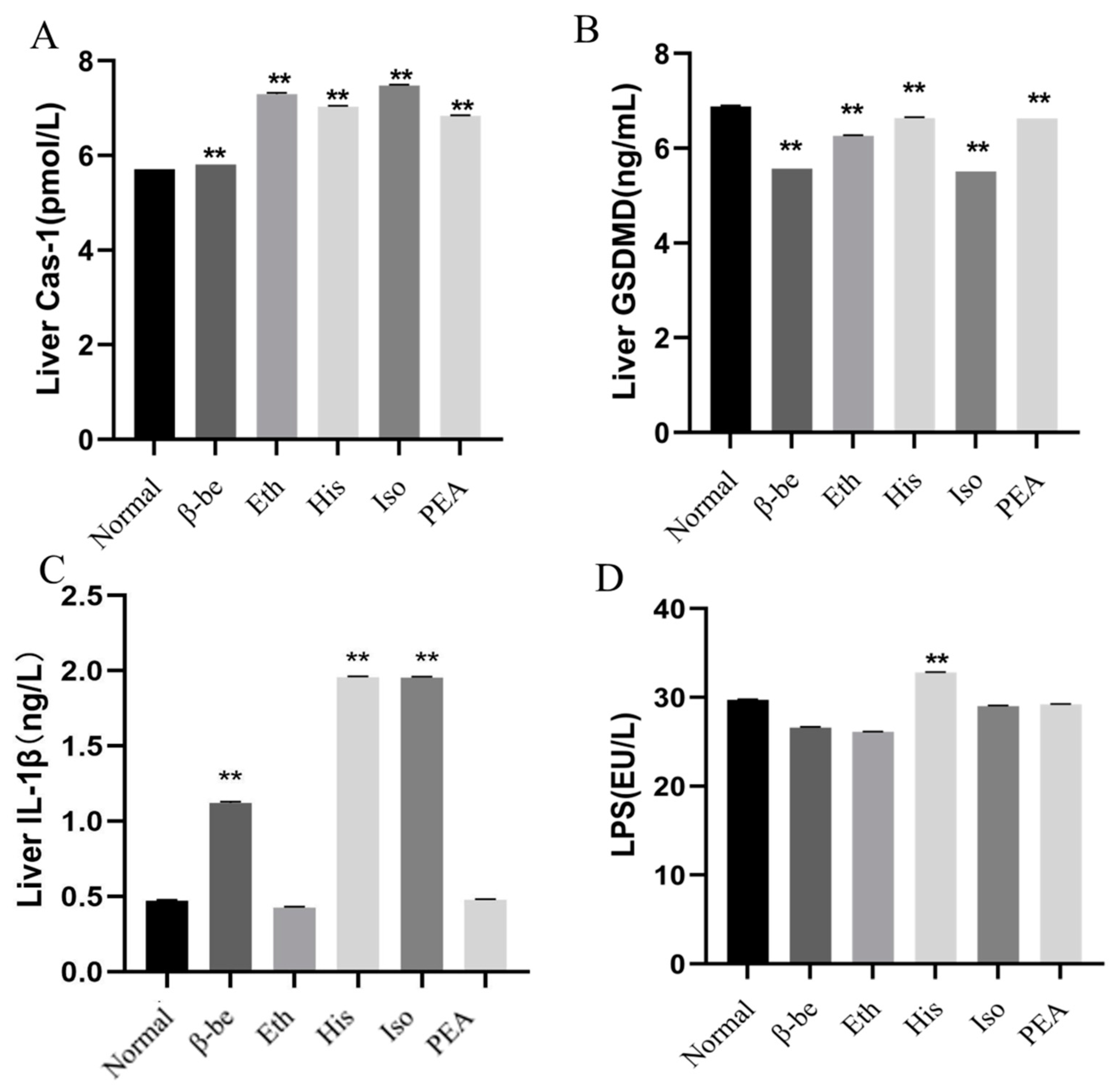
2.3. Effects of His, PEA, β-Be, and Iso on Pyroptosis in the Liver
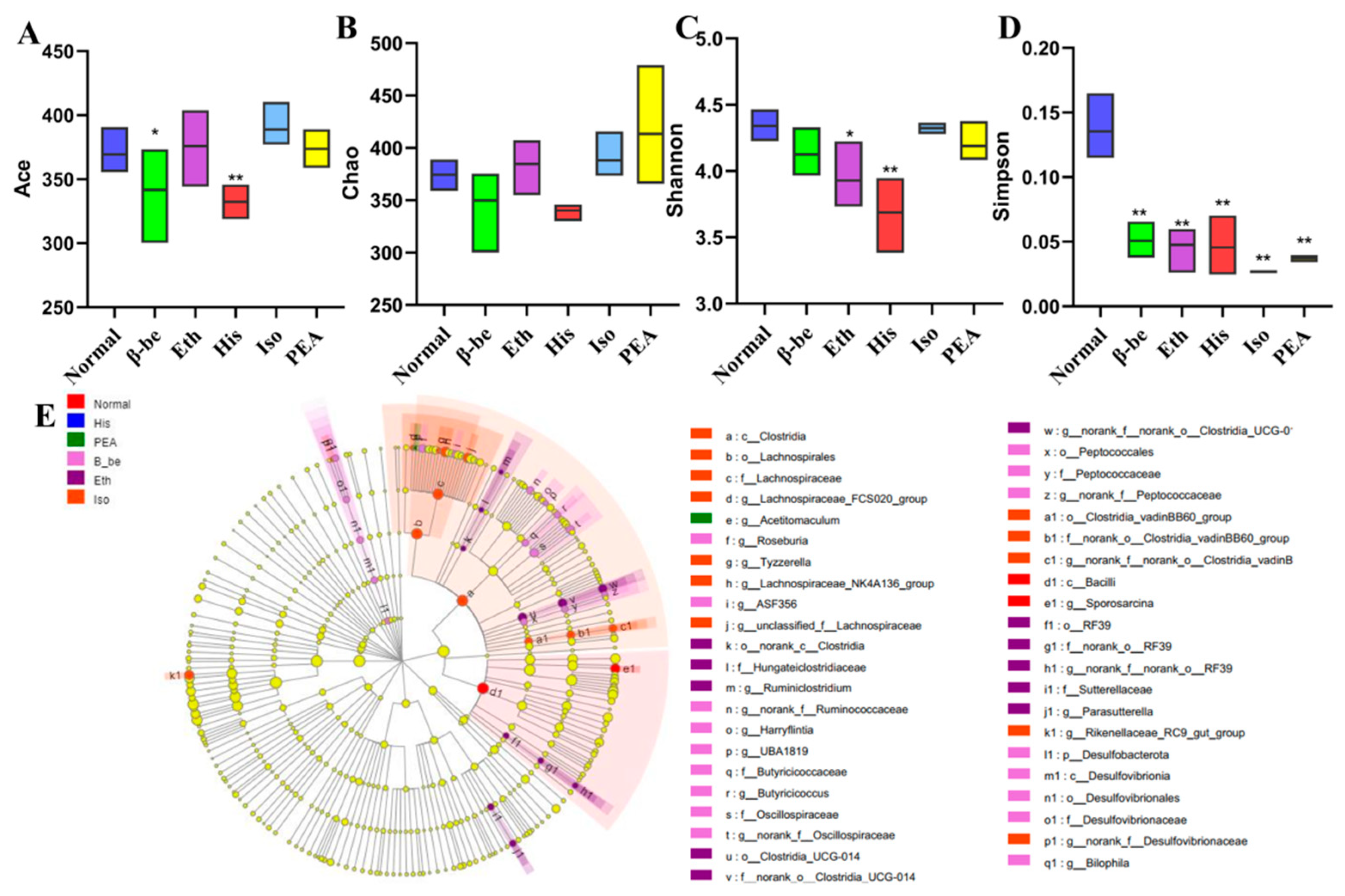
2.4. Effects of His, PEA, β-Be, and Iso on Gut Microbial Community Structure and Composition
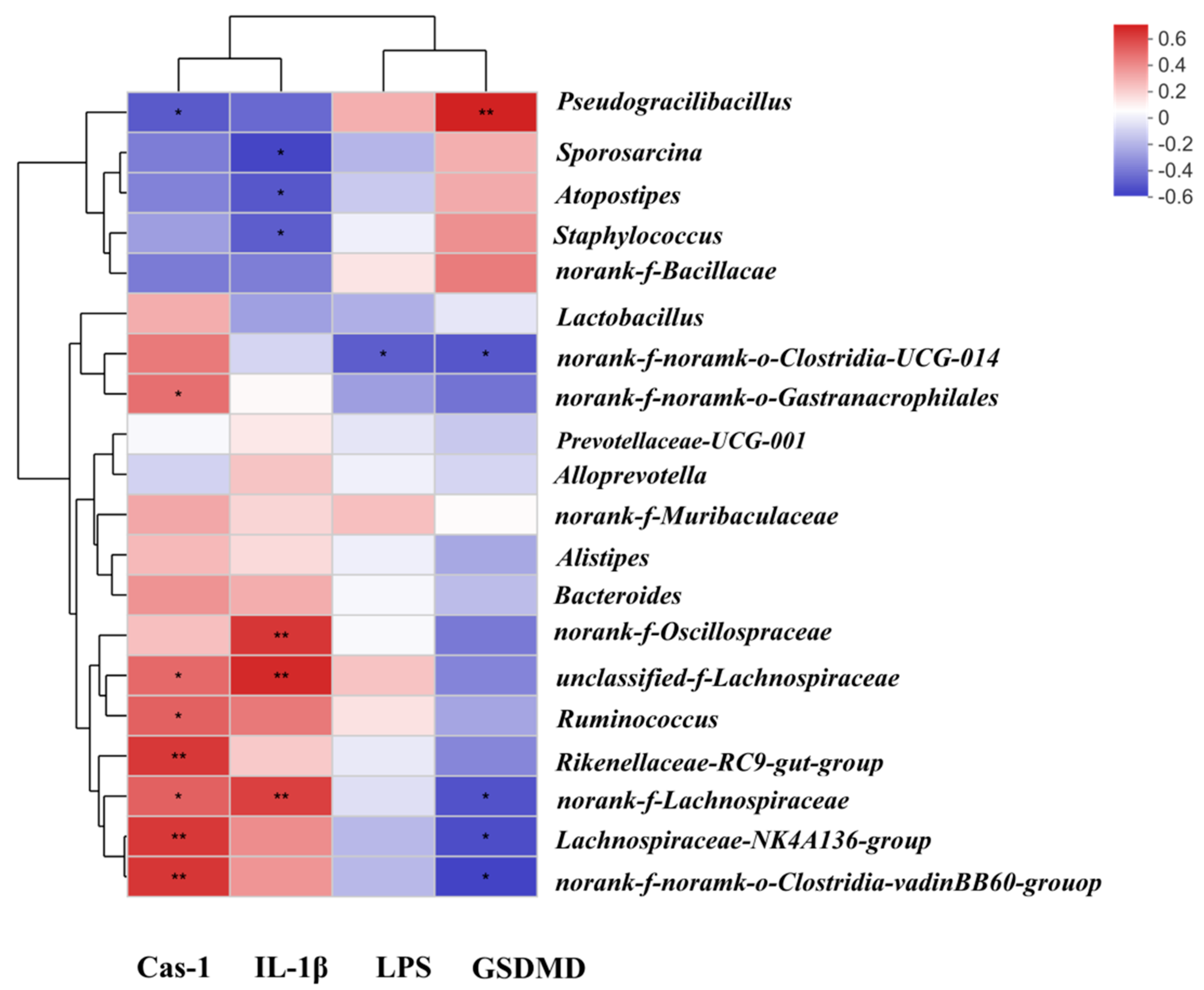
2.5. Correlation among Gut Microbiota, Liver Pyroptosis and Microbial Metabolism
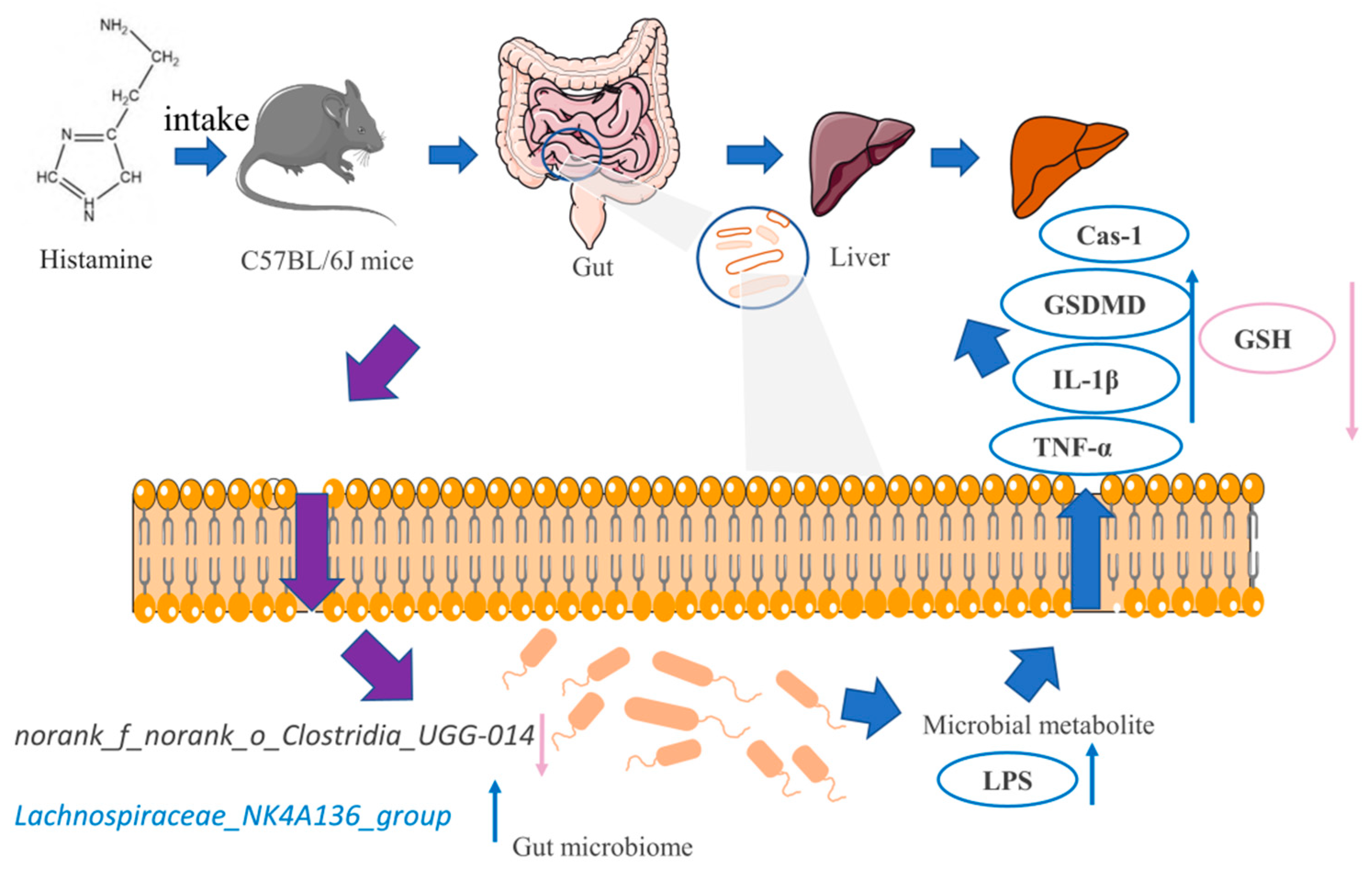
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Experimental Treatment
4.3. Biomarker Analysis of Serum and Liver
4.4. Histopathological Analysis of Liver Tissues
4.5. Validation of the Cellular Pyroptosis Pathway
4.6. Determination of LPS in Gut Tissues
4.7. Analysis of Gut Microbiome
4.8. Correlation Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Fan, G.; Fu, Z.; Wang, W.; Xu, Y.; Teng, C.; Zhang, C.; Yang, R.; Sun, B.; Li, X. Effects of fortification of Daqu with various yeasts on microbial community structure and flavor metabolism. Food. Res. Int. 2020, 129, 108837. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Ye, G.; Zhang, K. Diversity, function, and application of Clostridium in Chinese strong flavor Baijiu ecosystem: A review. J. Food. Sci. 2018, 83, 1193–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Xu, E.; Long, J.; Wang, F.; Xu, X.; Jin, Z.; Jiao, A. Measurement of fermentation parameters of Chinese rice wine using Raman spectroscopy combined with linear and non-linear regression methods. Food. Cont. 2015, 56, 95–102. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y.; Qian, M.C. Aroma characterization of Chinese rice wine by gas chromatography-olfactometry, chemical quantitative analysis, and aroma reconstitution. J. Agric. Food. Chem. 2013, 61, 11295–11302. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Yu, J.X.; Wei, X.L.; Ji, Z.W.; Zhou, Z.L.; Meng, X.Y.; Mao, J. Sequencing-based screening of functional microorganisms to decrease the formation of biogenic amines in Chinese rice wine. Food. Cont. 2016, 64, 98–103. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Mao, J.; Yu, Z.; Lin, Z.; Mao, J. New insights into the impacts of Huangjiu components on intoxication. Food. Chem. 2020, 317, 126420. [Google Scholar] [CrossRef] [PubMed]
- Krymchantowski, A.V.; Jevoux, C.D.C. Wine and headache. Headache 2014, 54, 967–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Jiang, Y.; Wang, M.; Melaku, M.; Liu, L.; Zhao, Y.; Everaert, N.; Yi, B.; Zhang, H. Intestinal dysbiosis in nonalcoholic fatty liver disease (NAFLD): Focusing on the gut-liver axis. Crit. Rev. Food. Sci. Nutr. 2021, 18, 1–18. [Google Scholar] [CrossRef]
- Brandl, K.; Hartmann, P.; Jih, L.J.; Pizzo, D.P.; Argemi, J.; Ventura-Cots, M.; Coulter, S.; Liddle, C.; Ling, L.; Rossi, S.J.; et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J. Hepatol. 2018, 69, 396–405. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, H.H.L.; Kim, Y.; Lee, H.-B.; Lee, E.; Park, J.H.; Park, H.-Y. Corchorus olitorius L. ameliorates alcoholic liver disease by regulating gut-liver axis. J. Funct. Foods. 2021, 85, 10468. [Google Scholar] [CrossRef]
- Chu, H.; Duan, Y.; Yang, L.; Schnabl, B. Small metabolites, possible big changes: A microbiota-centered view of non-alcoholic fatty liver disease. Gut 2019, 68, 359–370. [Google Scholar] [CrossRef]
- Sharpton, S.; Schnabl, B.; Knight, R.; Loomba, R. Current concepts, opportunities, and challenges of gut microbiome-based personalized medicine in nonalcoholic fatty liver disease. Cell. Metab. 2021, 33, 21–32. [Google Scholar] [CrossRef]
- Friedman, E.S.; Li, Y.; Shen, T.C.D.; Jiang, J.; Chau, L.; Adorini, L.; Babakhani, F.; Edwards, J.; Shapiro, D.; Zhao, C.; et al. FXR-dependent modulation of the human small intestinal microbiome by the bile acid derivative obeticholic acid. Gastroenterology 2018, 155, 1741–1752.e5. [Google Scholar] [CrossRef] [Green Version]
- He, W.-T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell. Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Wang, S.; Wang, J. Mechanism and regulation of pyroptosis-mediated in cancer cell death. Chem. Biol. Int. 2020, 323, 109052. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wang, R.; Tan, H. Role of pyroptosis in cardiovascular diseases and its therapeutic implications. Int. J. Biol. Sci. 2019, 15, 1345–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, B.; Jeon, K.; Moon, S.; Lee, K.; Kim, W.-K.; Jeong, H.; Cha, K.H.; Lim, M.Y.; Kang, W.; Kweon, M.-N.; et al. Roseburia spp. abundance associates with alcohol consumption in humans and its administration ameliorates alcoholic fatty liver in mice. Cell. Host. Microbe. 2020, 27, 25–40.e6. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Szego, É.M.; Leonov, A.; Benito, E.; Becker, S.; Fischer, A.; Zweckstetter, M.; Outeiro, T.; Schneider, A. Translocator protein-ligand protects against neurodegeneration in the MPTP mouse model of parkinsonism. J. Neurosci. 2019, 39, 3752–3769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Zhan, L.; Lu, T.; Zhou, C.; Chen, X.; Dong, Y.; Lv, G.; Chen, S. Dendrobium officinale polysaccharides protected against ethanol-induced acute liver injury in vivo and in vitro via the TLR4/NF-kappaB signaling pathway. Cytokine 2020, 130, 155058. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Masaki, T.; Seike, M.; Yoshimatsu, H. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element-binding protein-1c (SREBP-1c). Exp. Bio. Med. 2007, 232, 614–621. [Google Scholar]
- Karatayli, E.; Hall, R.A.; Weber, S.N.; Dooley, S.; Lammert, F. Effect of alcohol on the interleukin 6-mediated inflammatory response in a new mouse model of acute-on-chronic liver injury. Biochim. Biophys. Acta. Mol. Basis. Dis. 2019, 1865, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netea, M.G.; Simon, A.; van de Veerdonk, F.; Kullberg, B.-J.; Van der Meer, J.W.M.; Joosten, L.A.B. IL-1beta processing in host defense: Beyond the inflammasomes. PLoS. Pathog. 2010, 6, e1000661. [Google Scholar] [CrossRef] [Green Version]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef] [PubMed]
- von Moltke, J.; Ji, M.; Liang, H.-E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, X.; Stamova, B.; Sharp, F.R. Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer’s disease brain: A review. Front. Aging. Neurosci. 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends. Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Liu, X.; Jin, M.; Yang, X.; Hu, X.; Li, C.; Zhao, K. Fubrick tea attenuates high-fat diet induced fat deposition and metabolic disorder by regulating gut microbiota and caffeine metabolism. Food. Funct. 2020, 11, 6971–6986. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Hu, R.; Wu, L.; Lv, X.; Li, X.; Zhao, L.; Liu, B. Ganoderma polysaccharide and chitosan synergistically ameliorate lipid metabolic disorders and modulate gut microbiota composition in high fat diet-fed golden hamsters. J. Food. Biochem. 2020, 44, e13109. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liu, S.; Ji, Z.; Chen, S.; Mao, J. Structure characterization of polysaccharide isolated from Huangjiu and its anti-inflammatory activity through MAPK signaling. Int. J. Food. Sci. Tech. 2019, 54, 1874–1883. [Google Scholar] [CrossRef]
- Mehmood, A.; Zhao, L.; Wang, C.; Hossen, I.; Raka, R.N.; Zhang, H. Stevia residue extract increases intestinal uric acid excretion via interactions with intestinal urate transporters in hyperuricemic mice. Food. Funct. 2019, 10, 7900–7912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Couvigny, B.; de Wouters, T.; Kaci, G.; Jacouton, E.; Delorme, C.; Dore, J.; Renault, P.; Blottière, H.M.; Guédon, E.; Lapaque, N. Commensal streptococcus salivarius modulates PPAR gamma transcriptional activity in human intestinal epithelial cells. PLoS ONE 2015, 10, e0125371. [Google Scholar] [CrossRef] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Shi, R.; Liu, Y.; Huang, L.; Chen, W.; Wang, C. Histamine Causes Pyroptosis of Liver by Regulating Gut-Liver Axis in Mice. Int. J. Mol. Sci. 2022, 23, 3710. https://doi.org/10.3390/ijms23073710
Luo Q, Shi R, Liu Y, Huang L, Chen W, Wang C. Histamine Causes Pyroptosis of Liver by Regulating Gut-Liver Axis in Mice. International Journal of Molecular Sciences. 2022; 23(7):3710. https://doi.org/10.3390/ijms23073710
Chicago/Turabian StyleLuo, Qiaoqiao, Ruoyu Shi, Yutong Liu, Libo Huang, Wei Chen, and Chengtao Wang. 2022. "Histamine Causes Pyroptosis of Liver by Regulating Gut-Liver Axis in Mice" International Journal of Molecular Sciences 23, no. 7: 3710. https://doi.org/10.3390/ijms23073710
APA StyleLuo, Q., Shi, R., Liu, Y., Huang, L., Chen, W., & Wang, C. (2022). Histamine Causes Pyroptosis of Liver by Regulating Gut-Liver Axis in Mice. International Journal of Molecular Sciences, 23(7), 3710. https://doi.org/10.3390/ijms23073710







