Hippo Signaling in the Endometrium
Abstract
:1. Introduction
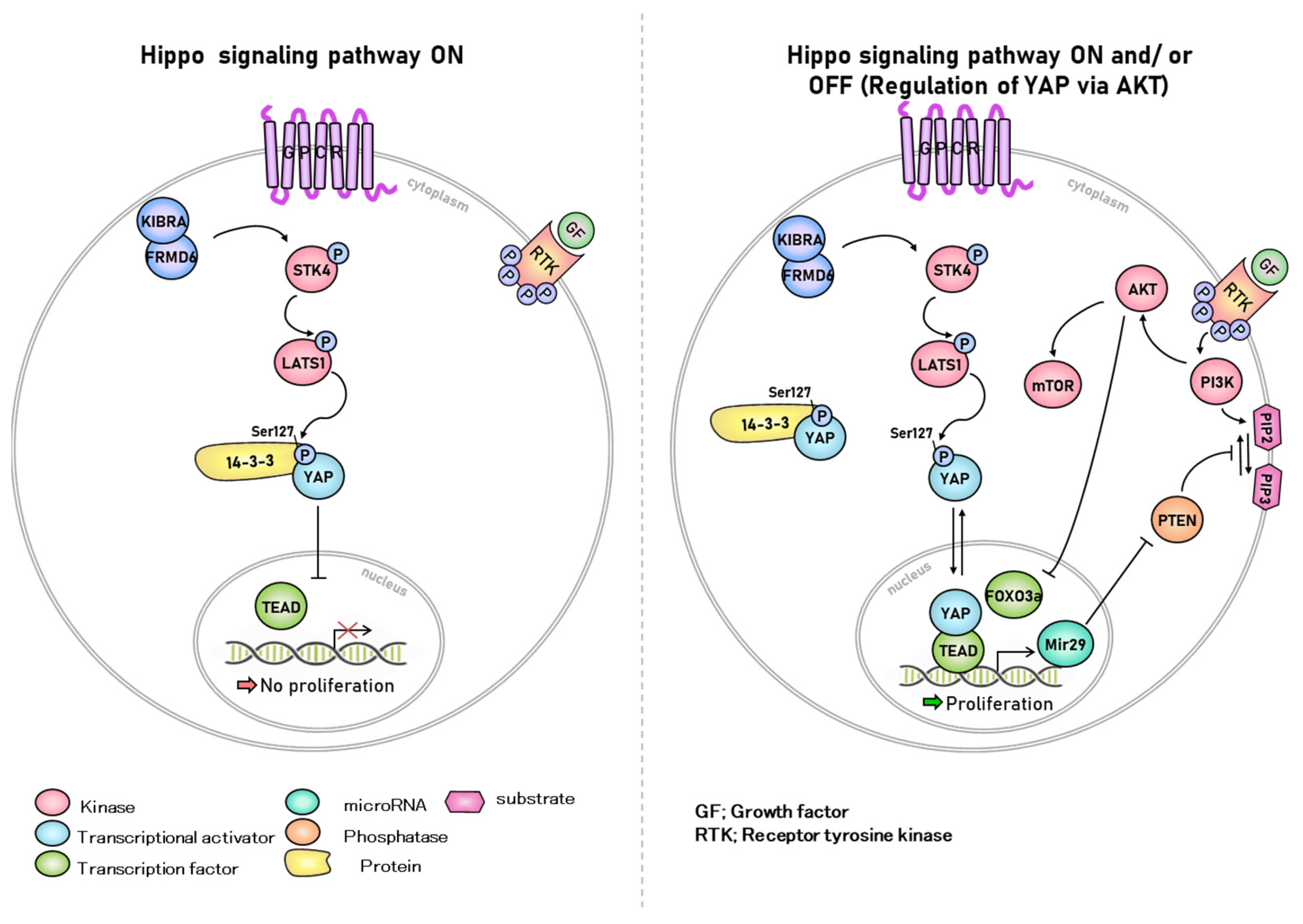
2. Hippo Signaling in Various Cellular Functions
2.1. Kinase Reaction Related to Hippo Signaling Pathway in Cellular Functions
2.2. Cell-to-Cell Contact Interaction
2.3. Hippo Signaling in Regulation of Cell Density and Cancer
2.4. STK4 in Apoptosis
2.5. Rules of Hippo Signaling in an In Vitro System
3. Hippo Signaling Factors in the Uterine Endometrium
3.1. STK3/4 in the Uterine Endometrium
3.2. LATS1/2 in the Uterine Endometrium
3.3. YAP in the Uterine Endometrium
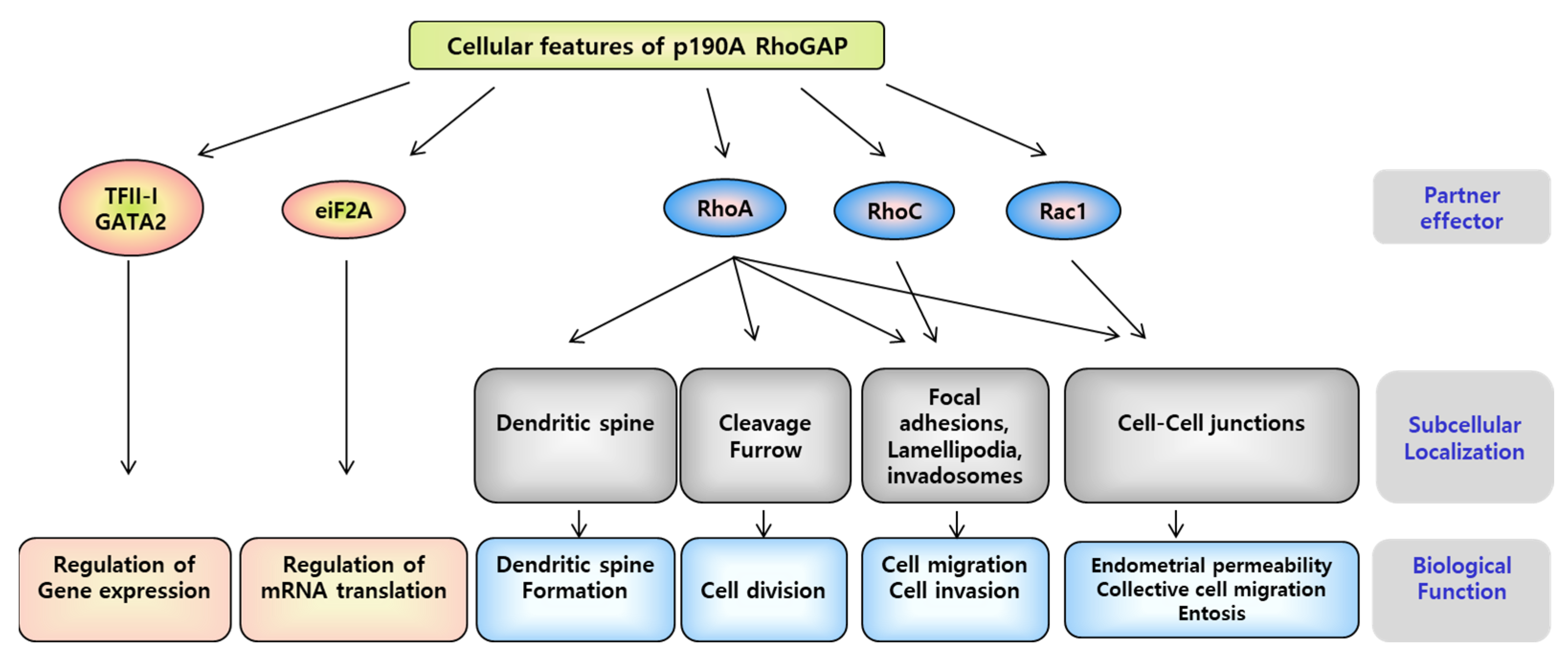
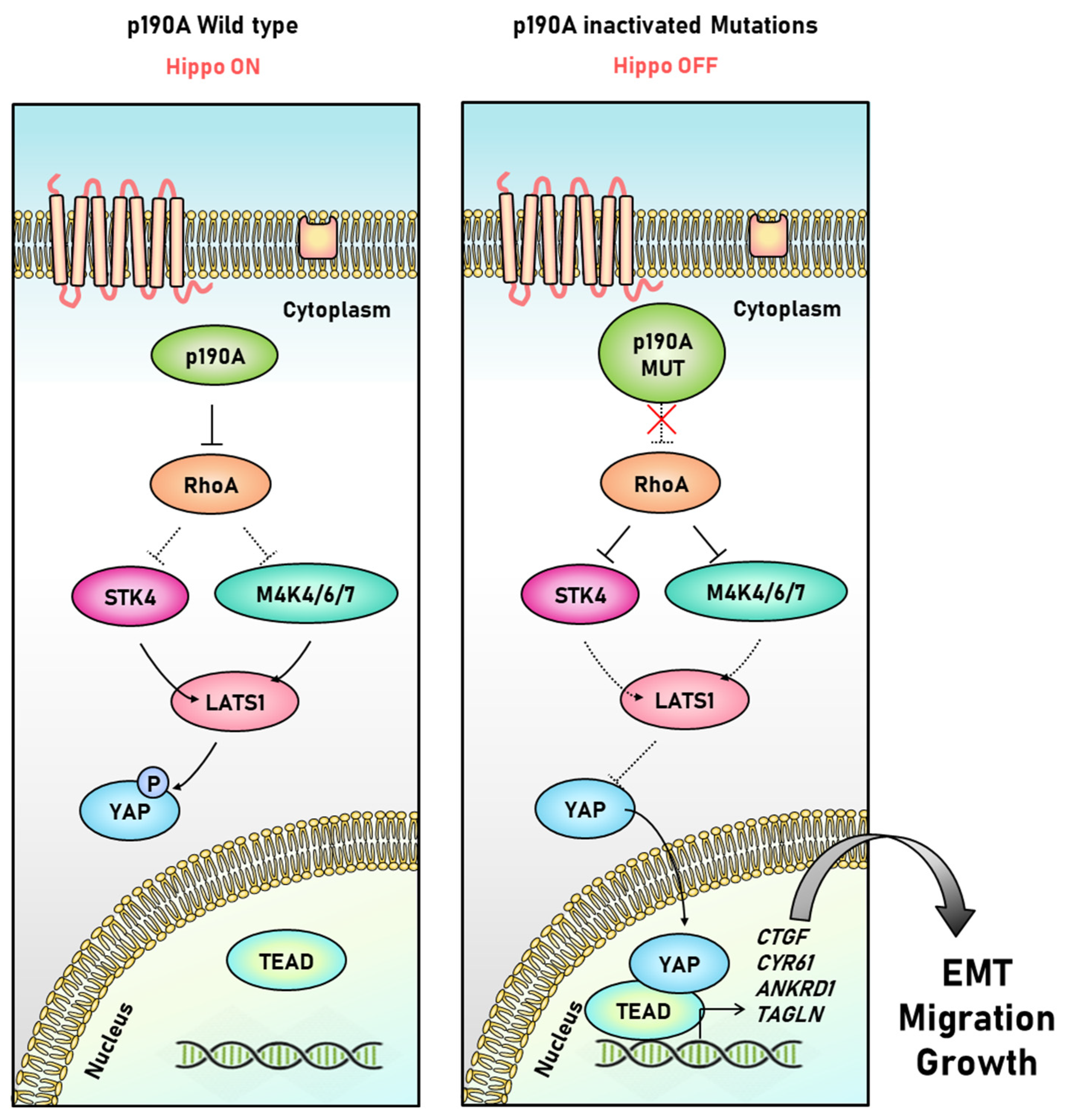
3.4. P190A in the Uterine Endometrium
4. Downstream Targets of Hippo Signaling in the Endometrium
4.1. Connective Tissue Growth Factor (CTGF)
4.2. Cysteine-Rich Angiogenesis Inducer 61 (CYR61)
4.3. Thrombospondin-1 (THBS1)
4.4. Cyclin D1 (CCND1)
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumgartner, R.; Poernbacher, I.; Buser, N.; Hafen, E.; Stocker, H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev. Cell 2010, 18, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genevet, A.; Wehr, M.C.; Brain, R.; Thompson, B.J.; Tapon, N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev. Cell 2010, 18, 300–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, K.F.; Pfleger, C.M.; Hariharan, I.K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 2003, 114, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Zheng, Y.; Dong, J.; Klusza, S.; Deng, W.M.; Pan, D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev. Cell 2010, 18, 288–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, G.; Johnson, R.L. Hippo signaling: Growth control and beyond. Development 2011, 138, 9–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.G.; Koh, E.; Chen, X.; Gumbiner, B.M. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc. Natl. Acad. Sci. USA 2011, 108, 11930–11935. [Google Scholar] [CrossRef] [Green Version]
- Gumbiner, B.M.; Kim, N.G. The Hippo-YAP signaling pathway and contact inhibition of growth. J. Cell Sci. 2014, 127, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Nishioka, N.; Inoue, K.; Adachi, K.; Kiyonari, H.; Ota, M.; Ralston, A.; Yabuta, N.; Hirahara, S.; Stephenson, R.O.; Ogonuki, N.; et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 2009, 16, 398–410. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.; Choi, Y. Role of estrogen and RAS signaling in repeated implantation failure. BMB Rep. 2018, 51, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauviel, A.; Nallet-Staub, F.; Varelas, X. Integrating developmental signals: A Hippo in the (path)way. Oncogene 2012, 31, 1743–1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boggiano, J.C.; Vanderzalm, P.J.; Fehon, R.G. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev. Cell 2011, 21, 888–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udan, R.S.; Kango-Singh, M.; Nolo, R.; Tao, C.; Halder, G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 2003, 5, 914–920. [Google Scholar] [CrossRef]
- Xiang, C.; Li, J.; Hu, L.; Huang, J.; Luo, T.; Zhong, Z.; Zheng, Y.; Zheng, L. Hippo signaling pathway reveals a spatio-temporal correlation with the size of primordial follicle pool in mice. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 35, 957–968. [Google Scholar] [CrossRef]
- Avruch, J.; Zhou, D.; Fitamant, J.; Bardeesy, N.; Mou, F.; Barrufet, L.R. Protein kinases of the Hippo pathway: Regulation and substrates. Semin. Cell Dev. Biol. 2012, 23, 770–784. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; Halder, G. The two faces of Hippo: Targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 2014, 13, 63–79. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.G.; Moroishi, T.; Guan, K.L. YAP and TAZ: A nexus for Hippo signaling and beyond. Trends Cell Biol. 2015, 25, 499–513. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Conrad, C.; Xia, F.; Park, J.S.; Payer, B.; Yin, Y.; Lauwers, G.Y.; Thasler, W.; Lee, J.T.; Avruch, J.; et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 2009, 16, 425–438. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Jho, E.H. The history and regulatory mechanism of the Hippo pathway. BMB Rep. 2018, 51, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Settleman, J. Rho GTPases in development. Prog. Mol. Subcell. Biol. 1999, 22, 201–229. [Google Scholar] [PubMed]
- Heraud, C.; Pinault, M.; Lagree, V.; Moreau, V. p190RhoGAPs, the ARHGAP35- and ARHGAP5-Encoded Proteins, in Health and Disease. Cells 2019, 8, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sordella, R.; Classon, M.; Hu, K.Q.; Matheson, S.F.; Brouns, M.R.; Fine, B.; Zhang, L.; Takami, H.; Yamada, Y.; Settleman, J. Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev. Cell 2002, 2, 553–565. [Google Scholar] [CrossRef] [Green Version]
- Frank, S.R.; Kollmann, C.P.; Luong, P.; Galli, G.G.; Zou, L.; Bernards, A.; Getz, G.; Calogero, R.A.; Frodin, M.; Hansen, S.H. p190 RhoGAP promotes contact inhibition in epithelial cells by repressing YAP activity. J. Cell Biol. 2018, 217, 3183–3201. [Google Scholar] [CrossRef]
- Ouyang, H.; Luong, P.; Frodin, M.; Hansen, S.H. p190A RhoGAP induces CDH1 expression and cooperates with E-cadherin to activate LATS kinases and suppress tumor cell growth. Oncogene 2020, 39, 5570–5587. [Google Scholar] [CrossRef]
- Zeng, Q.; Hong, W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 2008, 13, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Mayor, R.; Carmona-Fontaine, C. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 2010, 20, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Cooper, J.; Zhou, L.; Yang, C.; Erdjument-Bromage, H.; Zagzag, D.; Snuderl, M.; Ladanyi, M.; Hanemann, C.O.; Zhou, P.; et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell 2014, 26, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.Y.; Zhang, X.P.; Wu, J.H.; Qiu, X.S.; Wang, E.H. Expression of LATS1 contributes to good prognosis and can negatively regulate YAP oncoprotein in non-small-cell lung cancer. Tumour Biol. J. Int. Soc. Oncodevel. Biol. Med. 2014, 35, 6435–6443. [Google Scholar] [CrossRef]
- McClatchey, A.I.; Fehon, R.G. Merlin and the ERM proteins—Regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009, 19, 198–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladden, A.B.; Hebert, A.M.; Schneeberger, E.E.; McClatchey, A.I. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev. Cell 2010, 19, 727–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhang, N.; Gray, R.S.; Li, H.; Ewald, A.J.; Zahnow, C.A.; Pan, D. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 2014, 28, 432–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamar, J.M.; Stern, P.; Liu, H.; Schindler, J.W.; Jiang, Z.G.; Hynes, R.O. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl. Acad. Sci. USA 2012, 109, E2441–E2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nallet-Staub, F.; Marsaud, V.; Li, L.; Gilbert, C.; Dodier, S.; Bataille, V.; Sudol, M.; Herlyn, M.; Mauviel, A. Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in cutaneous melanoma. J. Investig. Dermatol. 2014, 134, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, G.M.; Schmidt, M.O.; Yi, C.; Hu, Z.; Haddad, B.R.; Glasgow, E.; Riegel, A.T.; Wellstein, A. Cell growth density modulates cancer cell vascular invasion via Hippo pathway activity and CXCR2 signaling. Oncogene 2015, 34, 5879–5889. [Google Scholar] [CrossRef] [Green Version]
- Hajra, K.M.; Fearon, E.R. Cadherin and catenin alterations in human cancer. Genes Chromosomes Cancer 2002, 34, 255–268. [Google Scholar] [CrossRef]
- Jeanes, A.; Gottardi, C.; Yap, A. Cadherins and cancer: How does cadherin dysfunction promote tumor progression? Oncogene 2008, 27, 6920–6929. [Google Scholar] [CrossRef] [Green Version]
- Henkart, P.A. ICE family proteases: Mediators of all apoptotic cell death? Immunity 1998, 4, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Graves, J.D.; Gotoh, Y.; Draves, K.E.; Ambrose, D.; Han, D.K.; Wright, M.; Chernoff, J.; Clark, E.A.; Krebs, E.G. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 1998, 17, 2224–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montenarh, M.; Gotz, C. Protein kinase CK2 and ion channels (Review). Biomed. Rep. 2020, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Servas, C.; Kiehlmeier, S.; Hach, J.; Gross, R.; Gotz, C.; Montenarh, M. The mammalian STE20-like kinase 1 (MST1) is a substrate for the apoptosis inhibiting protein kinase CK2. Cell. Signal. 2017, 36, 163–175. [Google Scholar] [CrossRef]
- Intemann, J.; Saidu, N.E.B.; Schwind, L.; Montenarh, M. ER stress signaling in ARPE-19 cells after inhibition of protein kinase CK2 by CX-4945. Cell. Signal. 2014, 26, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Turowec, J.P.; Duncan, J.S.; Gloor, G.B.; Litchfield, D.W. Regulation of caspase pathways by protein kinase CK2: Identification of proteins with overlapping CK2 and caspase consensus motifs. Mol. Cell. Biochem. 2011, 356, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, W.; Wang, B.; Trinko, R.; Jiang, J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003, 17, 2514–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kango-Singh, M.; Nolo, R.; Tao, C.; Verstreken, P.; Hiesinger, P.R.; Bellen, H.J.; Halder, G. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 2002, 129, 5719–5730. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Huang, J.; Dong, J.; Pan, D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 2003, 114, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Ji, C.; Tan, L.; Lin, S.; Zhu, Y.; Long, M.; Luo, D.; Li, H. Long non-coding RNA TNRC6C-AS1 promotes methylation of STK4 to inhibit thyroid carcinoma cell apoptosis and autophagy via Hippo signalling pathway. J. Cell. Mol. Med. 2020, 24, 304–316. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, F.; Cui, Y.; Liu, S.; Sun, H. Mst1 overexpression combined with Yap knockdown augments thyroid carcinoma apoptosis via promoting MIEF1-related mitochondrial fission and activating the JNK pathway. Cancer Cell Int. 2019, 19, 143. [Google Scholar] [CrossRef]
- Chang, Y.; Fu, X.R.; Cui, M.; Li, W.M.; Zhang, L.; Li, X.; Li, L.; Sun, Z.C.; Zhang, X.D.; Li, Z.M.; et al. Activated hippo signal pathway inhibits cell proliferation and promotes apoptosis in NK/T cell lymphoma cells. Cancer Med. 2019, 8, 3892–3904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.; Yang, J.; DeRan, M.; Wu, C.; Su, A.I.; Bonamy, G.M.; Liu, J.; Peters, E.C.; Wu, X. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem. Biol. 2012, 19, 955–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, S.; von Otte, S.; Robenek, H.; Diedrich, K.; Nofer, J.-R. Follicular fluid high-density lipoprotein-associated sphingosine 1-phosphate (S1P) promotes human granulosa lutein cell migration via S1P receptor type 3 and small G-protein RAC1. Biol. Reprod. 2011, 84, 604–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Moolenaar, W.H. Lysophosphatidic Acid, a Multifunctional Phospholipid Messenger. J. Biol. Chem. 1995, 270, 12949–12952. [Google Scholar] [CrossRef] [Green Version]
- Pustilnik, T.B.; Estrella, V.; Wiener, J.R.; Mao, M.; Eder, A.; Watt, M.-A.V.; Bast, R.C.; Mills, G.B. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin. Cancer Res. 1999, 5, 3704–3710. [Google Scholar]
- Fukushima, N.; Weiner, J.A.; Kaushal, D.; Contos, J.J.; Rehen, S.K.; Kingsbury, M.A.; Kim, K.Y.; Chun, J. Lysophosphatidic acid influences the morphology and motility of young, postmitotic cortical neurons. Mol. Cell. Neurosci. 2002, 20, 271–282. [Google Scholar] [CrossRef]
- Kim, K.-S.; Sengupta, S.; Berk, M.; Kwak, Y.-G.; Escobar, P.F.; Belinson, J.; Mok, S.C.; Xu, Y. Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res. 2006, 66, 7983–7990. [Google Scholar] [CrossRef] [Green Version]
- Eder, A.M.; Sasagawa, T.; Mao, M.; Aoki, J.; Mills, G.B. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: Role of phospholipase D and phospholipase A2. Clin. Cancer Res. 2000, 6, 2482–2491. [Google Scholar]
- Kowalczyk-Zieba, I.; Boruszewska, D.; Saulnier-Blache, J.S.; Da Costa, L.L.; Jankowska, K.; Skarzynski, D.J.; Woclawek-Potocka, I. Lysophosphatidic acid action in the bovine corpus luteum—An in vitro study. J. Reprod. Dev. 2012, 58, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Luquain, C.; Singh, A.; Wang, L.; Natarajan, V.; Morris, A.J. Role of phospholipase D in agonist-stimulated lysophosphatidic acid synthesis by ovarian cancer cells. J. Lipid Res. 2003, 44, 1963–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.; Belinson, J.; Morton, R.E.; Xu, Y. Phorbol 12-myristate 13-acetate stimulates lysophosphatidic acid secretion from ovarian and cervical cancer cells but not from breast or leukemia cells. Gynecol. Oncol. 1998, 71, 364–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woclawek-Potocka, I.; Brzezicka, E.; Skarzynski, D.J. Lysophosphatic acid modulates prostaglandin secretion in the bovine endometrial cells differently on days 8–10 of the estrous cycle and early pregnancy. J. Reprod. Dev. 2009, 55, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.-C.; Wang, E.Y.; Yi, Y.; Thakur, A.; Tsai, S.-H.; Hoodless, P.A. S1P stimulates proliferation by upregulating CTGF expression through S1PR2-mediated YAP activation. Mol. Cancer Res. 2018, 16, 1543–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, H.; Terai, K.; Nakajima, H.; Chiba, A.; Fukuhara, S.; Mochizuki, N. S1P-Yap1 signaling regulates endoderm formation required for cardiac precursor cell migration in zebrafish. Dev. Cell 2014, 31, 128–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Feng, Y.; Jansson, L.; Sato, Y.; Deguchi, M.; Kawamura, K.; Hsueh, A.J. Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the Hippo signaling effector YAP. FASEB J. 2015, 29, 2423–2430. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, I.; Chaqour, B. Regulation of connective tissue growth factor (CTGF/CCN2) gene transcription and mRNA stability in smooth muscle cells: Involvement of RhoA GTPase and p38 MAP kinase and sensitivity to actin dynamics. Eur. J. Biochem. 2004, 271, 4436–4450. [Google Scholar] [CrossRef]
- Fan, Q.; Cheng, Y.; Chang, H.-M.; Deguchi, M.; Hsueh, A.J.; Leung, P.C. Sphingosine-1-phosphate promotes ovarian cancer cell proliferation by disrupting Hippo signaling. Oncotarget 2017, 8, 27166. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-H.; Sanchez, T.; Pappalardo, A.; Lynch, K.R.; Hla, T.; Ferrer, F. Induction of antiproliferative connective tissue growth factor expression in Wilms’ tumor cells by sphingosine-1-phosphate receptor 2. Mol. Cancer Res. 2008, 6, 1649–1656. [Google Scholar] [CrossRef] [Green Version]
- Young, N.; Pearl, D.K.; Van Brocklyn, J.R. Sphingosine-1-phosphate regulates glioblastoma cell invasiveness through the urokinase plasminogen activator system and CCN1/Cyr61. Mol. Cancer Res. 2009, 7, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.; Lee, O.-H.; Lee, S.; Lee, J.; Park, H.; Park, M.; Chang, E.M.; Park, K.-H.; Choi, Y. STK3/4 Expression Is Regulated in Uterine Endometrial Cells during the Estrous Cycle. Cells 2019, 8, 1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britschgi, A.; Duss, S.; Kim, S.; Couto, J.P.; Brinkhaus, H.; Koren, S.; De Silva, D.; Mertz, K.D.; Kaup, D.; Varga, Z.; et al. The Hippo kinases LATS1 and 2 control human breast cell fate via crosstalk with ERalpha. Nature 2017, 541, 541–545. [Google Scholar] [CrossRef]
- St John, M.A.; Tao, W.; Fei, X.; Fukumoto, R.; Carcangiu, M.L.; Brownstein, D.G.; Parlow, A.F.; McGrath, J.; Xu, T. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat. Genet. 1999, 21, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Pepling, M.E.; Diaz, F.J. Lats1 Deletion Causes Increased Germ Cell Apoptosis and Follicular Cysts in Mouse Ovaries. Biol. Reprod. 2015, 93, 22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Aikawa, S.; Deng, W.; Bartos, A.; Walz, G.; Grahammer, F.; Huber, T.B.; Sun, X.; Dey, S.K. Primary decidual zone formation requires Scribble for pregnancy success in mice. Nat. Commun. 2019, 10, 5425. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Bachman, J.; Lai, Z.C. Mutation analysis of large tumor suppressor genes LATS1 and LATS2 supports a tumor suppressor role in human cancer. Protein Cell 2015, 6, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Song, Y.; Yang, S.; Fu, J.; Feng, X.; Huang, W. YAP mediates human decidualization of the uterine endometrial stromal cells. Placenta 2017, 53, 30–35. [Google Scholar] [CrossRef]
- Pei, T.; Huang, X.; Long, Y.; Duan, C.; Liu, T.; Li, Y.; Huang, W. Increased expression of YAP is associated with decreased cell autophagy in the eutopic endometrial stromal cells of endometriosis. Mol. Cell. Endocrinol. 2019, 491, 110432. [Google Scholar] [CrossRef]
- Song, Y.; Fu, J.; Zhou, M.; Xiao, L.; Feng, X.; Chen, H.; Huang, W. Activated hippo/yes-associated protein pathway promotes cell proliferation and anti-apoptosis in endometrial stromal cells of endometriosis. J. Clin. Endocrinol. Metab. 2016, 101, 1552–1561. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, M.; Yang, J.-X.; Cao, D.-Y.; Zhang, Y.; Zhou, H.-M.; Yuan, Z.; Shen, K. Genomic comparison of endometrioid endometrial carcinoma and its precancerous lesions in Chinese patients by high-depth next generation sequencing. Front. Oncol. 2019, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Wan, J.; He, Q.; Wang, M.; Li, S.; Jiang, M.; Qian, Z.; Liu, B.; Lu, W.; Wang, K.; et al. p190A inactivating mutations cause aberrant RhoA activation and promote malignant transformation via the Hippo-YAP pathway in endometrial cancer. Signal Transduct. Target. Ther. 2020, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Shome, D.; von Woedtke, T.; Riedel, K.; Masur, K. The HIPPO Transducer YAP and Its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing. Oxidative Med. Cell. Longev. 2020, 2020, 4910280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haskins, J.W.; Nguyen, D.X.; Stern, D.F. Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway target genes and promote cell migration. Sci. Signal. 2014, 7, ra116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Li, L.; Lei, Q.; Guan, K.L. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010, 24, 862–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.Y.; Chinnaiyan, A.M.; et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, S.W.; Lim, C.J.; Chong, Y.F.; Pobbati, A.V.; Huang, C.; Hong, W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J. Biol. Chem. 2011, 286, 7018–7026. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Huang, J.; Chen, J. Angiomotin-like proteins associate with and negatively regulate YAP1. J. Biol. Chem. 2011, 286, 4364–4370. [Google Scholar] [CrossRef] [Green Version]
- Reardon, S.N.; King, M.L.; MacLean, J.A., 2nd; Mann, J.L.; DeMayo, F.J.; Lydon, J.P.; Hayashi, K. CDH1 is essential for endometrial differentiation, gland development, and adult function in the mouse uterus. Biol. Reprod. 2012, 86, 141-1–141-10. [Google Scholar] [CrossRef]
- Herington, J.L.; Bi, J.; Martin, J.D.; Bany, B.M. Beta-catenin (CTNNB1) in the mouse uterus during decidualization and the potential role of two pathways in regulating its degradation. J. Histochem. Cytochem. 2007, 55, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Kim, G.N.; Fellman, B.M.; Urbauer, D.L.; Mills, G.B.; Zhang, W.; Broaddus, R.R. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod. Pathol. 2017, 30, 1032–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaitseva, M.; Holdsworth-Carson, S.J.; Waldrip, L.; Nevzorova, J.; Martelotto, L.; Vollenhoven, B.J.; Rogers, P.A. Aberrant expression and regulation of NR2F2 and CTNNB1 in uterine fibroids. Reproduction 2013, 146, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Keightley, M.C.; Sales, K.J.; Jabbour, H.N. PGF2alpha-F-prostanoid receptor signalling via ADAMTS1 modulates epithelial cell invasion and endothelial cell function in endometrial cancer. BMC Cancer 2010, 10, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, B.; Koshi, K.; Kizaki, K.; Ushizawa, K.; Takahashi, T.; Hosoe, M.; Sato, T.; Ito, A.; Hashizume, K. Expression of ADAMTS1 mRNA in bovine endometrium and placenta during gestation. Domest. Anim. Endocrinol. 2013, 45, 43–48. [Google Scholar] [CrossRef]
- Almodovar-Garcia, K.; Kwon, M.; Samaras, S.E.; Davidson, J.M. ANKRD1 acts as a transcriptional repressor of MMP13 via the AP-1 site. Mol. Cell. Biol. 2014, 34, 1500–1511. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Ruggiero, C.L.; Bauman, W.A.; Cardozo, C. Ankrd1 is a transcriptional repressor for the androgen receptor that is downregulated by testosterone. Biochem. Biophys. Res. Commun. 2013, 437, 355–360. [Google Scholar] [CrossRef]
- Honda, H.; Barrueto, F.F.; Gogusev, J.; Im, D.D.; Morin, P.J. Serial analysis of gene expression reveals differential expression between endometriosis and normal endometrium. Possible roles for AXL and SHC1 in the pathogenesis of endometriosis. Reprod. Biol. Endocrinol. 2008, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Findakly, D.; Wang, J. Molecular Profiling of Benign Metastasizing Leiomyoma of the Uterus Revealing Unique Novel Therapeutic Targets. Cureus 2020, 12, e7701. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Ge, L.; Dong, S.; Liu, Y.; Wang, D.; Zhou, C.; Ma, C.; Wang, Y.; Su, F.; Jiang, Y. Global miRNA, lncRNA, and mRNA Transcriptome Profiling of Endometrial Epithelial Cells Reveals Genes Related to Porcine Reproductive Failure Caused by Porcine Reproductive and Respiratory Syndrome Virus. Front. Immunol. 2019, 10, 1221. [Google Scholar] [CrossRef] [Green Version]
- Gashaw, I.; Stiller, S.; Boing, C.; Kimmig, R.; Winterhager, E. Premenstrual regulation of the pro-angiogenic factor CYR61 in human endometrium. Endocrinology 2008, 149, 2261–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLaughlan, S.D.; Palomino, W.A.; Mo, B.; Lewis, T.D.; Lininger, R.A.; Lessey, B.A. Endometrial expression of Cyr61: A marker of estrogenic activity in normal and abnormal endometrium. Obstet. Gynecol. 2007, 110, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ball, D.K.; Surveyor, G.A.; Diehl, J.R.; Steffen, C.L.; Uzumcu, M.; Mirando, M.A.; Brigstock, D.R. Characterization of 16- to 20-kilodalton (kDa) connective tissue growth factors (CTGFs) and demonstration of proteolytic activity for 38-kDa CTGF in pig uterine luminal flushings. Biol. Reprod. 1998, 59, 828–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigstock, D.R.; Steffen, C.L.; Kim, G.Y.; Vegunta, R.K.; Diehl, J.R.; Harding, P.A. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids. Identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J. Biol. Chem. 1997, 272, 20275–20282. [Google Scholar] [CrossRef] [Green Version]
- Maybin, J.A.; Barcroft, J.; Thiruchelvam, U.; Hirani, N.; Jabbour, H.N.; Critchley, H.O. The presence and regulation of connective tissue growth factor in the human endometrium. Hum. Reprod. 2012, 27, 1112–1121. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.; Lee, S.; Caesar, J.A.; Pruchenko, S.; Leask, A.; Knowles, J.A.; Sinon, J.; Chaqour, B. A CTGF-YAP regulatory pathway is essential for angiogenesis and barriergenesis in the retina. Iscience 2020, 23, 101184. [Google Scholar] [CrossRef]
- Harding, P.A.; Surveyor, G.A.; Brigstock, D.R. Characterization of pig connective tissue growth factor (CTGF) cDNA, mRNA and protein from uterine tissue. DNA Seq. J. DNA Seq. Mapp. 1998, 8, 385–390. [Google Scholar] [CrossRef]
- Moussad, E.E.; Rageh, M.A.; Wilson, A.K.; Geisert, R.D.; Brigstock, D.R. Temporal and spatial expression of connective tissue growth factor (CCN2; CTGF) and transforming growth factor beta type 1 (TGF-beta1) at the utero-placental interface during early pregnancy in the pig. Mol. Pathol. MP 2002, 55, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Surveyor, G.A.; Wilson, A.K.; Brigstock, D.R. Localization of connective tissue growth factor during the period of embryo implantation in the mouse. Biol. Reprod. 1998, 59, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Uzumcu, M.; Homsi, M.F.; Ball, D.K.; Coskun, S.; Jaroudi, K.; Hollanders, J.M.; Brigstock, D.R. Localization of connective tissue growth factor in human uterine tissues. Mol. Hum. Reprod. 2000, 6, 1093–1098. [Google Scholar] [CrossRef]
- Winterhager, E.; Gellhaus, A. The role of the CCN family of proteins in female reproduction. Cell. Mol. Life Sci. CMLS 2014, 71, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Rageh, M.A.; Moussad, E.E.; Wilson, A.K.; Brigstock, D.R. Steroidal regulation of connective tissue growth factor (CCN2; CTGF) synthesis in the mouse uterus. Mol. Pathol. MP 2001, 54, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiodoni, C.; Colombo, M.P.; Sangaletti, S. Matricellular proteins: From homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010, 29, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.-I.; Lau, L.F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 2010, 12, 676–685. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Bornstein, P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb. Haemost. 2003, 90, 986–992. [Google Scholar] [CrossRef]
- Lau, L.F. CCN1/CYR61: The very model of a modern matricellular protein. Cell. Mol. Life Sci. 2011, 68, 3149–3163. [Google Scholar] [CrossRef] [Green Version]
- Sampath, D.; Zhu, Y.; Winneker, R.C.; Zhang, Z. Aberrant expression of Cyr61, a member of the CCN (CTGF/Cyr61/Cef10/NOVH) family, and dysregulation by 17β-estradiol and basic fibroblast growth factor in human uterine leiomyomas. J. Clin. Endocrinol. Metab. 2001, 86, 1707–1715. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Stiller, S.; Gashaw, I. Epidermal growth factor upregulates endometrial CYR61 expression via activation of the JAK2/STAT3 pathway. Reprod. Fertil. Dev. 2012, 24, 482–489. [Google Scholar] [CrossRef]
- Li, Z.; Yan, G.; Diao, Q.; Yu, F.; Sheng, X.; Liu, Y.; Dai, Y.; Zhou, H.; Zhen, X.; Hu, Y. Transplantation of human endometrial perivascular cells with elevated CYR61 expression induces angiogenesis and promotes repair of a full-thickness uterine injury in rat. Stem Cell Res. Ther. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Park, M.-H.; Kim, A.k.; Manandhar, S.; Oh, S.-Y.; Jang, G.-H.; Kang, L.; Lee, D.-W.; Lee, S.-H.; Lee, H.E.; Huh, T.-L. CCN1 interlinks integrin and hippo pathway to autoregulate tip cell activity. Elife 2019, 8, e46012. [Google Scholar] [CrossRef]
- Hilfiker-Kleiner, D.; Kaminski, K.; Kaminska, A.; Fuchs, M.; Klein, G.; Podewski, E.; Grote, K.; Kiian, I.; Wollert, K.C.; Hilfiker, A. Regulation of proangiogenic factor CCN1 in cardiac muscle: Impact of ischemia, pressure overload, and neurohumoral activation. Circulation 2004, 109, 2227–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Absenger, Y.; Hess-Stumpp, H.; Kreft, B.; Kratzschmar, J.; Haendler, B.; Schutze, N.; Regidor, P.A.; Winterhager, E. Cyr61, a deregulated gene in endometriosis. Mol. Hum. Reprod. 2004, 10, 399–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, W.; Kumagai, T.; Miller, C.W.; Desmond, J.C.; Frank, J.M.; Said, J.W.; Koeffler, H.P. Cyr61 suppresses growth of human endometrial cancer cells. J. Biol. Chem. 2004, 279, 53087–53096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gashaw, I.; Hastings, J.M.; Jackson, K.S.; Winterhager, E.; Fazleabas, A.T. Induced endometriosis in the baboon (Papio anubis) increases the expression of the proangiogenic factor CYR61 (CCN1) in eutopic and ectopic endometria. Biol. Reprod. 2006, 74, 1060–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witek, L.; Janikowski, T.; Bodzek, P.; Olejek, A.; Mazurek, U. Expression of tumor suppressor genes related to the cell cycle in endometrial cancer patients. Adv. Med. Sci. 2016, 61, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell. Mol. Med. 2002, 6, 1–12. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- Zaslavsky, A.; Baek, K.-H.; Lynch, R.C.; Short, S.; Grillo, J.; Folkman, J.; Italiano, J.E., Jr.; Ryeom, S. Platelet-derived thrombospondin-1 is a critical negative regulator and potential biomarker of angiogenesis. Blood J. Am. Soc. Hematol. 2010, 115, 4605–4613. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Manzaneque, J.C.; Lane, T.F.; Ortega, M.A.; Hynes, R.O.; Lawler, J.; Iruela-Arispe, M.L. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc. Natl. Acad. Sci. USA 2001, 98, 12485–12490. [Google Scholar] [CrossRef] [Green Version]
- Seki, N.; Kodama, J.; Hashimoto, I.; Hongo, A.; Yoshinouchi, M.; Kudo, T. Thrombospondin-1 and-2 messenger RNA expression in normal and neoplastic endometrial tissues: Correlation with angiogenesis and prognosis. Int. J. Oncol. 2001, 19, 305–310. [Google Scholar] [CrossRef]
- Bonagura, T.W.; Aberdeen, G.W.; Babischkin, J.S.; Koos, R.D.; Pepe, G.J.; Albrecht, E.D. Divergent regulation of angiopoietin-1 and-2, Tie-2, and thrombospondin-1 expression by estrogen in the baboon endometrium. Mol. Reprod. Dev. Inc. Gamete Res. 2010, 77, 430–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukas, J.; Müller, H.; Bartkova, J.; Spitkovsky, D.; Kjerulff, A.A.; Jansen-Dürr, P.; Strauss, M.; Bartek, J. DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell’s requirement for cyclin D1 function in G1. J. Cell Biol. 1994, 125, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. D-type cyclins. Trends Biochem. Sci. 1995, 20, 187–190. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Hu, L.; Hu, D.; Ma, S.; Sun, X.; Jiang, L.; Song, J.; Ji, L.; Masau, J.F. CDKN1C (P57): One of the determinants of human endometrial stromal cell decidualization. Biol. Reprod. 2018, 98, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhu, J.-f.; Liu, Y.-y.; Han, G.-p. An analysis of cyclin D1, cytokeratin 5/6 and cytokeratin 8/18 expression in breast papillomas and papillary carcinomas. Diagn. Pathol. 2013, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Horvai, A.E.; Kramer, M.J.; O’Donnell, R. β-catenin nuclear expression correlates with cyclin D1 expression in primary and metastatic synovial sarcoma: A tissue microarray study. Arch. Pathol. Lab. Med. 2006, 130, 792–798. [Google Scholar] [CrossRef]
- Kim, J.K.; Diehl, J.A. Nuclear cyclin D1: An oncogenic driver in human cancer. J. Cell. Physiol. 2009, 220, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Hicks, D.; Xu, B.; Sigel, J.E.; Bergfeld, W.F.; Montgomery, E.; Fisher, C.; Hartke, M.; Tubbs, R.; Goldblum, J.R. Expression profile and molecular genetic regulation of cyclin D1 expression in epithelioid sarcoma. Mod. Pathol. 2005, 18, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Khabaz, M.N.; Abdelrahman, A.S.; Butt, N.S.; Al-Maghrabi, B.; Al-Maghrabi, J. Cyclin D1 is significantly associated with stage of tumor and predicts poor survival in endometrial carcinoma patients. Ann. Diagn. Pathol. 2017, 30, 47–51. [Google Scholar] [CrossRef]
- Liang, S.; Mu, K.; Wang, Y.; Zhou, Z.; Zhang, J.; Sheng, Y.; Zhang, T. CyclinD1, a prominent prognostic marker for endometrial diseases. Diagn. Pathol. 2013, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- McCormick, D.; Chong, H.; Hobbs, C.; Datta, C.; Hall, P. Detection of the Ki-67 antigen in fixed and wax-embedded sections with the monoclonal antibody MIB1. Histopathology 1993, 22, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Shevra, C.; Ghosh, A.; Kumar, M. Cyclin D1 and Ki-67 expression in normal, hyperplastic and neoplastic endometrium. J. Postgrad. Med. 2015, 61, 15. [Google Scholar] [PubMed]
- Shawana, S.; Kehar, S.I.; Masood, S.; Aamir, I. Immunoexpression of cyclin D1 and PTEN in various endometrial pathologies. J. Coll. Physicians Surg. Pak. 2016, 26, 277–282. [Google Scholar] [PubMed]
- Cirpan, T.; Terek, M.; Mgoyi, L.; Zekioglu, O.; Iscan, O.; Ozsaran, A. Immunohistochemical evaluation of PTEN protein in patients with endometrial intraepithelial neoplasia compared to endometrial adenocarcinoma and proliferative phase endometrium. Eur. J. Gynaecol. Oncol. 2006, 27, 389–392. [Google Scholar]
- Lee, K.-K.; Yonehara, S. Identification of mechanism that couples multisite phosphorylation of Yes-associated protein (YAP) with transcriptional coactivation and regulation of apoptosis. J. Biol. Chem. 2012, 287, 9568–9578. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Choi, H.J.; Kang, C.S.; Lee, H.J.; Lee, W.S.; Park, C.S. Expression of miRNAs and PTEN in endometrial specimens ranging from histologically normal to hyperplasia and endometrial adenocarcinoma. Mod. Pathol. 2012, 25, 1508–1515. [Google Scholar] [CrossRef] [Green Version]
- Tantbirojn, P.; Triratanachat, S.; Trivijitsilp, P.; Niruthisard, S. Detection of PTEN Immunoreactivity in Endmetrial Hyperplasia and Adenocarcinoma. Med. J. Med. Assoc. Thail. 2008, 91, 1161–1165. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.; Hwang, S.; Kim, B.; Lee, S.; Kim, H.; Lee, G.; Hong, K.; Song, H.; Choi, Y. Hippo Signaling in the Endometrium. Int. J. Mol. Sci. 2022, 23, 3852. https://doi.org/10.3390/ijms23073852
Moon S, Hwang S, Kim B, Lee S, Kim H, Lee G, Hong K, Song H, Choi Y. Hippo Signaling in the Endometrium. International Journal of Molecular Sciences. 2022; 23(7):3852. https://doi.org/10.3390/ijms23073852
Chicago/Turabian StyleMoon, Sohyeon, Semi Hwang, Byeongseok Kim, Siyoung Lee, Hyoukjung Kim, Giwan Lee, Kwonho Hong, Hyuk Song, and Youngsok Choi. 2022. "Hippo Signaling in the Endometrium" International Journal of Molecular Sciences 23, no. 7: 3852. https://doi.org/10.3390/ijms23073852
APA StyleMoon, S., Hwang, S., Kim, B., Lee, S., Kim, H., Lee, G., Hong, K., Song, H., & Choi, Y. (2022). Hippo Signaling in the Endometrium. International Journal of Molecular Sciences, 23(7), 3852. https://doi.org/10.3390/ijms23073852







