Optimization and Validation of Dosage Regimen for Ceftiofur against Pasteurella multocida in Swine by Physiological Based Pharmacokinetic–Pharmacodynamic Model
Abstract
:1. Introduction
2. Results
2.1. MIC, MBC, MPC and PAE of HB13
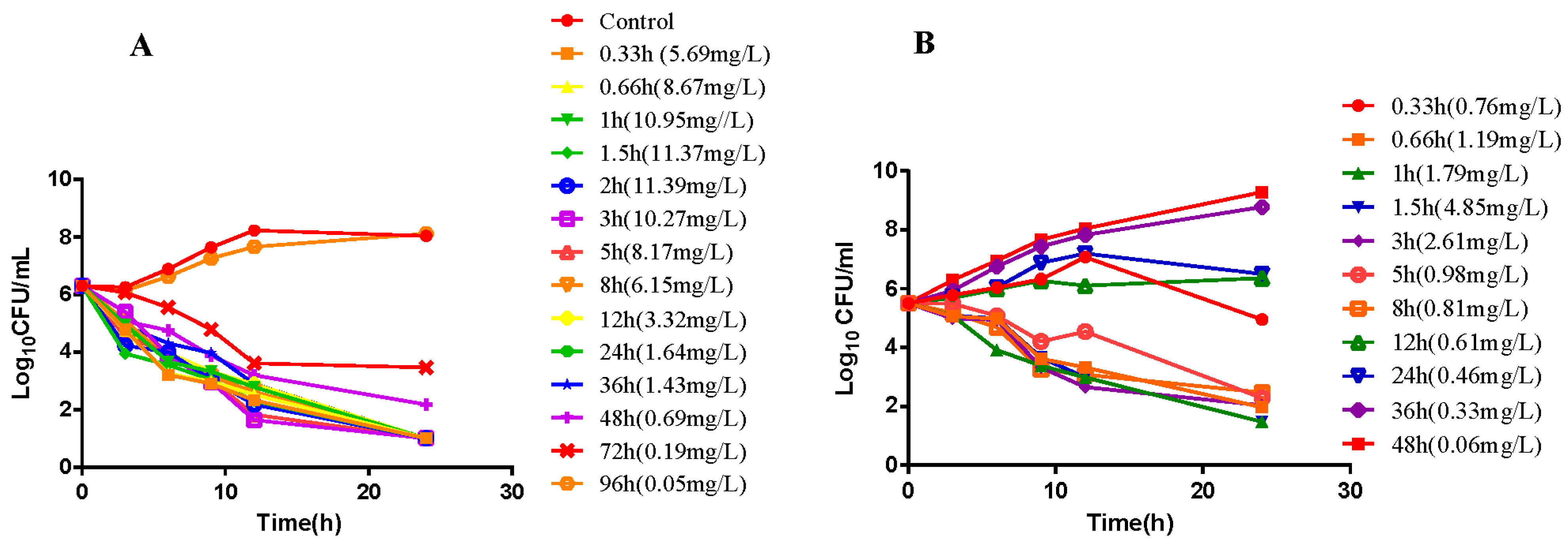
2.2. In Vitro and Ex Vivo Time-Killing Curves
2.3. Pharmacokinetic of Ceftiofur in Infected Pigs
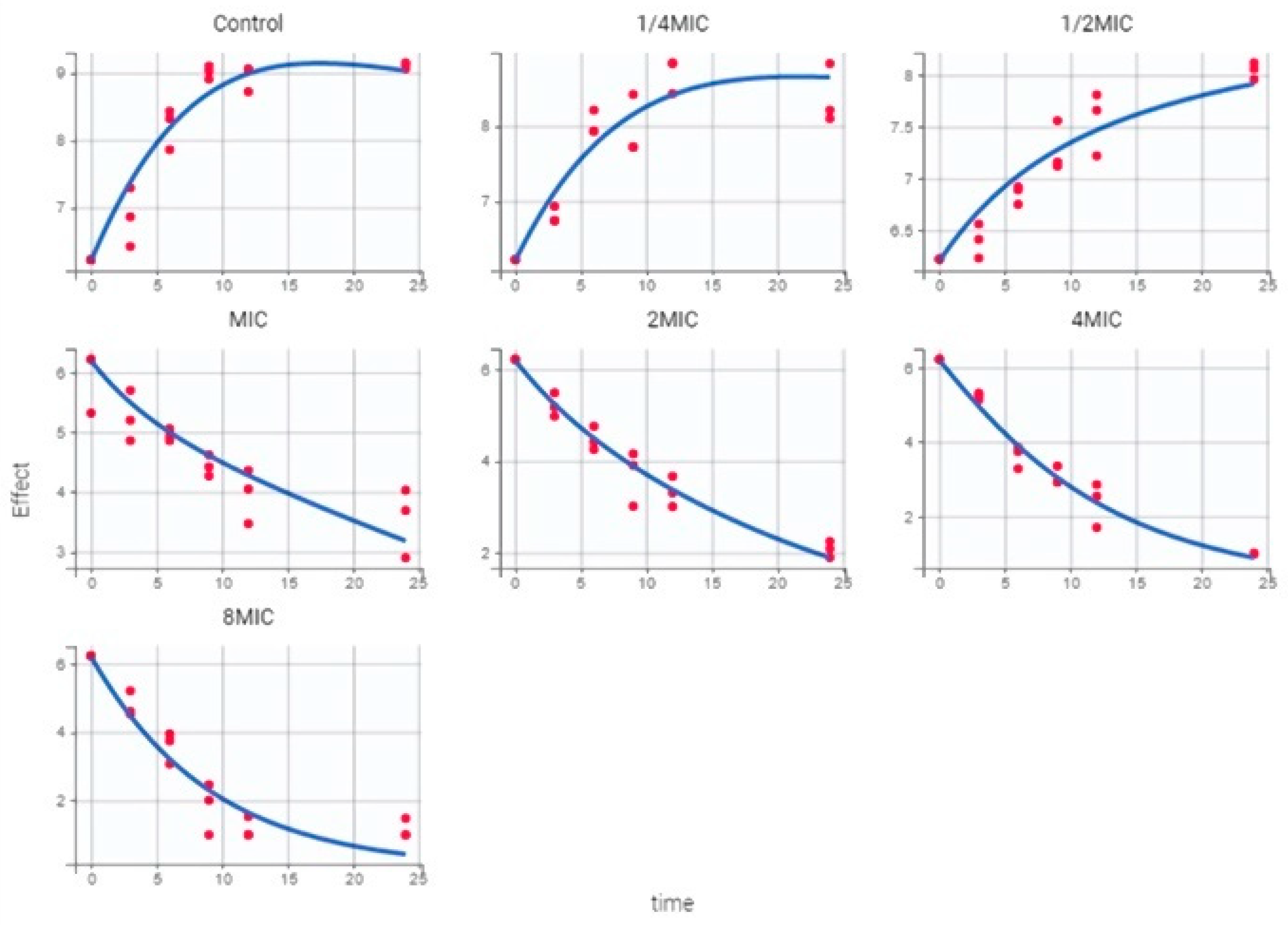
2.4. PK/PD Model and Dosing Regimen
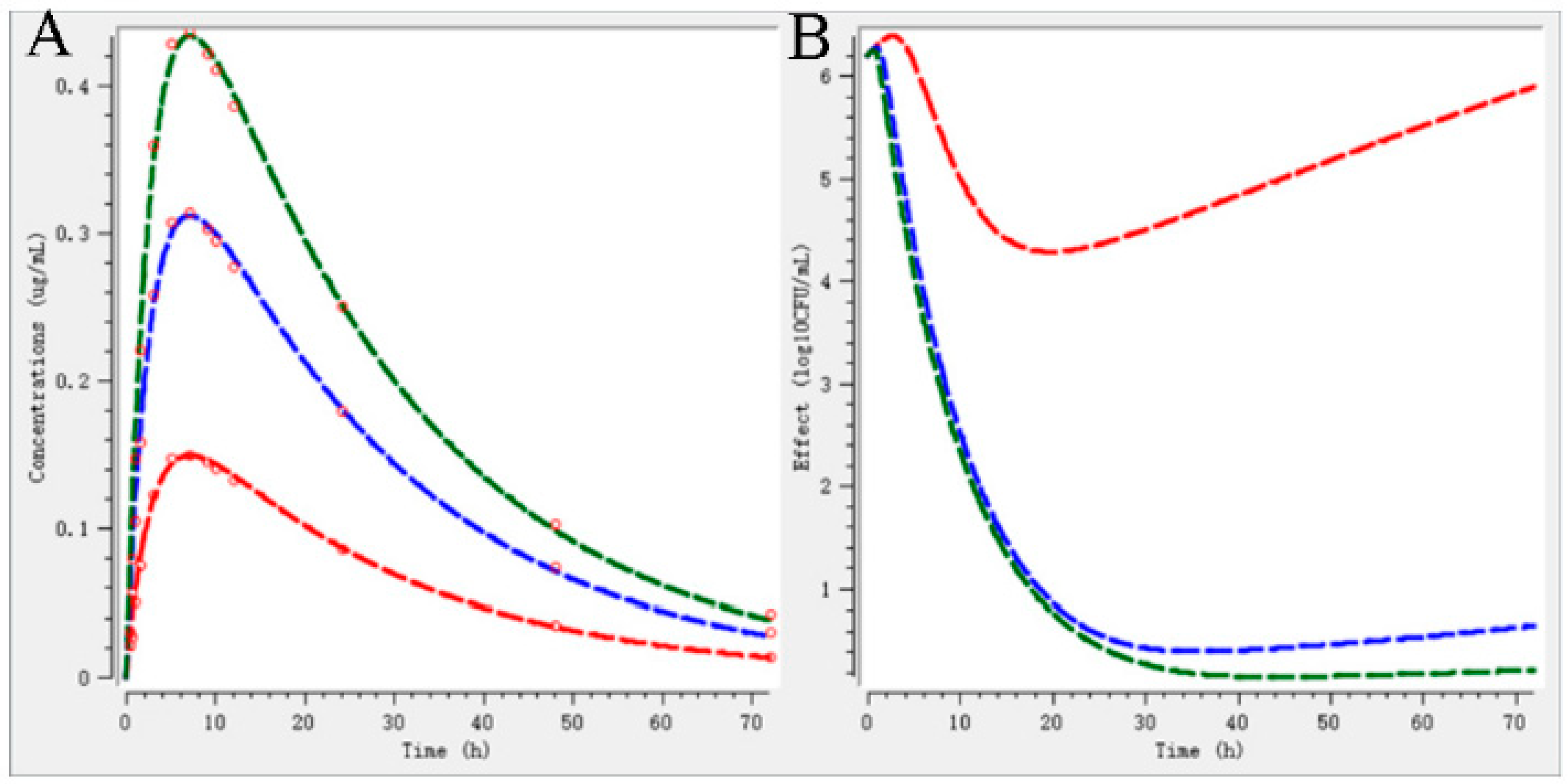
2.5. Validation of Dosage Regimen
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Bacteria and Antibiotic
4.3. Pharmacodynamics
4.3.1. Determination of MIC
4.3.2. In Vitro and Ex Vivo Pharmacodynamic of CEF against P. multocida
4.4. Pharmacokinetics
4.5. HPLC Analysis
4.6. PK/PD Model
4.7. Dose Determination
4.8. Evaluation of Dosage Regimen
4.8.1. PBPK Model
4.8.2. Semi-Mechanistic PD Model
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bardou, M.; Honnorat, E.; Dubourg, G.; Couderc, C.; Fournier, P.E.; Seng, P.; Stein, A. Meningitis caused by Pasteurella multocida in a dog owner without a dog bite: Clonal lineage identification by MALDI-TOF mass spectrometry. BMC Res. Notes 2015, 8, 626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilaró, A.; Novell, E.; Enrique-Tarancón, V.; Balielles, J.; Vilalta, C.; Martinez, S.; Fraile Sauce, L.J. Antimicrobial Susceptibility Pattern of Porcine Respiratory Bacteria in Spain. Antibiotics 2020, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Zwe, Y.H.; Yuk, H.G. Prior exposure of agriculture cephalosporin ceftiofur impaired conjugation of bla(CTX-M-65) gene-bearing plasmid in Salmonella Saintpaul. J. Appl. Microbiol. 2020, 129, 1552–1565. [Google Scholar] [CrossRef]
- Volkova, V.V.; Lanzas, C.; Lu, Z.; Gröhn, Y.T. Mathematical model of plasmid-mediated resistance to ceftiofur in commensal enteric Escherichia coli of cattle. PLoS ONE 2012, 7, e36738. [Google Scholar] [CrossRef] [Green Version]
- Boxall, A.B.; Johnson, P.; Smith, E.J.; Sinclair, C.J.; Stutt, E.; Levy, L.S. Uptake of veterinary medicines from soils into plants. J. Agric. Food Chem. 2006, 54, 2288–2297. [Google Scholar] [CrossRef]
- Aguirre, L.; Vidal, A.; Seminati, C.; Tello, M.; Redondo, N.; Darwich, L.; Martín, M. Antimicrobial resistance profile and prevalence of extended-spectrum beta-lactamases (ESBL), AmpC beta-lactamases and colistin resistance (mcr) genes in Escherichia coli from swine between 1999 and 2018. Porc. Health Manag. 2020, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Zhao, Z.; Hu, J.; Wu, B.; Cai, X.; He, Q.; Chen, H. Isolation, antimicrobial resistance, and virulence genes of Pasteurella multocida strains from swine in China. J. Clin. Microbiol. 2009, 47, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Sou, T.; Kukavica-Ibrulj, I.; Levesque, R.C.; Friberg, L.E.; Bergström, C.A.S. Model-Informed Drug Development in Pulmonary Delivery: Semimechanistic Pharmacokinetic-Pharmacodynamic Modeling for Evaluation of Treatments against Chronic Pseudomonas aeruginosa Lung Infections. Mol. Pharm. 2020, 17, 1458–1469. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Madabushi, R.; Liu, Q.; Huang, S.M.; Zineh, I. Model-Informed Drug Development: Current US Regulatory Practice and Future Considerations. Clin. Pharm. Therap. 2019, 105, 899–911. [Google Scholar] [CrossRef]
- Toutain, P.-L. Pharmacokinetic/pharmacodynamic integration in drug development and dosage-regimen optimization for veterinary medicine. Aaps. Pharmsci. 2002, 4, 160–188. [Google Scholar] [CrossRef]
- Ahmad, I.; Hao, H.; Huang, L.; Sanders, P.; Wang, X.; Chen, D.; Tao, Y.; Xie, S.; Xiuhua, K.; Li, J. Integration of PK/PD for dose optimization of Cefquinome against Staphylococcus aureus causing septicemia in cattle. Front. Microbiol. 2015, 6, 588. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhang, H.; Li, M.; Ahmad, I.; Wang, Y.; Yuan, Z. Pharmacokinetic-pharmacodynamic modeling of tylosin against Streptococcus suis in pigs. BMC Vet. Res. 2018, 14, 319. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Cheng, P.; Xiao, T.; Ulziikhutag, J.; Yu, H.; Li, J.; Liu, R.; Muhammad, I.; Zhang, X. Pharmacokinetics and pharmacodynamics integration of danofloxacin against Eschrichia coli in piglet ileum ultrafiltration probe model. Sci. Rep. 2021, 11, 681. [Google Scholar] [CrossRef]
- DeDonder, K.D.; Apley, M.D.; Li, M.; Gehring, R.; Harhay, D.M.; Lubbers, B.V.; White, B.J.; Capik, S.F.; KuKanich, B.; Riviere, J.E.; et al. Pharmacokinetics and pharmacodynamics of gamithromycin in pulmonary epithelial lining fluid in naturally occurring bovine respiratory disease in multisource commingled feedlot cattle. J. Vet. Pharmacol. Ther. 2016, 39, 157–166. [Google Scholar] [CrossRef]
- Iqbal, K.; Broeker, A.; Nowak, H.; Rahmel, T.; Nussbaumer-Pröll, A.; Österreicher, Z.; Zeitlinger, M.; Wicha, S.G. A pharmacometric approach to define target site-specific breakpoints for bacterial killing and resistance suppression integrating microdialysis, time-kill curves and heteroresistance data: A case study with moxifloxacin. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 1255.e1251–1255.e1258. [Google Scholar] [CrossRef]
- Seeger, J.; Michelet, R.; Kloft, C. Quantification of persister formation of Escherichia coli leveraging electronic cell counting and semi-mechanistic pharmacokinetic/pharmacodynamic modelling. J. Antimicrob. Chemother. 2021, 76, 2088–2096. [Google Scholar] [CrossRef]
- Pelligand, L.; Lees, P.; Sidhu, P.K.; Toutain, P.L. Semi-Mechanistic Modeling of Florfenicol Time-Kill Curves and in silico Dose Fractionation for Calf Respiratory Pathogens. Front. Microbiol. 2019, 10, 1237. [Google Scholar] [CrossRef]
- Ronaghinia, A.A.; Birch, J.M.; Frandsen, H.L.; Toutain, P.L.; Damborg, P.; Struve, T. Evaluating a tylosin dosage regimen for treatment of Staphylococcus delphini infection in mink (Neovison vison): A pharmacokinetic-pharmacodynamic approach. Vet. Res. 2021, 52, 34. [Google Scholar] [CrossRef]
- Lin, Z.; Vahl, C.I.; Riviere, J.E. Human Food Safety Implications of Variation in Food Animal Drug Metabolism. Sci. Rep. 2016, 6, 27907. [Google Scholar] [CrossRef] [Green Version]
- Petrocchi-Rilo, M.; Gutiérrez-Martín, C.B.; Pérez-Fernández, E.; Vilaró, A.; Fraile, L.; Martínez-Martínez, S. Antimicrobial Resistance Genes in Porcine Pasteurella multocida Are Not Associated with Its Antimicrobial Susceptibility Pattern. Antibiotics 2020, 9, 614. [Google Scholar] [CrossRef]
- Sun, D.; Mi, K.; Hao, H.; Xie, S.; Chen, D.; Huang, L. Optimal regimens based on PK/PD cutoff evaluation of ceftiofur against Actinobacillus pleuropneumoniae in swine. BMC Vet. Res. 2020, 16, 366. [Google Scholar] [CrossRef]
- Tantituvanont, A.; Yimprasert, W.; Werawatganone, P.; Nilubol, D. Pharmacokinetics of ceftiofur hydrochloride in pigs infected with porcine reproductive and respiratory syndrome virus. J. Antimicrob. Chemother. 2009, 63, 369–373. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Lan, W.; Wang, Y.; Jiang, L.; Jiang, Y.; Wang, Z. Comparative pharmacokinetics of danofloxacin in healthy and Pasteurella multocida infected ducks. J. Vet. Pharmacol. Ther. 2018, 41, 912–918. [Google Scholar] [CrossRef]
- Elazab, S.T.; Schrunk, D.E.; Griffith, R.W.; Ensley, S.M.; Dell’Anna, G.; Mullin, K.; Elsayed, M.G.; Amer, M.S.; El-Nabtity, S.M.; Hsu, W.H. Pharmacokinetics of cefquinome in healthy and Pasteurella multocida-infected rabbits. J. Vet. Pharmacol. Ther. 2018, 41, 374–377. [Google Scholar] [CrossRef]
- Li, X.D.; Chi, S.Q.; Wu, L.Y.; Liu, C.; Sun, T.; Hong, J.; Chen, X.; Chen, X.G.; Wang, G.S.; Yu, D.J. PK/PD modeling of Ceftiofur Sodium against Haemophilus parasuis infection in pigs. BMC Vet. Res. 2019, 15, 272. [Google Scholar] [CrossRef] [Green Version]
- Toutain, P.L.; Pelligand, L.; Lees, P.; Bousquet-Mélou, A.; Ferran, A.A.; Turnidge, J.D. The pharmacokinetic/pharmacodynamic paradigm for antimicrobial drugs in veterinary medicine: Recent advances and critical appraisal. J. Vet. Pharmacol. Ther. 2021, 44, 172–200. [Google Scholar] [CrossRef]
- Li, M.; Gehring, R.; Riviere, J.E.; Lin, Z. Probabilistic Physiologically Based Pharmacokinetic Model for Penicillin G in Milk From Dairy Cows Following Intramammary or Intramuscular Administrations. Toxicol. Sci. Off. J. Soc. Toxicol. 2018, 164, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Susla, G.; Atkinson, A. Effect of Liver Disease on Pharmacokinetics; Academic Press: Cambridge, MA, USA, 2007; pp. 73–87. [Google Scholar] [CrossRef]
- Jones, H.M.; Mayawala, K.; Poulin, P. Dose selection based on physiologically based pharmacokinetic (PBPK) approaches. AAPS J. 2013, 15, 377–387. [Google Scholar] [CrossRef]
- Zhou, K.; Huo, M.; Ma, W.; Mi, K.; Xu, X.; Algharib, S.A.; Xie, S.; Huang, L. Application of a Physiologically Based Pharmacokinetic Model to Develop a Veterinary Amorphous Enrofloxacin Solid Dispersion. Pharmaceutics 2021, 13, 602. [Google Scholar] [CrossRef]
- Sadiq, M.W.; Nielsen, E.I.; Khachman, D.; Conil, J.M.; Georges, B.; Houin, G.; Laffont, C.M.; Karlsson, M.O.; Friberg, L.E. A whole-body physiologically based pharmacokinetic (WB-PBPK) model of ciprofloxacin: A step towards predicting bacterial killing at sites of infection. J. Pharmacokinet. Pharmacodyn. 2017, 44, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, E.I.; Viberg, A.; Löwdin, E.; Cars, O.; Karlsson, M.O.; Sandström, M. Semimechanistic pharmacokinetic/pharmacodynamic model for assessment of activity of antibacterial agents from time-kill curve experiments. Antimicrob. Agents Chemother. 2007, 51, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, E.I.; Friberg, L.E. Pharmacokinetic-pharmacodynamic modeling of antibacterial drugs. Pharmacol. Rev. 2013, 65, 1053–1090. [Google Scholar] [CrossRef] [Green Version]
- Kamp, E.M.; Bokken, G.C.; Vermeulen, T.M.; de Jong, M.F.; Buys, H.E.; Reek, F.H.; Smits, M.A. A specific and sensitive PCR assay suitable for large-scale detection of toxigenic Pasteurella multocida in nasal and tonsillar swabs specimens of pigs. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. 1996, 8, 304–309. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, H.J.; Kim, Y.K.; Yu, S.; Chong, Y.P.; Kim, S.-H.; Sung, H.; Lee, S.-O.; Kim, M.-N.; Lim, C.-M. Cellular profiles of bronchoalveolar lavage fluid and their prognostic significance for non-HIV-infected patients with Pneumocystis jirovecii pneumonia. J. Clin. Microbiol. 2015, 53, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Rennard, S.; Basset, G.; Lecossier, D.; O’donnell, K.; Pinkston, P.; Martin, P.; Crystal, R. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 1986, 60, 532–538. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, Q.; Yang, S.; Yang, B.; Khaliq, H.; Li, K.; Ahmed, S.; Sajid, A.; Zhang, B.; Chen, P.; et al. PK-PD Integration Modeling and Cutoff Value of Florfenicol against Streptococcus suis in Pigs. Front. Pharmacol. 2018, 9, 2. [Google Scholar] [CrossRef] [Green Version]




| Ceftiofur (µg/Ml) | PAE (h) | |
|---|---|---|
| 1 h Exposure | 2 h Exposure | |
| 1 × MIC | 0.28 | 0.33 |
| 2 × MIC | 0.43 | 0.62 |
| 4 × MIC | 0.60 | 1.01 |
| Parameter | Units | Plasma (n = 6) | BALF (n = 4) |
|---|---|---|---|
| Tmax | h | 1.92 ± 0.68 | 1.38 ± 0.18 |
| Cmax | µg/mL | 11.81 ± 3.09 | 5.05 ± 3.22 |
| AUC0-t | µg·h/mL | 159.05 ± 15.98 | 30.31 ± 16.01 |
| AUC0-∞ | µg·h/mL | 163.04 ± 15.57 | 31.45 ± 16.79 |
| CL/F | L/kg/h | 0.03 ± 0.00 | 0.24 ± 0.10 |
| Vd/F | L/kg | 0.60 ± 0.16 | 3.97 ± 1.99 |
| MRT | h | 15.88 ± 1.36 | 13.85 ± 0.55 |
| Ke | h−1 | 0.06 ± 0.01 | 0.06 ± 0.01 |
| T1/2 | h | 13.28 ± 3.23 | 11.59 ± 1.97 |
| Parameters | Unit | Plasma | BALF |
|---|---|---|---|
| Imax | Log10 CFU/mL | 9.78 ± 0.37 | 7.41 ± 0.12 |
| E0 | Log10 CFU/mL | 3.57 ± 0.43 | 3.59 ± 0.11 |
| IC50 | h | 60.14 ± 3.48 | 59.84 ± 0.52 |
| N | - | 1.94 ± 0.55 | 4.10 ± 0.08 |
| AUC24 h/MIC (E = 0) | h | 44.02 ± 3.17 | 58.99 ± 4.82 |
| AUC24 h/MIC (E = −3) | h | 89.40 ± 4.48 | 99.69 ± 2.81 |
| AUC24 h/MIC (E = −4) | h | 119.90 ± 19.75 | - |
| Abbreviation | Distribution | Mean | SD | CV | Upper Bound | Lower Bound |
|---|---|---|---|---|---|---|
| QCC | Normal | 5 | 1.5 | 0.3 | 7.940 | 2.060 |
| QKC | Normal | 0.12 | 0.036 | 0.3 | 0.191 | 0.049 |
| BW | Normal | 40 | 12 | 0.3 | 63.520 | 16.480 |
| VLC | Normal | 0.0247 | 0.00741 | 0.3 | 0.039 | 0.010 |
| PL | Lognormal | 0.13 | 0.052 | 0.4 | 0.002 | 0.810 |
| PK | Lognormal | 0.4 | 0.16 | 0.4 | 0.055 | 1.446 |
| PM | Lognormal | 0.06 | 0.024 | 0.4 | 0.0002 | 0.410 |
| PL1 | Lognormal | 0.13 | 0.052 | 0.4 | 0.002 | 0.810 |
| PK1 | Lognormal | 0.4 | 0.16 | 0.4 | 0.055 | 1.446 |
| PM1 | Lognormal | 0.06 | 0.024 | 0.4 | 0.107 | 0.013 |
| KmC | Normal | 1 | 0.3 | 0.3 | 1.588 | 0.412 |
| Frac | Normal | 0.7 | 0.21 | 0.3 | 1.112 | 0.288 |
| Kurine1C | Normal | 0.01 | 0.003 | 0.3 | 0.016 | 0.004 |
| Parameter | Units | P. multacia | |
|---|---|---|---|
| Mean | SE | ||
| kgrowth | 1/h | 0.2 | 0.28 |
| kdeath | 1/h | 0.179 | (fixed) |
| Bmax | 1/h | 8.48 | 0.28 |
| Emax | 1/h | 0.11 | 0.027 |
| EC50 | mg/L | 0.14 | 0.031 |
| γ | - | 8.54 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, K.; Pu, S.; Hou, Y.; Sun, L.; Zhou, K.; Ma, W.; Xu, X.; Huo, M.; Liu, Z.; Xie, C.; et al. Optimization and Validation of Dosage Regimen for Ceftiofur against Pasteurella multocida in Swine by Physiological Based Pharmacokinetic–Pharmacodynamic Model. Int. J. Mol. Sci. 2022, 23, 3722. https://doi.org/10.3390/ijms23073722
Mi K, Pu S, Hou Y, Sun L, Zhou K, Ma W, Xu X, Huo M, Liu Z, Xie C, et al. Optimization and Validation of Dosage Regimen for Ceftiofur against Pasteurella multocida in Swine by Physiological Based Pharmacokinetic–Pharmacodynamic Model. International Journal of Molecular Sciences. 2022; 23(7):3722. https://doi.org/10.3390/ijms23073722
Chicago/Turabian StyleMi, Kun, Shanju Pu, Yixuan Hou, Lei Sun, Kaixiang Zhou, Wenjin Ma, Xiangyue Xu, Meixia Huo, Zhenli Liu, Changqing Xie, and et al. 2022. "Optimization and Validation of Dosage Regimen for Ceftiofur against Pasteurella multocida in Swine by Physiological Based Pharmacokinetic–Pharmacodynamic Model" International Journal of Molecular Sciences 23, no. 7: 3722. https://doi.org/10.3390/ijms23073722






