The Mammary Gland: Basic Structure and Molecular Signaling during Development
Abstract
1. Introduction
2. Development of Rudimentary Structure of Mammary Gland
2.1. Human Rudimentary Structure of the Mammary Gland
2.2. Mouse Rudimentary Structure of the Mammary Gland
2.3. Regulators of Embryonic Rudimentary Mammary Development
2.4. Transcriptional Factors
3. Journey of Pubertal Mammary Gland
3.1. Terminal End Buds (TEB)
3.2. Extracellular Matrix (ECM)
Biochemical Composition of ECM
3.3. Stroma
3.3.1. Glycosaminoglycans (GAG)
3.3.2. Actin and Tubulin
3.3.3. Lysyl Oxidase (LOX)
3.3.4. Cadherins
3.3.5. Integrins
3.3.6. Adipocytes and Fibroblasts
3.3.7. Macrophages and Eosinophils
3.4. Basement Membrane (BM)
3.5. Role of Hormones and Growth Factors in Regulation of Pubertal Mammary Gland
3.5.1. Estrogen (E2)
3.5.2. Growth Hormone (GH) and Insulin like Growth Factor-1 (IGF-1)
3.5.3. Wnt, Hedgehog (Hh), and Fibroblast Growth Factor (FGF) Signaling
3.5.4. Epidermal Growth Factors Signaling
3.5.5. Hormonal Regulation of Transcription Factors
3.5.6. Matrix Metalloproteinases (MMPs)
3.5.7. Transforming Growth Factor β (TGFβ)
3.5.8. Axonal Guidance Molecules
3.6. Pattern Formation during Pubertal Mammary Morphogenesis
4. Anatomy of Adult Mammary Gland
4.1. Differentiation of Mammary Gland during Pregnancy
4.2. Regulators of Mammary Development during Pregna
4.2.1. Progesterone (P4) and Progesterone Receptor (PR)
4.2.2. Prolactin (PRL) and Prolactin Receptor (PRLR)
4.2.3. Cortisol
4.2.4. ErbB Receptors
4.2.5. Inhibitor of Differentiation-1 (Id-1)
4.2.6. Activin
4.2.7. Extracellular Matrix (ECM)
4.2.8. Protein Kinase Cδ, Multiple B-Cell Leukemia/Lymphoma 2 (Bcl-2), and Ductal Lumen Formation
5. The Lactating Mammary Glands
6. Stromal Regulation of Involution
7. Mammary Gland at Single Cell Resolution
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hovey, R.C.; Trott, J.F.; Vonderhaar, B.K. Establishing a framework for the functional mammary gland: From endocrinology to morphology. J. Mammary Gland Biol. Neoplasia 2002, 7, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Peaker, M. The mammary gland in mammalian evolution: A brief commentary on some of the concepts. J. Mammary Gland Biol. Neoplasia 2002, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Muschler, J.; Streuli, C.H. Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a003202. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Khaled, W.T. Mammary development in the embryo and adult: A journey of morphogenesis and commitment. Development 2008, 135, 995–1003. [Google Scholar] [CrossRef]
- Sternlicht, M.D. Key stages in mammary gland development: The cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006, 8, 201. [Google Scholar] [CrossRef]
- Visvader, J.E. Keeping a breast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009, 23, 2563–2577. [Google Scholar] [CrossRef]
- Streuli, C.H. Cell adhesion in mammary gland biology and neoplasia. J. Mammary Gland Biol. Neoplasia 2003, 8, 375–381. [Google Scholar] [CrossRef]
- Cowin, P.; Wysolmerski, J. Molecular mechanisms guiding embryonic mammary gland development. Cold Spring Harb. Perspect. Biol. 2010, 2, a003251. [Google Scholar] [CrossRef]
- Parmar, H.; Cunha, G.R. Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr.-Relat. Cancer 2004, 11, 437. [Google Scholar] [CrossRef]
- Javed, A.; Lteif, A. Development of the Human Breast. Semin. Plast. Surg. 2013, 27, 5–12. [Google Scholar]
- Vorherr, H. The Breast: Morphology, Physiology and Lactation; Academic Press: London, UK, 1974. [Google Scholar]
- Howard, B.A.; Gusterson, B.A. Human breast development. J. Mammary Gland Biol. Neoplasia 2000, 5, 119. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Russo, I.H. The Mammary Gland: Development, Regulation and Function; Neville, M.C., Daniel, C.W., Eds.; Plenum Publishing Corporation: New York, NY, USA, 1987; pp. 67–93. [Google Scholar]
- Jolicoeur, F. Intrauterine breast development and the mammary myoepithelial lineage. J. Mammary Gland Biol. Neoplasia 2005, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, T. Mammary embryogenesis. In The Mammary Gland: Development, Regulation and Function; Neville, M.C., Daniel, C.W., Eds.; Plenum Publishing Corporation: New York, NY, USA, 1987; pp. 37–66. [Google Scholar]
- Hens, J.R.; Wysolmerski, J.J. Key stages of mammary gland development: Molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res. 2005, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Hinck, L.; Silberstein, G.B. Key stages in mammary gland development: The mammary end bud as a motile organ. Breast Cancer Res. 2005, 7, 245. [Google Scholar] [CrossRef]
- Chu, E.Y.; Hens, J.; Andl, T.; Kairo, A.; Yamaguchi, T.P.; Brisken, C.; Glick, A.; Wysolmerski, J.J.; Millar, S.E. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development 2004, 131, 4819. [Google Scholar] [CrossRef]
- Veltmaat, J.M.; Van Veelen, W.; Thiery, J.P.; Bellusci, S. Identification of the mammary line in mouse by Wnt10b expression. Dev. Dyn. 2004, 229, 349. [Google Scholar] [CrossRef]
- Foley, J.; Dann, P.; Hong, J.; Cosgrove, J.; Dreyer, B.; Rimm, D.; Dunbar, M.; Philbrick, W.; Wysolmerski, J. Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development 2001, 128, 513. [Google Scholar] [CrossRef]
- Kratochwil, K. Tissue combination and organ culture studies in the development of the embryonic mammary gland. In Developmental Biology: A Comprehensive Synthesis; Gwatkin, R.B.L., Ed.; Plenum Press: New York, NY, USA, 1987; pp. 315–334. [Google Scholar]
- Masso-Welch, P.A.; Darcy, K.M.; Stangle-Castor, N.C.; Ip, M.M. A developmental atlas of rat mammary gland histology. J. Mammary Gland Biol. Neoplasia 2000, 5, 165. [Google Scholar] [CrossRef]
- Hennighausen, L.; Robinson, G.W. Signaling pathways in mammary gland development. Dev. Cell 2001, 1, 467. [Google Scholar] [CrossRef]
- Staal, F.J.; Chhatta, A.; Mikkers, H. Caught in a Wnt storm: Complexities of Wnt signalling in hematopoiesis. Exp. Hematol. 2016, 44, 451–457. [Google Scholar] [CrossRef]
- McNeill, H.; Woodgett, J.R. When pathways collide: Collaboration and connivance among signalling proteins in development. Nat. Rev. Mol. Cell Biol. 2010, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- Brennan, K.R.; Brown, A.M. Wnt proteins in mammary development and cancer. J. Mammary Gland Biol. Neoplasia 2004, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Bouras, T.; Pal, B.; Vaillant, F.; Harburg, G.; Asselin-Labat, M.L.; Oakes, S.R.; Lindeman, G.J.; Visvader, J.E. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 2008, 3, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, C.; Evans, N.C.; Zylstra, C.R.; Li, Y.; Alexander, C.M.; Williams, B.O. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J. Biol. Chem. 2006, 281, 35081–35087. [Google Scholar] [CrossRef]
- Lindvall, C.; Zylstra, C.R.; Evans, N.; West, R.A.; Dykema, K.; Furge, K.A.; Williams, B.O. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS ONE 2009, 4, e5813. [Google Scholar] [CrossRef]
- Eblaghie, M.C.; Song, S.J.; Kim, J.Y.; Akita, K.; Tickle, C.; Jung, H.S. Interactions between FGF and Wnt signals and Tbx3 gene expression in mammary gland initiation in mouse embryos. J. Anat. 2004, 205, 1. [Google Scholar] [CrossRef]
- Incassati, A.; Chandramouli, A.; Eelkema, R.; Cowin, P. Key signaling nodes in mammary gland development and cancer: β-catenin. Breast Cancer Res. 2010, 12, 213. [Google Scholar] [CrossRef]
- Christiansen, J.H.; Dennis, C.L.; Wicking, C.A.; Monkley, S.J.; Wilkinson, D.G.; Wainwright, B.J. Murine Wnt-11 and Wnt-12 have temporally and spatially restricted expression patterns during embryonic development. Mech. Dev. 1995, 51, 341. [Google Scholar] [CrossRef]
- Hens, J.; Dann, P.; Hiremath, M.; Pan, T.C.; Chodosh, L.; Wysolmerski, J. Analysis of gene expression in PTHrP−/− mammary buds supports a role for BMP signaling and MMP2 in the initiation of ductal morphogenesis. Dev. Dyn. 2009, 238, 2713. [Google Scholar] [CrossRef] [PubMed]
- Hynes, N.E.; Watson, C.J. Mammary gland growth factors: Roles in normal development and in cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a003186. [Google Scholar] [CrossRef] [PubMed]
- Pond, A.C.; Bin, X.; Batts, T.; Roarty, K.; Hilsenbeck, S.; Rosen, J.M. Fibroblast growth factor receptor signaling is essential for normal mammary gland development and stem cell function. Stem Cells 2013, 31, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Mailleux, A.A.; Spencer-Dene, B.; Dillon, C.; Ndiaye, D.; Savona-Baron, C.; Itoh, N.; Kato, S.; Dickson, C.; Thiery, J.P.; Bellusci, S. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development 2002, 129, 53. [Google Scholar] [CrossRef] [PubMed]
- Veltmaat, J.M.; Relaix, F.; Le, L.T.; Kratochwil, K.; Sala, F.G.; van Veelen, W.; Rice, R.; Spencer-Dene, B.; Mailleux, A.A.; Rice, D.P.; et al. Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development 2006, 133, 2325. [Google Scholar] [CrossRef] [PubMed]
- Walterhouse, D.O.; Lamm, M.L.; Villavicencio, E.; Iannaccone, P.M. Emerging roles for hedgehog-patched-Gli signal transduction in reproduction. Biol. Reprod. 2003, 69, 8. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Akimaru, H.; Tanaka, Y.; Maekawa, T.; Nakafuku, M.; Ishii, S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J. Biol. Chem. 1999, 274, 8143. [Google Scholar] [CrossRef]
- Ikram, M.S.; Neill, G.W.; Regl, G.; Eichberger, T.; Frischauf, A.M.; Aberger, F.; Quinn, A.; Philpott, M. GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J. Investig. Dermatol. 2004, 122, 1503. [Google Scholar] [CrossRef]
- Hatsell, S.J.; Cowin, P. Gli3-mediated repression of Hedgehog targets is required for normal mammary development. Development 2006, 133, 3661. [Google Scholar] [CrossRef]
- Kleinberg, D.L.; Wood, T.L.; Furth, P.A.; Lee, A.V. Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocr. Rev. 2009, 30, 51. [Google Scholar] [CrossRef]
- Heckman, B.M.; Chakravarty, G.; Vargo-Gogola, T.; Gonzales-Rimbau, M.; Hadsell, D.L.; Lee, A.V.; Settleman, J.; Rosen, J.M. Crosstalk between the p190-B RhoGAP and IGF signaling pathways is required for embryonic mammary bud development. Dev. Biol. 2007, 309, 137. [Google Scholar] [CrossRef] [PubMed]
- Hens, J.R.; Dann, P.; Zhang, J.P.; Harris, S.; Robinson, G.W.; Wysolmerski, J. BMP4 and PTHrP interact to stimulate ductal outgrowth during embryonic mammary development and to inhibit hair follicle induction. Development 2007, 134, 1221. [Google Scholar] [CrossRef] [PubMed]
- Wysolmerski, J.J.; Philbrick, W.M.; Dunbar, M.E.; Lanske, B.; Kronenberg, H.; Broadus, A.E. Rescue of the parathyroid hormone-related protein knockout mouse demonstrates that parathyroid hormone-related protein is essential for mammary gland development. Development 1998, 125, 1285. [Google Scholar] [CrossRef] [PubMed]
- Gritli-Linde, A.; Hallberg, K.; Harfe, B.D.; Reyahi, A.; Kannius-Janson, M.; Nilsson, J.; Cobourne, M.T.; Sharpe, P.T.; McMahon, A.P.; Linde, A. Abnormal hair development and apparent follicular transformation to mammary gland in the absence of hedgehog signaling. Dev. Cell 2007, 12, 99. [Google Scholar] [CrossRef]
- Howard, B.; Panchal, H.; McCarthy, A.; Ashworth, A. Identification of the scaramanga gene implicates Neuregulin3 in mammary gland specification. Genes Dev. 2005, 19, 2078. [Google Scholar] [CrossRef]
- Hardy, K.M.; Booth, B.W.; Hendrix, M.J.; Salomon, D.S.; Strizzi, L. ErbB/EGF signaling and EMT in mammary development and breast cancer. J. Mammary Gland Biol. Neoplasia 2010, 15, 191. [Google Scholar] [CrossRef]
- Wansbury, O.; Panchal, H.; James, M.; Parry, S.; Ashworth, A.; Howard, B. Dynamic expression of Erbb pathway members during early mammary gland morphogenesis. J. Investig. Dermatol. 2008, 128, 1009. [Google Scholar] [CrossRef]
- Tidcombe, H.; Jackson-Fisher, A.; Mathers, K.; Stern, D.F.; Gassmann, M.; Golding, J.P. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc. Natl. Acad. Sci. USA 2003, 100, 8281. [Google Scholar] [CrossRef]
- Harris, H.A.; Albert, L.M.; Leathurby, Y.; Malamas, M.S.; Mewshaw, R.E.; Miller, C.P.; Kharode, Y.P.; Marzolf, J.; Komm, B.S.; Winneker, R.C.; et al. Evaluation of an estrogen receptor-β agonist in animal models of human disease. Endocrinology 2003, 144, 4241. [Google Scholar] [CrossRef]
- Davenport, T.G.; Jerome-Majewska, L.A.; Papaioannou, V.E. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development 2003, 130, 2263. [Google Scholar] [CrossRef]
- Bamshad, M.; Lin, R.C.; Law, D.J.; Watkins, W.C.; Krakowiak, P.A.; Moore, M.E.; Franceschini, P.; Lala, R.; Holmes, L.B.; Gebuhr, T.C.; et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat. Genet. 1997, 16, 311. [Google Scholar] [CrossRef] [PubMed]
- Phippard, D.J.; Weber-Hall, S.J.; Sharpe, P.T.; Naylor, M.S.; Jayatalake, H.; Maas, R.; Woo, I.; Roberts-Clark, D.; Francis-West, P.H.; Liu, Y.H.; et al. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development 1996, 122, 2729. [Google Scholar] [CrossRef] [PubMed]
- Satokata, I.; Ma, L.; Ohshima, H.; Bei, M.; Woo, I.; Nishizawa, K.; Maeda, T.; Takano, Y.; Uchiyama, M.; Heaney, S.; et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat. Genet. 2000, 24, 391. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, M.E.; Dann, P.R.; Robinson, G.W.; Hennighausen, L.; Zhang, J.P.; Wysolmerski, J.J. Parathyroid hormone-related protein signaling is necessary for sexual dimorphism during embryonic mammary development. Development 1999, 126, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Russo, I.H. Development of the human breast. Maturitas 2004, 49, 2. [Google Scholar] [CrossRef] [PubMed]
- Capuco, A.V.; Ellis, S.E. Comparative aspects of mammary gland development and homeostasis. Annu. Rev. Anim. Biosci. 2013, 1, 179–202. [Google Scholar] [CrossRef] [PubMed]
- Michael Akers, R. A 100-Year Review: Mammary development and lactation. J. Dairy Sci. 2017, 100, 10332–10352. [Google Scholar] [CrossRef]
- Williams, J.M.; Daniel, C.W. Mammary ductal elongation: Differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev. Biol. 1983, 97, 274. [Google Scholar] [CrossRef]
- Daniel, C.W.; Smith, G.H. The mammary gland: A model for development. J. Mammary Gland Biol. Neoplasia 1999, 4, 3–8. [Google Scholar] [CrossRef]
- Bai, L.; Rohrschneider, L.R. s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev. 2010, 24, 1882. [Google Scholar] [CrossRef]
- Rios, A.C.; Fu, N.Y.; Lindeman, G.L.; Visvader, J.E. In situ identification of bipotent stem cells in the mammary gland. Nature 2014, 506, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, R.C.; Krajewska, M.; Krnacik, S.; Jaeger, R.; Weiher, H.; Krajewski, S.; Reed, J.C.; Rosen, J.M. Apoptosis in the terminal endbud of the murine mammary gland: A mechanism of ductal morphogenesis. Development 1996, 122, 4013. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.J.; Huebner, R.J.; Palsdottir, H.; Lee, J.K.; Perez, M.J.; Jorgens, D.M.; Tauscher, A.N.; Cheung, K.J.C.-Z.; Auer, M. Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. J. Cell Sci. 2012, 125 Pt 11, 2638–2654. [Google Scholar]
- Paine, I.; Chauviere, A.; Landua, J.; Sreekumar, A.; Cristini, V.; Rosen, J.; Lewis, M.T. A geometrically-constrained mathematical model of mammary gland ductal elongation reveals novel cellular dynamics within the terminal end Bud. PLoS Comput. Biol. 2016, 12, e1004839. [Google Scholar] [CrossRef] [PubMed]
- Fata, J.E.; Chaudhary, V.; Khokha, R. Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17β-estradiol during the estrous cycle. Biol. Reprod. 2001, 65, 680. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.E.; Schor, A.M.; Howell, A.; Ferguson, M.W. Changes in the extracellular matrix of the normal human breast during the menstrual cycle. Cell Tissue Res. 1992, 268, 167. [Google Scholar] [CrossRef]
- Potten, C.S.; Watson, R.J.; Williams, G.T.; Ticle, S.; Roberts, S.A.; Harris, M.; Howell, A. The effect of age and the menstrual cycle upon proliferative activity of the normal human breast. Br. J. Cancer 1988, 58, 163–170. [Google Scholar] [CrossRef]
- Lester, S.C. Differential diagnosis of granulomatous mastitis. Breast J. 2005, 11, 534. [Google Scholar] [CrossRef]
- Metcalfe, A.D.; Gilmore, A.; Klinowska, T.; Oliver, J.; Valentijn, A.J.; Brown, R.; Ross, A.; MacGregor, G.; Hickman, J.A.; Streuli, C.H. Developmental regulation of Bcl-2 family protein expression in the involuting mammary gland. J. Cell Sci. 1999, 112, 1771. [Google Scholar] [CrossRef]
- Gjorevski, N.; Nelson, C.M. Integrated morphodynamic signalling of the mammary gland. Nat. Rev. Mol. Cell Biol. 2011, 12, 581. [Google Scholar] [CrossRef]
- McNally, S.; Martin, F. Molecular regulators of pubertal mammary gland development. Ann. Med. 2011, 43, 212. [Google Scholar] [CrossRef] [PubMed]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Fata, J.E.; Werb, Z.; Bissell, M.J. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ekblom, P.; Lonai, P.; Talts, J.F. Expression and biological role of laminin-1. Matrix Biol. 2003, 22, 35. [Google Scholar] [CrossRef]
- Jalkanen, M.; Rapraeger, A.; Bernfield, M. Mouse mammary epithelial cells produce basement membrane and cell surface heparan sulfate proteoglycans containing distinct core proteins. J. Cell Biol. 1988, 106, 953. [Google Scholar] [CrossRef]
- Kalluri, R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 2003, 3, 422. [Google Scholar] [CrossRef]
- Maller, O.; Martinson, H.; Schedin, P. Extracellular matrix composition reveals complex and dynamic stromal-epithelial interactions in the mammary gland. J. Mammary Gland Biol. Neoplasia 2010, 15, 301. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Patton, B.L. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 2009, 15, 1277. [Google Scholar] [CrossRef]
- Tzu, J.; Marinkovich, M.P. Bridging structure with function: Structural, regulatory, and developmental role of laminins. Int. J. Biochem. Cell Biol. 2008, 40, 199. [Google Scholar] [CrossRef]
- Aumailley, M.; Bruckner-Tuderman, L.; Carter, W.G.; Deutzmann, R.; Edgar, D.; Ekblom, P.; Engel, J.; Engvall, E.; Hohenester, E.; Jones, J.C.; et al. A simplified laminin nomenclature. Matrix Biol. 2005, 24, 326. [Google Scholar] [CrossRef] [PubMed]
- Prince, J.M.; Klinowska, T.C.; Marshman, E.; Lowe, E.T.; Mayer, U.; Miner, J.; Aberdam, D.; Vestweber, D.; Gusterson, B.; Streuli, C.H. Cell-matrix interactions during development and apoptosis of the mouse mammary gland in vivo. Dev. Dyn. 2002, 223, 497. [Google Scholar] [CrossRef] [PubMed]
- Delehedde, M.; Lyon, M.; Sergeant, N.; Rahmoune, H.; Fernig, D.G. Poteoglycans: Pericellular and cell surface multireceptors that integrate external stimuli in the mammary gland. J. Mammary Gland Biol. Neoplasia 2001, 6, 253. [Google Scholar] [CrossRef] [PubMed]
- Barresi, R.; Campbell, K.P. Dystroglycan: From biosynthesis to pathogenesis of human disease. J. Cell Sci. 2006, 119, 199. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.R.; Humphries, M.J.; Bass, M.D. Synergist.tic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 2007, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Vogel, W.F.; Aszodi, A.; Alves, F.; Pawson, T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol. Cell Biol. 2001, 21, 2906. [Google Scholar] [CrossRef]
- Midwood, K.S.; Valenick, L.V.; Hsia, H.C.; Schwarzbauer, J.E. Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol. Biol. Cell. 2004, 15, 5670. [Google Scholar] [CrossRef]
- Shattil, S.J.; Kim, C.; Ginsberg, M.H. The final steps of integrin activation: The end game. Nat. Rev. Mol. Cell Biol. 2010, 11, 288. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef]
- Naylor, M.J.; Ginsburg, E.; Iismaa, T.P.; Vonderhaar, B.K.; Wynick, D.; Ormandy, C.J. The neuropeptide galanin augments lobuloalveolar development. J. Biol. Chem. 2003, 278, 29145. [Google Scholar] [CrossRef]
- Friedrich, M.V.; Gohring, W.; Morgelin, M.; Brancaccio, A.; David, G.; Timpl, R. Structural basis of glycosaminoglycan modification and of heterotypic interactions of perlecan domain V. J. Mol. Biol. 1999, 294, 259. [Google Scholar] [CrossRef] [PubMed]
- Schedin, P.; Mitrenga, T.; McDaniel, S.; Kaeck, M. Mammary ECM composition and function are altered by reproductive state. Mol. Carcinog. 2004, 41, 207. [Google Scholar] [CrossRef] [PubMed]
- Gouon-Evans, V.; Rothenberg, M.E.; Pollard, J.W. Postnatal mammary gland development requires macrophages and eosinophils. Development 2000, 127, 2269–2282. [Google Scholar] [CrossRef] [PubMed]
- Ingman, W.V.; Wyckoff, J.; Gouon-Evans, V.; Condeelis, J.; Pollard, J.W. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev. Dyn. 2006, 235, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, A.; Anderson, R.; Britt, K. Stromal fibroblasts and the immune microenvironment: Partners in mammary gland biology and pathology? J. Mammary Gland Biol. Neoplasia 2014, 19, 169–182. [Google Scholar] [CrossRef]
- Zhang, X.; Martinez, D.; Koledova, Z.; Qiao, G.; Streuli, C.H.; Lu, P. FGF ligands of the postnatal mammary stroma regulate distinct aspects of epithelial morphogenesis. Development 2014, 141, 3352–3362. [Google Scholar] [CrossRef]
- Liu, J.; Esmailpour, T.; Shang, X.; Gulsen, G.; Liu, A.; Huang, T. TBX3 over-expression causes mammary gland hyperplasia and increases mammary stem-like cells in an inducible transgenic mouse model. BMC Dev. Biol. 2011, 11, 65. [Google Scholar] [CrossRef]
- Sakai, T.; Larsen, M.; Yamada, K.M. Fibronectin requirement in branching morphogenesis. Nature 2003, 423, 876. [Google Scholar] [CrossRef]
- Shin, D.H.; Jang, S.H.; Kang, B.C.; Kim, H.J.; Oh, S.H.; Kong, G. Constitutive overexpression of Id-1 in mammary glands of transgenic mice results in precocious and increased formation of terminal end buds, enhanced alveologenesis, delayed involution. J. Cell Physiol. 2011, 226, 1340. [Google Scholar] [CrossRef]
- Fata, J.E.; Mori, H.; Ewald, A.J.; Zhang, H.; Yao, E.; Werb, Z.; Bissell, M.J. The MAPK (ERK-1,2) pathway integrates distinct and antagonistic signals from TGFα and FGF7 in morphogenesis of mouse mammary epithelium. Dev. Biol. 2007, 306, 193. [Google Scholar] [CrossRef]
- Barker, T.H.; Baneyx, G.; Cardó-Vila, M.; Workman, G.A.; Weaver, M.; Menon, P.M.; Dedhar, S.; Rempel, S.A.; Arap, W.; Pasqualini, R.; et al. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J. Biol. Chem. 2005, 280, 36483. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Crowley, D.; Bronson, R.T.; Hynes, R.O. Analyses of the role of endogenous SPARC in mouse models of prostate and breast cancer. Clin. Exp. Metastasis 2008, 25, 109. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Iozzo, R.V. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J. Biol. Chem. 2008, 283, 21305. [Google Scholar] [CrossRef] [PubMed]
- Minor, K.; Tang, X.; Kahrilas, G.; Archibald, S.J.; Davies, J.E.; Davies, S.J. Decorin promotes robust axon growth on inhibitory CSPGs and myelin via a direct effect on neurons. Neurobiol. Dis. 2008, 32, 88. [Google Scholar] [CrossRef] [PubMed]
- Wight, T.N.; Heinegard, D.K.; Hascall, V.C. Proteoglycans Structure and Function. In Cell Biology of Extracellular Matrix; Hay, E.D., Ed.; Plenum Press: New York, NY, USA, 1991; pp. 45–78. [Google Scholar]
- Zhu, J.X.; Goldoni, S.; Bix, G.; Owens, R.T.; McQuillan, D.J.; Reed, C.C.; Iozzo, R.V. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J. Biol. Chem. 2005, 280, 32468–32479. [Google Scholar] [CrossRef]
- Xu, T.; Bianco, P.; Fisher, L.W.; Longenecker, G.; Smith, E.; Goldstein, S.; Bonadio, J.; Boskey, A.; Heegaard, A.M.; Sommer, B.; et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat. Genet. 1998, 20, 78. [Google Scholar] [CrossRef]
- Schaefer, L.; Mihalik, D.; Babelova, A.; Krzyzankova, M.; Gröne, H.J.; Iozzo, R.V.; Young, M.F.; Seidler, D.G.; Lin, G.; Reinhardt, D.P.; et al. Regulation of fibrillin-1 by biglycan and decorin is important for tissue preservation in the kidney during pressure-induced injury. Am. J. Pathol. 2004, 165, 383. [Google Scholar] [CrossRef]
- Mecham, R.P.; Heuser, J.E. Cell Biology of Extracellular Matrix, 2nd ed.; Hay, E.D., Ed.; Plenum Press: New York, NY, USA, 1991; pp. 79–109. [Google Scholar]
- Reinboth, B.; Hanssen, E.; Cleary, E.G.; Gibson, M.A. Molecular interactions of biglycan and decorin with elastic fiber components: Biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1. J. Biol. Chem. 2002, 277, 3950. [Google Scholar] [CrossRef]
- Trask, B.C.; Trask, T.M.; Broekelmann, T.; Mecham, R.P. The microfibrillar proteins MAGP-1 and fibrillin-1 form a ternary complex with the chondroitin sulfate proteoglycan decorin. Mol. Biol. Cell 2000, 11, 1499. [Google Scholar] [CrossRef][Green Version]
- Silberstein, G.B.; Daniel, C.W. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev. Biol. 1982, 90, 215. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009, 28, 5. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208. [Google Scholar] [CrossRef] [PubMed]
- Caswell, P.T.; Vadrevu, S.; Norman, J.C. Integrins: Masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 2009, 10, 843. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891. [Google Scholar] [CrossRef]
- Lorand, L.; Graham, R.M. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003, 4, 140. [Google Scholar] [CrossRef]
- Pullan, S.; Wilson, J.; Metcalfe, A.; Edwards, G.M.; Goberdhan, N.; Tilly, J.; Hickman, J.A.; Dive, C.; Streuli, C.H. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J. Cell Sci. 1996, 109, 631. [Google Scholar] [CrossRef]
- Runswick, S.K.; O’Hare, M.J.; Jones, L.; Streuli, C.H.; Garrod, D.R. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat. Cell Biol. 2001, 3, 823. [Google Scholar] [CrossRef]
- Klinowska, T.C.; Soriano, J.V.; Edwards, G.M.; Oliver, J.M.; Valentijn, A.J.; Montesano, R.; Streuli, C.H. Laminin and β1 integrins are crucial for normal mammary gland development in the mouse. Dev. Biol. 1999, 215, 13. [Google Scholar] [CrossRef]
- Naylor, M.J.; Li, N.; Cheung, J.; Lowe, E.T.; Lambert, E.; Marlow, R.; Wang, P.; Schatzmann, F.; Wintermantel, T.; Schüetz, G.; et al. Ablation of β1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J. Cell Biol. 2005, 171, 717. [Google Scholar] [CrossRef] [PubMed]
- Gehler, S.; Baldassarre, M.; Lad, Y.; Leight, J.L.; Wozniak, M.A.; Riching, K.M.; Eliceiri, K.W.; Weaver, V.M.; Calderwood, D.A.; Keely, P.J. Filamin A-β1 integrin complex tunes epithelial cell response to matrix tension. Mol. Biol. Cell 2009, 20, 3224. [Google Scholar] [CrossRef] [PubMed]
- Glukhova, M.A.; Streuli, C.H. How integrins control breast biology. Curr. Opin. Cell Biol. 2013, 25, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Taddei, I.; Deugnier, M.A.; Faraldo, M.M.; Petit, V.; Bouvard, D.; Medina, D.; Fässler, R.; Thiery, J.P.; Glukhova, M.A. β1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat. Cell Biol. 2008, 10, 716. [Google Scholar] [CrossRef]
- Rebustini, I.T.; Patel, V.N.; Stewart, J.S.; Layvey, A.; Georges-Labouesse, E.; Miner, J.H.; Hoffman, M.P. Laminin α5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through β1 integrin signaling. Dev. Biol. 2007, 308, 15. [Google Scholar] [CrossRef]
- Iozzo, R.V. Basement membrane proteoglycans: From cellar to ceiling. Nat. Rev. Mol. Cell Biol. 2005, 6, 646. [Google Scholar] [CrossRef]
- Liu, Y.; Chattopadhyay, N.; Qin, S.; Szekeres, C.; Vasylyeva, T.; Mahoney, Z.X.; Taglienti, M.; Bates, C.M.; Chapman, H.A.; Miner, J.H.; et al. Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis. Development 2009, 136, 843. [Google Scholar] [CrossRef]
- Castets, M.; Coissieux, M.M.; Delloye-Bourgeois, C.; Bernard, L.; Delcros, J.G.; Bernet, A.; Laudet, V.; Mehlen, P. Inhibition of endothelial cell apoptosis by netrin-1 during angiogenesis. Dev. Cell 2009, 16, 614. [Google Scholar] [CrossRef]
- Hagedorn, E.J.; Yashiro, H.; Ziel, J.W.; Ihara, S.; Wang, Z.; Sherwood, D.R. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell’s invasive membrane in C. elegans. Dev. Cell 2009, 17, 187. [Google Scholar] [CrossRef]
- Strizzi, L.; Postovit, L.M.; Margaryan, N.V.; Seftor, E.A.; Abbott, D.E.; Seftor, R.E.; Salomon, D.S.; Hendrix, M.J. Emerging roles of nodal and Cripto-1: From embryogenesis to breast cancer progression. Breast Dis. 2008, 29, 91. [Google Scholar] [CrossRef]
- Couldrey, C.; Moitra, J.; Vinson, C.; Anver, M.; Nagashima, K.; Green, J. Adipose tissue: A vital in vivo role in mammary gland development but not differentiation. Dev. Dyn. 2002, 223, 459. [Google Scholar] [CrossRef] [PubMed]
- Kamikawa, A.; Ichii, O.; Yamaji, D.; Imao, T.; Suzuki, C.; Okamatsu-Ogura, Y.; Terao, A.; Kon, Y.; Kimura, K. Diet-induced obesity disrupts ductal development in the mammary glands of nonpregnant mice. Dev. Dyn. 2009, 238, 1092. [Google Scholar] [CrossRef] [PubMed]
- Landskroner-Eiger, S.; Park, J.; Israel, D.; Pollard, J.W.; Scherer, P.E. Morphogenesis of the developing mammary gland: Stage-dependent impact of adipocytes. Dev. Biol. 2010, 344, 968. [Google Scholar] [CrossRef] [PubMed]
- Kamalati, T.; Niranjan, B.; Yant, J.; Buluwela, L. HGF/SF in mammary epithelial growth and morphogenesis: In vitro and in vivo models. J. Mammary Gland Biol. Neoplasia 1999, 4, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kleinberg, D.L.; Feldman, M.; Ruan, W. IGF-I: An essential factor in terminal end bud formation and ductal morphogenesis. J. Mammary Gland Biol. Neoplasia 2000, 5, 7. [Google Scholar] [CrossRef]
- Knox, S.M.; Lombaert, I.M.; Reed, X.; Vitale-Cross, L.; Gutkind, J.S.; Hoffman, M.P. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 2010, 329, 1645. [Google Scholar] [CrossRef]
- Atabai, K.; Sheppard, D.; Werb, Z. Roles of the innate immune system in mammary gland remodeling during involution. J. Mammary Gland Biol. Neoplasia 2007, 12, 37. [Google Scholar] [CrossRef]
- Lilla, J.N.; Werb, Z. Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Dev. Biol. 2010, 337, 124. [Google Scholar] [CrossRef]
- Russell, J.S.; McGee, S.O.; Ip, M.M.; Kuhlmann, D.; Masso-Welch, P.A. Conjugated linoleic acid induces mast cell recruitment during mouse mammary gland stromal remodeling. J. Nutr. 2007, 137, 1200. [Google Scholar] [CrossRef][Green Version]
- Aupperlee, M.D.; Zhao, Y.; Tan, Y.S.; Leipprandt, J.R.; Bennett, J.; Haslam, S.Z.; Schwartz, R.C. Epidermal growth factor receptor (EGFR) signaling is a key mediator of hormone-induced leukocyte infiltration in the pubertal female mammary gland. Endocrinology 2014, 155, 2301–2313. [Google Scholar] [CrossRef]
- Horiuchi, T.; Weller, P.F. Expression of vascular endothelial growth factor by human eosinophils: Upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am. J. Respir Cell Mol. Biol. 1997, 17, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Gusterson, B.A.; Warburton, M.J.; Mitchell, D.; Ellison, M.; Munro Neville, A.; Rudland, P.S. Distribution of Myoeithelial Cells and Basement Membrane Proteins in the Normal Breast and in Benign and Malignant Breast Diseases. Cancer Res. 1982, 42, 4763–4770. [Google Scholar] [PubMed]
- Silberstein, G.B. Postnatal mammary gland morphogenesis. Microsc. Res. Tech. 2001, 52, 155. [Google Scholar] [CrossRef]
- Bocchinfuso, W.P.; Lindzey, J.K.; Hewitt, S.C.; Clark, J.A.; Myers, P.H.; Cooper, R.; Korach, K.S. Induction of mammary gland development in estrogen receptor-α knockout mice. Endocrinology 2000, 141, 2982. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.L.; Rosen, J.M. The role of C/EBPβ in mammary gland development and breast cancer. J. Mammary Gland Biol. Neoplasia 2003, 8, 191. [Google Scholar] [CrossRef]
- Kleinberg, D.L.; Ruan, W. IGF-I, GH, and sex steroid effects in normal mammary gland development. J. Mammary Gland Biol. Neoplasia 2008, 13, 353. [Google Scholar] [CrossRef]
- Rusidz, M.; Adlanmrini, M.; Chantalat, E.; Letron, I.R.; Cayre, S.; Arnal, J.F.; Deugnier, M.A.; Lenfant, F. Estrogen receptor-α signaling in postnatal mammary development and breast cancers. Cell. Mol. Life Sci. 2021, 78, 5681–5705. [Google Scholar] [CrossRef]
- Fisher, C.R.; Graves, K.H.; Parlow, A.F.; Simpson, E.R. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc. Natl. Acad. Sci. USA 1998, 95, 6965. [Google Scholar] [CrossRef]
- Bocchinfuso, W.P.; Korach, K.S. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J. Mammary Gland Biol. Neoplasia 1997, 2, 323. [Google Scholar] [CrossRef]
- Cheng, G.; Weihua, Z.; Warner, M.; Gustafsson, J.A. Estrogen receptors ERα and ERβ in proliferation in the rodent mammary gland. Proc. Natl. Acad. Sci. USA 2004, 101, 3739. [Google Scholar] [CrossRef]
- Curtis Hewitt, S.; Couse, J.F.; Korach, K.S. Estrogen receptor transcription and transactivation: Estrogen receptor knockout mice: What their phenotypes reveal about mechanisms of estrogen action. Breast Cancer Res. 2000, 2, 345. [Google Scholar] [CrossRef] [PubMed]
- Mallepell, S.; Krust, A.; Chambon, P.; Brisken, C. Paracrine signaling through the epithelial estrogen receptor α is required for proliferation and morphogenesis in the mammary gland. Proc. Natl. Acad. Sci. USA 2006, 103, 2196. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Manka, D.; Wagner, K.U.; Khan, S.A. Estrogen receptor-α expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 14718. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Kumar, R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2006, 238, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C.; O’Malley, B. Hormone action in the mammary gland. Cold Spring Harb. Perspect. Biol. 2010, 2, a003178. [Google Scholar] [CrossRef]
- Castoria, G.; Migliaccio, A.; Bilancio, A.; Di Domenico, M.; de Falco, A.; Lombardi, M.; Fiorentino, R.; Varricchio, L.; Barone, M.V.; Auricchio, F. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 2001, 20, 6050–6059. [Google Scholar] [CrossRef]
- Lombardi, M.; Castoria, G.; Migliaccio, A.; Barone, M.V.; Di Stasio, R.; Ciociola, A.; Bottero, D.; Yamaguchi, H.; Appella, E.; Auricchio, F. Hormone-dependent nuclear export of estradiol receptor and DNA synthesis in breast cancer cells. J. Cell Biol. 2008, 182, 327–340. [Google Scholar] [CrossRef]
- Migliaccio, A.; Di Domenico, M.; Castoria, G.; de Falco, A.; Bontempo, P.; Nola, E.; Auricchio, F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996, 15, 1292–1300. [Google Scholar] [CrossRef]
- Simoncini, T.; Hafezi-Moghadam, A.; Brazil, D.P.; Ley, K.; Chin, W.W.; Liao, J.K. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 2000, 407, 538–541. [Google Scholar] [CrossRef]
- Ciarloni, L.; Mallepell, S.; Brisken, C. Amphiregulin is an essential mediator of estrogen receptor α function in mammary gland development. Proc. Natl. Acad. Sci. USA 2007, 104, 5455–5460. [Google Scholar] [CrossRef]
- Kenney, N.J.; Bowman, A.; Korach, K.S.; Barrett, J.C.; Salomon, D.S. Effect of exogenous epidermal-like growth factors on mammary gland development and differentiation in the estrogen receptor-α knockout (ERKO) mouse. Breast Cancer Res. Treat. 2003, 79, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Sunnarborg, S.W. The ADAM17-amphiregulin-EGFR axis in mammary development and cancer. J. Mammary Gland Biol. Neoplasia 2008, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ewald, A.J.; Martin, G.R.; Werb, Z. Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis. Dev. Biol. 2008, 321, 77. [Google Scholar] [CrossRef] [PubMed]
- Woodward, T.L.; Mienaltowski, A.S.; Modi, R.R.; Bennett, J.M.; Haslam, S.Z. Fibronectin and the α5β1 integrin are under developmental and ovarian steroid regulation in the normal mouse mammary gland. Endocrinology 2001, 142, 3214. [Google Scholar] [CrossRef][Green Version]
- Liang, Y.; Brekken, R.A.; Hyder, S.M. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr. Relat. Cancer 2006, 13, 905. [Google Scholar] [CrossRef]
- Dabrosin, C.; Margetts, P.J.; Gauldie, J. Estradiol increases extracellular levels of vascular endothelial growth factor in vivo in murine mammary cancer. Int. J. Cancer 2003, 107, 535. [Google Scholar] [CrossRef]
- Feldman, M.; Ruan, W.; Cunningham, B.C.; Wells, J.A.; Kleinberg, D.L. Evidence that the growth hormone receptor mediates differentiation and development of the mammary gland. Endocrinology 1993, 133, 1602. [Google Scholar] [CrossRef]
- Argetsinger, L.S.; Campbell, G.S.; Yang, X.; Witthuhn, B.A.; Silvennoinen, O.; Ihle, J.N.; Carter-Su, C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 1993, 74, 237. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Kopchick, J.J.; Frank, S.J. Disulfide linkage of growth hormone (GH) receptors (GHR) reflects GH-induced GHR dimerization. Association of JAK2 with the GHR is enhanced by receptor dimerization. J. Biol. Chem. 1999, 274, 33072. [Google Scholar] [CrossRef]
- Carter-Su, C.; Schwartz, J.; Smit, L.S. Molecular mechanism of growth hormone action. Annu. Rev. Physiol. 1996, 58, 187. [Google Scholar] [CrossRef]
- Frank, S.J. Mechanistic aspects of crosstalk between GH and PRL and ErbB receptor family signaling. J. Mammary Gland Biol. Neoplasia 2008, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.W.; Spencer, S.A.; Cachianes, G.; Hammonds, R.G.; Collins, C.; Henzel, W.J.; Barnard, R.; Waters, M.J.; Wood, W.I. Growth hormone receptor and serum binding protein: Purification, cloning and expression. Nature 1987, 330, 537. [Google Scholar] [CrossRef] [PubMed]
- Zeps, N.; Bentel, J.M.; Papadimitriou, J.M.; Antuono, M.F.D.; Dawkins, H.J. Estrogen receptor-negative epithelial cells in mouse mammary gland development and growth. Differentiation 1998, 62, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef]
- Cullen, K.J.; Allison, A.; Martire, I.; Ellis, M.; Singer, C. Insulin-like growth factor expression in breast cancer epithelium and stroma. Breast Cancer Res. Treat. 1992, 22, 21–29. [Google Scholar] [CrossRef]
- Cullen, K.J.; Smith, H.S.; Hill, S.; Rosen, N.; Lippman, M.E. Growth factor messenger RNA expression by human breast fibroblasts from benign and malignant lesions. Cancer Res. 1991, 51, 4978–4985. [Google Scholar]
- Paik, S. Expression of IGF-I and IGF-II mRNA in breast tissue. Breast Cancer Res. Treat. 1992, 22, 31–38. [Google Scholar] [CrossRef]
- Gallego, M.I.; Binart, N.; Robinson, G.W.; Okagaki, R.; Coschigano, K.T.; Perry, J.; Kopchick, J.J.; Oka, T.; Kelly, P.A.; Hennighausen, L. Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev. Biol. 2001, 229, 163. [Google Scholar] [CrossRef]
- Green, K.A.; Streuli, C.H. Apoptosis regulation in the mammary gland. Cell. Mol. Life Sci. 2004, 61, 1867. [Google Scholar] [CrossRef]
- Corbit, K.C.; Aanstad, P.; Singla, V.; Norman, A.R.; Stainier, D.Y.; Reiter, J.F. Vertebrate Smoothened functions at the primary cilium. Nature 2005, 437, 1018. [Google Scholar] [CrossRef]
- Milenkovic, L.; Scott, M.P.; Rohatgi, R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J. Cell Biol. 2009, 187, 365. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, R.; Milenkovic, L.; Scott, M.P. Patched1 regulates hedgehog signaling at the primary cilium. Science 2007, 317, 372. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.E.; Scott, M.P. Communicating with Hedgehogs. Nat. Rev. Mol. Cell Biol. 2005, 6, 306. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, D.; Liu, A.; Rakeman, A.S.; Murcia, N.S.; Niswander, L.; Anderson, K.V. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 2003, 426, 83–87. [Google Scholar] [CrossRef]
- Varjosalo, M.; Taipale, J. Hedgehog signaling. J. Cell Sci. 2007, 120, 3. [Google Scholar] [CrossRef]
- Kogerman, P.; Grimm, T.; Kogerman, L.; Krause, D.; Undén, A.B.; Sandstedt, B.; Toftgård, R.; Zaphiropoulos, P.G. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat. Cell Biol. 1999, 1, 312. [Google Scholar] [CrossRef]
- Bühler, T.A.; Dale, T.C.; Kieback, C.; Humphreys, R.C.; Rosen, J.M. Localization and quantification of Wnt-2 gene expression in mouse mammary development. Dev. Biol. 1993, 155, 87. [Google Scholar] [CrossRef]
- Lane, T.F.; Leder, P. Wnt-10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene 1997, 15, 2133–2144. [Google Scholar] [CrossRef]
- Roarty, K.; Shore, A.N.; Creighton, C.J.; Rosen, J.M. Ror2 regulates branching, differentiation, and actin-cytoskeletal dynamics within the mammary epithelium. J. Cell Biol. 2015, 208, 351–366. [Google Scholar] [CrossRef]
- Neugebauer, J.M.; Amack, J.D.; Peterson, A.G.; Bisgrove, B.W.; Yost, H.J. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature 2009, 458, 651. [Google Scholar] [CrossRef]
- Van Genderen, C.; Okamura, R.M.; Fariñas, I.; Quo, R.G.; Parslow, T.G.; Bruhn, L.; Grosschedl, R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994, 8, 2691. [Google Scholar] [CrossRef] [PubMed]
- Roarty, K.; Serra, R. Wnt5a is required for proper mammary gland development and TGF-β-mediated inhibition of ductal growth. Development 2007, 134, 3929. [Google Scholar] [CrossRef] [PubMed]
- Astigiano, S.; Damonte, P.; Barbieri, O. Inhibition of ductal morphogenesis in the mammary gland of WAP-fgf4 transgenic mice. Anat. Embryol. 2003, 206, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, Q. Expression and functions of fibroblast growth factor 10 in the mouse mammary gland. Int. J. Mol. Sci. 2013, 14, 4094–4105. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N. FGF10: A multifunctional mesenchymal-epithelial signaling growth factor in development, health, and disease. Cytokine Growth Factor Rev. 2016, 28, 63–69. [Google Scholar] [CrossRef]
- Parsa, S.; Ramasamy, S.K.; Langhe, S.D.; Gupte, V.V.; Haigh, J.J.; Medina, D.; Bellusci, S. Terminal end bud maintenance in mammary gland is dependent upon FGFR2b signaling. Dev. Biol. 2008, 317, 121–131. [Google Scholar] [CrossRef]
- Wiesen, J.F.; Young, P.; Werb, Z.; Cunha, G.R. Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development 1999, 126, 335. [Google Scholar] [CrossRef]
- Schroeder, J.A.; Lee, D.C. Dynamic expression and activation of ERBB receptors in the developing mouse mammary gland. Cell Growth Differ. 1998, 9, 451. [Google Scholar]
- Coleman, S.; Silberstein, G.B.; Daniel, C.W. Ductal morphogenesis in the mouse mammary gland: Evidence supporting a role for epidermal growth factor. Dev. Biol. 1988, 127, 304. [Google Scholar] [CrossRef]
- Sebastian, J.; Richards, R.G.; Walker, M.P.; Wiesen, J.F.; Werb, Z.; Derynck, R.; Hom, Y.K.; Cunha, G.R.; DiAugustine, R.P. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth Differ. 1998, 9, 777. [Google Scholar]
- Xie, W.; Paterson, A.J.; Chin, E.; Nabell, L.M.; Kudlow, J.E. Targeted expression of a dominant negative epidermal growth factor receptor in the mammary gland of transgenic mice inhibits pubertal mammary duct development. Mol. Endocrinol. 1997, 11, 1766–1781. [Google Scholar] [CrossRef] [PubMed]
- Howlin, J.; McBryan, J.; Napoletano, S.; Lambe, T.; McArdle, E.; Shioda, T.; Martin, F. CITED1 homozygous null mice display aberrant pubertal mammary ductal morphogenesis. Oncogene 2006, 25, 1532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sternlicht, M.D.; Sunnarborg, S.W.; Kouros-Mehr, H.; Yu, Y.; Lee, D.C.; Werb, Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development 2005, 132, 3923. [Google Scholar] [CrossRef] [PubMed]
- Linggi, B.; Carpenter, G. ErbB receptors: New insights on mechanisms and biology. Trends Cell Biol. 2006, 16, 649. [Google Scholar] [CrossRef]
- Boeri Erba, E.; Bergatto, E.; Cabodi, S.; Silengo, L.; Tarone, G.; Defilippi, P.; Jensen, O.N. Systematic analysis of the epidermal growth factor receptor by mass spectrometry reveals stimulation-dependent multisite phosphorylation. Mol. Cell. Proteomics 2005, 4, 1107. [Google Scholar] [CrossRef]
- Guo, L.; Kozlosky, C.J.; Ericsson, L.H.; Daniel, T.O.; Cerretti, D.P.; Johnson, R.S. Studies of ligand-induced site-specific phosphorylation of epidermal growth factor receptor. J. Am. Soc. Mass Spectrom. 2003, 14, 1022. [Google Scholar] [CrossRef]
- Wu, S.L.; Kim, J.; Bandle, R.W.; Liotta, L.; Petricoin, E.; Karger, B.L. Dynamic profiling of the post-translational modifications and interaction partners of epidermal growth factor receptor signaling after stimulation by epidermal growth factor using Extended Range Proteomic Analysis (ERPA). Mol. Cell. Proteomics 2006, 5, 1610. [Google Scholar] [CrossRef]
- Schulze, W.X.; Deng, L.; Mann, M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol. Syst. Biol. 2005, 1, 2005-0008. [Google Scholar] [CrossRef]
- Jorissen, R.N.; Walker, F.; Pouliot, N.; Garrett, T.P.; Ward, C.W.; Burgess, A.W. Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp. Cell Res. 2003, 284, 31. [Google Scholar] [CrossRef]
- Patel, V.N.; Knox, S.M.; Likar, K.M.; Lathrop, C.A.; Hossain, R.; Eftekhari, S.; Whitelock, J.M.; Elkin, M.; Vlodavsky, I.; Hoffman, M.P. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development 2007, 134, 4177. [Google Scholar] [CrossRef]
- Zcharia, E.; Metzger, S.; Chajek-Shaul, T.; Aingorn, H.; Elkin, M.; Friedmann, Y.; Weinstein, T.; Li, J.P.; Lindahl, U.; Vlodavsky, I. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB J. 2004, 18, 252. [Google Scholar] [CrossRef] [PubMed]
- Luetteke, N.C.; Qiu, T.H.; Fenton, S.E.; Troyer, K.L.; Riedel, R.F.; Chang, A.; Lee, D.C. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 1999, 126, 2739. [Google Scholar] [CrossRef] [PubMed]
- Aupperlee, M.D.; Leipprandt, J.R.; Bennett, J.M.; Schwartz, R.C.; Haslam, S.Z. Amphiregulin mediates progesterone-induced mammary ductal development during puberty. Breast Cancer Res. 2013, 15, R44. [Google Scholar] [CrossRef] [PubMed]
- McBryan, J.; Howlin, J.; Napoletano, S.; Martin, F. Amphiregulin: Role in mammary gland development and breast cancer. J. Mammary Gland Biol. Neoplasia 2008, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Inazu, T.; Kawai, Y.; Masamura, K.; Yoshida, M.; Tanaka, N.; Miyamoto, K.; Miyamori, I. Amphiregulin is a potent mitogen for the vascular smooth muscle cell line, A7r5. Biochem. Biophys. Res. Commun. 2003, 301, 1109. [Google Scholar] [CrossRef]
- Meyer, S.E.; Zinser, G.M.; Stuart, W.D.; Pathrose, P.; Waltz, S.E. The Ron receptor tyrosine kinase negatively regulates mammary gland branching morphogenesis. Dev. Biol. 2009, 333, 173. [Google Scholar] [CrossRef][Green Version]
- Vaught, D.; Chen, J.; Brantley-Sieders, D.M. Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol. Biol. Cell 2009, 20, 2572. [Google Scholar] [CrossRef]
- Allen-Petersen, B.L.; Miller, M.R.; Neville, M.C.; Anderson, S.M.; Nakayama, K.I.; Reyland, M.E. Loss of protein kinase C delta alters mammary gland development and apoptosis. Cell Death Dis. 2010, 1, e17. [Google Scholar] [CrossRef][Green Version]
- Jäger, R.; Schäfer, S.; Hau-Liersch, M.; Schorle, H. Loss of transcription factor AP-2γ/TFAP2C impairs branching morphogenesis of the murine mammary gland. Dev. Dyn. 2010, 239, 1027. [Google Scholar] [CrossRef]
- Asselin-Labat, M.L.; Shackleton, M.; Stingl, J.; Vaillant, F.; Forrest, N.C.; Eaves, C.J.; Visvader, J.E.; Lindeman, G.J. Steroid hormone receptor status of mouse mammary stem cells. J. Natl. Cancer Inst. 2006, 98, 1011. [Google Scholar] [CrossRef]
- Kouros-Mehr, H.; Werb, Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev. Dyn. 2006, 235, 3404. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.J.; Park, J.S.; Hayashi, S.; Majumdar, A.; McMahon, A.P. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell. 2005, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Matsuda, M.; Furuse, M.; Tsukita, S. Regulation of tight junctions during the epithelium-mesenchyme transition: Direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 2003, 116, 1959. [Google Scholar] [CrossRef] [PubMed]
- De Boer, T.P.; van Veen, T.A.; Bierhuizen, M.F.; Kok, B.; Rook, M.B.; Boonen, K.J.; Vos, M.A.; Doevendans, P.A.; de Bakker, J.M.; van der Heyden, M.A. Connexin43 repression following epithelium-to-mesenchyme transition in embryonal carcinoma cells requires Snail1 transcription factor. Differentiation 2007, 75, 208. [Google Scholar] [CrossRef]
- Savanger, P.; Yamada, K.M.; Thiery, J.P. The Zinc-Finger Protein Slug Causes Desmosome Dissociation, an Initial and Necessary Step for Growth Factor–induced Epithelial–Mesenchymal Transition. J. Cell Biol. 1997, 137, 1403–1419. [Google Scholar]
- Ciruna, B.; Rossant, J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell. 2001, 1, 37. [Google Scholar] [CrossRef]
- Yook, J.I.; Li, X.Y.; Ota, I.; Hu, C.; Kim, H.S.; Kim, N.H.; Cha, S.Y.; Ryu, J.K.; Choi, Y.J.; Kim, J.; et al. A Wnt-Axin2-GSK3β cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 2006, 8, 1398. [Google Scholar] [CrossRef]
- Yu, M.; Smolen, G.A.; Zhang, J.; Wittner, B.; Schott, B.J.; Brachtel, E.; Ramaswamy, S.; Maheswaran, S.; Haber, D.A. A developmentally regulated inducer of EMT, LBX1, contributes to breast cancer progression. Genes Dev. 2009, 23, 1737. [Google Scholar] [CrossRef]
- Peinado, H.; Quintanilla, M.; Cano, A. Transforming growth factor β-1 induces snail transcription factor in epithelial cell lines: Mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 2003, 278, 21113. [Google Scholar] [CrossRef]
- Laffin, B.; Wellberg, E.; Kwak, H.I.; Burghardt, R.C.; Metz, R.P.; Gustafson, T.; Schedin, P.; Porter, W.W. Loss of singleminded-2s in the mouse mammary gland induces an epithelial-mesenchymal transition associated with up-regulation of slug and matrix metalloprotease 2. Mol. Cell Biol. 2008, 28, 1936. [Google Scholar] [CrossRef]
- Cannito, S.; Novo, E.; Compagnone, A.; Valfrè di Bonzo, L.; Busletta, C.; Zamara, E.; Paternostro, C.; Povero, D.; Bandino, A.; Bozzo, F.; et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis 2008, 29, 2267. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117, 927. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Hamada, S.; Kimura, K.; Kanno, A.; Hirota, M.; Umino, J.; Fujibuchi, W.; Masamune, A.; Tanaka, N.; Miura, K.; et al. Up-regulation of MSX2 enhances the malignant phenotype and is associated with twist 1 expression in human pancreatic cancer cells. Am. J. Pathol. 2008, 172, 926. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Wu, K.J. TWIST activation by hypoxia inducible factor-1 (HIF-1): Implications in metastasis and development. Cell Cycle 2008, 7, 2090. [Google Scholar] [CrossRef] [PubMed]
- Coletta, R.D.; McCoy, E.L.; Burns, V.; Kawakami, K.; McManaman, J.L.; Wysolmerski, J.J.; Ford, H.L. Characterization of the Six1 homeobox gene in normal mammary gland morphogenesis. BMC Dev. Biol. 2010, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Moffett, P.; Reece, M.; Pelletier, J. The murine Sim-2 gene product inhibits transcription by active repression and functional interference. Mol. Cell Biol. 1997, 17, 4933. [Google Scholar] [CrossRef]
- Micalizzi, D.S.; Farabaugh, S.M.; Ford, H.L. Epithelial-mesenchymal transition in cancer: Parallels between normal development and tumor progression. J. Mammary Gland Biol. Neoplasia 2010, 15, 117. [Google Scholar] [CrossRef]
- LaMarca, H.L.; Visbal, A.P.; Creighton, C.J.; Liu, H.; Zhang, Y.; Behbod, F.; Rosen, J.M. CCAAT/enhancer binding protein β regulates stem cell activity and specifies luminal cell fate in the mammary gland. Stem. Cells 2010, 28, 535. [Google Scholar] [CrossRef]
- Siegel, P.M.; Muller, W.J. Transcription factor regulatory networks in mammary epithelial development and tumorigenesis. Oncogene 2010, 29, 2753. [Google Scholar] [CrossRef][Green Version]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221. [Google Scholar] [CrossRef]
- Khokha, R.; Werb, Z. Mammary gland reprogramming: Metalloproteinases couple form with function. Cold Spring Harb. Perspect. Biol. 2011, 3, a004333. [Google Scholar] [CrossRef]
- Mori, H.; Lo, A.T.; Inman, J.L.; Alcaraz, J.; Ghajar, C.M.; Mott, J.D.; Nelson, C.M.; Chen, C.S.; Zhang, H.; Bascom, J.L.; et al. Transmembrane/cytoplasmic, rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary branching morphogenesis via binding to integrin β1. Development 2013, 140, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Varner, V.D.; Voronov, D.A.; Taber, L.A. Mechanics of head fold formation: Investigating tissue-level forces during early development. Development 2010, 137, 3801. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, B.S.; Sternlicht, M.D.; Lund, L.R.; Alexander, C.M.; Mott, J.; Bissell, M.J.; Soloway, P.; Itohara, S.; Werb, Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J. Cell Biol. 2003, 162, 1123. [Google Scholar] [CrossRef] [PubMed]
- Szabova, L.; Yamada, S.S.; Birkedal-Hansen, H.; Holmbeck, K. Expression pattern of four membrane-type matrix metalloproteinases in the normal and diseased mouse mammary gland. J. Cell Physiol. 2005, 205, 123. [Google Scholar] [CrossRef]
- Schenk, S.; Hintermann, E.; Bilban, M.; Koshikawa, N.; Hojilla, C.; Khokha, R.; Quaranta, V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J. Cell Biol. 2003, 161, 197. [Google Scholar] [CrossRef]
- Rebustini, I.T.; Myers, C.; Lassiter, K.S.; Surmak, A.; Szabova, L.; Holmbeck, K.; Pedchenko, V.; Hudson, B.G.; Hoffman, M.P. MT2-MMP-dependent release of collagen IV NC1 domains regulates submandibular gland branching morphogenesis. Dev. Cell 2009, 17, 482. [Google Scholar] [CrossRef]
- Giannelli, G.; Falk-Marzillier, J.; Schiraldi, O.; Stetler-Stevenson, W.G.; Quaranta, V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 1997, 277, 225. [Google Scholar] [CrossRef]
- Ucar, A.; Vafaizadeh, V.; Jarry, H.; Fiedler, J.; Klemmt, P.A.; Thum, T.; Groner, B.; Chowdhury, K. miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nat. Genet. 2010, 42, 1101. [Google Scholar] [CrossRef]
- Gomes, A.M.; Bhat, R.; Correia, A.L.; Mott, J.D.; Ilan, N.; Vlodavsky, I.; Pavão, M.S.G.; Bissell, M. Mammary branching morphogenesis requires reciprocal signaling by heparanase and MMP-14. J. Cell. Biochem. 2015, 116, 1668–1679. [Google Scholar] [CrossRef]
- Fernandez-Valdivia, R.; Mukherjee, A.; Ying, Y.; Li, J.; Paquet, M.; DeMayo, F.J.; Lydon, J.P. The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev. Biol. 2009, 328, 127. [Google Scholar] [CrossRef] [PubMed]
- Mulac-Jericevic, B.; Lydon, J.P.; DeMayo, F.J.; Conneely, O.M. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. USA 2003, 100, 9744. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; Vanduijn, M.M.; Inman, J.L.; Fletcher, D.A.; Bissell, M.J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 2006, 314, 298. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Miyazono, K.; Heldin, C.H.; Keski-Oja, J. Latent transforming growth factor-β 1 associates to fibroblast extracellular matrix via latent TGF-β binding protein. J. Cell Biol. 1994, 124, 171. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.D.; Silberstein, G.B.; Roberts, A.B.; Flanders, K.C.; Daniel, C.W. Regulated expression and growth inhibitory effects of transforming growth factor-β isoforms in mouse mammary gland development. Development 1991, 113, 867. [Google Scholar] [CrossRef]
- Silberstein, G.B.; Daniel, C.W. Reversible inhibition of mammary gland growth by transforming growth factor-β. Science 1987, 237, 291–293. [Google Scholar] [CrossRef]
- Munger, J.S.; Huang, X.; Kawakatsu, H.; Griffiths, M.J.; Dalton, S.L.; Wu, J.; Pittet, J.F.; Kaminski, N.; Garat, C.; Matthay, M.A.; et al. The integrin αvβ6 binds and activates latent TGF β1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999, 96, 319. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163. [Google Scholar] [CrossRef]
- Wipff, P.J.; Rifkin, D.B.; Meister, J.J.; Hinz, B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 2007, 179, 1311. [Google Scholar] [CrossRef]
- Massague, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783. [Google Scholar] [CrossRef]
- Lee, W.C.; Davies, J.A. Epithelial branching: The power of self-loathing. Bioessays 2007, 29, 205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pierce, D.F., Jr.; Johnson, M.D.; Matsui, Y.; Robinson, S.D.; Gold, L.I.; Purchio, A.F.; Daniel, C.W.; Hogan, B.L.; Moses, H.L. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-β 1. Genes Dev. 1993, 7, 2308. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, G.B.; Strickland, P.; Coleman, S.; Daniel, C.W. Epithelium-dependent extracellular matrix synthesis in transforming growth factor-β 1-growth-inhibited mouse mammary gland. J. Cell Biol. 1990, 110, 2209. [Google Scholar] [CrossRef]
- Soriano, J.V.; Pepper, M.S.; Orci, L.; Montesano, R. Roles of hepatocyte growth factor/scatter factor and transforming growth factor-β1 in mammary gland ductal morphogenesis. J. Mammary Gland Biol. Neoplasia 1998, 3, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, R.K.; Howard, J. Slow local movements of collagen fibers by fibroblasts drive the rapid global self-organization of collagen gels. J. Cell Biol. 2002, 157, 1083. [Google Scholar] [CrossRef] [PubMed]
- Ballard, M.S.; Zhu, A.; Iwai, N.; Stensrud, M.; Mapps, A.; Postiglione, M.P.; Knoblich, J.A.; Hinck, L. Mammary stem cell self-renewal is regulated by Slit2/Robo1 signaling through SNAI1 and mINSC. Cell Rep. 2015, 13, 290–301. [Google Scholar] [CrossRef]
- Morris, J.S.; Stein, T.; Pringle, M.A.; Davies, C.R.; Weber-Hall, S.; Ferrier, R.K.; Bell, A.K.; Heath, V.J.; Gusterson, B.A. Involvement of axonal guidance proteins and their signaling partners in the developing mouse mammary gland. J. Cell Physiol. 2006, 206, 16–24. [Google Scholar] [CrossRef]
- Strickland, P.; Shin, G.C.; Plump, A.; Tessier-Lavigne, M.; Hinck, L. Slit2 and netrin 1 act synergistically as adhesive cues to generate tubular bi-layers during ductal morphogenesis. Development 2006, 133, 823–832. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Thompson, E.W.; Williams, E.D. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs 2007, 185, 7. [Google Scholar] [CrossRef]
- Chrzanowska-Wodnicka, M.; Burridge, K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996, 133, 1403–1415. [Google Scholar] [CrossRef]
- Landsverk, M.L.; Epstein, H.F. Genetic analysis of myosin II assembly and organization in model organisms. Cell. Mol. Life Sci. 2005, 62, 2270. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; de Castro, K.; Barnes, H.E.; Parks, W.T.; Stewart, L.; Böttinger, E.P.; Danielpour, D.; Wakefield, L.M. Loss of responsiveness to transforming growth factor β induces malignant transformation of nontumorigenic rat prostate epithelial cells. Cancer Res. 1999, 59, 4834. [Google Scholar] [PubMed]
- Wozniak, M.A.; Desai, R.; Solski, P.A.; Der, C.J.; Keely, P.J. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J. Cell Biol. 2003, 163, 583. [Google Scholar] [CrossRef]
- Nagy, T.; Wie, H.; Shen, T.L.; Peng, X.; Liang, C.C.; Gan, B.; Guan, J.L. Mammary epithelial-specific deletion of the focal adhesion kinase gene leads to severe lobulo-alveolar hypoplasia and secretory immaturity of the murine mammary gland. J. Biol. Chem. 2007, 282, 31766. [Google Scholar] [CrossRef]
- Van Miltenburg, M.H.; Lalai, R.; de Bont, H.; van Waaij, E.; Beggs, H.; Danen, E.H.; van de Water, B. Complete focal adhesion kinase deficiency in the mammary gland causes ductal dilation and aberrant branching morphogenesis through defects in Rho kinase-dependent cell contractility. FASEB J. 2009, 23, 3482. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871. [Google Scholar] [CrossRef]
- Revenu, C.; Gilmour, D. EMT 2.0: Shaping epithelia through collective migration. Curr. Opin. Genet. Dev. 2009, 19, 338. [Google Scholar] [CrossRef]
- Brahmbhatt, A.A.; Klemke, R.L. ERK and RhoA differentially regulate pseudopodia growth and retraction during chemotaxis. J. Biol. Chem. 2003, 278, 13016. [Google Scholar] [CrossRef]
- Webb, D.J.; Parsons, J.T.; Horwitz, A.F. Adhesion assembly, disassembly and turnover in migrating cells—Over and over and over again. Nat. Cell Biol. 2002, 4, E97. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.J.; Brenot, A.; Duong, M.; Chan, B.S.; Werb, Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell. 2008, 14, 570. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Werb, Z. Patterning mechanisms of branched organs. Science 2008, 322, 1506. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Takeichi, M. Remodeling of the adherens junctions during morphogenesis. Curr. Top Dev. Biol. 2009, 89, 33. [Google Scholar] [PubMed]
- Schedin, P.; Keely, P.J. Mammary gland ECMremodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb. Perspect. Biol. 2011, 3, a003228. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Khan, S.A.; Badve, S. Morphological changes in breast tissue with mestrual cycle. Mod. Pathol. 2002, 15, 1348–1356. [Google Scholar] [CrossRef]
- Hartmann, P.E. The breast and breast-feeding. In Scientific Foundations of Obstetrics and Gynaecology, 4th ed.; Butterworth Heinemann: Oxford, UK, 1991. [Google Scholar]
- Moffatt, D.F.; Going, J.J. Three dimensional anatomy of complete duct systems in the human breast: Pathological and developmental implications. J. Clin. Pathol. 1996, 49, 48–52. [Google Scholar] [CrossRef]
- Love, S.M.; Barsky, S.H. Anatomy of the nipple and breast ducts revisited. Cancer 2004, 101, 1947–1957. [Google Scholar] [CrossRef]
- Bannister, L.H.; Berry, M.M.; Collins, P.; Dyson, M.; Dussek, J.E. Gray’s Anatomy, 38th ed.; Churchill Livingstone: New York, NY, USA, 1995; pp. 417–424. [Google Scholar]
- Jamal, N.; Ng, K.H.; McLean, D.; Looi, L.M.; Moosa, F. Mammographic breast glandularity in malaysian women: Data derived from radiography. Am. J. Roentgenol. 2004, 182, 713–717. [Google Scholar] [CrossRef]
- Cunningham, L. The anatomy of the arteries and veins of the breast. J. Surg. Oncol. 1977, 9, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Schlenz, I.; Kuzbari, R.; Gruber, H.; Holle, J. The sensitivity of the nipple-areola complex: An anatomic study. Plast. Reconstr. Surg. 2000, 105, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Vonderhaar, B.K. Prolactin as a mitogen in mammary cells. J. Mammary Gland Biol. Neoplasia 1997, 2, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Haslam, S.Z. The ontogeny of mouse mammary gland responsiveness to ovarian steroid hormones. Endocrinology 1989, 125, 2766. [Google Scholar] [CrossRef] [PubMed]
- Lydon, J.P.; DeMayo, F.J.; Funk, C.R.; Mani, S.K.; Hughes, A.R.; Montgomery, C.A., Jr.; Shyamala, G.; Conneely, O.M.; O’Malley, B.W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995, 9, 2266. [Google Scholar] [CrossRef]
- Oakes, S.R.; Robertson, F.G.; Kench, J.G.; Gardiner-Garden, M.; Wand, M.P.; Green, J.E.; Ormandy, C.J. Loss of mammary epithelial prolactin receptor delays tumor formation by reducing cell proliferation in low-grade preinvasive lesions. Oncogene 2007, 26, 543. [Google Scholar] [CrossRef][Green Version]
- Oakes, S.R.; Rogers, R.L.; Naylor, M.J.; Ormandy, C.J. Prolactin regulation of mammary gland development. J. Mammary Gland Biol. Neoplasia 2008, 13, 13. [Google Scholar] [CrossRef]
- Ormandy, C.J.; Binart, N.; Kelly, P.A. Mammary gland development in prolactin receptor knockout mice. J. Mammary Gland Biol. Neoplasia 1997, 2, 355. [Google Scholar] [CrossRef]
- Russo, J.; Moral, R.; Balogh, G.A.; Mailo, D.; Russo, I.H. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005, 7, 131. [Google Scholar] [CrossRef]
- Blackman, B.; Russell, T.; Nordeenm, S.K.; Medina, D.; Neville, M.C. Claudin 7 expression and localization in the normal murine mammary gland and murine mammary tumors. Breast Cancer Res. 2005, 7, R248. [Google Scholar] [CrossRef]
- Rudolph, M.C.; McManaman, J.L.; Hunter, L.; Phang, T.; Neville, M.C. Functional development of the mammary gland: Use of expression profiling and trajectory clustering to reveal changes in gene expression during pregnancy, lactation, and involution. J. Mammary Gland Biol. Neoplasia 2003, 8, 287. [Google Scholar] [CrossRef]
- Hennighausen, L. The genetics and pathology of mouse mammary cancer. Semin. Cancer Biol. 2001, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.C.; Medina, C.; Monks, J.; Hovey, R.C. Editorial Commentary: The mammary fat pad. J. Mammary Gland Biol. Neoplasia 1998, 3, 109. [Google Scholar] [CrossRef] [PubMed]
- Traurig, H.H. A radioautographic study of cell proliferation in the mammary gland of the pregnant mouse. Anat. Rec. 1967, 157, 489–504. [Google Scholar] [CrossRef]
- Thoresen, M.; Wesche, J. Doppler measurements of changes in human mammary and uterine blood flow during pregnancy and lactation. Obstet. Gynecol. Scand 1988, 67, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Hennighausen, L.; Robinson, G.W. Information networks in the mammary gland. Nat. Rev. Mol. Cell Biol. 2005, 6, 715. [Google Scholar] [CrossRef]
- D’Cruz, C.M.; Moody, S.E.; Master, S.R.; Hartman, J.L.; Keiper, E.A.; Imielinski, M.B.; Cox, J.D.; Wang, J.Y.; Ha, S.I.; Keister, B.A.; et al. Persistent parity-induced changes in growth factors, TGF-β3, and differentiation in the rodent mammary gland. Mol. Endocrinol. 2002, 16, 2034. [Google Scholar] [CrossRef]
- Djonov, V.; Andres, A.C.; Ziemiecki, A. Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc. Res. Tech. 2001, 52, 182. [Google Scholar] [CrossRef]
- McCready, J.; Arendt, L.M.; Rudnick, J.A.; Kuperwasser, C. The contribution of dynamic stromal remodeling during mammary development to breast carcinogenesis. Breast Cancer Res. 2010, 12, 205. [Google Scholar] [CrossRef]
- Milanese, T.R.; Hartmann, L.C.; Sellers, T.A.; Frost, M.H.; Vierkant, R.A.; Maloney, S.D.; Shane Pankratz, V.; Degnim, A.C.; Vachon, C.M.; Reynolds, C.A.; et al. Age-related lobular involution and risk of breast cancer. J. Natl. Cancer Inst. 2006, 98, 1600. [Google Scholar] [CrossRef]
- Radisky, D.C. Defining a role for the homeoprotein Six1 in EMT and mammary tumorigenesis. J. Clin. Investig. 2009, 119, 2528. [Google Scholar] [CrossRef]
- Brisken, C.; Ayyannan, A.; Nguyen, C.; Heineman, A.; Reinhardt, F.; Tan, J.; Dey, S.K.; Dotto, G.P.; Weinberg, R.A. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev. Cell. 2002, 3, 877. [Google Scholar] [CrossRef]
- Haslam, S.Z.; Woodward, T.L. Host microenvironment in breast cancer development: Epithelial-cell-stromal-cell interactions and steroid hormone action in normal and cancerous mammary gland. Breast Cancer Res. 2003, 5, 208. [Google Scholar] [CrossRef] [PubMed]
- Streuli, C.H.; Akhtar, N. Signal co-operation between integrins and other receptor systems. Biochem. J. 2009, 418, 491. [Google Scholar] [CrossRef] [PubMed]
- Haslam, S.Z.; Levely, M.L. Estrogen responsiveness of normal mouse mammary cells in primary cell culture: Association of mammary fibroblasts with estrogenic regulation of progesterone receptors. Endocrinology 1985, 116, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, R.C.; Lydon, J.; O’Malley, B.W.; Rosen, J.M. Mammary gland development is mediated by both stromal and epithelial progesterone receptors. Mol. Endocrinol. 1997, 11, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, R.C.; Lydon, J.; O’Malley, B.W.; Rosen, J.M. Use of PRKO mice to study the role of progesterone in mammary gland development. J. Mammary Gland Biol. Neoplasia 1997, 2, 343–354. [Google Scholar] [CrossRef]
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 2000, 80, 1–29. [Google Scholar] [CrossRef]
- Brisken, C.; Park, S.; Vass, T.; Lydon, J.P.; O’Malley, B.W.; Weinberg, R.A. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl. Acad. Sci. USA 1998, 95, 5076. [Google Scholar] [CrossRef]
- Humphreys, R.C.; Hennighausen, L. Signal transducer and activator of transcription 5a influences mammary epithelial cell survival and tumorigenesis. Cell Growth Differ. 1999, 10, 685–694. [Google Scholar]
- Hiremath, M.; Lydon, J.P.; Cowin, P. The pattern of β-catenin responsiveness within the mammary gland is regulated by progesterone receptor. Development 2007, 134, 3703. [Google Scholar] [CrossRef]
- Teissedre, B.; Pinderhughes, A.; Incassati, A.; Hatsell, S.J.; Hiremath, M.; Cowin, P. MMTV-Wnt1 and -ΔN89β-catenin induce canonical signaling in distinct progenitors and differentially activate Hedgehog signaling within mammary tumors. PLoS ONE 2009, 4, e4537. [Google Scholar] [CrossRef]
- Brisken, C.; Heineman, A.; Chavarria, T.; Elenbaas, B.; Tan, J.; Dey, S.K.; McMahon, J.A.; McMahon, A.P.; Weinberg, R.A. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000, 14, 650. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C.; Kaur, S.; Chavarria, T.E.; Binart, N.; Sutherland, R.L.; Weinberg, R.A.; Kelly, P.A.; Ormandy, C.J. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev. Biol. 1999, 210, 96. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Monaco, M.E.; Kleinberg, D.L. Progesterone stimulates mammary gland ductal morphogenesis by synergizing with and enhancing insulin-like growth factor-I action. Endocrinology 2005, 146, 1170. [Google Scholar] [CrossRef]
- Schramek, D.; Leibbrandt, A.; Sigl, V.; Kenner, L.; Pospisilik, J.A.; Lee, H.J.; Hanada, R.; Joshi, P.A.; Aliprantis, A.; Glimcher, L.; et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 2010, 468, 98. [Google Scholar] [CrossRef]
- Theill, L.E.; Boyle, W.J.; Penninger, J.M. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 2002, 20, 795. [Google Scholar] [CrossRef]
- Lamberti, C.; Lin, K.M.; Yamamoto, Y.; Verma, U.; Verma, I.M.; Byers, S.; Gaynor, R.B. Regulation of β-catenin function by the IκB kinases. J. Biol. Chem. 2001, 276, 42276. [Google Scholar] [CrossRef]
- Gavin, B.J.; McMahon, A.P. Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol. Cell Biol. 1992, 12, 2418. [Google Scholar]
- Weber-Hall, S.J.; Phippard, D.J.; Niemeyer, C.C.; Dale, T.C. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation 1994, 57, 205. [Google Scholar] [CrossRef]
- Kim, Y.C.; Clark, R.J.; Pelegri, F.; Alexander, C.M. Wnt4 is not sufficient to induce lobuloalveolar mammary development. BMC Dev. Biol. 2009, 9, 55. [Google Scholar] [CrossRef]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. 2000 Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef] [PubMed]
- Hennighausen, L.; Robinson, G.W. Think globally, act locally: The making of a mouse mammary gland. Genes Dev. 1998, 12, 449. [Google Scholar] [CrossRef]
- Horseman, N.D.; Zhao, W.; Montecino-Rodriguez, E.; Tanaka, M.; Nakashima, K.; Engle, S.J.; Smith, F.; Markoff, E.; Dorshkind, K. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997, 16, 6926. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Robinson, G.W.; Wagner, K.U.; Garrett, L.; Wynshaw-Boris, A.; Hennighausen, L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997, 11, 179. [Google Scholar] [CrossRef]
- Miyoshi, K.; Cui, Y.; Riedlinger, G.; Robinson, P.; Lehoczky, J.; Zon, L.; Oka, T.; Dewar, K.; Hennighausen, L. Structure of the mouse Stat 3/5 locus: Evolution from Drosophila to zebrafish to mouse. Genomics 2001, 71, 150. [Google Scholar] [CrossRef]
- Udy, G.B.; Towers, R.P.; Snell, R.G.; Wilkins, R.J.; Park, S.H.; Ram, P.A.; Waxman, D.J.; Davey, H.W. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc. Natl. Acad. Sci. USA 1997, 94, 7239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chehab, R.; Tkalcevic, J.; Naylor, M.J.; Harris, J.; Wilson, T.J.; Tsao, S.; Tellis, I.; Zavarsek, S.; Xu, D.; et al. Elf5 is essential for early embryogenesis and mammary gland development during pregnancy and lactation. EMBO J. 2005, 24, 635. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Stanford, P.M.; Sutherland, K.; Oakes, S.R.; Naylor, M.J.; Robertson, F.G.; Blazek, K.D.; Kazlauskas, M.; Hilton, H.N.; Wittlin, S.; et al. Socs2 and elf5 mediate prolactin-induced mammary gland development. Mol. Endocrinol. 2006, 20, 1177–1187. [Google Scholar] [CrossRef]
- Kubo, M.; Hanada, T.; Yoshimura, A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003, 4, 1169. [Google Scholar] [CrossRef]
- Lindeman, G.J.; Wittlin, S.; Lada, H.; Naylor, M.J.; Santamaria, M.; Zhang, J.G.; Starr, R.; Hilton, D.J.; Alexander, W.S.; Ormandy, C.J.; et al. SOCS1 deficiency results in accelerated mammary gland development and rescues lactation in prolactin receptor-deficient mice. Genes Dev. 2001, 15, 1631. [Google Scholar] [CrossRef]
- Park, D.S.; Lee, H.; Frank, P.G.; Razani, B.; Nguyen, A.V.; Parlow, A.F.; Russell, R.G.; Hulit, J.; Pestell, R.G.; Lisanti, M.P. Caveolin-1-deficient mice show accelerated mammary gland development during pregnancy, premature lactation, and hyperactivation of the Jak-2/STAT5a signaling cascade. Mol. Biol. Cell 2002, 13, 3416. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Matsuda, M.; Hou, Z.; Bailey, J.P.; Kitazawa, R.; Herbst, M.P.; Horseman, N.D. Receptor activator of NF-κB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J. Biol. Chem. 2003, 278, 46171. [Google Scholar] [CrossRef] [PubMed]
- Fantl, V.; Stamp, G.; Andrews, A.; Rosewell, I.; Dickson, C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995, 9, 2364. [Google Scholar] [CrossRef] [PubMed]
- Sicinski, P.; Donaher, J.L.; Parker, S.B.; Li, T.; Fazeli, A.; Gardner, H.; Haslam, S.Z.; Bronson, R.T.; Elledge, S.J.; Weinberg, R.A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 1995, 82, 621. [Google Scholar] [CrossRef]
- Li, S.; Rosen, J.M. Distal regulatory elements required for rat whey acidic protein gene expression in transgenic mice. J. Biol. Chem. 1994, 269, 14235. [Google Scholar] [CrossRef]
- Pittius, C.W.; Sankaran, L.; Topper, Y.J.; Hennighausen, L. Comparison of the regulation of the whey acidic protein gene with that of a hybrid gene containing the whey acidic protein gene promoter in transgenic mice. Mol. Endocrinol. 1988, 2, 1027. [Google Scholar] [CrossRef]
- Walton, K.D.; Wagner, K.U.; Rucker, E.B., 3rd; Shillingford, J.M.; Miyoshi, K.; Hennighausen, L. Conditional deletion of the bcl-x gene from mouse mammary epithelium results in accelerated apoptosis during involution but does not compromise cell function during lactation. Mech. Dev. 2001, 109, 281. [Google Scholar] [CrossRef]
- Khaled, W.T.; Read, E.K.; Nicholson, S.E.; Baxter, F.O.; Brennan, A.J.; Came, P.J.; Sprigg, N.; McKenzie, A.N.; Watson, C.J. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell development. Development 2007, 134, 2739. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Aggeler, J.; Ram, T.G.; Bissell, M.J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 1989, 105, 223. [Google Scholar] [CrossRef]
- Katz, E.; Streuli, C.H. The extracellular matrix as an adhesion checkpoint for mammary epithelial function. Int. J. Biochem. Cell Biol. 2007, 39, 715. [Google Scholar] [CrossRef]
- Streuli, C.H.; Bissell, M.J. Expression of extracellular matrix components is regulated by substratum. J. Cell Biol. 1991, 110, 1405. [Google Scholar] [CrossRef] [PubMed]
- Streuli, C.H.; Edwards, G.M.; Delcommenne, M.; Whitelaw, C.B.; Burdon, T.G.; Schindler, C.; Watson, C.J. Stat5 as a target for regulation by extracellular matrix. J. Biol. Chem. 1995, 270, 21639. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Chakrabarti, R.; Escamilla-Hernandez, R.; Sinha, S. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: Failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev. Biol. 2009, 329, 227. [Google Scholar] [CrossRef] [PubMed]
- Lapinskas, E.J.; Palmer, J.; Ricardo, S.; Hertzog, P.J.; Hammacher, A.; Pritchard, M.A. A major site of expression of the ets transcription factor Elf5 is epithelia of exocrine glands. Histochem. Cell Biol. 2004, 122, 521. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F. Developmental Biology, 6th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2006. [Google Scholar]
- Antonova, L.; Aronson, K.; Mueller, C.R. Stress and breast cancer: From epidemiology to molecular biology. Breast Cancer Res. 2011, 13, 208. [Google Scholar] [CrossRef]
- Homo-Delarche, F.; Fitzpatrick, F.; Christeff, N.; Nunez, E.A.; Bach, J.F.; Dardenne, M. Sex steroids, glucocorticoids, stress and autoimmunity. J. Steroid Biochem. Mol. Biol. 1991, 40, 619. [Google Scholar] [CrossRef]
- VanItallie, T. Stress: A risk factor for serious illness. Metabolism 2002, 51, 40. [Google Scholar] [CrossRef]
- Majumder, P.; Joshi, J.; Banerjee, M. Correlation between nuclear glucocorticoid receptor levels and casein gene expression in murine mammary gland in vitro. J. Biol. Chem. 1983, 258, 6793. [Google Scholar] [CrossRef]
- Reichardt, H.; Horsch, K.; Grone, H.; Kolbus, A.; Beug, H.; Hynes, N.; Schutz, G. Mammary gland development and lactation are controlled by diff erent glucocorticoid receptor activities. Eur. J. Endocrinol. 2001, 145, 519. [Google Scholar] [CrossRef]
- Wintermantel, T.; Bock, D.; Fleig, V.; Greiner, E.; Schutz, G. The epithelial glucocorticoid receptor is required for the normal timing of cell proliferation during mammary lobuloalveolar development but is dispensable for milk production. Mol. Endocrinol. 2005, 19, 340. [Google Scholar] [CrossRef]
- Jones, F.E.; Welte, T.; Fu, X.Y.; Stern, D.F. ErbB4 signaling in the mammary gland is required for lobuloalveolar development and Stat5 activation during lactation. J. Cell Biol. 1999, 147, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Muraoka-Cook, R.S.; Sandahl, M.; Hunter, D.; Miraglia, L.; Earp, H.S. Prolactin and ErbB4/HER4 signaling interact via Janus kinase 2 to induce mammary epithelial cell gene expression differentiation. Mol. Endocrinol. 2008, 22, 2307–2321. [Google Scholar] [CrossRef] [PubMed]
- Ball, E.M.; Risbridger, G.P. Activins as regulators of branching morphogenesis. Dev. Biol. 2001, 238, 1. [Google Scholar] [CrossRef] [PubMed]
- Bloise, E.; Cassali, G.D.; Ferreira, M.C.; Ciarmela, P.; Petraglia, F.; Reis, F.M. Activin-related proteins in bovine mammary gland: Localization and differential expression during gestational development and differentiation. J. Dairy Sci. 2010, 93, 4592. [Google Scholar] [CrossRef] [PubMed]
- Muttukrishna, S.; Hyett, J.; Paine, M.; Moodley, J.; Groome, N.; Rodeck, C. Uterine vein and maternal urinary levels of activin A and inhibin A in pre-eclampsia patients. Clin. Endocrinol. 2006, 64, 469. [Google Scholar] [CrossRef]
- Luisi, S.; Calonaci, G.; Florio, P.; Lombardi, I.; De Felice, C.; Bagnoli, F.; Petraglia, F. Identification of activin A and follistatin in human milk. Growth Factors 2002, 20, 147. [Google Scholar] [CrossRef]
- Bussmann, U.A.; Lanuza, G.M.; Bussmann, L.E. Activin and follistatin in rat mammary gland. Mol. Cell Endocrinol. 2004, 221, 9. [Google Scholar] [CrossRef]
- Gorska, A.E.; Jensen, R.A.; Shyr, Y.; Aakre, M.E.; Bhowmick, N.A.; Moses, H.L. Transgenic mice expressing a dominant-negative mutant type II transforming growth factor-β receptor exhibit impaired mammary development and enhanced mammary tumor formation. Am. J. Pathol. 2003, 163, 1539. [Google Scholar] [CrossRef]
- Adair-Kirk, T.L.; Senior, R.M. Fragments of extracellular matrix as mediators of inflammation. Int. J. Biochem. Cell Biol. 2008, 40, 1101. [Google Scholar] [CrossRef]
- Huang, W.; Chiquet-Ehrismann, R.; Moyano, J.V.; Garcia-Pardo, A.; Orend, G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 2001, 61, 8586. [Google Scholar]
- Li, N.; Zhang, Y.; Naylor, M.J.; Schatzmann, F.; Maurer, F.; Wintermantel, T.; Schuetz, G.; Mueller, U.; Streuli, C.H.; Hynes, N.E. β1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J. 2005, 24, 1942. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Marlow, R.; Lambert, E.; Schatzmann, F.; Lowe, E.T.; Cheung, J.; Katz, E.; Li, W.; Wu, C.; Dedhar, S.; et al. Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Development 2009, 136, 1019. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Streuli, C.H. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J. Cell Biol. 2006, 173, 781. [Google Scholar] [CrossRef] [PubMed]
- Schedin, P.; O’Brien, J.; Rudolph, M.; Stein, T.; Borges, V. Microenvironment of the involuting mamJmary gland mediates mammary cancer progression. J. Mammary Gland Biol. Neoplasia 2007, 12, 71. [Google Scholar] [CrossRef]
- O’Brien, J.; Lyons, T.; Monks, J.; Lucia, M.S.; Wilson, R.S.; Hines, L.; Man, Y.G.; Borges, V.; Schedin, P. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am. J. Pathol. 2010, 176, 1241. [Google Scholar] [CrossRef]
- Humphreys, R.C.; Bierie, B.; Zhao, L.; Raz, R.; Levy, D.; Hennighausen, L. Deletion of Stat3 blocks mammary gland involution and extends functional competence of the secretory epithelium in the absence of lactogenic stimuli. Endocrinology 2002, 143, 3641. [Google Scholar] [CrossRef]
- Mailleux, A.A.; Overholtzer, M.; Schmelzle, T.; Bouillet, P.; Strasser, A.; Brugge, J.S. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev. Cell 2007, 12, 221. [Google Scholar] [CrossRef]
- Wang, N.; Kudryavtseva, E.; Chen, I.L.; McCormick, J.; Sugihara, T.M.; Ruiz, R.; Andersen, B. Expression of an engrailed-LMO4 fusion protein in mammary epithelial cells inhibits mammary gland development in mice. Oncogene 2004, 23, 1507. [Google Scholar] [CrossRef][Green Version]
- Whyte, J.; Bergin, O.; Bianchi, A.; McNally, S.; Martin, F. Key signalling nodes in mammary gland development and cancer. Mitogen-activated protein kinase signalling in experimental models of breast cancer progression and in mammary gland development. Breast Cancer Res. 2009, 11, 209. [Google Scholar] [CrossRef]
- Pearson, J.F.; Hughes, S.; Chambers, K.; Lang, S.H. Polarized fluid movement and not cell death, creates luminal spaces in adult prostate epithelium. Cell Death Differ. 2009, 16, 475. [Google Scholar] [CrossRef]
- Weir, M.L.; Oppizzi, M.L.; Henry, M.D.; Onishi, A.; Campbell, K.P.; Bissell, M.J.; Muschler, J.L. Dystroglycan loss disrupts polarity and β-casein induction in mammary epithelial cells by perturbing laminin anchoring. J. Cell Sci. 2006, 119, 4047. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, J.; Xu, R.; Mori, H.; Nelson, C.M.; Mroue, R.; Spencer, V.A.; Brownfield, D.; Radisky, D.C.; Bustamante, C.; Bissell, M.J. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 2008, 27, 2829. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.T.; Chavally-Mis, B.; Herbage, D.; Eastwood, M.; Brown, R.A. Mechanical loading regulates protease production by fibroblasts in three-dimensional collagen substrates. Wound Repair Regen. 2000, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, J.M.; Jones, J.A.; Stroud, R.E.; Mukherjee, R.; Spinale, F.G.; Ikonomidis, J.S. Differential effects of mechanical and biological stimuli on matrix metalloproteinase promoter activation in the thoracic aorta. Circulation 2009, 120, S262. [Google Scholar] [CrossRef]
- McManaman, J.L.; Neville, M.C. Mammary physiology and milk secretion. Adv. Drug Deliv. Rev. 2003, 55, 629–641. [Google Scholar] [CrossRef]
- Monks, J.; Rosner, D.; Jon Geske, F.; Lehman, L.; Hanson, L.; Neville, M.C.; Fadok, V.A. Epithelial cells as phagocytes: Apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ. 2005, 12, 107–114. [Google Scholar] [CrossRef]
- Monks, J.; Smith-Steinhart, C.; Kruk, E.R.; Fadok, V.A.; Henson, P.M. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol. Reprod. 2008, 78, 586–594. [Google Scholar] [CrossRef]
- Lim, C.L.; Yu Zuan Or, Y.Z.; Ong, Z.; Chung, H.H.; Hayashi, H. Estrogen exacerbates mammary involution through neutrophil-dependent and –independent mechanism. eLife 2020, 9, e57274. [Google Scholar] [CrossRef]
- Chapman, R.S.; Lourenco, P.; Tonner, E.; Flint, D.; Selbert, S.; Takeda, K.; Akira, S.; Clarke, A.R.; Watson, C.J. The role of Stat3 in apoptosis and mammary gland involution. Conditional deletion of Stat3. Adv. Exp. Med. Biol. 2000, 480, 129–138. [Google Scholar]
- Håkansson, A.; Andréasson, J.; Zhivotovsky, B.; Karpman, D.; Orrenius, S.; Svanborg, C. Multimeric α-lactalbumin from human milk induces apoptosis through a direct effect on cell nuclei. Exp Cell Res. 1999, 246, 451. [Google Scholar] [CrossRef]
- Håkansson, A.; Zhivotovsky, B.; Orrenius, S.; Sabharwal, H.; Svanborg, C. Apoptosis induced by a human milk protein. Proc. Natl. Acad. Sci. USA 1995, 92, 8064. [Google Scholar] [CrossRef] [PubMed]
- Kritikou, E.A.; Sharkey, A.; Abell, K.; Came, P.J.; Anderson, E.; Clarkson, R.W.; Watson, C.J. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development 2003, 130, 3459. [Google Scholar] [CrossRef] [PubMed]
- Schere-Levy, C.; Buggiano, V.; Quaglino, A.; Gattelli, A.; Cirio, M.C.; Piazzon, I.; Vanzulli, S.; Kordon, E.C. Leukemia inhibitory factor induces apoptosis of the mammary epithelial cells and participates in mouse mammary gland involution. Exp. Cell Res. 2003, 282, 35. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.S.; Lourenco, P.C.; Tonner, E.; Flint, D.J.; Selbert, S.; Takeda, K.; Akira, S.; Clarke, A.R.; Watson, C.J. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 1999, 13, 2604–2616. [Google Scholar] [CrossRef]
- Yang, Y.A.; Tang, B.; Robinson, G.; Hennighausen, L.; Brodie, S.G.; Deng, C.X.; Wakefield, L.M. Smad3 in the mammary epithelium has a nonredundant role in the induction of apoptosis, but not in the regulation of proliferation or differentiation by transforming growth factor-β. Cell Growth Differ. 2002, 13, 123. [Google Scholar]
- Roberts, A.W.; Robb, L.; Rakar, S.; Hartley, L.; Cluse, L.; Nicola, N.A.; Metcalf, D.; Hilton, D.J.; Alexander, W.S. Placental defects and embryonic lethality in mice lacking suppressor of cytokine signaling. Proc. Natl. Acad. Sci. USA 2001, 98, 9324. [Google Scholar] [CrossRef]
- Zhao, L.; Hart, S.; Cheng, J.; Melenhorst, J.J.; Bierie, B.; Ernst, M.; Stewart, C.; Schaper, F.; Heinrich, P.C.; Ullrich, A.; et al. Mammary gland remodeling depends on gp130 signaling through Stat3 and MAPK. J. Biol. Chem. 2004, 279, 44093. [Google Scholar] [CrossRef]
- Zhao, L.; Melenhorst, J.J.; Hennighausen, L. Loss of interleukin 6 results in delayed mammary gland involution: A possible role for mitogen-activated protein kinase and not signal transducer and activator of transcription. Mol. Endocrinol. 2002, 16, 2902. [Google Scholar] [CrossRef]
- Takahashi, Y.; Carpino, N.; Cross, J.C.; Torres, M.; Parganas, E.; Ihle, J.N. SOCS3: An essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. EMBO J. 2003, 22, 372–384. [Google Scholar] [CrossRef]
- Sun, H.; Miao, Z.; Zhang, X.; In Chan, U.; Su, S.M.; Guo, S.; Ho Wong, C.K.; Xiaoling, X.; Chu-Xia Deng, X. Single-cell RNA-Seq reveals cell heterogeneity and hierarchy within mouse mammary epithelia. J. Biol. Chem. 2018, 293, 8315–8329. [Google Scholar] [CrossRef]
- Chung, C.Y.; Ma, Z.; Dravis, C.; Preissl, S.; Poirion, O.; Luna, G.; Hou, X.; Giraddi, R.R.; Ren, B.; Wahl, G.M. Single-Cell Chromatin Analysis of Mammary Gland Development Reveals Cell-State Transcriptional Regulators and Lineage Relationships. Cell Rep. 2019, 29, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.; Pensa, S.; Grzelak, M.; Hadfield, J.; Adams, D.J.; Marioni, J.C.; Khaled, W.T. Differentiation dynamics of mammary epithelial cells revealed by single-cell RNA sequencing. Nat. Commun. 2017, 8, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Twigger, A.J.; Khaled, W.T. Mammary gland development from a single cell omics view. Semin. Cell Devel. Biol. 2021, 114, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, T.; Villadsen, R.; Nielsen, H.L.; Rønnov-Jessen, L.; Bissell, M.J.; Petersen, O.W. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002, 16, 693. [Google Scholar] [CrossRef] [PubMed]

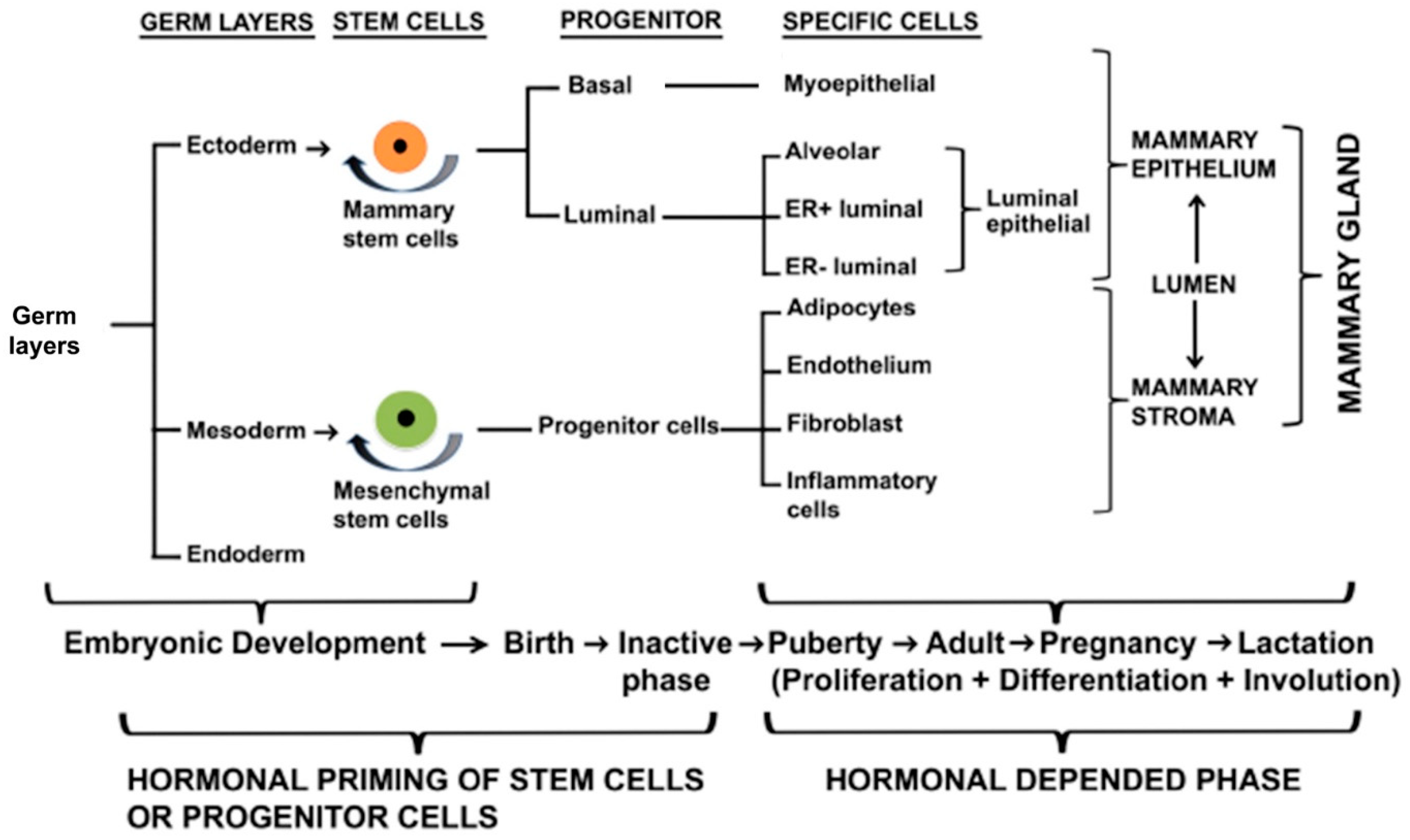
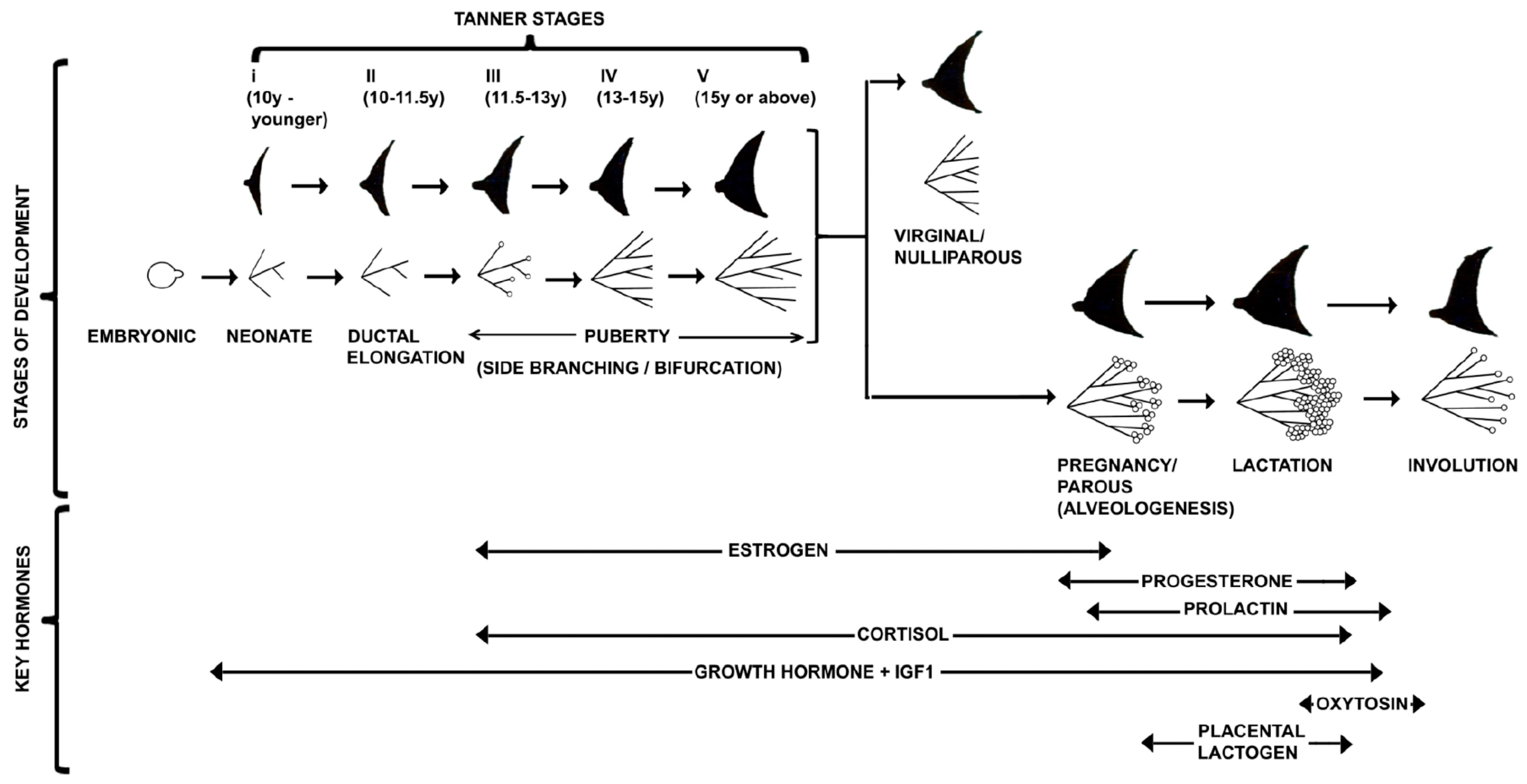
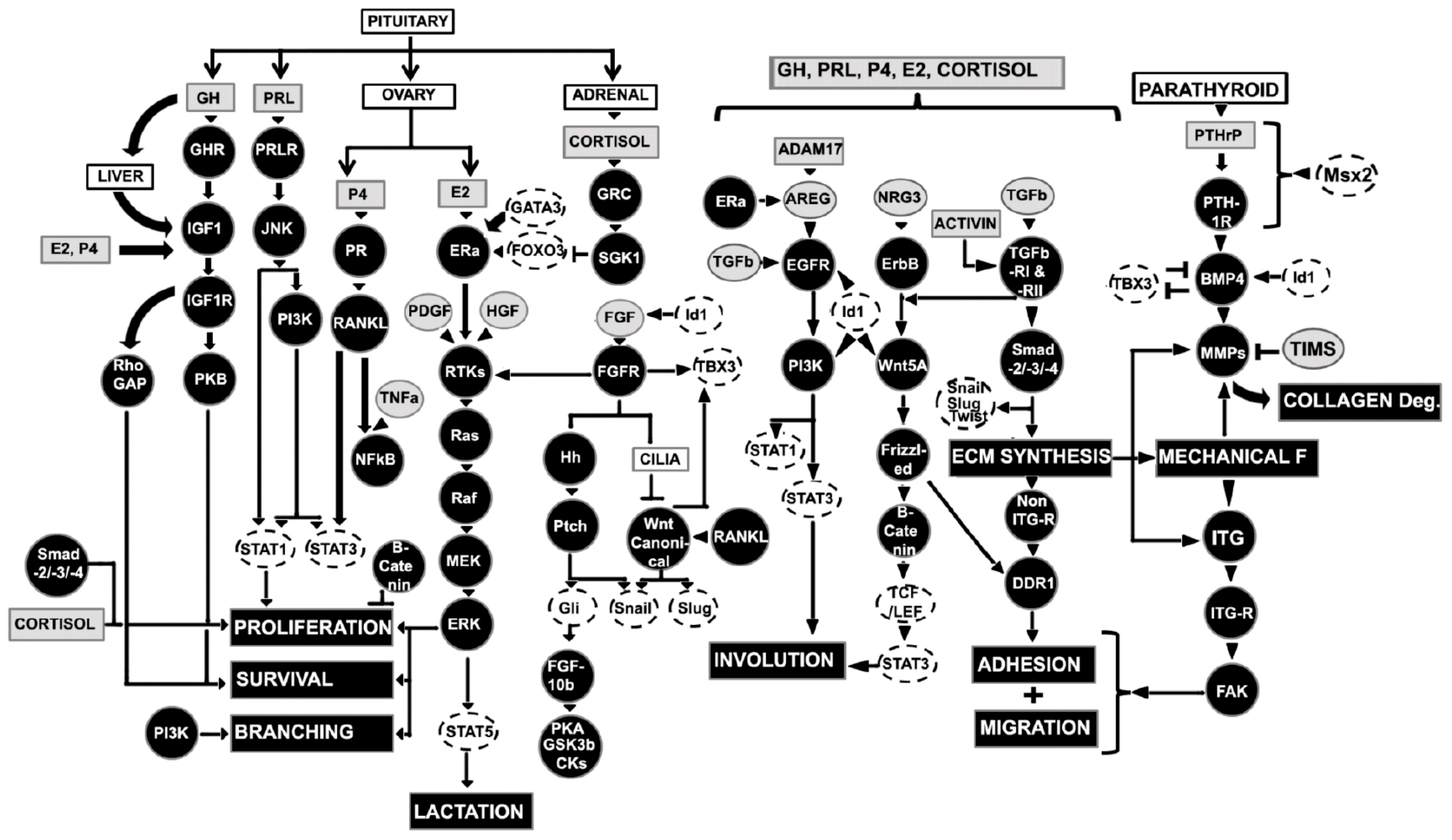
| Stage | Embryo or Fetus Size | Gestational Stage (in Week, W) |
|---|---|---|
| Ridge stage | <5 mm embryo | 4 W, around day 35 |
| Milk hill stage | >5.5 mm embryo | 4–6 W |
| Mammary disc stage | ~10–11 mm embryo | 7–8 W |
| Lobule type stage | 11–25 mm embryo | 8–9 W |
| Cone stage | 25–30 mm embryo | 9–10 W |
| Budding stage | 30–68 mm embryo | 11–12 W |
| Identification stage | 68 mm–10 cm embryo | 12–15 W |
| Branching stage | 10 cm fetus | 15–20 W |
| Canalization stage | 28 cm fetus | 22–32 W |
| End vesicle/Newborn stage (Monolayer of epithelium and contain colostrum) | 6 months |
| Stage | Age | Characteristics |
|---|---|---|
| Tanner I | 10 y or younger | Glandular tissues absent, areola follows the skin contour of chest |
| Tanner II | 10–11.5 y | Mammary bud forms with small area of surrounding glandular tissue and areola begins to widen |
| Tanner III | 11.5–13 y | Mammary begins to more elevated and extends beyond the borders of the areola, which continues to widen but remains in contour with surrounding breast |
| Tanner IV | 13–15 y | Increased mammary size and elevation, areola and papilla form a secondary mound projecting from the contour of the surrounding mammary gland |
| Tanner V | 15 y or above | Mammary reaches final adult size, areola returns to contour of the surrounding mammary with a projecting central papilla |
| Type | Women Stage | Characteristics |
|---|---|---|
| I | Virginal or Nulliparous (never given birth to a viable or live infant) | Alveolar bud cluster around a terminal duct, and terminal ducts/alveolar buds are surrounded by bilayer epithelium (~11 ductules) |
| II | Parous (given birth to a viable or live infant) | Ductules increases in number (~47 ductules), sprouting of new alveolar buds |
| III | Parous | Ductules increases in number (~80 ductules) |
| IV | Lactating | Maximal functional differentiation and development, milk secreting lobules |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, S.K.; Banerjee, S.; Baker, G.W.; Kuo, C.-Y.; Chowdhury, I. The Mammary Gland: Basic Structure and Molecular Signaling during Development. Int. J. Mol. Sci. 2022, 23, 3883. https://doi.org/10.3390/ijms23073883
Biswas SK, Banerjee S, Baker GW, Kuo C-Y, Chowdhury I. The Mammary Gland: Basic Structure and Molecular Signaling during Development. International Journal of Molecular Sciences. 2022; 23(7):3883. https://doi.org/10.3390/ijms23073883
Chicago/Turabian StyleBiswas, Swarajit Kumar, Saswati Banerjee, Ginger Wendolyn Baker, Chieh-Yin Kuo, and Indrajit Chowdhury. 2022. "The Mammary Gland: Basic Structure and Molecular Signaling during Development" International Journal of Molecular Sciences 23, no. 7: 3883. https://doi.org/10.3390/ijms23073883
APA StyleBiswas, S. K., Banerjee, S., Baker, G. W., Kuo, C.-Y., & Chowdhury, I. (2022). The Mammary Gland: Basic Structure and Molecular Signaling during Development. International Journal of Molecular Sciences, 23(7), 3883. https://doi.org/10.3390/ijms23073883






