Progesterone Can Directly Inhibit the Life Activities of Toxoplasma gondii In Vitro through the Progesterone Receptor Membrane Component (PGRMC)
Abstract
:1. Introduction
2. Results
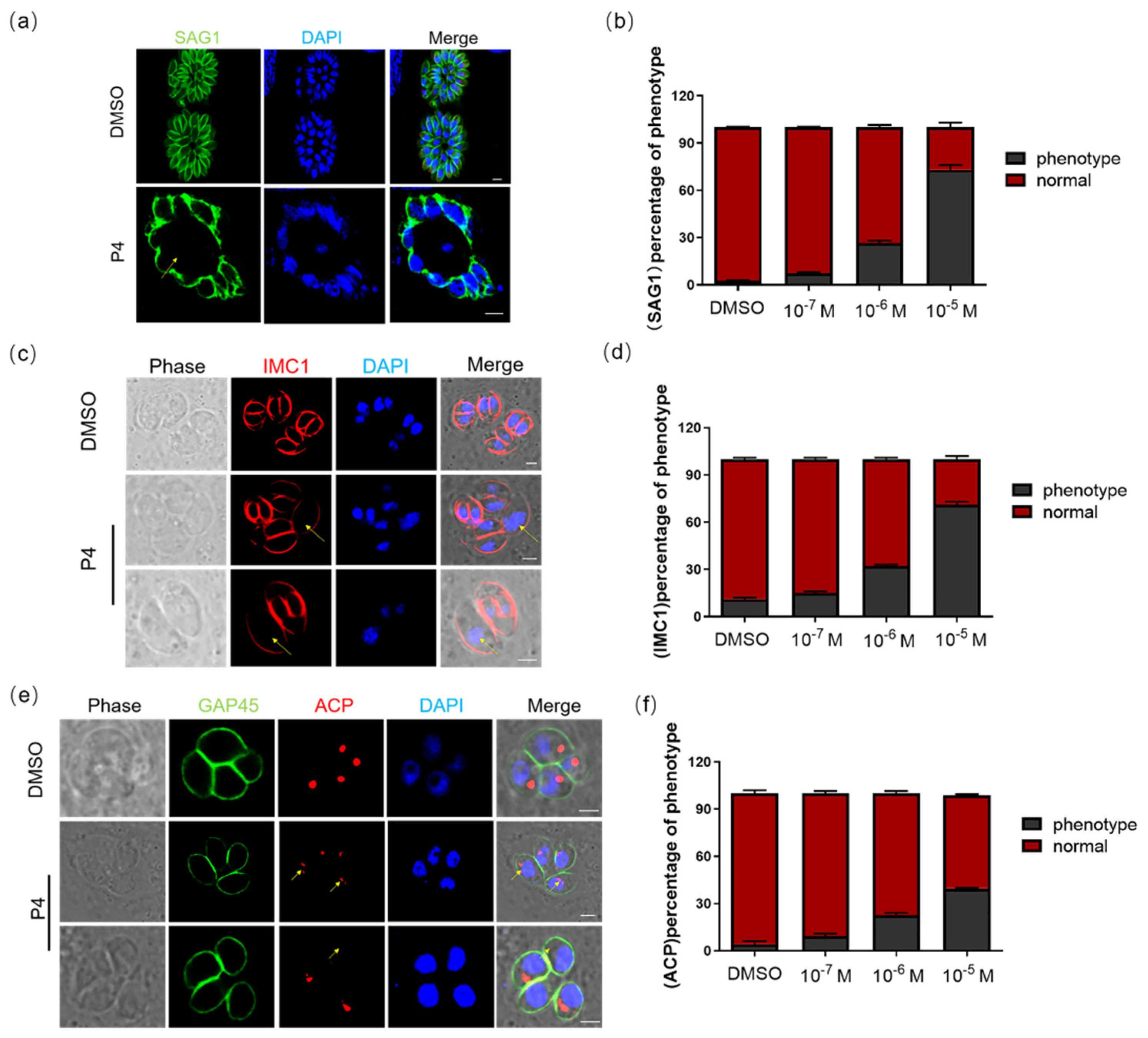
2.1. Progesterone Inhibits the Invasion of T. gondii
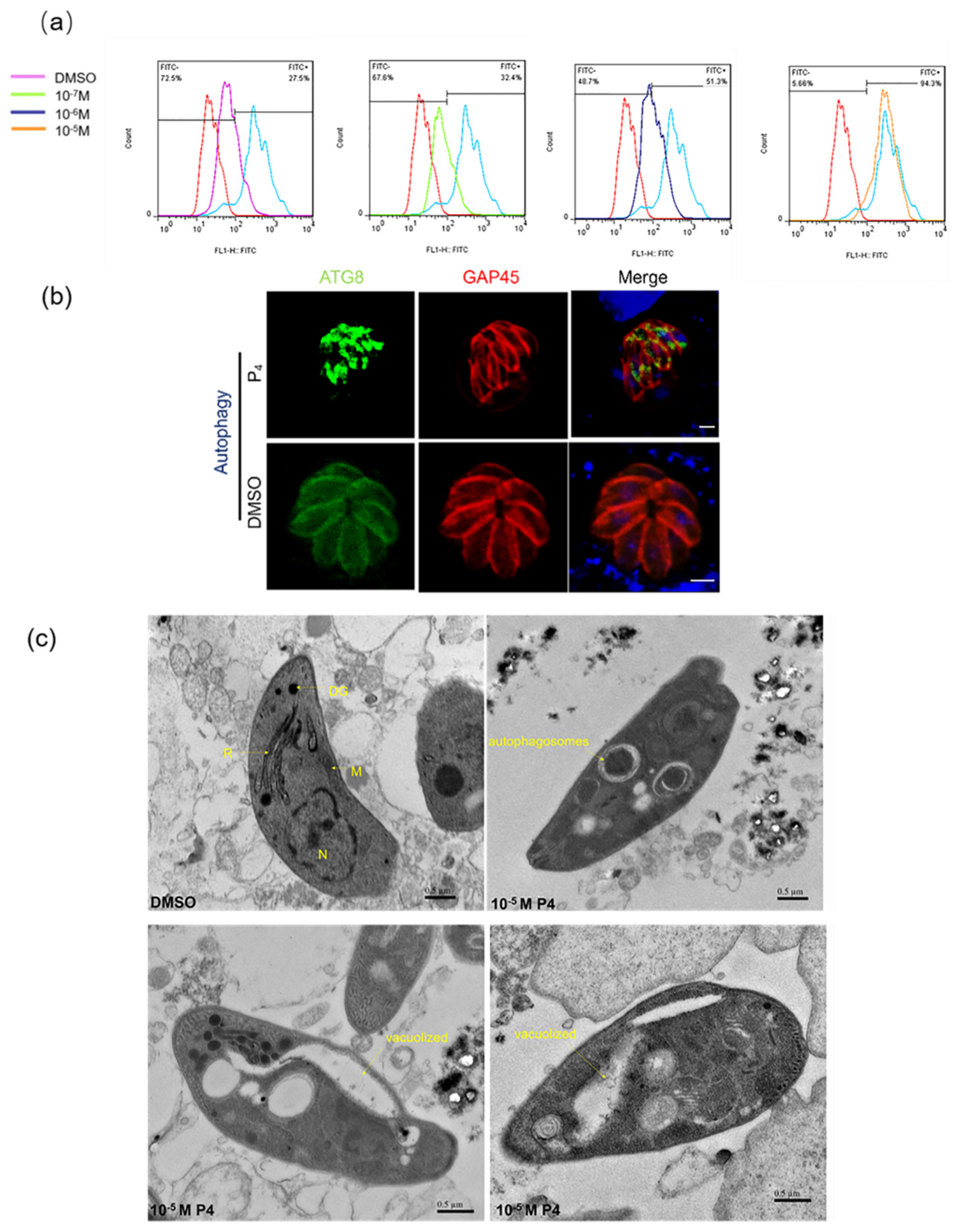
2.2. Progesterone Affects the Morphology and Division of T. gondii and Induces Autophagy
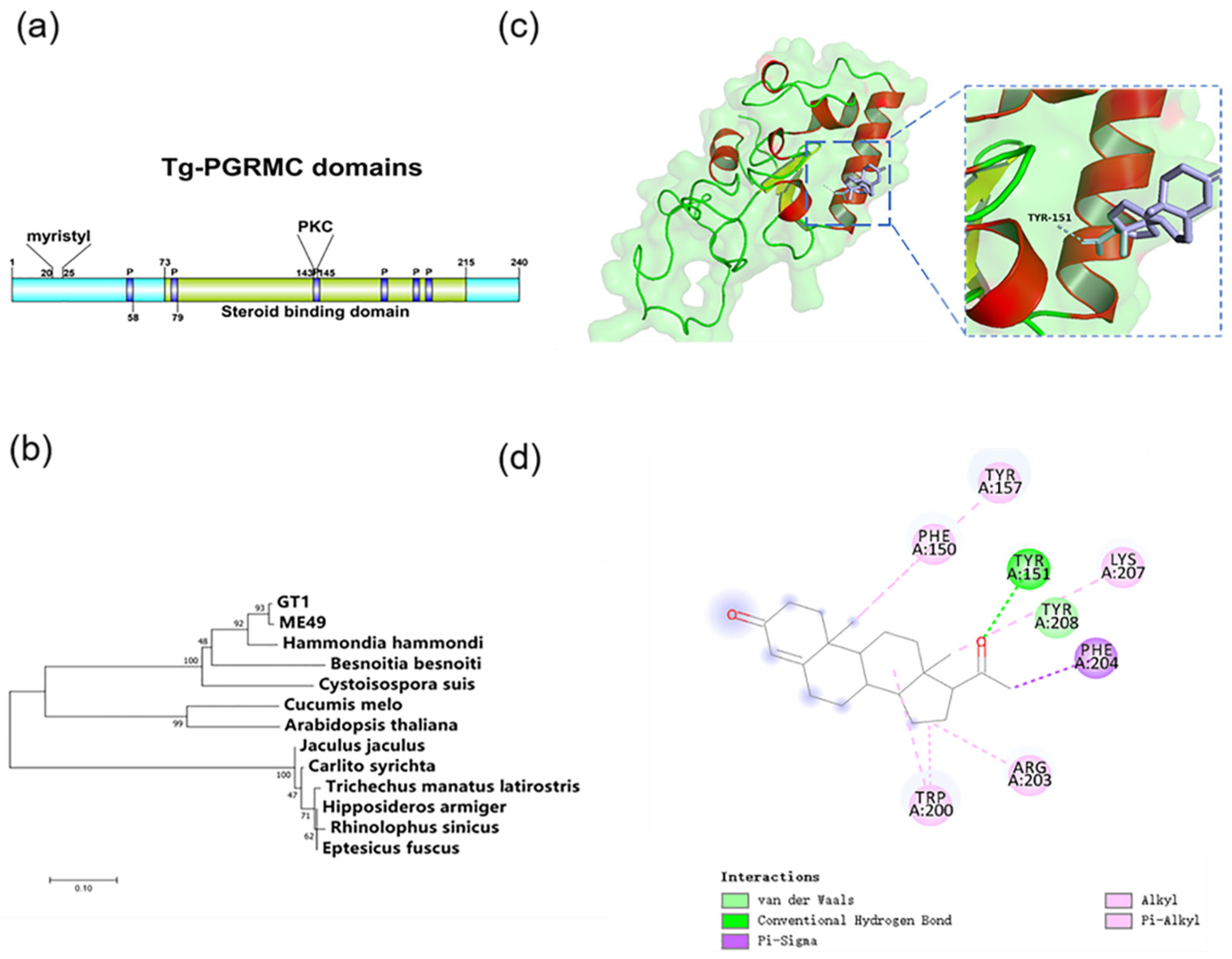
2.3. Progesterone Membrane Receptor in T. gondii
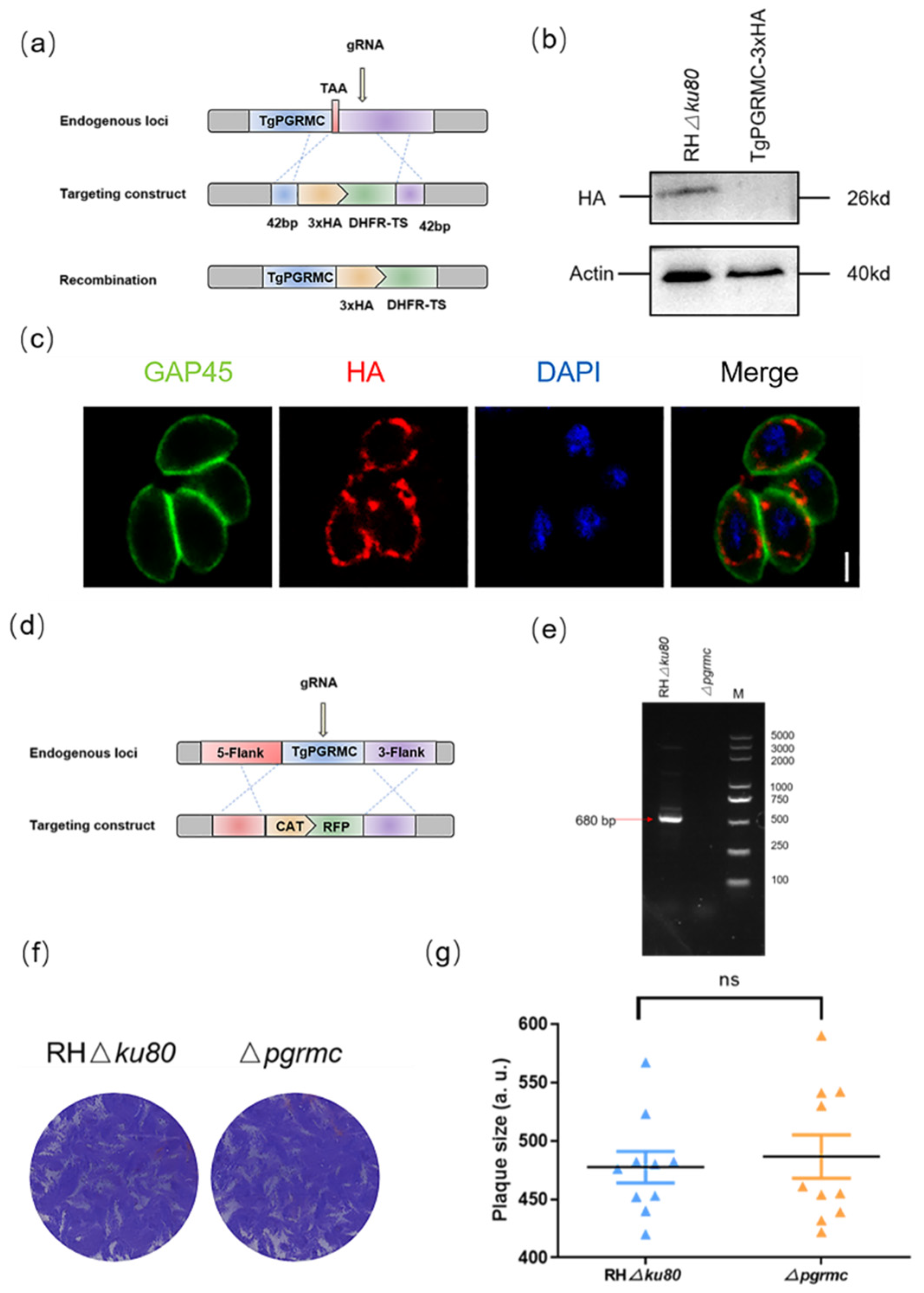
2.4. TgPGRMC Localizes in the Mitochondria and Is Not Necessary for the Parasite Lytic Cycle
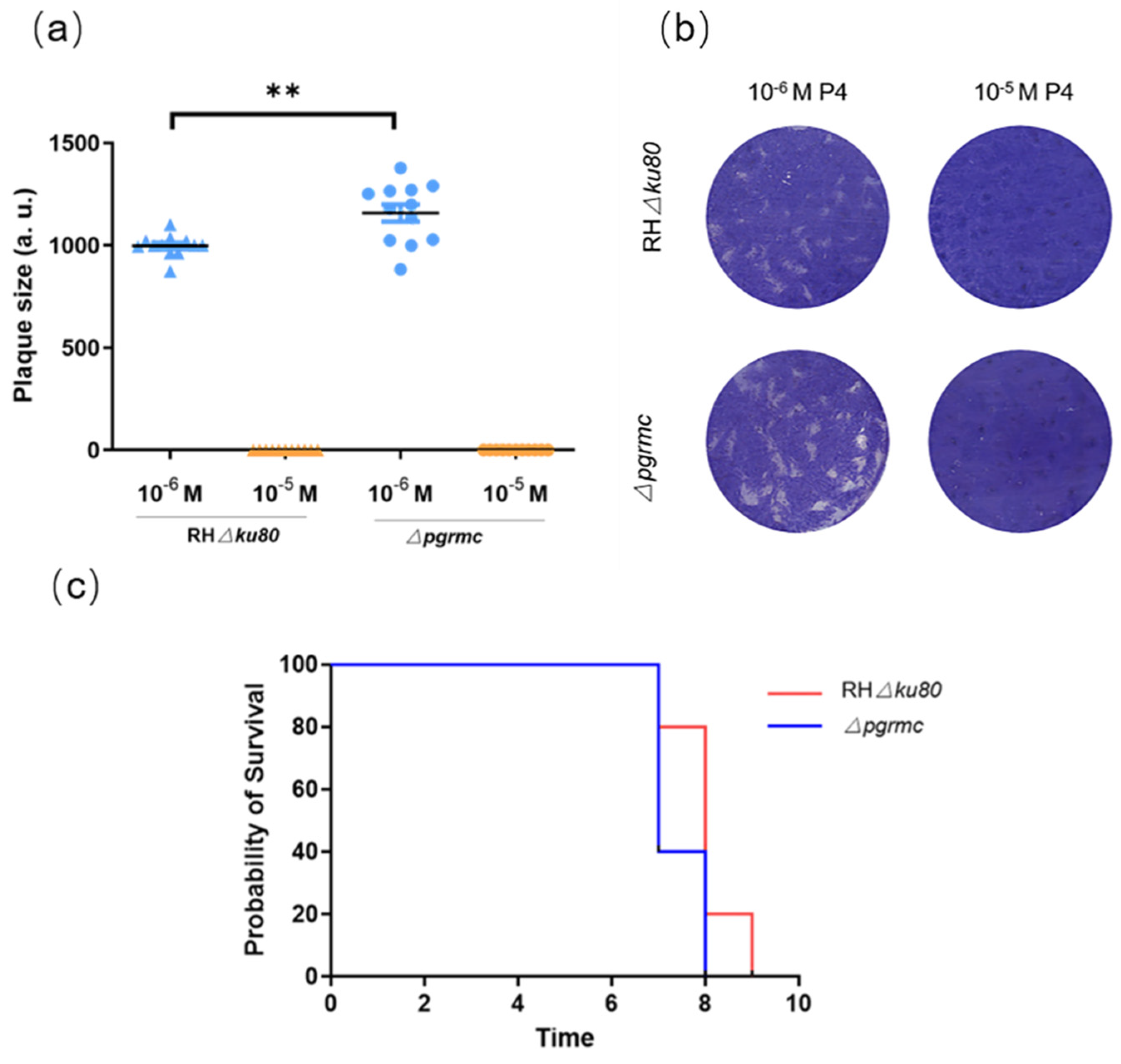
2.5. Knockout pgrmc Gene Alleviates the Inhibitory Effect of Progesterone on T. gondii
3. Discussion
4. Materials and Methods
4.1. Ethics Approval and Consent to Participate
4.2. Progesterone Treatment
4.3. Parasite and Culture
4.4. Plaque Assays
4.5. Invasion Assay
4.6. Mice and Experimental Infections
4.7. Molecular Docking Test
4.8. TUNEL Assay
4.9. Construction of Transgenic Parasite Lines
4.10. Transmission Electron Microscope
4.11. Immunofluorescence Assays and Western Blot
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lourido, S. Toxoplasma gondii. Trends Parasitol. 2019, 35, 944–945. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Goldstein, E.; Montoya, J.G.; Remington, J.S. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. 2008, 47, 554–566. [Google Scholar]
- Zhang, X.; Liu, J.; Li, M.; Fu, Y.; Zhang, T.; Han, Q.; Liu, Q. Role of an estradiol regulatory factor-hydroxysteroid dehydrogenase (HSD) in Toxoplasma gondii infection and pathogenicity. J. Steroid Biochem. Mol. Biol. 2017, 174, 176–182. [Google Scholar] [CrossRef]
- Desmonts, G.D.; Couvreur, J. Congenital toxoplasmosis. A prospective study of 378 pregnancies. N. Engl. J. Med. 1974, 290, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Settiyanan, T.; Wanapirak, C.; Sirichotiyakul, S.; Tongprasert, F.; Srisupundit, K.; Luewan, S.; Traisrisilp, K.; Tongsong, T. Association between isolated abnormal levels of maternal serum unconjugated estriol in the second trimester and adverse pregnancy outcomes. J. Matern. -Fetal Neonatal Med. 2016, 29, 2093–2097. [Google Scholar] [CrossRef]
- Batra, S.; Bengtsson, L.P.; Grundsell, H.; Sjöberg, N.-O. Levels of Free and Protein-Bound Progesterone in Plasma during Late Pregnancy. J. Clin. Endocrinol. Metab. 1976, 42, 1041–1047. [Google Scholar] [CrossRef]
- Kauppila, A.; Järvinen, P.A. Peripheral blood concentrations of progesterone and oestradiol during human pregnancy and delivery. Acta Physiol. Hung. 1985, 65, 473–478. [Google Scholar]
- Szekeresbartho, J.; Polgar, B. Progesterone, Pregnancy, and Innate Immunity. In Sex Hormones and Immunity to Infection; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Roberts, C.W.; Walker, W.; Alexander, J. Sex-Associated Hormones and Immunity to Protozoan Parasites. Clin. Microbiol. Rev. 2001, 14, 476–488. [Google Scholar] [CrossRef] [Green Version]
- Druckmann, R.; Druckmann, M.-A. Progesterone and the immunology of pregnancy. J. Steroid Biochem. Mol. Biol. 2005, 97, 389–396. [Google Scholar] [CrossRef]
- Raghupathy, R. Th 1-type immunity is incompatible with successful pregnancy. Immunol. Today 1997, 18, 478–482. [Google Scholar] [CrossRef]
- Lin, H.; Mosmann, T.R.; Guilbert, L.; Tuntipopipat, S.; Wegmann, T.G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 1993, 151, 4562–4573. [Google Scholar] [PubMed]
- Kalu, E.; Bhaskaran, S.; Thum, M.Y.; Vishwanatha, R.; Croucher, C.; Sherriff, E.; Ford, B.; Bansal, A.S. Serial Estimation of Th1:Th2 Cytokines Profile in Women Undergoing In-Vitro Fertilization-Embryo Transfer. Am. J. Reprod. Immunol. 2008, 59, 206–211. [Google Scholar] [CrossRef]
- Thouvenin, M.; Candolfi, E.; Villard, O.; Kien, T. Exploration of immune response in a murine model of congenital toxoplasmosis. Ann. Biol. Clin. 1997, 55, 460–464. [Google Scholar]
- Luft, B.J.; Remington, J.S. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infections in mice. Infect. Immun. 1982, 38, 1164–1171. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.A. Controversies in reproductive immunology. Crit. Rev. Immunol. 1991, 11, 215–247. [Google Scholar]
- Lingnau, A.; Margos, G.; Maier, W.A.; Seitz, H.M. The effects of hormones on the gametocytogenesis of Plasmodium falciparum in vitro. Appl. Parasitol. 1993, 34, 153–160. [Google Scholar]
- Zhang, H.; Liu, J.; Ying, Z.; Li, S.; Wu, Y.; Liu, Q. Toxoplasma gondii UBL-UBA shuttle proteins contribute to the degradation of ubiquitinylated proteins and are important for synchronous cell division and virulence. FASEB J. 2020, 34, 13711–13725. [Google Scholar] [CrossRef]
- Vaishnava, S.; Striepen, B. The cell biology of secondary endosymbiosis? how parasites build, divide and segregate the apicoplast. Mol. Microbiol. 2006, 61, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Sahani, M.H. ATG8 localization in apicomplexan parasites: Apicoplast and more? Autophagy 2014, 10, 1487–1494. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, J.; Lv, K.; Li, B.; Yan, B.; Gai, L.; Shi, C.; Wang, X.; Si, H.; Zhang, J. Myrislignan Induces Redox Imbalance and Activates Autophagy in Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2021, 11, 730222. [Google Scholar] [CrossRef]
- Delascio, D.; Kulay, L.; Netto, E.F. The hormonology of pregnancy. Matern. Infanc. 1968, 27, 103–124. [Google Scholar]
- Macdonald, P.C.; Gant, N.F.; Duenhoelter, J.H.; Porter, J.C. Physiology of hormones during pregnancy and hormone monitoring. Prog. Clin. Biol. Res. 1976, 10, 249–257. [Google Scholar]
- Li, X.; Lonard, D.M.; O’Malley, B.W. A contemporary understanding of progesterone receptor function. Mech. Ageing Dev. 2004, 125, 669–678. [Google Scholar] [CrossRef]
- Zhu, Y.; Bond, J.; Thomas, P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 2237–2242. [Google Scholar] [CrossRef] [Green Version]
- Cahill, M.A. Progesterone receptor membrane component 1: An integrative review. J. Steroid Biochem. Mol. Biol. 2007, 105, 16–36. [Google Scholar] [CrossRef]
- Lösel, R.; Wehling, M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–55. [Google Scholar] [CrossRef]
- Amsterdam, A.; Hanukoglu, I.; Suh, B.; Keren-Tal, I.; Plehn-Dujowich, D.; Sprengel, R.; Rennert, H.; Strauss, J. Oncogene-transformed granulosa cells as a model system for the study of steroidogenic processes. J. Steroid Biochem. Mol. Biol. 1992, 43, 875–884. [Google Scholar] [CrossRef]
- Pfeifer, S.M.; Furth, E.E.; Ohba, T.; Chang, Y.J.; Rennert, H.; Sakuragi, N.; Billheimer, J.T.; Strauss, J.F. Sterol carrier protein 2: A role in steroid hormone synthesis? J. Steroid Biochem. Mol. Biol. 1993, 47, 167–172. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Fu, Y.; Liu, J.; Liu, Q. Effects of Estradiol and Progesterone-Induced Intracellular Calcium Fluxes on Toxoplasma gondii Gliding, Microneme Secretion, and Egress. Front. Microbiol. 2018, 9, 1266. [Google Scholar] [CrossRef] [Green Version]
- Hoppler, S.; Kavanagh, C.L. Wnt signalling: Variety at the core. J. Cell Sci. 2007, 120, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Cui, X.; Fan, S.; Liu, J.; Zhang, X.; Wu, Y.; Liu, Q. Comprehensive Characterization of Toxoplasma Acyl Coenzyme A-Binding Protein TgACBP2 and Its Critical Role in Parasite Cardiolipin Metabolism. mBio 2018, 9, e01597-18. [Google Scholar] [CrossRef] [Green Version]
- Roos, D.S.; Donald, R.G.; Morrissette, N.S.; Moulton, A.L. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1995, 45, 27–63. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Liu, J.; Hao, P.; Ma, L.; Liu, Q. The Apoptotic Role of Metacaspase in Toxoplasma gondii. Front. Microbiol. 2016, 6, 1560. [Google Scholar] [CrossRef] [Green Version]
- Huet, D.; Rajendran, E.; van Dooren, G.G.; Lourido, S. Identification of cryptic subunits from an apicomplexan ATP synthase. eLife 2018, 7, e38097. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhang, X.; Fu, Y.; Liu, J.; Xue, Y.; Liu, Q. Progesterone Can Directly Inhibit the Life Activities of Toxoplasma gondii In Vitro through the Progesterone Receptor Membrane Component (PGRMC). Int. J. Mol. Sci. 2022, 23, 3843. https://doi.org/10.3390/ijms23073843
Wu Y, Zhang X, Fu Y, Liu J, Xue Y, Liu Q. Progesterone Can Directly Inhibit the Life Activities of Toxoplasma gondii In Vitro through the Progesterone Receptor Membrane Component (PGRMC). International Journal of Molecular Sciences. 2022; 23(7):3843. https://doi.org/10.3390/ijms23073843
Chicago/Turabian StyleWu, Yihan, Xiao Zhang, Yong Fu, Jing Liu, Yangfei Xue, and Qun Liu. 2022. "Progesterone Can Directly Inhibit the Life Activities of Toxoplasma gondii In Vitro through the Progesterone Receptor Membrane Component (PGRMC)" International Journal of Molecular Sciences 23, no. 7: 3843. https://doi.org/10.3390/ijms23073843
APA StyleWu, Y., Zhang, X., Fu, Y., Liu, J., Xue, Y., & Liu, Q. (2022). Progesterone Can Directly Inhibit the Life Activities of Toxoplasma gondii In Vitro through the Progesterone Receptor Membrane Component (PGRMC). International Journal of Molecular Sciences, 23(7), 3843. https://doi.org/10.3390/ijms23073843





