Sprague Dawley Rats Show More Severe Bone Loss, Osteophytosis and Inflammation Compared toWistar Han Rats in a High-Fat, High-Sucrose Diet Model of Joint Damage
Abstract
:1. Introduction
2. Results
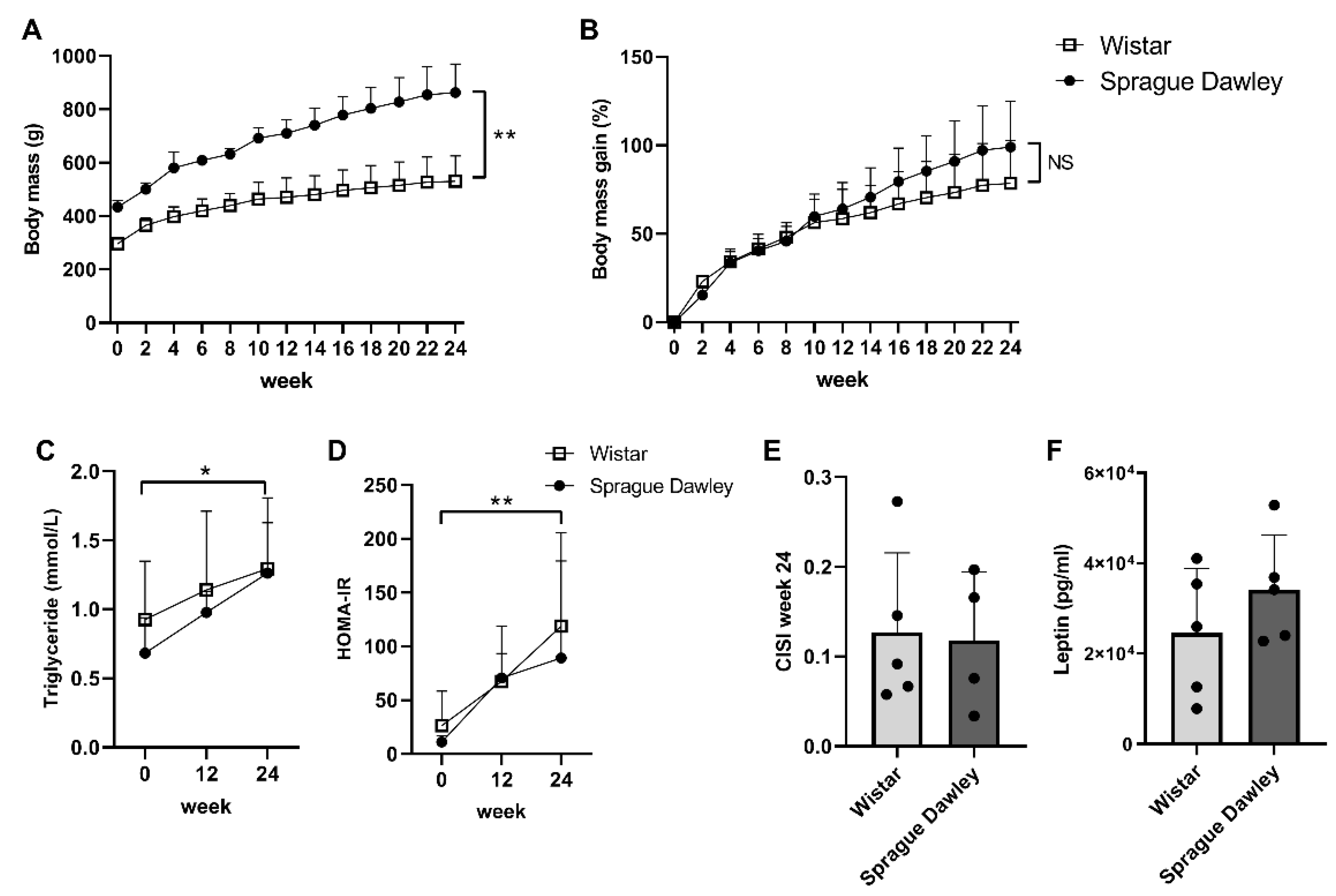
2.1. Body Mass and Metabolic Parameters
2.2. OARSI Histopathology Score and Inflammation Markers
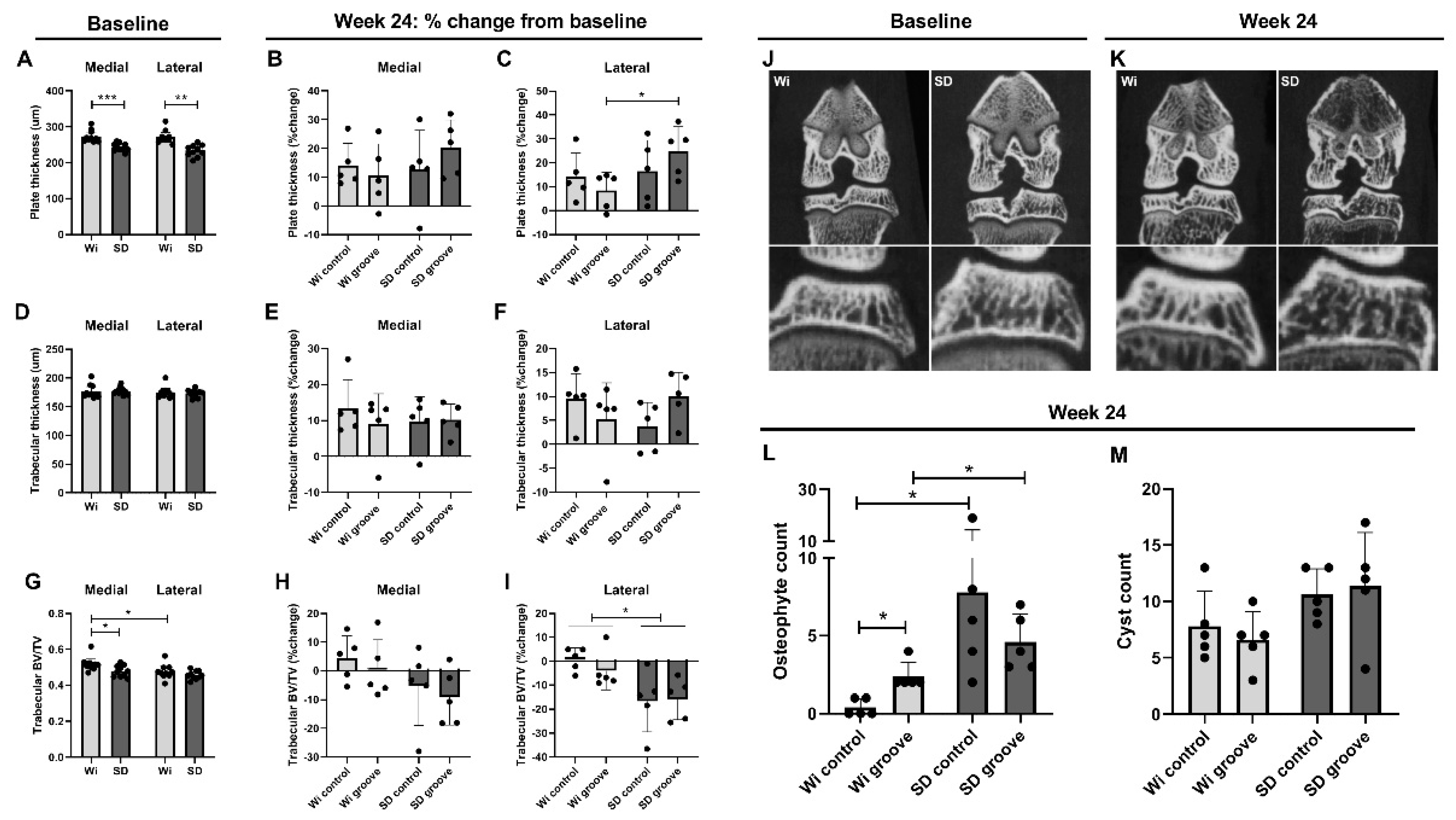
2.3. Micro Computed Tomography (μCT) Bone Measurements
3. Discussion
4. Materials and Methods
4.1. Animals and OA Model
4.2. Blood Measurements
4.3. Micro Computed Tomography (μCT)
4.4. Histology
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poole, A.R. Osteoarthritis as a whole joint disease. HSS J. 2012, 8, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef]
- Botter, S.M.; van Osch, G.J.; Waarsing, J.H.; van der Linden, J.C.; Verhaar, J.A.; Pols, H.A.; van Leeuwen, J.P.; Weinans, H. Cartilage damage pattern in relation to subchondral plate thickness in a collagenase-induced model of osteoarthritis. Osteoarthr. Cartil. 2008, 16, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botter, S.M.; van Osch, G.J.; Clockaerts, S.; Waarsing, J.H.; Weinans, H.; van Leeuwen, J.P. Osteoarthritis induction leads to early and temporal subchondral plate porosity in the tibial plateau of mice: An in vivo microfocal computed tomography study. Arthritis Rheum. 2011, 63, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- de Visser, H.M.; Korthagen, N.M.; Muller, C.; Ramakers, R.M.; Krijger, G.C.; Lafeber, F.; Beekman, F.J.; Mastbergen, S.C.; Weinans, H. Imaging of Folate Receptor Expressing Macrophages in the Rat Groove Model of Osteoarthritis: Using a New DOTA-Folate Conjugate. Cartilage 2018, 9, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebelt, M.; Korthagen, N.; Wei, W.; Groen, H.; Bastiaansen-Jenniskens, Y.; Muller, C.; Waarsing, J.H.; de Jong, M.; Weinans, H. Triamcinolone acetonide activates an anti-inflammatory and folate receptor-positive macrophage that prevents osteophytosis in vivo. Arthritis Res. Ther. 2015, 17, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioan-Facsinay, A.; Kloppenburg, M. Osteoarthritis: Inflammation and fibrosis in adipose tissue of osteoarthritic joints. Nat. Rev. Rheumatol. 2017, 13, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Warmink, K.; Kozijn, A.E.; Bobeldijk, I.; Stoop, R.; Weinans, H.; Korthagen, N.M. High-fat feeding primes the mouse knee joint to develop osteoarthritis and pathologic infrapatellar fat pad changes after surgically induced injury. Osteoarthr. Cartil. 2020, 28, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Berenbaum, F.; Sellam, J. The Phenotypic Approach to Osteoarthritis: A Look at Metabolic Syndrome-Associated Osteoarthritis. Jt. Bone Spine 2019, 86, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Eymard, F.; Houard, X. Osteoarthritis, inflammation and obesity. Curr. Opin. Rheumatol. 2013, 25, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Gabay, O.; Berenbaum, F. Adipokines in arthritis: New kids on the block. Curr. Rheumatol. Rev. 2009, 5, 226–232. [Google Scholar] [CrossRef]
- de Visser, H.M.; Mastbergen, S.C.; Kozijn, A.E.; Coeleveld, K.; Pouran, B.; van Rijen, M.H.; Lafeber, F.; Weinans, H. Metabolic dysregulation accelerates injury-induced joint degeneration, driven by local inflammation; an in vivo rat study. J. Orthop. Res. 2018, 36, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Kozijn, A.E.; Gierman, L.M.; van der Ham, F.; Mulder, P.; Morrison, M.C.; Kuhnast, S.; van der Heijden, R.A.; Stavro, P.M.; van Koppen, A.; Pieterman, E.J.; et al. Variable cartilage degradation in mice with diet-induced metabolic dysfunction: Food for thought. Osteoarthr. Cartil. 2018, 26, 95–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hans, J.H. The Laboratory Rat, Chapter 1—History, Strains and Models; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Kuhn, E.R.; Bellon, K.; Huybrechts, L.; Heyns, W. Endocrine differences between the Wistar and Sprague-Dawley laboratory rat: Influence of cold adaptation. Horm. Metab. Res. 1983, 15, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity Rat model: A comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2016, 5, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, I.; Mowat, V. Comparison of longevity and common tumor profiles between Sprague-Dawley and Han Wistar rats. J. Toxicol. Pathol. 2020, 33, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Yang, L.; Zhang, R.; Zhu, X.; Wang, P. Are there differences between Sprague-Dawley and Wistar rats in long-term effects of ovariectomy as a model for postmenopausal osteoporosis? Int. J. Clin. Exp. Pathol. 2015, 8, 1491–1502. [Google Scholar]
- Rios, J.L.; Bomhof, M.R.; Reimer, R.A.; Hart, D.A.; Collins, K.H.; Herzog, W. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci. Rep. 2019, 9, 3893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tellegen, A.R.; Rudnik-Jansen, I.; Pouran, B.; de Visser, H.M.; Weinans, H.H.; Thomas, R.E.; Kik, M.J.L.; Grinwis, G.C.M.; Thies, J.C.; Woike, N.; et al. Controlled release of celecoxib inhibits inflammation, bone cysts and osteophyte formation in a preclinical model of osteoarthritis. Drug Deliv. 2018, 25, 1438–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Lent, P.L.; Blom, A.B.; van der Kraan, P.; Holthuysen, A.E.; Vitters, E.; van Rooijen, N.; Smeets, R.L.; Nabbe, K.C.; van den Berg, W.B. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum. 2004, 50, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.B.; van Lent, P.L.; Holthuysen, A.E.; van der Kraan, P.M.; Roth, J.; van Rooijen, N.; van den Berg, W.B. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr. Cartil. 2004, 12, 627–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Visser, H.M.; Weinans, H.; Coeleveld, K.; van Rijen, M.H.; Lafeber, F.P.; Mastbergen, S.C. Groove model of tibia-femoral osteoarthritis in the rat. J. Orthop. Res. 2017, 35, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Waarsing, J.H.; Day, J.S.; Weinans, H. An improved segmentation method for in vivo microCT imaging. J. Bone Miner. Res. 2004, 19, 1640–1650. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S24–S34. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warmink, K.; Rios, J.L.; van Valkengoed, D.R.; Korthagen, N.M.; Weinans, H. Sprague Dawley Rats Show More Severe Bone Loss, Osteophytosis and Inflammation Compared toWistar Han Rats in a High-Fat, High-Sucrose Diet Model of Joint Damage. Int. J. Mol. Sci. 2022, 23, 3725. https://doi.org/10.3390/ijms23073725
Warmink K, Rios JL, van Valkengoed DR, Korthagen NM, Weinans H. Sprague Dawley Rats Show More Severe Bone Loss, Osteophytosis and Inflammation Compared toWistar Han Rats in a High-Fat, High-Sucrose Diet Model of Joint Damage. International Journal of Molecular Sciences. 2022; 23(7):3725. https://doi.org/10.3390/ijms23073725
Chicago/Turabian StyleWarmink, Kelly, Jaqueline L. Rios, Devin R. van Valkengoed, Nicoline M. Korthagen, and Harrie Weinans. 2022. "Sprague Dawley Rats Show More Severe Bone Loss, Osteophytosis and Inflammation Compared toWistar Han Rats in a High-Fat, High-Sucrose Diet Model of Joint Damage" International Journal of Molecular Sciences 23, no. 7: 3725. https://doi.org/10.3390/ijms23073725
APA StyleWarmink, K., Rios, J. L., van Valkengoed, D. R., Korthagen, N. M., & Weinans, H. (2022). Sprague Dawley Rats Show More Severe Bone Loss, Osteophytosis and Inflammation Compared toWistar Han Rats in a High-Fat, High-Sucrose Diet Model of Joint Damage. International Journal of Molecular Sciences, 23(7), 3725. https://doi.org/10.3390/ijms23073725







