Physiological Electric Field: A Potential Construction Regulator of Human Brain Organoids
Abstract
1. Introduction
2. Traditional Approaches to Obtain Brain Organoid
2.1. Following the In Vivo Procedures of Neural Development as a Strategy for Constructing Brain Organoids
2.2. Challenges for Constructing Brain Organoids
3. Could Adding EFs Enhance the Efficacy of Constructing Human Brain Organoids?
3.1. Using EFs Influences the Formation of Brain Organoids: Thinking Differently
3.2. Endogenous EFs Regulate Neural Development In Vivo
| Main Results | Species | References |
|---|---|---|
| Xenopus embryos maintain an inwardly positive electrical potential across their skin throughout most of their early development. | xenopus | [58] |
| EF exists in the inner side of the nerve plate and nerve folds and the side-wall of the neural tube during embryonic development. In the process of neural tube formation, blocking endogenous EF will cause neural development defects. | axolotl | [59] |
| Altering the internal field results in defects in the tail, limb bud, and head development. | chick | [27] |
| The physiological EFs direct and stimulate the migration of the SVZ neuroblasts along the rostral migratory path. | mouse | [61] |
| Subplate neurons extend neurites toward the ventricular side of the subplate and form transient glutamatergic synapses. | mouse | [55] |
| An electrically active boundary organizes neuronal migration during cortical development. | mouse | [60] |
| Cells manifest high electrical activity as they establish afferent and efferent synaptic connections within the developing cortex. | mouse/human | [62] |
| Ion flow and voltage difference are generated among different zones in the brain cortex. |
4. EFs Affect the In Vitro Behavior of Neural Cells
4.1. Types of Exogenous EFs
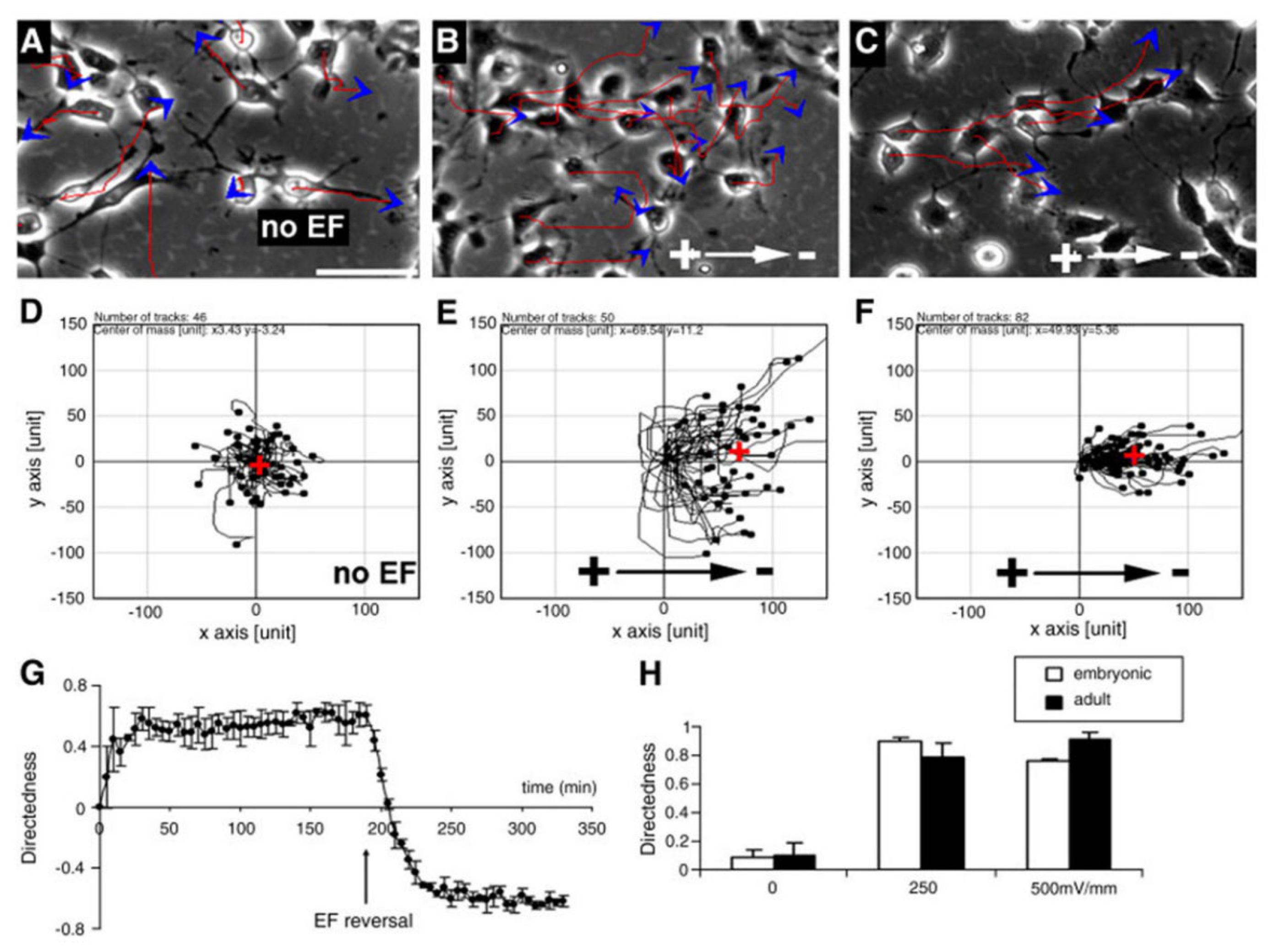
4.2. EFs Induce the Directional Migration of Neural Lineage Cells In Vitro
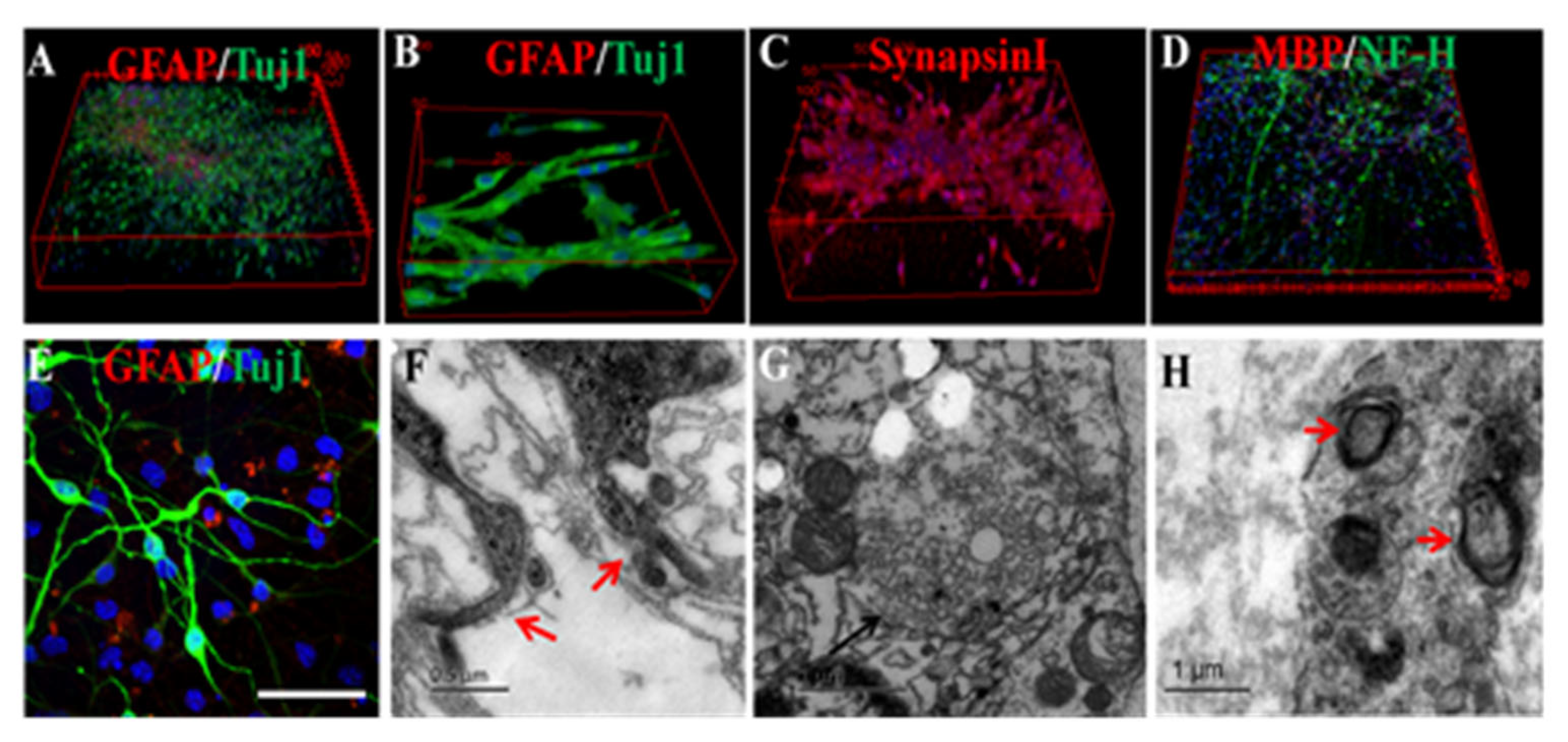
4.3. EFs Regulate In Vitro Neuronal Differentiation
| Cell Type | Tuj1% | MAP2% | GFAP% | Oligo% | Intensity | Time | Species | References |
|---|---|---|---|---|---|---|---|---|
| NPCs | 16.9 ± 5.3 | — | 61.6 ± 2.7 | 10.1 ± 0.7 | 115 V/m | 2 h/day | mouse | [70] |
| NSCs | ~2.3 | ~1.5 | — | — | 100 Hz | 7 days | mouse | [84] |
| NPCs | 42 | — | 15 | — | 437 mV/mm | 16–24 h/day | rat | [79] |
| MSCs | ↑ 4.5 folds | ↑ 4 folds | — | — | 250 mV | 1000 s | human | [80] |
| NSCs | ↑~2.5 folds | — | ↑~1.5 folds | — | 20–30 μA | — | mouse | [81] |
| NPCs | 18.3 ± 12.0 — — | — — — | — 54.9 ± 23.5 — | — — 0.7 ± 0.2 | 10 Hz 1 Hz 10 Hz | 3 DIV 14 DIV 14 DIV | porcine | [82] |
| iPSCs | — | 29 | — | — | 30 μA | 10 min | human | [74] |
| NPCs | 20.9 | — | 69.4 | 29 | 300 mV/mm | 48 h | Mouse (E13.5) | [75] |
| NSCs | 79.5 | — | 11.6 | — | 75 mV | 7 days | human | [53] |
| NSCs | the ratio of neurons to astrocytes 1:2 | 0.1~10 Hz | 1/7/14/21 days | mouse | [85] | |||
4.4. EFs Promote Axon Outgrowth and Alignment
| Species | Cell Type | EF Type | Intensity | Time | Main Results | Reference |
|---|---|---|---|---|---|---|
| Chicken | embryo dorsal root ganglion | DC | 70~140 mV/mm | 20 h | The growth rate of the protrusion on the cathode surface is several times faster than that on the anode surface. | [86] |
| Xenopus | neurons nerve duct | DC | 1~10 V/cm 7~190 mV/mm | 6 h 16–18 h | Neurites facing the cathode grew faster, while those facing the anode grew slower. When the field intensity is 7~190 mV/mm, axons preferentially grow towards the negative or cathode. | [87,88] |
| Chick | DRG neurons | DC | 400~500 mV/mm | 3~6 h | Retraction was followed by the re-extension of fibers to a preferred orientation perpendicular to the voltage gradient. | [89] |
| Rat | hippocampal neurons | DC | 28, 80 or 219 mV/mm | 24 h | Neurites lay perpendicular to the field after exposure to 28, 80, or 219 mV/mm. | [90] |
| Rat | cortical neurons | AC | 0.5~2000 Hz 80 mV/mm at 0.5 Hz to 2 kHz | 24 h 4 Days | The direction of the axon was perpendicular to the adjacent electrode. Promoting axon 3D length growth and alternating electric fields orient large-scale axon alignment in 3D. | [52] |
| Rat | * FT-NPCs | DC | 150 mV/mm | 2 h/Day | EFs significantly increase the neuronal differentiation rate of FT-derived NPCs and align neurite outgrowth and promote the length of neurite processes. | [82] |
| Mouse | PC12 | AgNWs/PDMS | 240 mV and 20 Hz | 120 h | The proliferation rate and axon growth of PC12 cells increased only under electrical stimulation. PC12 cells showed axonal orientation perpendicular to the stretching direction. | [53] |
| Embryonic rat | hippocampal neurons | DC | 0.58~4.73 mV/pm | 24 h | The growth cones on the single long process (the putative axon) of cultured hippocampal neurons failed to orient themselves with the electric field applied by focusing, while the growth cones on the short and straight process (the putative dendrite) were oriented toward the cathode. | [91] |
| Rat | Neural Stem/Progenitor Cells | DC, AC | DC EF:437mV/mm AC EF:46 mV/mm | — | The overall arrangement of NPCs grew obviously perpendicular to the electric field axis. | [92] |
| Rat | DRG—astrocyte culture | DC | 10 mVmm−1 or 500 mV mm−1 | 24 h | The growth of neurites was in the same direction and along the same process of the aligned astrocytes. | [93] |
| Mouse | NPCs/NSCs | DC | 150 mV mm−1 | 1 h per day | Induced neuronal differentiation and neurite extension | [94] |
| Mouse | PC12 | DC | 0.07 mA | 2 h | Electrical stimulation results in longer neurites, more growth, and alignment of cells and neurites at an angle to the applied current. | [95] |
| Guinea-pig | spinal cord | DC | ~100 μV/mm | 3 weeks | EF initiate regeneration of central axon in adult guinea pig spinal cord transects. | [96] |
| Mouse | Primary pre-frontal cortical | two-electrode | ±0.25 mA/cm2 | 8 h per 24-h period for 3 days | Electrical stimulation using conductive polymer polypyrrole counters reduced the neurite outgrowth of primary prefrontal cortical neurons from NRG1-KO and DISC1-LI mice. | [97] |
| Mouse | Primary Cortical Neurons | — | 1 ± 0.25 mA/cm2 | 8 h per 24 h period for 3 days | 3D electrical stimulation improved the neurite outgrowth in 3D neuronal cultures from both wild-type and NRG1-knockout (NRG1-KO) mice. | [98] |
4.4.1. Electrical Stimulation Promotes Neurites Growing toward the Negative Pole
4.4.2. Axons Exhibit a Parallel Arrangement
4.4.3. Electrical Stimulation Activates Perpendicular Neurite Arrangement in 3D Conditions
5. Electrical Stimulation Potentially Promotes Neuronal Network Formation and Constructing Layer-Specific Connections
6. Signaling Pathways Potentially Induced by EFs in Neuronal Cultures
7. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Qian, X.Y.; Song, H.J. Ming G Brain organoids: Advances, applications and challenges. Development 2019, 146, v166074. [Google Scholar] [CrossRef] [PubMed]
- Eiraku, M.; Watanabe, K.; Matsuo-Takasaki, M.; Kawada, M.; Yonemura, S.; Matsumura, M.; Wataya, T.; Nishiyama, A.; Muguruma, K.; Sasai, Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 2018, 3, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. FOXG1-dependent dysregulation of GABA/Glutamate neuron differentiation in autism spectrum disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Groveman, B.R.; Ferreira, N.C.; Foliaki, S.T.; Walters, R.O.; Winkler, C.W.; Race, B.; Hughson, A.G.; Zanusso, G.; Haigh, C.L. Human cerebral organoids as a therapeutic drug screening model for Creutzfeldt–Jakob disease. Sci. Rep. 2021, 11, 5165. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jang, S.; Lee, D.; Lee, J.; Kang, U.; Chang, H.J.; Kim, H.J.; Han, S.; Seo, J.; Choi, M.; et al. logical network-based drug-screening platform for Alzheimer’s disease representing pathological features of human brain organoids. Nat. Commun. 2021, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Giandomenico, S.L.; Mierau, S.B.; Gibbons, G.M.; Wenger, L.M.D.; Masullo, L.; Sit, T.; Sutcliffe, M.; Boulanger, J.; Tripodi, M.; Derivery, E.; et al. Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 2019, 22, 669–679. [Google Scholar]
- Jo, J.; Xiao, Y.X.; Sun, A.X.; Cukuroglu, E.; Tran, H.; Göke, J.; Tan, Z.Y.; Saw, T.Y.; Tan, C.; Lokman, H.; et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem. Cell 2016, 19, 248–257. [Google Scholar] [CrossRef]
- Monzel, A.S.; Smits, L.M.; Hemmer, K.; Hachi, S.; Moreno, E.L.; van Wuellen, T.; Jarazo, J.; Walter, J.; Brüggemann, I.; Boussaad, I.; et al. Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem. Cell Rep. 2017, 8, 1144–1154. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Kadoshima, T.; Soen, M.; Narii, N.; Ishida, Y.; Ohgushi, M.; Takahashi, J.; Eiraku, M.; Sasai, Y. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun. 2015, 6, 8896. [Google Scholar]
- Qian, X.Y.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Muguruma, K.; Nishiyama, A.; Kawakami, H.; Hashimoto, K.; Sasai, Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015, 10, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Eiraku, M.; Sasai, Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Neurosci. Res. 2011, 472, 5. [Google Scholar]
- Avior, Y.; Sagi, I.; Benvenisty, N. Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 2016, 17, 170–182. [Google Scholar] [CrossRef]
- Bershteyn, M.; Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Nene, A.; Wynshaw-Boris, A.; Kriegstein, A.R. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem. Cell 2017, 20, 435–449. [Google Scholar] [CrossRef]
- Hotta, A.; Yamanaka, S. From genomics to gene therapy: Induced pluripotent stem cells meet genome editing. Annu. Rev. Genet. 2015, 49, 47–70. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Corsini, N.S.; Wolfinger, S.; Gustafson, E.H.; Phillips, A.W.; Burkard, T.R.; Otani, T.; Livesey, F.J.; Knoblich, J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017, 35, 659–666. [Google Scholar]
- Zhu, R.; Sun, Z.Q.; Li, C.P.; Ramakrishna, S.; Chiu, K.; He, L.M. Electrical stimulation affects neural stem cell fate and function in vitro. Exp. Neurol. 2019, 319, 112963. [Google Scholar] [CrossRef]
- Huang, Y.H.; Li, Y.; Chen, J.; Zhou, H.X.; Tan, S. Electrical stimulation elicits neural stem cells activation: New perspectives in CNS repair. Front. Hum. Neurosci. 2015, 9, 586. [Google Scholar] [CrossRef]
- Bertucci, C.; Koppes, R.; Dumont, C.; Koppes, A. Neural responses to electrical stimulation in 2D and 3D in vitro environments. Brain Res. Bull. 2019, 152, 265–284. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.B.; Singh, A. Cellular self-assembly and biomaterials-based organoid models of development and diseases. Acta Biomater. 2017, 53, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Cederquist, G.Y.; Asciolla, J.J.; Tchieu, J.; Walsh, R.M.; Cornacchia, D.; Resh, M.D.; Studer, L. Specification of positional identity in forebrain organoids. Nat. Biotechnol. 2019, 37, 436–444. [Google Scholar]
- Chojnacki, A.; Weiss, S. Production of neurons, astrocytes and oligodendrocytes from mammalian CNS stem cells. Nat. Protoc. 2008, 3, 935–940. [Google Scholar] [CrossRef]
- Kelava, I.; Lancaster, M. A Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev. Biol. 2016, 420, 199–209. [Google Scholar] [CrossRef]
- Hotary, K.B.; Robinson, K.R. Endogenous electrical currents and the resultant voltage gradients in the chick embryo. Dev. Biol. 1990, 140, 149–160. [Google Scholar] [CrossRef]
- Hotary, K.B.; Robinson, K.R. Evidence of a role for endogenous electrical fields in chick embryo development. Development 1992, 114, 985–996. [Google Scholar] [CrossRef]
- Hotary, K.B.; Robinson, K.R. Endogenous electrical currents and voltage gradients in xenopus embryos and the consequences of their disruption. Dev. Biol. 1994, 166, 789–800. [Google Scholar] [CrossRef]
- Duchesne, A.; Dong, J.; Bayne, A.N.; Mohamed, N.V.; Yi, W.; Mathur, M.; Fon, E.A.; Durcan, T.M.; Trempe, J.F. Trempe An Approach to Measuring Protein Turnover in Human Induced Pluripotent Stem Cell Organoids by Mass Spectrometry. Methods 2022. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Sutcliffe, M.; Lancaster, M.A. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat. Protoc. 2021, 16, 579–602. [Google Scholar] [CrossRef]
- Mostajo-Radji, M.A.; Schmitz, M.T.; Montoya, S.T.; Pollen, A.A. Reverse engineering human brain evolution using organoid models. Brain Res. 2020, 1729, 146582. [Google Scholar] [CrossRef] [PubMed]
- Kadoshima, T.; Sakaguchi, H.; Nakano, T.; Soen, M.; Ando, S.; Eiraku, M.; Sasai, Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA 2013, 110, 20284–20289. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Sakaguchi, H.; Miyamoto, S.; Takahashi, J. Three-dimensional induction of dorsal, intermediate and ventral spinal cord tissues from human pluripotent stem cells. Development 2018, 145, v162214. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Xiang, Y.F.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Benito Kwiecinski, S.; Lancaster, M.A. Brain Organoids: Human Neurodevelopment in a Dish. Cold Spring Harb Perspect. Biol. 2020, 12, a035709. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.N.; Xu, T.Y.; Hong, C.; Cheng, M.H.; Zhu, P.X.; Lin, J.S.; Su, D.F.; Miao, C.Y. Cerebral organoids transplantation improves neurological motor function in rat brain injury. CNS Neurosci. Ther. 2020, 26, 682–697. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef]
- Ham, O.; Jin, Y.B.; Kim, J.; Lee, M. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem. Bioph. Res. Commun. 2020, 521, 84–90. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef]
- Miyamoto, A.; Wake, H.; Moorhouse, A.J.; Nabekura, J. Microglia and synapse interactions: Fine tuning neural circuits and candidate molecules. Front. Cell. Neurosci. 2013, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, A.; Wake, H.; Ishikawa, A.W.; Eto, K.; Shibata, K.; Murakoshi, H.; Koizumi, S.; Moorhouse, A.J.; Yoshimura, Y.; Nabekura, J. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun. 2016, 7, 12540. [Google Scholar]
- Ormel, P.R.; Vieira De Sá, R.; van Bodegraven, E.J.; Karst, H.; Harschnitz, O.; Sneeboer, M.A.M.; Johansen, L.E.; van Dijk, R.E.; Scheefhals, N.; Berdenis Van Berlekom, A.; et al. Microglia innately develop within cerebral organoids. Nat. Commun. 2018, 9, 4167. [Google Scholar]
- Arslan, O.E. Chapter 3—Computational Basis of Neural Elements. In Artificial Neural Network for Drug Design, Delivery and Disposition Boston; Puri, M., Pathak, Y., Sutariya, V., Tipparaju, S., Moreno, W., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 29–82. [Google Scholar]
- Kitahara, T.; Sakaguchi, H.; Morizane, A.; Kikuchi, T.; Miyamoto, S.; Takahashi, J. Axonal extensions along corticospinal tracts from transplanted human cerebral organoids. Stem. Cell Rep. 2020, 15, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Cullen, D.K.; Gordián-Vélez, W.J.; Struzyna, L.A.; Jgamadze, D.; Lim, J.; Wofford, K.L.; Browne, K.D.; Chen, H.I. Bundled three-dimensional human axon tracts derived from brain organoids. iScience 2019, 21, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.; Nevin, Z.S.; Shick, H.E.; Garrison, E.; Clarkson-Paredes, C.; Karl, M.; Clayton BL, L.; Factor, D.C.; Allan, K.C.; Barbar, L.; et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods 2018, 15, 700–706. [Google Scholar] [CrossRef]
- Blackiston, D.J.; Levin, M. Ectopic eyes outside the head inXenopus tadpoles provide sensory data for light-mediated learning. J. Exp. Biol. 2013, 216, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.N.; Doulgkeroglou, M.N.; Zeugolis, D.I. Electric field stimulation for tissue engineering applications. BMC Biomed. Eng. 2021, 3, 1. [Google Scholar] [CrossRef]
- Chang, H.; Chou, S.; Cheng, J. Electric-field-induced neural precursor cell differentiation in microfluidic devices. J. Vis. Exp. 2021, 170, e61917. [Google Scholar] [CrossRef]
- Dong, Z.; Pei, Z.; Wang, Y.; Li, Z.; Khan, A.; Meng, X. Ascl1, regulates electric field-induced neuronal differentiation through PI3K/Akt pathway. Neuroscience 2019, 404, 141–152. [Google Scholar] [CrossRef]
- Tang-Schomer, M.D. 3D axon growth by exogenous electrical stimulus and soluble factors. Brain Res. 2018, 1678, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.L.; Liu, X.X.; Li, X.K.; Yin, J.; Wei, P.; Qian, J. Sun J Effect of electrical and electromechanical stimulation on PC12, cell proliferation and axon outgrowth. Front. Bioeng. Biotechnol. 2021, 9, 757906. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Huang, W.; Chen, L.; Liang, N.; Wang, H.; Lu, J.J.; Wang, X.; Wang, T. Neural tissue engineering: The influence of scaffold surface topography and extracellular matrix microenvironment. J. Mater. Chem. B 2021, 9, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Hotary, K.B.; Robinson, K.R. The neural tube of the Xenopus embryo maintains a potential difference across itself. Dev. Brain Res. 1991, 59, 65–73. [Google Scholar] [CrossRef]
- Levin, M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 2014, 25, 3835–3850. [Google Scholar] [CrossRef]
- Cao, L.; Wei, D.G.; Reid, B.; Zhao, S.; Pu, J.; Pan, T.; Yamoah, E.N.; Zhao, M. Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep. 2013, 14, 184–190. [Google Scholar] [CrossRef]
- Ohtaka-Maruyama, C. Subplate neurons as an organizer of mammalian neocortical development. Front. Neuroanat. 2020, 14, 8. [Google Scholar] [CrossRef]
- Ohtaka-Maruyama, C.; Okamoto, M.; Endo, K.; Oshima, M.; Kaneko, N.; Yura, K.; Okado, H.; Miyata, T.; Maeda, N. Synaptic transmission from subplate neurons controls radial migration of neocortical neurons. Science 2018, 360, 313–317. [Google Scholar] [CrossRef]
- Medvedeva, V.P.; Pierani, A. How do electric fields coordinate neuronal migration and maturation in the developing cortex? Front. Cell Dev. Biol. 2020, 8, 580657. [Google Scholar] [CrossRef]
- Moore, S.W.; Sheetz, M.P. Biophysics of substrate interaction: Influence on neural motility, differentiation, and repair. Dev. Neurobiol. 2011, 71, 1090–1101. [Google Scholar] [CrossRef]
- Mccaig, C.D.; Robinson, K.R. The ontogeny of the transepidermal potential difference in frog embryos. Dev. Biol. 1982, 90, 335–339. [Google Scholar] [CrossRef]
- Meng, X.T.; Arocena, M.; Penninger, J.; Gage, F.H.; Zhao, M.; Song, B. PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp. Neurol. 2011, 227, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Abdolmaleki, P.; Behmanesh, M.; Satari, M. Stable morphological-physiological and neural protein expression changes in rat bone marrow mesenchymal stem cells treated with electromagnetic field and nitric oxide. Bioelectromagnetics 2017, 38, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Han, X.; Chen, H.; Zheng, C.; Yang, Y.; Huang, X. Repetitive magnetic stimulation promotes neural stem cells proliferation by upregulating MiR-106b in vitro. J. Huazhong Univ. Sci. Technol. 2015, 35, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Abbasnia, K.; Ghanbari, A.; Abedian, M.; Ghanbari, A.; Sharififar, S.; Azari, H. The effects of repetitive transcranial magnetic stimulation on proliferation and differentiation of neural stem cells. Anat. Cell Biol. 2015, 48, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Arias-Carrión, O.; Verdugo-Díaz, L.; Feria-Velasco, A.; Millán-Aldaco, D.; Gutiérrez, A.A.; Hernández-Cruz, A.; Drucker-Colín, R. Neurogenesis in the subventricular zone following transcranial magnetic field stimulation and nigrostriatal lesions. J. Neurosci. Res. 2004, 78, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Czéh, B.; Welt, T.; Fischer, A.K.; Erhardt, A.; Schmitt, W.; Müller, M.B.; Toschi, N.; Fuchs, E.; Keck, M.E. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: Effects on stress hormone levels and adult hippocampal neurogenesis. Biol. Psychiat. 2002, 52, 1057–1065. [Google Scholar] [CrossRef]
- Ma, Q.L.; Chen, C.H.; Deng, P.; Zhu, G.; Lin, M.; Zhang, L.; Xu, S.C.; He, M.D.; Lu, Y.H.; Duan, W.X.; et al. Extremely low-frequency electromagnetic fields promote in vitro neuronal differentiation and neurite outgrowth of embryonic neural stem cells via up-regulating TRPC1. PLoS ONE 2016, 11, e150923. [Google Scholar] [CrossRef]
- Serena, E.; Figallo, E.; Tandon, N.; Cannizzaro, C.; Gerecht, S.; Elvassore, N.; Vunjak-Novakovic, G. Electrical stimulation of human embryonic stem cells: Cardiac differentiation and the generation of reactive oxygen species. Exp. Cell Res. 2009, 315, 3611–3619. [Google Scholar] [CrossRef]
- Feng, J.; Liu, J.; Zhang, X.; Zhang, L.; Jiang, J.; Nolta, J.; Zhao, M. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells 2012, 30, 349–355. [Google Scholar] [CrossRef]
- Yao, L.; Shanley, L.; Mccaig, C.; Zhao, M. Small applied electric fields guide migration of hippocampal neurons. J. Cell. Physiol. 2008, 216, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.P.; Steiger, A.; Nohner, M.; Ye, H. Specific intensity direct current (DC) electric field improves neural stem cell migration and enhances differentiation towards βIII-Tubulin+ neurons. PLoS ONE 2015, 10, e129625. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; El-Hayek, Y.H.; Liu, B.S.; Chen, Y.H.; Gomez, E.; Wu, X.H.; Ning, K.; Li, L.; Chang, N.; Zhang, L.; et al. Direct-current electrical field guides neuronal Stem/Progenitor cell migration. Stem. Cells 2008, 26, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Yang, H.N.; Woo, D.G.; Jeon, S.Y.; Do, H.J.; Huh, S.H.; Kim, N.H.; Kim, J.H.; Park, K.H. Exogenous Nurr1 gene expression in electrically-stimulated human MSCs and the induction of neurogenesis. Biomaterials 2012, 33, 7300–7308. [Google Scholar] [CrossRef] [PubMed]
- Mckasson, M.J.; Huang, L.; Robinson, K.R. Chick embryonic Schwann cells migrate anodally in small electrical fields. Exp. Neurol. 2008, 211, 585–587. [Google Scholar] [CrossRef]
- Pires, F.; Ferreira, Q.; Rodrigues CA, V.; Morgado, J.; Ferreira, F.C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta 2015, 1850, 1158–1168. [Google Scholar] [CrossRef]
- Mccaig, C.D.; Song BRainicek, A.M. Electrical dimensions in cell science. J. Cell Sci. 2009, 12, 24267–24276. [Google Scholar] [CrossRef]
- Chang, K.; Kim, J.W.; Kim, J.A.; Lee, S.; Kim, S.; Suh, W.H.; Kim, H.; Kwon, S.; Kim, S.J.; Suh, Y. Biphasic electrical currents stimulation promotes both proliferation and differentiation of fetal neural stem cells. PLoS ONE 2011, 6, e18738. [Google Scholar]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 2011, 23, H263–H267. [Google Scholar] [CrossRef]
- Zhang, M.S.; Li, X.P.; Bai, L.M.; Uchida, K.; Bai, W.F.; Wu, B.; Xu, W.C.; Zhu, H.X.; Huang, H. Effects of low frequency electromagnetic field on proliferation of human epidermal stem cells: An in vitro study. Bioelectromagnetics 2013, 34, 74–80. [Google Scholar] [CrossRef]
- Yamada, M.; Tanemura, K.; Okada, S.; Iwanami, A.; Nakamura, M.; Mizuno, H.; Ozawa, M.; Ohyama-Goto, R.; Kitamura, N.; Kawano, M.; et al. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem Cells 2007, 25, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Sharpee, T.O.; Destexhe, A.; Kawato, M.; Sekulić, V.; Skinner, F.K.; Wójcik, D.K.; Chintaluri, C.; Cserpán, D.; Somogyvári, Z.; Kim, J.K.; et al. 25th annual computational neuroscience meeting: CNS-2016. In Seogwipo City, Jeju-do, South Korea. BMC Neuroscience 2016, 17 (Suppl. 1), 54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Nicholls, M.; Gu, Y.; Zhang, G.; Zhao, C.; Franklin, R.J.; Song, B. Electric Signals Regulate the Directional Migration of Oligodendrocyte Progenitor Cells (OPCs) via β1 Integrin. Int. J. Mol. Sci. 2016, 17, 1948. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Mccullen, S.D.; Piedrahita, J.A.; Loboa, E.G.; Olby, N.J. Alternating current electric fields of varying frequencies: Effects on proliferation and differentiation of porcine neural progenitor cells. Cell. Reprogram. 2013, 15, 405–412. [Google Scholar] [CrossRef]
- Patel, N.; Poo, M.M. Orientation of neurite growth by extracellular electric fields. J. Neurosci. 1982, 2, 483–496. [Google Scholar] [CrossRef]
- Hinkle, L.; Mccaig, C.D.; Robinson, K.R. The direction of growth of differentiating neurones and myoblasts from frog embryos in an applied electric field. J. Physiol. 1981, 314, 121–135. [Google Scholar] [CrossRef]
- Pan, L.; Borgens, R.B. Strict perpendicular orientation of neural crest-derived neurons in vitro is dependent on an extracellular gradient of voltage. J. Neurosci. Res. 2012, 90, 1335–1346. [Google Scholar] [CrossRef]
- Rajnicek, A.M.; Gow, N.A.; Mccaig, C.D. Electric field-induced orientation of rat hippocampal neurones in vitro. Exp. Physiol. 1992, 77, 229–232. [Google Scholar] [CrossRef]
- Davenport, R.W.; Mccaig, C.D. Hippocampal growth cone responses to focally applied electric fields. J. Neurobiol. 1993, 24, 89–100. [Google Scholar] [CrossRef]
- Ariza, C.A.; Fleury, A.T.; Tormos, C.J.; Petruk, V.; Sakaguchi, D.S.; Mallapragada, S.K. The influence of electric fields on hippocampal neural progenitor cells. Stem Cell 2010, 6, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.K.; Fuss, B.; Colello, R.J. Electric field-induced astrocyte alignment directs neurite outgrowth. Neuron Glia Biol. 2006, 2, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.T.; Du, Y.S.; Dong, Z.Y.; Wang, G.Q.; Dong, B.; Guan, X.W.; Yuan, Y.; Pan, H.; Wang, F. Combination of electrical stimulation and bFGF synergistically promote neuronal differentiation of neural stem cells and neurite extension to construct 3D engineered neural tissue. J. Neural Eng. 2020, 17, 56048. [Google Scholar] [CrossRef] [PubMed]
- Imaninezhad, M.; Pemberton, K.; Xu, F.; Kalinowski, K.; Bera, R.; Zustiak, S.P. Directed and enhanced neurite outgrowth following exogenous electrical stimulation on carbon nanotube-hydrogel composites. J. Neural Eng. 2018, 15, 56034. [Google Scholar] [CrossRef]
- Borgens, R.B. Electrically mediated regeneration and guidance of adult mammalian spinal axons into polymeric channels. Neuroscience 1999, 91, 251–264. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Esrafilzadeh, D.; Crook, J.M.; Kapsa, R.; Stewart, E.M.; Tomaskovic-Crook, E.; Wallace, G.G.; Huang, X. Electrical stimulation using conductive polymer polypyrrole counters reduced neurite outgrowth of primary prefrontal cortical neurons from NRG1-KO and DISC1-LI mice. Sci. Rep. 2017, 7, 42525. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Beirne, S.; Shu, K.; Esrafilzadeh, D.; Huang, X.; Wallace, G.G. Electrical stimulation with a conductive polymer promotes neurite outgrowth and synaptogenesis in primary cortical neurons in 3D. Sci. Rep. 2018, 8, 9855. [Google Scholar] [CrossRef]
- Jaffe, L.F.; Poo, M. Neurites grow faster towards the cathode than the anode in a steady field. J. Exp. Zool. 1979, 209, 115–128. [Google Scholar] [CrossRef]
- Zafeiriou, M.; Bao, G.; Hudson, J.; Halder, R.; Blenkle, A.; Schreiber, M.; Fischer, A.; Schild, D.; Zimmermann, W. Developmental GABA polarity switch and neuronal plasticity in bioengineered neuronal organoids. Nat. Commun. 2020, 11, 3791. [Google Scholar] [CrossRef]
- Dong, Z.; Pei, Z.; Li, Z.; Wang, Y.; Khan, A.; Meng, X. Electric field stimulation induced neuronal differentiation of filum terminale derived neural progenitor cells. Neurosci. Lett. 2017, 651, 109–115. [Google Scholar] [CrossRef]
- Catalano, S.M.; Shatz, C.J. Activity-dependent cortical target selection by thalamic axons. Science 1998, 281, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Dantzker, J.L.; Callaway, E.M. The development of local, layer-specific visual cortical axons in the absence of extrinsic influences and intrinsic activity. J. Neurosci. 1998, 18, 4145–4154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poli, D.; Massobrio, P. High-frequency electrical stimulation promotes reshaping of the functional connections and synaptic plasticity inin vitro cortical networks. Phys. Biol. 2018, 15, 1L–6L. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.T.; Eroglu, C. Molecular mechanisms of astrocyte-induced synaptogenesis. Curr. Opin. Neurobiol. 2017, 45, 113–120. [Google Scholar] [CrossRef]
- Lenk, K.; Satuvuori, E.; Lallouette, J.; Ladrón-De-Guevara, A.; Berry, H.; Hyttinen, J.A.K. A computational model of interactions between neuronal and astrocytic networks: The role of astrocytes in the stability of the neuronal firing rate. Front. Comput. Neurosc. 2020, 13, 1–19. [Google Scholar] [CrossRef]
- Cao, L.; Pu, J.; Scott, R.H.; Ching, J.; Mccaig, C.D. Physiological electrical signals promote chain migration of neuroblasts by up-regulating P2Y1, purinergic receptors and enhancing cell adhesion. Stem Cell Rev. Rep. 2015, 11, 75–86. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Boda, S.K.; Basu, B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. [Google Scholar] [CrossRef]
- Babona-Pilipos, R.; Droujinine, I.A.; Popovic, M.R.; Morshead, C.M. Adult subependymal neural precursors, but not differentiated cells, undergo rapid cathodal migration in the presence of direct current electric fields. PLoS ONE 2011, 6, e23808. [Google Scholar] [CrossRef][Green Version]
- Sato, M.J.; Kuwayama, H.; van Egmond, W.N.; Takayama, A.L.K.; Takagi, H.; van Haastert, P.J.M.; Yanagida, T.; Ueda, M. Switching direction in electric-signal-induced cell migration by cyclic guanosine monophosphate and phosphatidylinositol signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 6667–6672. [Google Scholar] [CrossRef]
- Özkucur, N.; Perike, S.; Sharma, P.; Funk, R.H. Persistent directional cell migration requires ion transport proteins as direction sensors and membrane potential differences in order to maintain directedness. BMC Cell Biol. 2011, 12, 4. [Google Scholar] [CrossRef]
- Jahanshahi, A.; Schönfeld, L.; Lemmens, E.; Hendrix, S.; Temel, Y. In vitro and in vivo neuronal electrotaxis: A potential mechanism for restoration? Mol. Neurobiol. 2014, 49, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Goldberg, T.; Barbul, A.; Ben-Dov, N.; Korenstein, R. Low electric fields induce ligand-independent activation of EGF receptor and ERK via electrochemical elevation of H+ and ROS concentrations. Biochim. Biophys. Acta 2013, 1833, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, H.; Li, L.; Huang, C.S.; Lin, J.; Zhu, G.; Chen, Z.; Wu, N.; Feng, H. Superoxide plays critical roles in electrotaxis of fibrosarcoma cells via activation of ERK and reorganization of the cytoskeleton. Free Radic. Bio. Med. 2012, 52, 1888–1896. [Google Scholar] [CrossRef]
- Li, F.; Chen, T.N.; Hu, S.L.; Lin, J.K.; Hu, R.; Feng, H. Superoxide mediates direct current electric Field-Induced directional migration of glioma cells through the activation of AKT and ERK. PLoS ONE 2013, 8, e61195. [Google Scholar] [CrossRef]
- Sheikh, A.Q.; Taghian, T.; Hemingway, B.; Cho, H.; Kogan, A.B.; Narmoneva, D.A. Regulation of endothelial MAPK/ERK signalling and capillary morphogenesis by low-amplitude electric field. J. R. Soc. Interface 2013, 10, 20120548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.; Wang, F.; Guo, A.; Walczysko, P.; Gu, Y.; et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Wu, H.C.; Tai, N.H.; Wang, T.W. Carbon Nanotube Rope with Electrical Stimulation Promotes the Differentiation and Maturity of Neural Stem Cells. Small 2012, 8, 2869–2877. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Meng, X.; Pei, Z.; Wang, G.; Liu, R.; Qi, M.; Zhou, J.; Wang, F. Physiological Electric Field: A Potential Construction Regulator of Human Brain Organoids. Int. J. Mol. Sci. 2022, 23, 3877. https://doi.org/10.3390/ijms23073877
Yu X, Meng X, Pei Z, Wang G, Liu R, Qi M, Zhou J, Wang F. Physiological Electric Field: A Potential Construction Regulator of Human Brain Organoids. International Journal of Molecular Sciences. 2022; 23(7):3877. https://doi.org/10.3390/ijms23073877
Chicago/Turabian StyleYu, Xiyao, Xiaoting Meng, Zhe Pei, Guoqiang Wang, Rongrong Liu, Mingran Qi, Jiaying Zhou, and Fang Wang. 2022. "Physiological Electric Field: A Potential Construction Regulator of Human Brain Organoids" International Journal of Molecular Sciences 23, no. 7: 3877. https://doi.org/10.3390/ijms23073877
APA StyleYu, X., Meng, X., Pei, Z., Wang, G., Liu, R., Qi, M., Zhou, J., & Wang, F. (2022). Physiological Electric Field: A Potential Construction Regulator of Human Brain Organoids. International Journal of Molecular Sciences, 23(7), 3877. https://doi.org/10.3390/ijms23073877






